Abstract
Adult autoimmune enteropathy (AIE) is a rare cause of malabsorption syndrome unresponsive to dietary restriction. Its diagnostic hallmarks are small-bowel villous atrophy and antienterocyte autoantibodies. Therapy is based mainly on nutritional support and immunosuppression. We treated a 61-year-old woman with corticosteroid-refractory AIE and life-threatening malabsorption syndrome with systemic infusions of autologous, bone marrow–derived, mesenchymal stromal cells (MSCs) as rescue therapy. The MSCs were expanded ex vivo following a previously used Good Manufacturing Practice procedure, and 2 intravenous infusions of 1.8 × 106 MSCs/kg body weight were administered 2 weeks apart. Analysis of circulating and mucosal regulatory T-and B-cell numbers, and of serum and secretory immunoglobulin levels, was performed before and after treatment. The MSC infusions were safe and effective, leading to disappearance of disease hallmarks and recovery from the life-threatening condition. Increases in mucosal regulatory T-cell numbers and secretory immunoglobulin levels were also observed. The benefit, however, was transient, and a further MSC infusion resulted in the same short efficacy. This case encourages the use of MSCs to treat patients with life-threatening, corticosteroid-refractory AIE and suggests that MSC infusion can attenuate, albeit transiently, the autoimmune attack.
Abbreviations and Acronyms: AIE, autoimmune enteropathy; FoxP3, forkhead box P3; MSC, mesenchymal stromal cell; sIgA, secretory immunoglobulin A
Cell-based therapy has gained attention for the cure of autoimmune diseases, with encouraging results obtained with the use of mesenchymal stromal cells (MSCs).1 These cells first attracted interest for their easy isolation and ex vivo expansion, their ability to undergo multilineage differentiation, and their lack of immunogenicity.1 More recently, they were reported to exert multifaceted action in vitro on the cells involved in the immune response, with the ultimate effect of dampening inflammation, although contradictory results have been obtained when MSCs were used in vivo.1
Adult autoimmune enteropathy (AIE) is a rare disorder characterized by the presence of severe malabsorption syndrome, unresponsive to dietary restriction, whose diagnostic hallmarks are the positivity of antienterocyte autoantibodies and the presence of villous atrophy with inflammatory infiltration of the small bowel mucosa, indistinguishable from that of celiac disease.2 Although the understanding of the pathogenesis of AIE has greatly improved in recent years,3,4 treatment is still not standardized, being mainly based on immunosuppressive or biological therapy and parenteral nutrition.5 In view of the successful use of MSCs for the treatment of AIE in a mouse model of multiorgan autoimmunity,6 we used this treatment as rescue therapy in a patient with AIE and life-threatening malabsorption syndrome; we also performed ancillary immunologic studies to gain insights into the mechanisms at the basis of MSC efficacy in vivo.
Case Report
In March 2009, a 61-year-old woman was hospitalized for severe malabsorption syndrome due to chronic diarrhea lasting 2 years. Findings from stool examinations for occult blood and pathogens were negative; findings from lower endoscopy were unremarkable, whereas upper endoscopy with biopsy showed villous atrophy and inflammatory infiltrate of the duodenal mucosa. Although the results of serologic screening for celiac disease (the search for antiendomysium and anti–tissue transglutaminase antibodies) proved to be negative, with normal levels of IgA, the patient was prescribed a gluten-free diet, assuming that she had seronegative celiac disease, and then was discharged. During the following 3 months, no clinical amelioration was observed but rather a worsening of diarrhea with the appearance of anasarca, which led to rehospitalization. Total parenteral nutrition was started, together with a course of antibiotic therapy (metronidazole and ciprofloxacin) following the suggestion of an unidentified infectious agent. Because of lack of clinical improvement, corticosteroid therapy (prednisone, 25 mg/d) was begun, and the patient was referred to the Department of Internal Medicine at the Istituto Di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo Foundation (Pavia, Italy) in November 2009 for evaluation for suspected refractory celiac disease.7 At that time, severe malnutrition was evident (body mass index of 14 [calculated as weight in kilograms divided by height in meters squared]), coupled with physical and laboratory signs of malabsorption, as confirmed by the D-xylose test (3 mg/dL; reference value, ≥30 mg/dL). The initial diagnosis of celiac disease and adherence to a gluten-free diet were reviewed, together with the search for those human leukocyte antigen (HLA) class II alleles known to be associated with genetic susceptibility, and serologic tests were performed to screen for other autoimmune conditions. The HLA genotyping showed the presence of the HLA-DQ2 haplotype (HLA-DQA1*0501-HLA-DQB1*0201), whereas all autoantibody tests yielded negative results. Histologic examination of new well-oriented duodenal biopsy specimens confirmed the presence of hyperplastic villous atrophy in the absence of lymphangiectasia, subepithelial collagenous membrane, and eosinophilic/lymphocytic or mast cell infiltrate. Wireless capsule endoscopy showed loss of intestinal folds and scalloping of the mucosa with visualization of the underlying vascular pattern up to the terminal ileum. After other causes of villous atrophy were ruled out, including drugs, infectious and toxic agents, food intolerance, and primary or secondary immunodeficiency, refractory sprue was suspected.7 At this point, immunophenotyping of intraepithelial and circulating lymphocytes and 18F-fluorodeoxyglucose positron emission tomography aimed at excluding the presence of diffuse T-cell lymphoma were performed, and the findings were negative.7 Therefore, AIE was investigated and confirmed by the presence of antienterocyte autoantibodies (Figure 1, A).2 Since hospital admission, total parenteral nutrition, albumin supplementation, and corticosteroid therapy (methylprednisolone, 40 mg/d intravenously) had been given to maintain stable vital signs and electrolyte balance because of the persistence of abundant liquid stools. Treatment with autologous MSCs was, therefore, proposed as rescue therapy and was approved by the local bioethics committee. After the patient had signed a written informed consent form, bone marrow–derived MSCs were isolated and expanded ex vivo following a previously reported protocol8 that took 4 weeks; 2 × 108 MSCs that satisfied the release criteria for clinical use were obtained. Two intravenous infusions of 1.8 × 106 MSCs/kg body weight were then administered 2 weeks apart; the cell dose was chosen in view of the clinical experience obtained using MSCs for corticosteroid-refractory intestinal acute graft vs host disease.8 In the interval between the 2 MSC infusions, corticosteroid therapy and parenteral nutrition were tapered and then discontinued on the day of the second infusion. Monitoring of clinical and serologic variables was scheduled monthly, and duodenal biopsy was scheduled 1, 3, and 6 months after the second MSC infusion. In addition, peripheral blood and saliva samples were collected before MSC treatment and at each scheduled visit to evaluate lymphocyte populations and secretory IgA (sIgA), respectively. Immunophenotyping of peripheral blood and mucosal lymphocytes was performed by flow cytometry (FACSCanto; BD Biosciences, San Diego, CA) and immunohistochemical analysis, respectively, following standard procedures. T cells expressing the forkhead box P3 (FoxP3) transcription factor were enumerated in peripheral blood, as previously reported,9 and at the mucosal level by using monoclonal antibodies against FoxP3 (Clone 236A/E7; BD Biosciences) and CD25 (Clone 4C9; Novocastra, Newcastle, UK) on serial sections according to the manufacturers' instructions. Differential counts of positive cells were estimated by a blinded pathologist and are expressed as a percentage of lamina propria mononuclear cells. Finally, sIgA levels were determined in saliva samples by electroimmunodiffusion assay, as previously described.10
FIGURE 1.
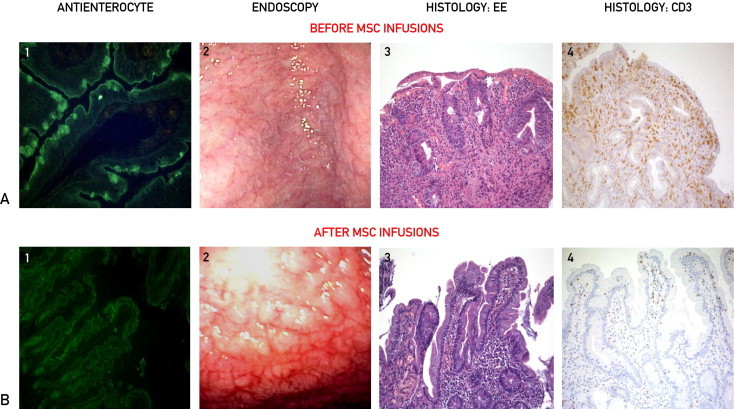
Serologic, endoscopic, and histologic features before (A) and after (B) 2 intravenous infusions of autologous bone marrow–derived mesenchymal stromal cells (MSCs) 2 weeks apart. 1, Immunofluorescent pattern of class A antienterocyte autoantibodies showing the strong positivity (A) (original magnification ×25) and negativity (B) (original magnification ×25) of the brush border on cryostat section of monkey jejunum. 2, Endoscopic appearance of duodenal mucosa before MSC infusions (A) showing flattening of intestinal folds with visualization of the underlying vascular pattern and after MSC infusions (B) showing the presence of islets of normal duodenal mucosa. 3, Histologic features of duodenal mucosa before MSC treatment (A) showing villous atrophy with crypt hyperplasia and heavy inflammatory infiltrate and after MSC treatment (B) indicating the recovery of normal architecture and disappearance of the infiltrate (hematoxylin-eosin, original magnification ×100). 4, Immunohistochemical characterization of the inflammatory infiltrate with anti-CD3 antibody (Clone PS1; Novocastra, Newcastle, UK) showing the presence of a heavy lymphocytic infiltrate at the lamina propria and epithelium levels before MSC infusions (A), which returned to normal levels after MSC infusions (B) (original magnification ×100).
