Abstract
Objective
To prospectively assess the safety and effectiveness of the investigational phosphodiesterase 5 inhibitor avanafil to treat erectile dysfunction in men with diabetes mellitus.
Patients and Methods
This 12-week, multicenter, double-blind, placebo-controlled study conducted between December 15, 2008, and February 11, 2010, randomized 390 men with diabetes and erectile dysfunction 1:1:1 to receive avanafil, 100 mg (n=129), avanafil, 200 mg (n=131), or placebo (n=130). Coprimary end points assessed changes in the percentage of sexual attempts in which men were able to maintain an erection of sufficient duration to have successful intercourse (Sexual Encounter Profile [SEP] 3), percentage of sexual attempts in which men were able to insert the penis into the partner's vagina (SEP 2), and International Index of Erectile Function erectile function domain score.
Results
Compared with placebo, least-squares mean change from baseline to study end in SEP 3, SEP 2, and International Index of Erectile Function erectile function domain score were significantly improved with both avanafil, 100 mg (P≤.002), and avanafil, 200 mg (P<.001). Additional analyses indicated that successful intercourse could be initiated in 15 minutes or less through more than 6 hours after avanafil dosing. Adverse events most commonly reported with avanafil treatment were headache, nasopharyngitis, flushing, and sinus congestion.
Conclusion
Avanafil was safe and effective for treating erectile dysfunction in men with diabetes and was effective as early as 15 minutes and more than 6 hours after dosing. The adverse events seen with avanafil were similar to those seen with other phosphodiesterase 5 inhibitors.
Trial Registration
clinicaltrials.gov Identifier NCT00809471.
Abbreviations and Acronyms: AE, adverse event; BP, blood pressure; ED, erectile dysfunction; IIEF-EF, International Index of Erectile Function erectile function; LS, least square; PDE, phosphodiesterase; SEP, Sexual Encounter Profile
Diagnosed and undiagnosed diabetes mellitus is estimated to affect approximately 25.8 million people in the United States 20 years or older, including approximately 13.0 million men.1 Complications of diabetes include heart disease, stroke, hypertension, neuropathy, and erectile dysfunction (ED).1,2 Erectile dysfunction, a consistent or recurrent inability to attain and/or maintain penile erection sufficient for sexual performance,3 affects approximately 18 million men in the United States.4 The prevalence of ED in men with diabetes has been reported to be as high as 71%5 and commonly presents within 10 years of diabetes diagnosis.6 Men with diabetes have a significantly greater prevalence of ED, and onset of ED generally occurs at an earlier age compared with men in the general population.4,5 Erectile dysfunction is positively correlated with greater duration and severity of diabetes and poor glycemic control.5,7-9
The pathophysiology of diabetic ED is multifactorial, making treatment of ED in men with diabetes challenging.6,10 Factors that contribute to the condition include advanced glycation end products and increased oxygen free radicals, impaired synthesis of nitric oxide and its effector molecule cyclic guanosine monophosphate, increased endothelin and endothelin-B receptor-binding sites, an up-regulated RhoA/Rho-kinase pathway, neuropathic damage, and impaired cyclic guanosine monophosphate–dependent protein kinase.6,11-13 Furthermore, the end-organ damage secondary to hyperglycemia can exacerbate symptoms of ED, as can comorbidities associated with diabetes, such as atherosclerosis,14 and adverse events (AEs) associated with such medications as antihypertensives.11
In addition to correcting modifiable risk factors for atherosclerotic vascular disease, treatment of ED in patients with diabetes is with phosphodiesterase type 5 (PDE5) inhibitors, such as sildenafil citrate, tadalafil, and vardenafil hydrochloride.7,8,15-19 These agents act to potentiate the intracellular effects of nitric oxide release by inhibiting the degradation of cyclic guanosine monophosphate by PDE5, providing a more sustained erection.20
Avanafil, an investigational PDE5 inhibitor, is highly selective for PDE5 and highly potent, with a 50% inhibitory concentration of 0.0043 to 0.0052 μM. The 50% inhibitory concentrations for PDE5 of other PDE5 inhibitors include 0.0035 to 0.0085 μM for sildenafil, 0.0009 to 0.0067 μM for tadalafil, and 0.0001 to 0.0007 μM for vardenafil.21 Avanafil also has relatively little cross-reactivity with other PDE isoenzymes.16 In an in vitro receptor-binding study comparing the inhibitory effects of avanafil on 11 PDE isozymes with those of sildenafil, vardenafil, and tadalafil, avanafil potently inhibited PDE5 activity without significant inhibition of other PDE isozymes. In contrast, sildenafil, vardenafil, and tadalafil produced inhibitory activity for other PDE isozymes (PDE1, PDE6, and PDE11; unpublished data). These differences in PDE isoenzyme activity are important given that greater selectivity for the PDE5 isoenzyme may diminish the potential for AEs (relating to inhibition of other PDE isoenzymes; eg, inhibition of PDE6 by other PDE5 inhibitors may lead to visual disturbances).21,22
In single-dose pharmacokinetic studies, avanafil had a time to maximum plasma drug concentration of 30 to 45 minutes and a half-life of approximately 3 to 5 hours. Other PDE5 inhibitors achieve time to maximum plasma drug concentration at approximately 60 (sildenafil and vardenafil) to 120 (tadalafil) minutes after dosing.23-25 Phase 2 studies that assessed penile tumescence and rigidity in response to visual stimulation in men with ED found that avanafil, 50, 100, and 200 mg, was statistically superior to placebo, with peak effects occurring 20 to 40 minutes after dosing, consistent with the drug's pharmacokinetic profile.26,27 No drug accumulation was observed in multiple-dose pharmacokinetic studies that evaluated once- and twice-daily dosing with avanafil for up to 2 weeks. As with other PDE5 inhibitors, with the exception of tadalafil, absorption of avanafil is reduced after a high-fat meal (maximum plasma concentration is reduced in the range of 20%-39% for PDE5 inhibitors; this reduction is considered of minimal clinical significance).24-27 A previous phase 3 study reported the safety and effectiveness of avanafil in a population of men with ED, which excluded men with diabetes.28 The aim of the present study was to evaluate the safety and effectiveness of avanafil in the treatment of ED in men with diabetes mellitus.
