Abstract
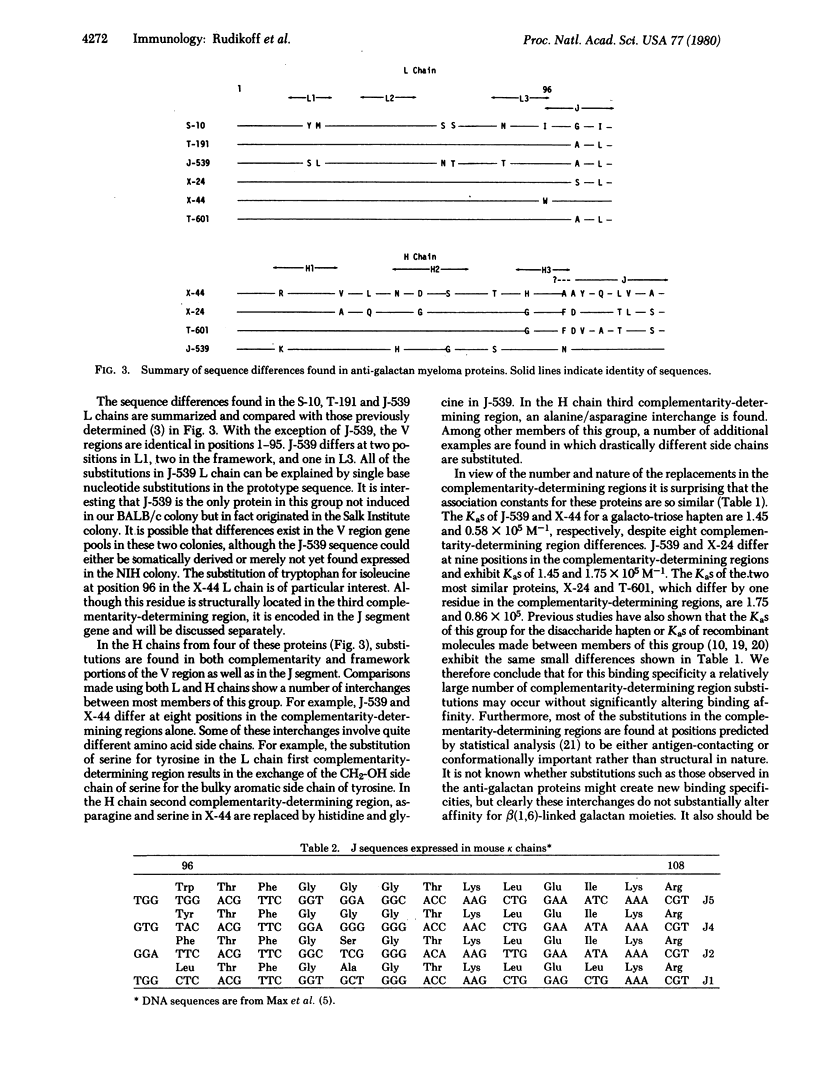
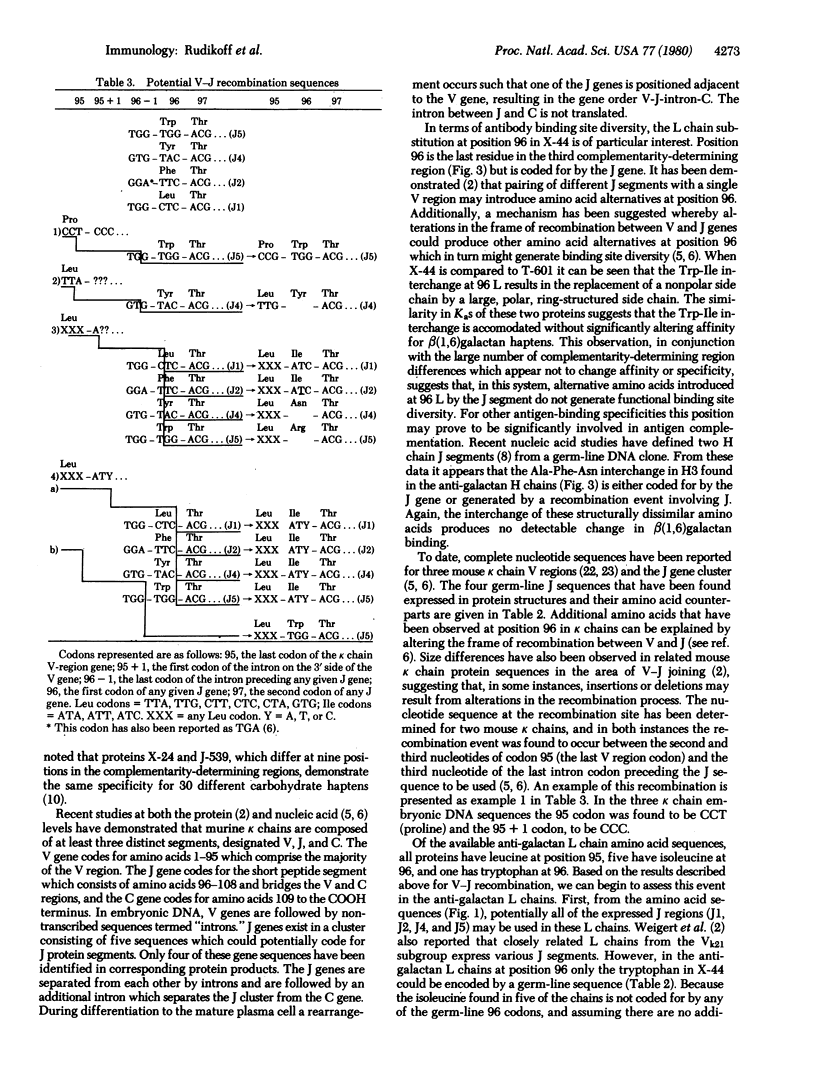
Immunoglobulin kappa light chains are coded for by at least three distinct gene segments designated variable, joining, and constant. The joining gene codes for the 13 amino acid segment linking the variable and constant regions. This peptide includes the last amino acid (96) in the third complementarity-determining region and thus could introduce structural diversity. We have determined the light chain variable region sequences from three myeloma proteins with beta(1,6)galactan-binding specificity, bringing to six the number of light chains sequenced from proteins demonstrating this specificity. Five of these have isoleucine at position 96 and the sixth tryptophan. This substitution appears to be accommodated with no significant change in association constant for a beta(1,6)galactan hapten. Additionally, as many as nine substitutions are found in both light and heavy chain complementarity-determining regions between members of this group although only minimal variations in hapten binding affinity are observed. The isoleucine found at position 96 in five of the kappa chains could not be coded for by any of the joining gene nucleotide sequences previously observed and would require a novel nucleotide sequence at the recombination site between variable and joining genes to produce the observed protein structure. Alternatively, there may exist joining gene segments not yet detected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M., Smith E. L. Action of staphylococcal proteinase on peptides of varying chain length and composition. Biochem Biophys Res Commun. 1976 Sep 20;72(2):411–417. doi: 10.1016/s0006-291x(76)80058-7. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Hengartner H., Meo T., Müller E. Assignment of genes for immunoglobulin kappa and heavy chains to chromosomes 6 and 12 in mouse. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4494–4498. doi: 10.1073/pnas.75.9.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley M. E., Glaudemans C. P., Rudikoff S., Potter M. Structural requirements for the binding of derivatives of D-galactose to two homogeneous murine immunoglobulins. Biochemistry. 1974 Jul 16;13(15):3179–3184. doi: 10.1021/bi00712a028. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Rudikoff S., Potter M., Glaudemans C. P. Spectral changes on binding of oligosaccharides to murine immunoglobulin A myeloma proteins. Biochemistry. 1973 Jul 31;12(16):3039–3044. doi: 10.1021/bi00740a015. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T., Bilofsky H. Unusual distributions of amino acids in complementarity-determining (hypervariable) segments of heavy and light chains of immunoglobulins and their possible roles in specificity of antibody-combining sites. J Biol Chem. 1977 Oct 10;252(19):6609–6616. [PubMed] [Google Scholar]