Patient Outcome
No adverse event was recorded during MSC infusion or in the following 22 months. A few days after the second infusion, the patient experienced a dramatic amelioration of her clinical condition and regularization of stool frequency, permitting her hospital discharge within 1 week. One month later, enterocyte autoantibodies were no longer detectable, and patchy recovery of duodenal mucosa at endoscopic and histologic examination was clearly evident (Figure 1, B), together with normalization of her nutritional status (body mass index of 20). A parallel improvement in absorptive function (xylosemia, 14 mg/dL) was also observed. However, 1 month later, the recurrence of malabsorption syndrome led to rehospitalization, during which we found weak positivity of enterocyte autoantibodies and worsening of absorptive test values (xylosemia, 5 mg/dL) and duodenal mucosa at endoscopic and histologic examination (not shown). An additional MSC infusion was performed using the same cell dose, and a few days later, a significant reduction in daily stool frequency was again recorded. However, this improvement also was transient; indeed, after 1 month, the recurrence of clinical, serologic, and histologic features of AIE required the reestablishment of total parenteral nutrition and corticosteroid therapy (prednisone, 40 mg/d). In view of a slow but progressive recovery of her general condition, parenteral nutrition was discontinued after 3 months, and prednisone therapy was initially tapered and then switched to budesonide therapy due to severe osteoporosis because this treatment had proved effective in another patient with AIE.11 This satisfactory clinical condition has continued, with persistent negativity of antienterocyte autoantibodies, improvement in D-xylose test values (25 mg/dL), and patchy recovery of duodenal mucosa as assessed after 6 and 12 months (not shown).
Regarding the immunologic findings, the analysis of peripheral T cells showed a consistent decrease in the percentage of cells expressing FoxP3, the master switch for the function of regulatory T cells,12 1 month after the first 2 MSC infusions and, although to a lesser extent, after the third infusion, which quickly returned to baseline levels and remained stable throughout follow-up (Figure 2, A). No modification of the percentage of other circulating T-cell subsets was found. In parallel, although FoxP3+CD25+ cells were almost undetectable at the mucosal level before treatment, these cells became detectable (2%) 1 month after the first 2 MSC infusions (Figure 2, B) but were no longer found at each subsequent evaluation. The levels of sIgA were found to be triple the preinfusion levels 1 month after the second MSC infusion, although they quickly returned to baseline values (Figure 2, C). In contrast, the levels of serum immunoglobulins showed an opposite pattern (Figure 2, D), and the percentage of peripheral B lymphocytes (18% before MSC infusion) decreased 1 month after both MSC treatments (to 8% and 3%, respectively) and remained unchanged at 14% throughout follow-up. At the mucosal level, the percentage of plasma cells increased as early as 1 month after both MSC treatments (60% and 70%, respectively, vs 40% before treatment), returning to baseline levels at the time of disease recurrence (30%), and remained stable during follow-up (40%).
FIGURE 2.
