Abstract
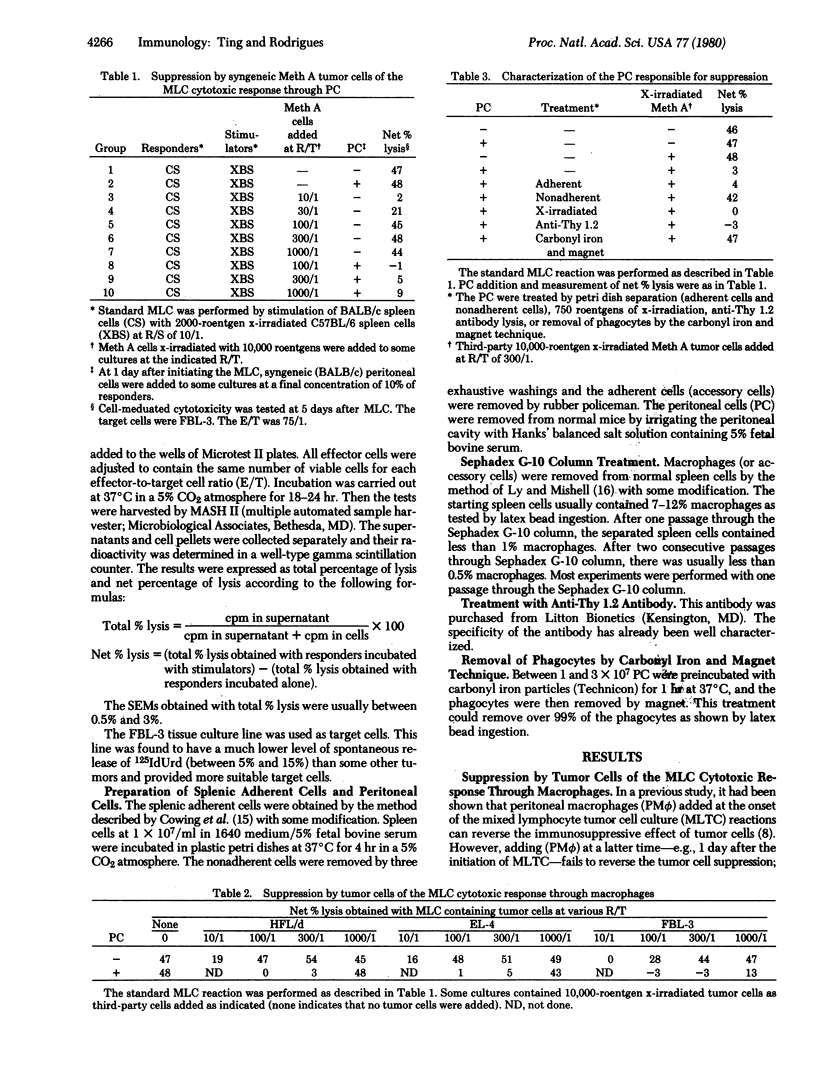
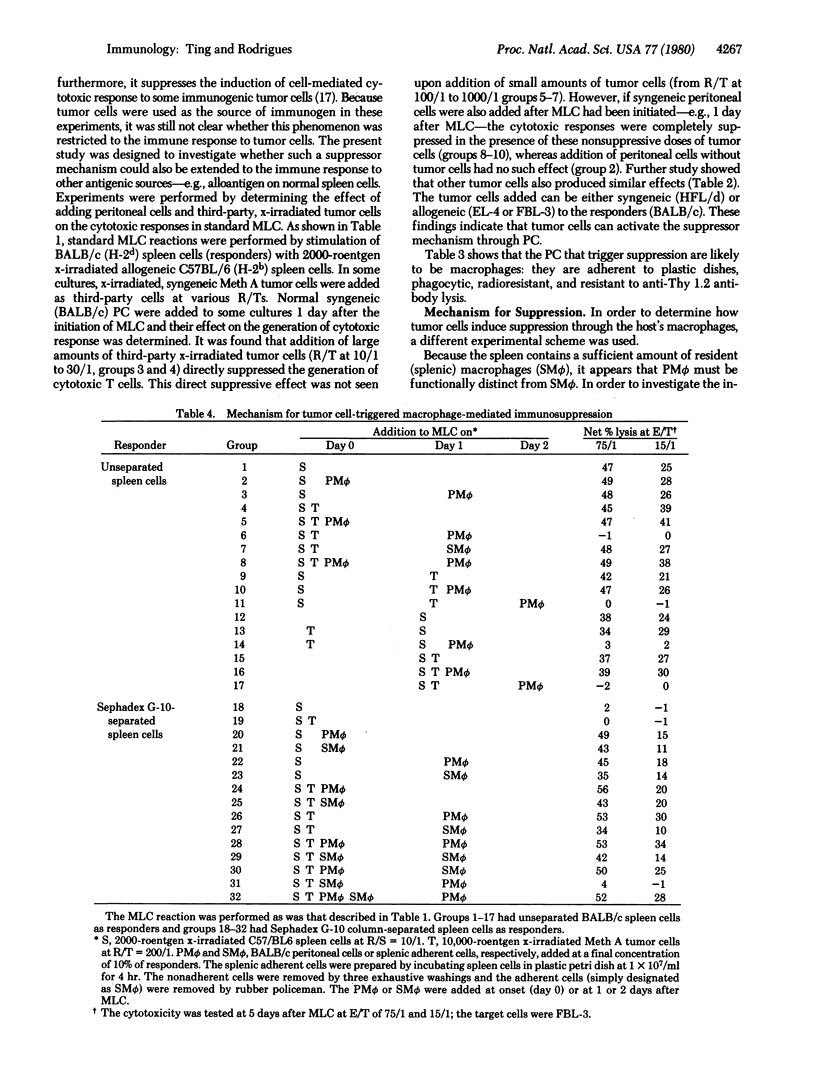
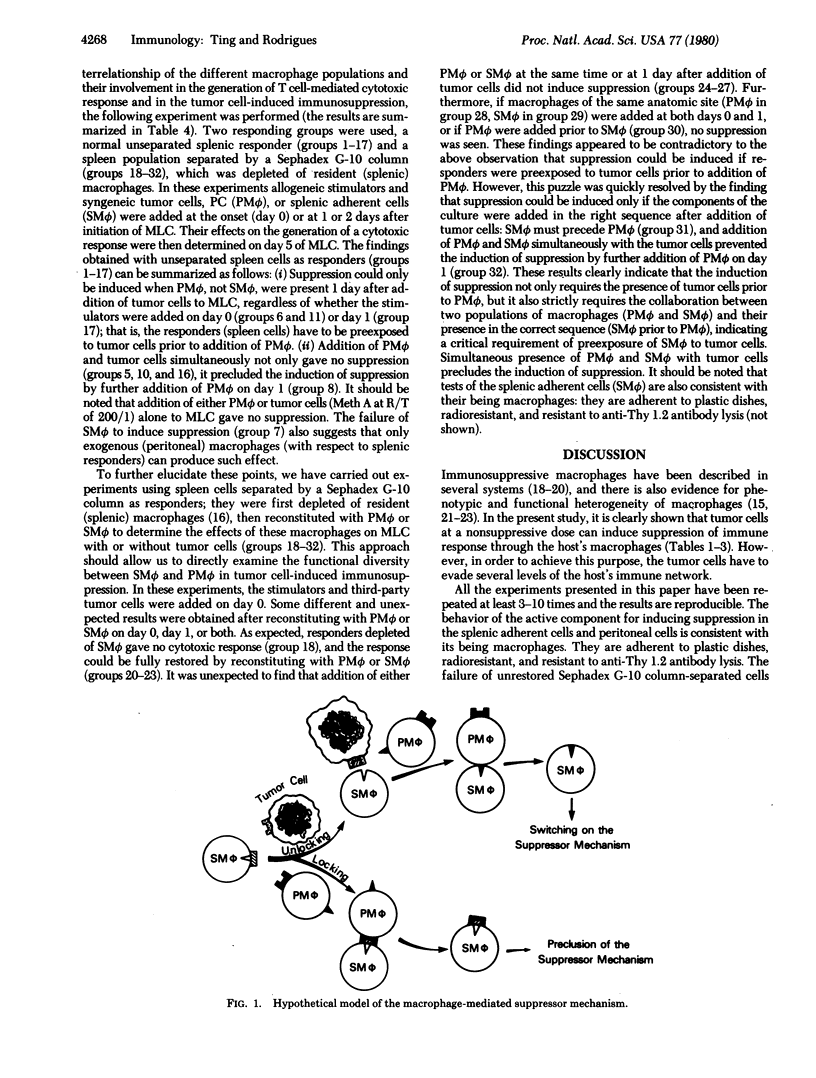
The present study demonstrates a unique mechanism for tumor cell-induced immunosuppression. In the presence of a nonsuppressive dose of tumor cells, generation of cytotoxic T cells in the mixed lymphocyte culture (MLC) is completely suppressed by adding exogenous (peritoneal) macrophages (PM phi) after the initiation of the MLC. This indicates that tumor cells can switch on a suppressor mechanism through host macrophages. It has further been determined that suppression can be induced only if resident (splenic) macrophages (SM phi) are exposed to tumor cells prior to addition of PM phi. If SM phi and PM phi are simultaneously present with the tumor cells, induction of suppression is completely precluded. These findings indicate that switching on of the suppressor mechanism by tumor cells has a critical requirement for the collaboration of two populations of macrophages, SM phi and PM phi, and their presence in a specific sequence (SM phi preceding PM phi). This may represent one of the mechanisms by which tumor cells evade host immune surveillance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R. Games parasites play: how parasites evade immune surveillance. Nature. 1979 May 3;279(5708):21–26. doi: 10.1038/279021a0. [DOI] [PubMed] [Google Scholar]
- Cantor H., Hugenberger J., McVay-Boudreau L., Eardley D. D., Kemp J., Shen F. W., Gershon R. K. Immunoregulatory circuits among T-cell sets. Identification of a subpopulation of T-helper cells that induces feedback inhibition. J Exp Med. 1978 Oct 1;148(4):871–877. doi: 10.1084/jem.148.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K. M., Elgert K. D. Concentration-dependent role of macrophages in mixed lymphocyte reaction regulation: elaboration of nondialyzable heat-stable inhibitor and heat-labile enhancing factors. J Reticuloendothel Soc. 1979 Mar;25(3):243–253. [PubMed] [Google Scholar]