Patients and Methods
Study Design
This randomized, double-blind, placebo-controlled, multicenter study was conducted at 39 clinical sites across the United States between December 15, 2008, and February 11, 2010. After initial screening, eligible men completed a 4-week run-in period during which baseline data on sexual function were collected. At the end of the run-in period, men with a 50% or greater failure rate in maintaining an erection of sufficient duration to permit successful intercourse and an International Index of Erectile Function erectile function (IIEF-EF) domain score of 5 through 25 who had made at least 4 attempts at intercourse during those 4 weeks were randomized 1:1:1 to receive avanafil, 100 mg, avanafil, 200 mg, or placebo.
Randomization was stratified on the basis of baseline severity of ED as determined by IIEF-EF domain score (mild, IIEF-EF score of 17-25; moderate, IIEF-EF score of 11-16; severe, IIEF-EF score ≤10), using a centralized, computer-generated randomization system. Site personnel contacted an interactive telephone and Web response system for the randomization treatment assignment. Investigators and study participants were masked to treatment assignment.
After the run-in period, randomized men entered a 12-week treatment period with follow-up visits at 4, 8, and 12 weeks. The men were instructed to take the study drug approximately 30 minutes before initiation of sexual activity and were permitted up to 2 doses in a 24-hour period, provided that the doses were separated by at least 12 hours. There were no restrictions on the intake of food or alcohol, and concurrent α-blocker use was also permitted. The men were required to answer questions on the dosing and timing of sexual activity, as well as specific questions from the Sexual Encounter Profile (SEP) in diary entries. The men also completed the IIEF questionnaire, a validated, self-administered, 15-item (5-domain) instrument designed to assess erectile function,29 at each study visit.
The study included men 18 years or older with documented type 1 or 2 diabetes and at least a 6-month history of mild to severe ED. The men were required to have been in a monogamous, heterosexual relationship for 3 months or longer and to agree to make at least 4 attempts at intercourse per month. Key exclusion criteria included uncontrolled diabetes (hemoglobin A1c >9%; to convert to a proportion of 1.0, multiply by 0.01), ED that resulted from spinal cord injury or radical prostatectomy, blood glucose level greater than 270 mg/dL (to convert to mmol/L, multiply by 0.0555), and untreated hypogonadism. Other disqualifiers included a prostate-specific antigen level greater than 4 ng/mL (to convert to μg/L, multiply by 1.0); clinically significant cardiac, hepatic, or renal disease; orthostatic hypotension; or uncontrolled hypertension or hypotension. Also excluded were men with an allergy or hypersensitivity to a PDE5 inhibitor or a history of consistent treatment failure or dose-limiting AEs during therapy with other PDE5 inhibitors. Previous or current antiandrogen therapy and the use of organic nitrates or any drugs known to inhibit the activity of cytochrome P450 3A4 within 28 days of initiation of therapy or during the study were not permitted.
The study was conducted in adherence with the principles of the Declaration of Helsinki, International Conference on Harmonisation Guidelines on Good Clinical Practice, and applicable US requirements. All men provided informed consent. Each investigator site was required to obtain institutional review board approval for the study protocol, informed consent form, and recruitment materials.
Efficacy End Points
The efficacy of treatment was assessed by 3 coprimary end points: (1) the change in the percentage of sexual attempts in which men were able to maintain an erection of sufficient duration to have successful intercourse (SEP 3) between the run-in and treatment periods, (2) the change in the percentage of sexual attempts in which the men were able to insert the penis into the partner's vagina (SEP 2) between the run-in and treatment periods, and (3) the change in the IIEF-EF domain score from baseline to the end of treatment.
Key secondary efficacy end points included the change in response to individual diary questions between run-in and the treatment period and other domains from the IIEF between baseline and the end of treatment. Planned subgroup analyses were performed based on baseline ED severity, duration of ED, type of diabetes, race, and duration of diabetes. Exploratory analyses included an assessment of SEP 3 and SEP 2 at various postdose time points and the percentage of men whose IIEF-EF domain score normalized (score of ≥26) during treatment.
Safety End Points
The safety population consisted of all men who took at least 1 dose of study drug and had safety data available. Safety was assessed by an evaluation of AEs and vital signs at each study visit; electrocardiogram, laboratory values, and physical examination results were evaluated at screening and the final study visit.