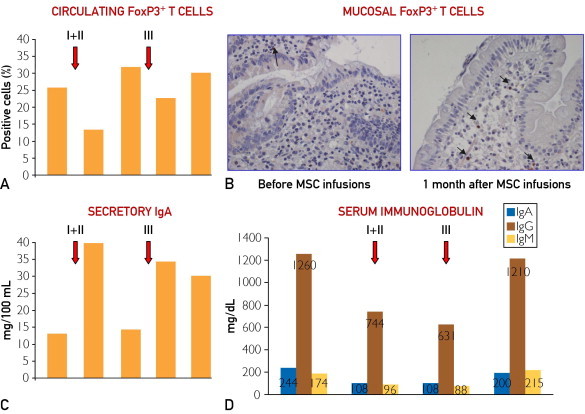
Immunologic findings. A, Flow cytometric assessment of circulating forkhead box P3 (FoxP3)+ T cells expressed as a percentage of CD4+CD25bright lymphocytes before and 1 and 3 months after mesenchymal stromal cell (MSC) infusions, indicated as “I+II” and “III.” B, Immunohistochemical detection of FoxP3+ T cells on duodenal mucosa before (left) and a month after (right) the first MSC treatment when an increase in the number of positive cells (those with brown nuclei, as indicated by black arrows) is evident (immunoperoxidase, hematoxylin counterstain, original magnification ×200). C, Quantification of secretory immunoglobulin A before and 1 and 3 months after MSC infusions, indicated as “I+II” and “III.” D, Quantification of serum IgA, IgG, and IgM before MSC treatment, 1 month after the 2 MSC treatments (indicated as “I+II” and “III”), and after another 6 months. In A, C, and D, the red arrows indicate the times of both MSC treatments consisting of 2 intravenous infusions (I+II) 2 weeks apart (first arrow) and 1 intravenous infusion (III) (second arrow).
Discussion
In the past decade, MSCs have been proposed and used as a new therapeutic strategy in immune-mediated disorders.13 Regarding chronic inflammatory bowel disease, we have already successfully treated fistulizing Crohn disease refractory to conventional treatments with local injections of autologous bone marrow–derived MSCs.14 After a benefit was observed in a mouse model of multiorgan autoimmunity,6 we used MSC infusions as described herein as rescue therapy in a patient with life-threatening malabsorption syndrome due to corticosteroid-refractory AIE. Ex vivo expansion of the patient's bone marrow–derived MSCs was feasible, and their infusion was safe. Although we cannot exclude a delayed effect of corticosteroid therapy on disease manifestations, the positive evolution of the clinical picture and the disappearance of disease hallmarks after MSC therapy point to the efficacy of this treatment. However, the benefit was transient, raising concerns about how long MSCs can maintain their immunologic activity in vivo and which mechanisms are involved.15 In this regard, studies on intestinal graft vs host disease showed that MSCs are beneficial when infused at the time of full-blown disease.8,15 This therapeutic window likely depends on the presence of an inflammatory milieu that attracts more MSCs and licenses them to exert their tolerogenic and immunosuppressive functions.16 The recruitment of other immune cells may contribute to the therapeutic effect of MSCs, thus suggesting that MSC persistence is not a crucial prerequisite for their efficacy.16 In this regard, in vitro studies demonstrated that MSCs favor the expansion of T cells with regulatory phenotype and down-regulate expansion of activated CD8+ T cells and natural killer cells.9 The present in vivo results are consistent with this experimental evidence since a mucosal enrichment, although transient, of FoxP3+ T cells after MSC infusions was observed. However, the simultaneous depletion of this cell population in peripheral blood suggests that MSCs direct these cells to the site of tissue injury rather than favoring their expansion. Moreover, we cannot rule out that these cells are effectory T cells because the main markers for the identification of regulatory T cells, CD25 and FoxP3, are also expressed by activated T cells and can be altered in inflammatory conditions.17
Remarkably, MSCs also modulate B-cell function, leading to inhibition of their activation, proliferation, and immunoglobulin secretion.18 When used in vivo, disappearance of pathogenic antibodies in an experimental model of autoimmune encephalomyelitis19 and resolution of a case of antibody-mediated autoimmune thrombocytopenic purpura refractory to conventional therapies20 have been reported. In the present patient, we observed increases, although transient, in intestinal plasma cell numbers and sIg levels with MSC infusions associated with (1) decreased circulating B cells, (2) decreased serum immunoglobulin levels, and (3) disappearance of pathogenic antibodies. Altogether, these findings seem to confirm the ability of MSCs to modulate B-cell function in vivo and to attenuate the autoimmune attack.
Finally, regarding the transient efficacy of MSCs, the ability of interleukin 2–activated natural killer and cytotoxic T cells to kill MSCs1 could account for the lack of sustained benefit. Another open issue that deserves further investigation concerns MSC interaction with immunomodulatory drugs. In the present patient, MSC infusion seemed to be able to restore the response to corticosteroid therapy, which had proved ineffective before MSC treatment.