- Cowing C., Pincus S. H., Sachs D. H., Dickler H. B. A subpopulation of adherent accessory cells bearing both I-A and I-E or C subregion antigens is required for antigen-specific murine T lymphocyte proliferation. J Immunol. 1978 Nov;121(5):1680–1686. [PubMed] [Google Scholar]
- Fernbach B. R., Kirchner H., Herberman R. B. Inhibition of the mixed lymphocyte culture by peritoneal exudate cells. Cell Immunol. 1976 Mar 15;22(2):399–403. doi: 10.1016/0008-8749(76)90043-5. [DOI] [PubMed] [Google Scholar]
- Freedman H. A., Lilly F. Properties of cell lines derived from tumors induced by Friend virus in BALB/c and BALB/c-H-2b mice. J Exp Med. 1975 Jul 1;142(1):212–223. doi: 10.1084/jem.142.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn J. P., McCoy J. L., Fefer A. Cross-resistance to the transplantation of syngeneic Friend, Moloney, and Rauscher virus-induced tumors. Cancer Res. 1968 Mar;28(3):434–439. [PubMed] [Google Scholar]
- Gorczynski R. M. Control of the immune response: role of macrophages in regulation of antibody-and cell-mediated immune responses. Scand J Immunol. 1976;5(9):1031–1047. doi: 10.1111/j.1365-3083.1976.tb03055.x. [DOI] [PubMed] [Google Scholar]
- Igarashi T., Rodrigues D., Ting C. C. Studies of the mechanisms for the induction of in vivo tumor immunity. IV. Enhancement of the in vitro generation of secondary cell-mediated cytotoxic response by normal peritoneal macrophages and their culture supernatants. J Immunol. 1979 Apr;122(4):1519–1527. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Monroy A., Rosati F. The evolution of the cell--cell recognition system. Nature. 1979 Mar 8;278(5700):165–166. doi: 10.1038/278165a0. [DOI] [PubMed] [Google Scholar]
- Montfort I., Pérez Tamayo R. Two antigenically different types of macrophages. Proc Soc Exp Biol Med. 1971 Oct;138(1):204–207. doi: 10.3181/00379727-138-35862. [DOI] [PubMed] [Google Scholar]