Statistical Analyses
All statistical procedures were completed using SAS statistical software, version 8.2 or higher (SAS Institute Inc, Cary, NC). The primary analysis population for efficacy end points was the intent-to-treat population, consisting of all men who were randomized, received 1 or more doses of study drug, and had 1 or more postdose efficacy assessments. Analyses of the primary efficacy variables were performed using an analysis of covariance model, with treatment and severity category as factors and baseline values as covariates; the difference of least-square (LS) means and 2-sided P values were derived from the analysis of covariance model for each comparison. For end points based on the IIEF data, the last observation carried forward convention was used; observed data were used for other analyses. A step-down procedure was used to compare the efficacy of each avanafil dose group with placebo. Subgroup interaction P values were derived using the same analysis of covariance model previously described, with additional terms for subgroup and treatment-by-subgroup interaction.
Using the average standard deviation for the same 3 coprimary efficacy end points from a previous phase 2 study, we determined the required sample size of each treatment group to be 125 men. This number provided a greater than 90% power to detect a mean difference of 13% by a 2-tailed t test, with a 5% type I error for the SEP 2 end point; a greater than 90% power to detect a mean difference of 29% between treatment groups for the SEP 3 end point; and a greater than 90% power to detect a mean difference of 5 points in ED domain score between 2 treatment groups.
Results
Of the 1378 men screened, 390 were randomized to treatment, with 129 receiving avanafil, 100 mg, 131 receiving avanafil, 200 mg, and 130 receiving placebo (Figure 1). Exclusionary laboratory test results accounted for most of the screening failures. Patient baseline characteristics were similar for all 3 treatment groups (Table 1). Most of the patients had type 2 diabetes (349/390 [89.5%]; randomized set) and presented with moderate or severe ED (365/390 [78.2%]; randomized set). Overall, patients had been diagnosed as having diabetes for approximately 11.3 years (randomized set). Most patients (239/388 [61.6%]; safety set) were taking concomitant medications to control blood pressure (BP), and 24 (6.2%; safety set) reported use of α-blockers (Table 1). In total, 224 patients (57.4%; randomized set) had dyslipidemia (including hypercholesterolemia, hyperlipidemia, and hypertriglyceridemia) at baseline. Most patients (292/388 [75.3%]; safety set) reported previous use of oral ED therapies.
FIGURE 1.
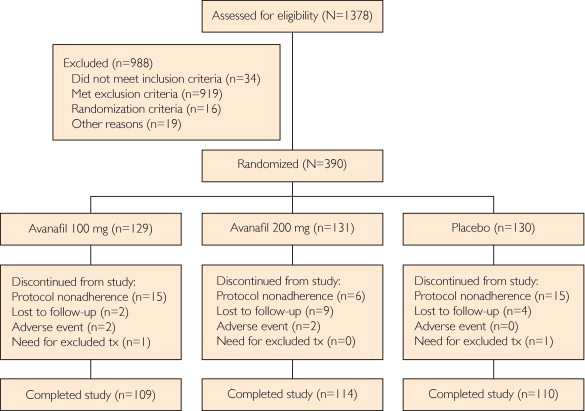
Patient disposition in the study. In total, 1378 patients were enrolled in the study and 390 were assigned randomly to treatment. Of the 390 randomized patients, 333 completed the study and 57 discontinued participation. tx = treatment.
TABLE 1.
Demographic and Baseline Characteristics of the Randomized Populationa
| Characteristic | Placebo (n=130) | Avanafil, 100 mg (n=129) | Avanafil, 200 mg (n=131) | Total (N=390) |
|---|---|---|---|---|
| Mean (SD) age (y) | 58.2 (8.6) | 58.2 (9.6) | 57.5 (9.0) | 58.0 (9.1) |
| Mean (SD) weight (kg) | 100.0 (19.9) | 98.6 (18.2) | 99.6 (18.7) | 99.4 (18.9) |
| Mean (SD) BMI (kg/m2) | 31.5 (5.9) | 31.3 (5.4) | 31.8 (5.5) | 31.5 (5.6) |
| No. (%) by race | ||||
| White | 103 (79.2) | 111 (86.0) | 100 (76.3) | 314 (80.5) |
| Black | 24 (18.5) | 16 (12.4) | 27 (20.6) | 67 (17.2) |
| Asian | 1 (0.8) | 2 (1.6) | 3 (2.3) | 6 (1.5) |
| Multiple | 1 (0.8) | 0 | 1 (0.8) | 2 (0.5) |
| Unknown | 1 (0.8) | 0 | 0 | 1 (0.3) |
| No. (%) by ED severityb | ||||
| Mild | 29 (22.3) | 28 (21.7) | 28 (21.4) | 85 (21.8) |
| Moderate | 40 (30.8) | 40 (31.0) | 42 (32.1) | 122 (31.3) |
| Severe | 61 (46.9) | 61 (47.3) | 61 (46.6) | 183 (46.9) |
| Mean (SD) ED duration (mo) | 78.7 (66.6) | 73.8 (53.1) | 64.6 (44.7) | 72.3 (55.7) |
| No. (%) by type of diabetes | ||||
| Type 1 | 14 (10.8) | 15 (11.6) | 12 (9.2) | 41 (10.5) |
| Type 2 | 116 (89.2) | 114 (88.4) | 119 (90.8) | 349 (89.5) |
| Mean (SD) diabetes duration (y) | 11.1 (8.7) | 11.2 (9.2) | 11.6 (10.4) | 11.3 (9.4) |
| No. (%) by diabetes duration category (y) | ||||
| <2 | 14 (10.8) | 13 (10.1) | 9 (6.9) | 36 (9.2) |
| ≥2 and <5 | 24 (18.5) | 30 (23.3) | 29 (22.1) | 83 (21.3) |
| ≥5 and <10 | 27 (20.8) | 30 (23.3) | 36 (27.5) | 93 (23.8) |
| ≥10 | 65 (50.0) | 56 (43.4) | 57 (43.5) | 178 (45.6) |
| No. (%) by history of hypertensionc | 86 (66.2) | 87 (68.5) | 87 (66.4) | 260 (67.0) |
| No. (%) by history of CADc | 20 (15.4) | 14 (11.0) | 20 (15.3) | 54 (13.9) |
| No. (%) by antihypertensive usec,d | 70 (53.8) | 87 (68.5) | 82 (62.6) | 239 (61.6) |
| No. (%) by α-blocker usec,d | 3 (2.3) | 13 (10.2) | 8 (6.1) | 24 (6.2) |
| Dyslipidemia at baseline | 68 (52.3) | 77 (59.7) | 79 (60.3) | 224 (57.4) |
BMI = body mass index; CAD = coronary artery disease; ED = erectile dysfunction.