Conclusion
These data suggest that MSC infusions are safe and useful in the short term for treating AIE, mainly when refractory to current therapies. The increase in regulatory T-cell and plasma cell numbers in the gut mucosa may play a role in silencing the pathogenic pathways leading to autoantibody production and tissue injury. However, further in vivo studies are needed to explain the lack of long-lasting benefits and to define the optimal conditions for the use of MSCs as immunotherapy.
Acknowledgments
We thank Dr Paola Bianchi for detection of antienterocyte antibody, Dr Sergio Rutella for critical reading of the manuscript, and Mrs Susan West for careful revision of the English language.
Footnotes
Grant Support: This study was supported in part by grants from Fondazione Celiachia Italia (“Studio di possibili fattori ambientali e sviluppo di nuove strategie terapeutiche nelle complicanze della malattia celiaca”) (G.R.C); Ministero dell'Istruzione, dell'Università e della Ricerca, Progetti di Rilevante Interesse Nazionale; Associazione Italiana per la Ricerca sul Cancro; Ospedale Pediatrico Bambino Gesù, Roma; and the Associazione Italiana per la Ricerca sul Cancro special project “5 per mille” (F.L.).
References
- 1.Bernardo M.E., Locatelli F., Fibbe W.E. Mesenchymal stromal cells: a novel treatment modality for tissue repair. Ann N Y Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 2.Corazza G.R., Biagi F., Volta U., Andreani M.L., De Franceschi L., Gasbarrini G. Autoimmune enteropathy and villous atrophy in adults. Lancet. 1997;350(9071):106–109. doi: 10.1016/S0140-6736(97)01042-8. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi I., Imamura K., Kubota M. Identification of an autoimmune enteropathy-related 75-kilodalton antigen. Gastroenterology. 1999;117(4):823–830. doi: 10.1016/s0016-5085(99)70340-9. [DOI] [PubMed] [Google Scholar]
- 4.Ciccocioppo R., D'Alò S., Di Sabatino A. Mechanisms of villous atrophy in autoimmune enteropathy and celiac disease. Clin Exp Immunol. 2002;128(1):88–93. doi: 10.1046/j.1365-2249.2002.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akram S., Murray J.A., Pardi D.S. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007;5(11):1282–1290. doi: 10.1016/j.cgh.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekkedan B., Tilles A.W., Yarmush M.L. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008;26:1913–1919. doi: 10.1634/stemcells.2007-0790. [DOI] [PubMed] [Google Scholar]
- 7.Verbeek W.H.M., Schreurs M.W.J., Visser O., von Blomberg M.E., Al-Toma A., Mulder C.J.J. Novel approaches in the management of refractory celiac disease. Expert Rev Clin Immunol. 2008;4:205–219. doi: 10.1586/1744666X.4.2.205. [DOI] [PubMed] [Google Scholar]
- 8.Le Blanc K., Frassoni F., Ball L., Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 9.Maccario R., Podestà M., Moretta A. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD34+ T cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90(4):516–525. [PubMed] [Google Scholar]
- 10.Plebani A., Mia E., Mevio E. IgM and IgD concentrations in the serum and secretions of children with selective IgA deficiency. Clin Exp Immunol. 1983;53(3):689–696. [PMC free article] [PubMed] [Google Scholar]
- 11.Daum S., Ipczynski R., Heine B., Schulzke J.D., Aeitz M., Ullrich R. Therapy with budesonide in patients with refractory sprue. Digestion. 2006;73(1):60–68. doi: 10.1159/000092639. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Dazzi F., Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract Res Clin Haematol. 2011;24(1):49–57. doi: 10.1016/j.beha.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Ciccocioppo R., Bernardo M.E., Sgarella A. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60(6):788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 15.Polchert D., Sobinski J., Douglas G. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38(6):1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uccelli A., Prockop D.J. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr Opin Immunol. 2010;22(6):768–774. doi: 10.1016/j.coi.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Ioan-Facsinay A., van der Voort E.I., Huizinga T.W., Toes R.E. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37(1):129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 18.Corcione A., Benvenuto F., Ferretti E. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 19.Gerdoni E., Gallo B., Casazza S. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61(3):219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 20.Fang B., Song Y.P., Li N., Li J., Han Q., Zhao R.C. Resolution of refractory chronic autoimmune thrombocytopenic purpura following mesenchymal stem cell transplantation: a case report. Transplant Proc. 2009;41(5):1827–1830. doi: 10.1016/j.transproceed.2008.12.031. [DOI] [PubMed] [Google Scholar]