- Manjula B. N., Glaudemans C. P., Mushinski E. B., Potter M. Subunit interactions in mouse myeloma proteins with anti-galactan activity. Proc Natl Acad Sci U S A. 1976 Mar;73(3):932–936. doi: 10.1073/pnas.73.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Mushinski E. B., Glaudemans C. P. The formation of active hybrid immunoglobulins from the heavy and light chains of beta(1, 6) D-galactan binding murine myeloma IgA's S10 and J539. J Immunol. 1977 Sep;119(3):867–871. [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushinski E. B., Potter M. Idiotypes on galactan binding myeloma proteins and anti-galactan antibodies in mice. J Immunol. 1977 Dec;119(6):1888–1893. [PubMed] [Google Scholar]
- Rao D. N., Rudikoff S., Krutzsch H., Potter M. Structural evidence for independent joining region gene in immunoglobulin heavy chains from anti-galactan myeloma proteins and its potential role in generating diversity in complementarity-determining regions. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2890–2894. doi: 10.1073/pnas.76.6.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D. N., Rudikoff S., Potter M. k Chain variable regions from three galactan binding myeloma proteins. Biochemistry. 1978 Dec 12;17(25):5555–5559. doi: 10.1021/bi00618a035. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Mushinski E. B., Potter M., Glaudemans C. P., Jolley M. E. Six BALB-c IgA myeloma proteins that bind beta-(1-6)-D-galactan. Partial amino acid sequences and idiotypes. J Exp Med. 1973 Nov 1;138(5):1095–1105. doi: 10.1084/jem.138.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Schilling J., Clevinger B., Davie J. M., Hood L. Amino acid sequence of homogeneous antibodies to dextran and DNA rearrangements in heavy chain V-region gene segments. Nature. 1980 Jan 3;283(5742):35–40. doi: 10.1038/283035a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder A., Nau M., Norman B., Leder P. Antibody diversity. Science. 1978 Oct 6;202(4363):11–17. doi: 10.1126/science.99815. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Weigert M., Gatmaitan L., Loh E., Schilling J., Hood L. Rearrangement of genetic information may produce immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):785–790. doi: 10.1038/276785a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]