Defined by International Index of Erectile Function erectile function domain score (mild, score of 17-25; moderate, score of 11-16; and severe, score ≤10).
On the basis of the safety population (n=388). Two men in the avanafil, 100 mg, group were missing values.
Concomitant medications were summarized according to Anatomical Therapeutic Chemical classifications.
Efficacy
From baseline to the treatment period, the percentage of sexual attempts in which patients were able to maintain an erection of sufficient duration for successful intercourse (SEP 3) was significantly improved after treatment with avanafil (100 and 200 mg; LS mean change for both doses, P<.001 vs placebo). On average, 20.5% of sexual attempts in patients receiving placebo resulted in successful intercourse compared with 34.4% and 40.0% of attempts in patients treated with avanafil, 100 and 200 mg, respectively. Patients receiving placebo experienced a LS mean increase from baseline to the treatment period of 13.6% in the percentage of successful intercourse attempts compared with increases of 28.7% and 34.0% for avanafil, 100 and 200 mg, respectively (P<.001 for both doses vs placebo and P<.001 for all 3 treatment arms vs baseline; Figure 2, A). The difference in SEP 3 efficacy observed between the avanafil, 100 and 200 mg, groups was not significant.
FIGURE 2.
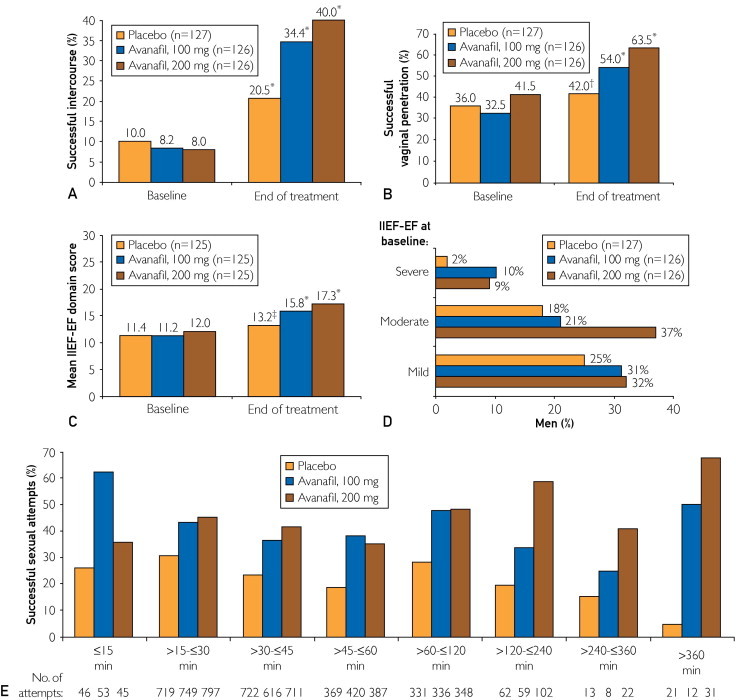
Effect of treatment between baseline and the treatment period on Sexual Encounter Profile (SEP) 3 (intent-to-treat [ITT] population) (A) and SEP 2 (ITT population) (B) and from baseline to the end of treatment on International Index of Erectile Function erectile function (IIEF-EF) domain score (ITT population) (C), normalization of IIEF-EF domain score (≥26) (ITT population) (D), and percentage of successful sexual attempts (SEP 3) over time after dosing (ITT last observation carried forward population) (E). *P<.001 vs baseline; †P=.009 vs baseline; ‡P=.007 vs baseline.
Avanafil treatment also resulted in a significant improvement between baseline and the treatment period in the percentage of sexual attempts in which patients were able to insert their penis into the partner's vagina (SEP 2). Patients taking avanafil, 100 and 200 mg, experienced a 21.5% and 25.9% LS mean increase in sexual attempts with successful vaginal penetration, respectively, whereas there was a 7.5% LS mean increase in successful penetration attempts in the placebo group (P<.001 and P<.001 for avanafil, 100 and 200 mg, vs placebo, respectively). Both avanafil treatment arms (P<.001) and the placebo arm (P=.009) were also significant vs baseline for the treatment period (Figure 2, B). As before, the difference between treatment with avanafil, 100 and 200 mg, was not significant.