- Nabholz M., Vives J., Young H. M., Meo T., Miggiano V., Rijnbeek A., Shreffler D. C. Cell-mediated cell lysis in vitro: genetic control of killer cell production and target specificities in the mouse. Eur J Immunol. 1974 May;4(5):378–387. doi: 10.1002/eji.1830040514. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Herberman R. B., Campbell D. A., Jr, Djeu J. Y. Inhibition of rat mixed lymphocyte cultures by suppressor macrophages. Cell Immunol. 1977 Mar 15;29(2):238–250. doi: 10.1016/0008-8749(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Stutman O. Immunodepression and malignancy. Adv Cancer Res. 1975;22:261–422. doi: 10.1016/s0065-230x(08)60179-7. [DOI] [PubMed] [Google Scholar]
- Ting C-C, Bushar G. S., Rodrigues D., Herberman R. B. Cell-mediated immunity to Friend virus-induced leukemia. I. Modification of 125IUdR release cytotoxicity assay for use with suspension target cells. J Immunol. 1975 Nov;115(5):1351–1356. [PubMed] [Google Scholar]
- Ting C. C., Rodrigues D., Igarashi T. Studies of the mechanisms for the induction of in vivo tumor immunity. III. Recruitment of host helper cells by donor T cells in adoptive transfer of cell-mediated immunity. J Immunol. 1979 Apr;122(4):1510–1518. [PubMed] [Google Scholar]
- Ting C. C., Rodrigues D. Immunoregulatory circuit among macrophage subsets for T cell-mediated cytotoxic response to tumor cells. J Immunol. 1980 Mar;124(3):1039–1044. [PubMed] [Google Scholar]
- Ting C. C., Rodrigues D. Reversal by peritoneal adherent cells of tumor cell suppression of T cell-mediated immunity. J Immunol. 1979 Aug;123(2):801–807. [PubMed] [Google Scholar]
- Ting C. C., Rodrigues D., Ting R. C., Wivel N., Collins M. J. Suppression of T cell-mediated immunity by tumor cells: immunogenicity versus immunosuppression and preliminary characterization of the suppressive factors. Int J Cancer. 1979 Nov 15;24(5):644–655. doi: 10.1002/ijc.2910240519. [DOI] [PubMed] [Google Scholar]
- Ting C. C., Tsai S. C., Rogers M. J. Host control of tumor growth. Science. 1977 Aug 5;197(4303):571–573. doi: 10.1126/science.267328. [DOI] [PubMed] [Google Scholar]