At end of treatment, the IIEF-EF domain scores were 13.2, 15.8, and 17.3 for placebo, avanafil, 100 mg, and avanafil, 200 mg, respectively. The LS mean change in IIEF-EF domain scores between baseline and end of treatment averaged 1.8, 4.5, and 5.4 for placebo, avanafil, 100 mg, and avanafil, 200 mg, respectively, with avanafil treatments providing significantly greater improvement when compared with placebo (P=.002 and P<.001 for avanafil, 100 and 200 mg, respectively; Figure 2, C). Consistent with other end points, the change in IIEF-EF domain score was not significantly different between the 2 avanafil treatment groups (P=.34). At the end of treatment, a numerically higher proportion of men who were receiving avanafil vs placebo had normalized IIEF-EF domain scores (≥26) for all baseline severity groups (Figure 2, D). In addition, significant improvements in the individual IIEF domain scores of orgasmic function (P=.003 and P=.002 for avanafil, 100 and 200 mg, vs placebo, respectively), intercourse satisfaction (P=.02 and P=.001 for avanafil, 100 and 200 mg, vs placebo, respectively), and overall satisfaction (P=.001 and P<.001 for avanafil, 100 and 200 mg, vs placebo, respectively) were observed for both avanafil treatment groups compared with placebo.
An exploratory analysis conducted to assess the number of successful intercourse attempts during various postdose periods revealed a numerically higher success rate for each interval tested in the avanafil treatment groups vs placebo (Figure 2, E). In total, 5634 of 6979 attempts (80.7%) were made within 1 hour of study drug dosing. For attempts initiated at 15 minutes or less after dosing, 33 of 53 (62.3%) and 16 of 45 (35.6%) were successful in men treated with avanafil, 100 and 200 mg, respectively, compared with 12 of 46 placebo-treated men (26.1%). When intercourse was attempted more than 6 hours after dosing, 6 of 12 attempts (50.0%) and 21 of 31 attempts (67.7%) were successful after treatment with avanafil, 100 and 200 mg, respectively, compared with 1 of 21 attempts (4.8%) in the placebo group. Similar results were observed for SEP 2 and for responses to diary questions regarding satisfaction with sexual experience.
The beneficial effects of avanafil treatment on all 3 coprimary efficacy end points were consistently observed regardless of diabetes type, diabetes duration, baseline ED severity, and duration of ED. However, the subgroup of men with type 1 diabetes was of insufficient sample size to make meaningful comparisons with the type 2 diabetes subgroup. The magnitude of the treatment effect was numerically greater in men with a longer duration of ED (≥60 months) than in those with a shorter duration of ED (<60 months) for SEP 2 and IIEF-EF scores. Although treatment with avanafil, 200 mg, resulted in similar improvements in the percentage of successful intercourse attempts for both white and black subgroups (34.9% and 29.3%, respectively; both P<.001 vs baseline), the small sample size of black men does not allow for definitive conclusions about potential differences in treatment effect between these groups.
Safety
Treatment with avanafil was safe and generally well tolerated. Overall, 86 of the 258 men (33.3%) treated with avanafil and 31 of the 130 men (23.8%) treated with placebo reported 1 or more treatment-emergent AEs (Table 2). Events that occurred in 2% or more of any treatment group included headache, nasopharyngitis, flushing, sinus congestion, back pain, sinusitis, dyspepsia, and influenza (Table 2). All drug-related AEs were mild to moderate in severity. Of the 57 men (14.6%) who discontinued participation in the study, 4 (1.0%) discontinued because of AEs. There were no reports of priapism, hemodynamic changes, or major cardiac events. One patient discontinued because of unstable angina; however, the investigators determined the event to be unrelated to study drug. One patient in the avanafil, 200 mg, group reported blurred vision. Eight patients reported serious AEs, which included spinal compression fracture (placebo), deep vein thrombosis, urinary tract infection, localized infection (avanafil, 100 mg), left arm pain and weakness, unstable angina, pneumonia, and bladder cancer (avanafil, 200 mg), none of which was deemed to be drug related. No deaths were reported during the study.
TABLE 2.
Overview of Adverse Events in the Safety Population
| Adverse event | Placebo (n=130), No. (%) | Avanafil, 100 mg (n=127), No. (%) | Avanafil, 200 mg (n=131), No. (%) | Total active (n=258), No. (%) | Total (N=388), No. (%) |
|---|---|---|---|---|---|
| Any TEAE | 31 (23.8) | 45 (35.4) | 42 (32.1) | 87 (33.7) | 118 (30.4) |
| Any drug-related TEAE | 5 (3.8) | 9 (7.1) | 20 (15.3) | 29 (11.2) | 34 (8.8) |
| Discontinued use of study medication because of TEAE | 0 | 1 (0.8) | 2 (1.5) | 3 (1.2) | 3 (0.8) |
| Discontinued use of study medication because of drug-related TEAE | 0 | 0 | 1 (0.8) | 1 (0.4) | 1 (0.3) |
| SAE | 1 (0.8) | 3 (2.4) | 4 (3.1) | 7 (2.7) | 8 (2.1) |
| Drug-related SAE | 0 | 0 | 0 | 0 | 0 |
| Death | 0 | 0 | 0 | 0 | 0 |
| TEAEs reported in ≥2% of men overall | |||||
| Headache | 2 (1.5) | 5 (3.9) | 15 (11.5) | 20 (7.8) | 22 (5.7) |
| Nasopharyngitis | 6 (4.6) | 4 (3.1) | 4 (3.1) | 8 (3.1) | 14 (3.6) |
| Flushing | 0 (0) | 2 (1.6) | 5 (3.8) | 7 (2.7) | 7 (1.8) |
| Sinus congestion | 1 (0.8) | 1 (0.8) | 4 (3.1) | 5 (1.9) | 6 (1.5) |
| Back pain | 3 (2.3) | 2 (1.6) | 1 (0.8) | 3 (1.2) | 6 (1.5) |
| Sinusitis | 0 | 4 (3.1) | 1 (0.8) | 5 (1.9) | 5 (1.3) |
| Dyspepsia | 0 | 0 | 4 (3.1) | 4 (1.6) | 4 (1.0) |
| Influenza | 0 | 3 (2.4) | 0 | 3 (1.2) | 3 (0.8) |
SAE = serious adverse event; TEAE = treatment-emergent adverse event.
No notable changes were found in vital signs, laboratory values, or physical examination results related to study treatment. For each treatment group, mean systolic BP, mean diastolic BP, and mean heart rate at the end of treatment were similar to those at randomization. Rates of abnormal systolic BP (systolic BP >140 mm Hg and an increase from baseline >20 mm Hg on 2 or more occasions or systolic BP >180 mm Hg at any time during the study) were consistent across all treatment groups (4 in the placebo group, 3 in the avanafil, 100 mg, group, and 3 in the avanafil, 200 mg, group). Three patients receiving avanafil, 100 mg, and 1 patient receiving placebo experienced an increase in diastolic BP of 15 mm Hg or greater from baseline on 2 or more occasions or diastolic BP greater than 110 mm Hg at any time during the study; no patients treated with avanafil, 200 mg, experienced abnormal diastolic BP values. No drug-drug interactions were reported with antidiabetic medications.
Discussion
In the current study, avanafil (100 or 200 mg) was an effective oral therapy for treatment of mild to severe ED in a group of heterosexual men with diabetes. After avanafil therapy, patients experienced significant improvements in the percentage of attempts that led to successful sexual intercourse (SEP 3), the percentage of attempts that resulted in successful vaginal penetration (SEP 2), and the IIEF-EF domain score compared with placebo. Improvement after avanafil treatment for each primary efficacy variable was consistently maintained at weeks 4, 8, and 12 of the study. The magnitude of treatment effect was generally greater in men with a longer duration of ED (≥60 months). The effects of avanafil on ED in men with diabetes is comparable to that found for other PDE5 inhibitors, including tadalafil (54%-57% and 42%-48% reported success as measured by SEP 2 and SEP 3, respectively),25 vardenafil (61%-64% and 49%-54% reported successful intercourse as measured by SEP 2 and SEP 3, respectively),26 and sildenafil (48% successful intercourse attempts as measured by IIEF subject diary entries).24
In a separate analysis, we evaluated success rates across various intervals from dosing to attempted intercourse, ranging from 15 minutes or less to more than 6 hours. For each interval tested, the avanafil group had a higher proportion of successful intercourse attempts (SEP 3), successful vaginal penetration (SEP 2), and satisfaction with the overall sexual experience than the placebo group. For currently approved PDE5 inhibitors, including avanafil, the prescribing information generally recommends dosing at least 30 to 60 minutes before the initiation of sexual activity, based on their respective pharmacokinetic profiles.22,24-26 However, the results of this study suggest that avanafil is effective as early as 15 minutes after dosing. This finding is consistent with its pharmacokinetic profile, with a relatively short time to maximum drug concentration and peak tumescent and rigidity effects occurring during the 20- to 40-minute postdosing assessment period.22,23 Furthermore, separate phase 3 trials of avanafil were performed in men with ED without type 1 or 2 diabetes and in men with ED after radical prostatectomy.28 Both studies, as well as previously performed phase 2 studies that included all 3 populations, found a similar efficacy and tolerability profile to the present study, including the rapid onset of action and persistence of effect.22
Treatment with avanafil was safe and well tolerated; only 1 patient treated with avanafil discontinued participation in the study because of a drug-related AE (headache). The incidence of common PDE5 effects, such as headache, flushing, dyspepsia, and nasal congestion, was low and dose dependent. Adverse events, such as priapism, visual disturbances, and hemodynamic change, which have been reported in studies of other PDE5 inhibitors, are thought to be related to the lack of stringent PDE5 specificity; although all of these agents are specific for the PDE5 isoenzyme, unintentional cross-reactivity may occur with other PDE isoenzymes, including inhibition of PDE6 and PDE11, which may lead to AEs.20,30-34 In this study, none of these types of events was reported, and there was only 1 report of blurred vision, supporting the high PDE5 specificity of avanafil.
Previous studies have found that the effect of PDE5 inhibitors on systemic vasodilatory properties may augment the BP-lowering effect of antihypertensive medications.35,36 However, for all treatment groups, mean systolic BP and mean diastolic BP values at the end of treatment were similar to those at randomization. Although 239 patients (61.6%; safety set) were receiving antihypertensive medications and 24 (6%; safety set) were receiving α-blockers, the incidence of marked changes in systolic BP and diastolic BP was low, and no major cardiac events were observed during the study. No issues were reported with concomitant antidiabetic medication use.
One limitation of the study was the small size of the subgroup of patients with type 1 diabetes, which was not sufficient to allow meaningful comparison with patients presenting with type 2 diabetes. Similarly, the subgroups of black, Asian, and multiracial patients were not sufficiently large to permit comparison with the white subgroup. In addition, although a significant number of the intercourse attempts were successful for the avanafil-treated men, fewer attempts were made 15 minutes or less and 6 hours or more after dosing than between 30 and 60 minutes after dosing. Despite the fewer attempts at intercourse, significant differences in the percentage of successful intercourse were achieved for avanafil-treated men compared with placebo at both the earliest (15 minutes after dosing) and latest (>6 hours after doing) time points. Considering that PDE5 inhibitors may be effective in the prevention of endothelial dysfunction37 and the treatment of lower urinary tract symptoms,38 conditions commonly found in patients with diabetes,37,39 future research should also aim to evaluate the effectiveness of avanafil for use in these conditions.
Conclusion
Avanafil has been found to be well tolerated and effective in the treatment of ED in this group of men with diabetes and mild to severe ED. During the 12-week treatment period, both the 100-mg and 200-mg doses of avanafil were significantly (P≤.002) more effective than placebo for all 3 coprimary end points. The rapid onset of efficacy observed is consistent with the pharmacokinetic profile of avanafil. These results suggest that avanafil may serve as an effective, on-demand therapy in men with diabetes and concurrent ED.
Acknowledgments
We acknowledge and thank the REVIVE-Diabetes investigators and study coordinators; the Quintiles team (study CRO); Sarah A. Odeh, CMPP, and Erin M. Falconer, PhD, from The Lockwood Group (editorial assistance, funded by VIVUS Inc); and VIVUS Inc, internal contributors. We also thank Andrew J. M. Boulton, MD, DSc (Hon), FRCP, for his critical review of the manuscript. We also gratefully acknowledge the manufacturer of the study drug, Mitsubishi Tanabe Pharma Corporation.
Footnotes
The participating investigators are listed at the end of the article.
Potential Competing Interests: Dr Goldstein has received grants and research support from various pharmaceutical companies, including but not limited to Absorption Pharmaceuticals, Astellas, Auxilium, BioSante, Boehringer Ingelheim, Emotional Brain, EndoCeutics, G&H Brands, GlaxoSmithKline, Medtronic, Palatin, Pfizer, Slate, Target Health, and VIVUS Inc; acted as a consultant for Absorption Pharmaceuticals, Auxilium, Emotional Brain, Fabre-Kramer, GlaxoSmithKline, Medtronic, Neocutis, Slate, Trimel, and VIVUS Inc; and has served as a speaker for Abbott, Ascend, Auxilium, Boehringer Ingelheim, Coloplast, Eli Lilly, Medtronic, and Slate. Dr Jones has served as a consultant for American Medical Systems and Coloplast Corporation, has served as a speaker for Astellas and GlaxoSmithKline, and has received research support from Auxilium, Lilly ICOS, VIVUS Inc, and Warner Chilcott. Dr Belkoff has served as a speaker for Dendreon, Ferring, GlaxoSmithKline, Sanofi-Aventis, VIVUS Inc, and Warner Chilcott. Dr Karlin has received research support from various pharmaceutical companies, including but not limited to Active Biotech, Allergan, Amgen, Astellas, Auxilium, Bayer, BioSante, Boehringer Ingelheim, Eli Lilly, Endo Pharmaceuticals, GTx, GlaxoSmithKline, Johnson and Johnson, Medtronic, Pfizer, Slate, VIVUS Inc, and Warner Chilcott and has served as a consultant for Amgen, Auxilium, Ferring, GP Pharma, Medtronic, Pfizer, and VIVUS Inc. Dr Karlin has participated in speaking presentations for multiple pharmaceutical companies. Dr Bowden, Dr Day, Mr Peterson, and Ms Trask are employees of VIVUS Inc.
Participating Investigators: Investigators who participated in this study are as follows: Miguel Trevino, MD, Clearwater, FL; James Clower, MD, Jacksonville, FL; Gerard Henry, MD, Shreveport, LA; George Adams, MD, Homewood, AL; Selwyn Spangenthal, MD, Charlotte, NC; James Barada, MD, Albany, NY; Laurence Belkoff, DO, Bala Cynwyd, PA; Gary Karlin, MD, Lawrenceville, NJ; Martin van Cleeff, MD, Cary, NC; Wilbur Wells, Jr, MD, Birmingham, AL; Richard Egelhof, MD, Wichita, KS; Bret Wittmer, MD, Madisonville, KY; Michael Cromer, MD, Tampa, FL; John Ervin, MD, Kansas City, MO; Larry Gilderman, DO, Pembroke Pines, FL; Steven Bowman, MD, Clearwater, FL; Richard Townsend, MD, Jacksonville, FL; Irwin Goldstein, MD, San Diego, CA; Brian Goldman, MD, Raleigh, NC; Dario Altamirano, DO, Hialeah, FL; Ronald Surowitz, DO, Jupiter, FL; Jeffrey Rosen, MD, Coral Gables, FL; Garth Denyer, MD, Spring, TX; Mira Baron, MD, Cleveland, OH; George Raad, MD, Charlotte, NC; Wayne Cline, MD, Salisbury, NC; Gladstone Sellers, MD, Sandy Springs, GA; Joseph Soufer, MD, Waterbury, CT; David Miller, MD, Harrisburg, NC; Sergio Rovner, MD, El Paso, TX; Douglas Young, MD, Sacramento, CA; Michael Bennett, MD, San Diego, CA; David Cook, MD, Winston-Salem, NC; LeRoy Jones, MD, San Antonio, TX; Maxwell Axler, MD, Houston, TX; Sidney Clevinger, MD, Ocala, FL; John McGettigan, MD, Tucson, AZ; Jonathan Staub, MD, Wilmington, NC; and Michael Warren, MD, Lancaster, PA.
References
- 1.http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Centers for Disease Control and Prevention Web site. Accessed March 28, 2012.
- 2.Vickers M.A., Satyanarayana R. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction in patients with diabetes mellitus. Int J Impot Res. 2002;14(6):466–471. doi: 10.1038/sj.ijir.3900910. [DOI] [PubMed] [Google Scholar]
- 3.Lewis R.W., Fugl-Meyer K.S., Bosch R. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1(1):35–39. doi: 10.1111/j.1743-6109.2004.10106.x. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E., Burnett A.L., Platz E.A. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120(2):151–157. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano F.A., Leriche A., Jaudinot E.O., de Gendre A.S. Prevalence of erectile dysfunction among 7689 patients with diabetes or hypertension, or both. Urology. 2004;64(6):1196–1201. doi: 10.1016/j.urology.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 6.Thorve V.S., Kshirsagar A.D., Vyawahare N.S., Joshi V.S., Ingale K.G., Mohite R.J. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25(2):129–136. doi: 10.1016/j.jdiacomp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Basu A., Ryder R.E. New treatment options for erectile dysfunction in patients with diabetes mellitus. Drugs. 2004;64(23):2667–2688. doi: 10.2165/00003495-200464230-00004. [DOI] [PubMed] [Google Scholar]
- 8.Chitaley K., Kupelian V., Subak L., Wessells H. Diabetes, obesity and erectile dysfunction: field overview and research priorities. J Urol. 2009;182(6, suppl):S45–S50. doi: 10.1016/j.juro.2009.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho S.Y., Park K., Paick J.S., Kim S.W. Change of erectile function and responsiveness to phosphodiesterase type 5 inhibitors at different stages of streptozotocin-induced diabetes in rats. J Sex Med. 2011;8(5):1352–1361. doi: 10.1111/j.1743-6109.2010.02099.x. [DOI] [PubMed] [Google Scholar]
- 10.Gratzke C., Angulo J., Chitaley K. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7(1, pt 2):445–475. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 11.Moore C.R., Wang R. Pathophysiology and treatment of diabetic erectile dysfunction. Asian J Androl. 2006;8(6):675–684. doi: 10.1111/j.1745-7262.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 12.Angulo J., Gonzalez-Corrochano R., Cuevas P. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J Sex Med. 2010;7(2, pt 1):758–768. doi: 10.1111/j.1743-6109.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferrini M.G., Rivera S., Moon J., Vernet D., Rajfer J., Gonzalez-Cadavid N.F. The genetic inactivation of inducible nitric oxide synthase (iNOS) intensifies fibrosis and oxidative stress in the penile corpora cavernosa in type 1 diabetes. J Sex Med. 2010;7(9):3033–3044. doi: 10.1111/j.1743-6109.2010.01884.x. [DOI] [PubMed] [Google Scholar]
- 14.Fukui M., Tanaka M., Okada H. Five-item version of the International Index of Erectile Function correlated with albuminuria and subclinical atherosclerosis in men with type 2 diabetes. J Atheroscler Thromb. 2011;18(11):991–997. doi: 10.5551/jat.9316. [DOI] [PubMed] [Google Scholar]
- 15.NIH Consensus Conference Impotence: NIH Consensus Development Panel on Impotence. JAMA. 1993;270(1):83–90. [PubMed] [Google Scholar]
- 16.Goldstein I., Young J.M., Fischer J., Bangerter K., Segerson T., Taylor T. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care. 2003;26(3):777–783. doi: 10.2337/diacare.26.3.777. [DOI] [PubMed] [Google Scholar]
- 17.Rendell M.S., Rajfer J., Wicker P.A., Smith M.D., Sildenafil Diabetes Study Group Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. JAMA. 1999;281(5):421–426. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 18.Saenz de Tejada I., Anglin G., Knight J.R., Emmick J.T. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25(12):2159–2164. doi: 10.2337/diacare.25.12.2159. [DOI] [PubMed] [Google Scholar]
- 19.Vardi M., Nini A. Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD002187.pub3. CD002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbin J.D. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16(suppl 1):S4–S7. doi: 10.1038/sj.ijir.3901205. [DOI] [PubMed] [Google Scholar]
- 21.Gresser U., Gleiter C.H. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil: review of the literature. Eur J Med Res. 2002;7(10):435–446. [PubMed] [Google Scholar]
- 22.Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004;16(suppl 1):S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- 23.Pfizer Labs; New York, NY: 2010. Viagra. [package insert] [Google Scholar]
- 24.Eli Lilly and Co; Indianapolis, IN: 2010. Cialis. [package insert] [Google Scholar]
- 25.Bayer HealthCare Pharmaceuticals Inc; Wayne, NJ: 2011. Levitra. [package insert] [Google Scholar]
- 26.Lewis R.W., Hellstrom W.J., Gittelman M. Rigiscan evaluation of TA-1790, a novel PDE5 inhibitor for the treatment of men with erectile dysfunction. J Urol. 2004;316:1196. [Google Scholar]
- 27.VIVUS Inc; Mountain View, CA: 2012. Stendra. [package insert] [Google Scholar]
- 28.Goldstein I., McCullough A.R., Jones L.A. A randomized double-blind, placebo-controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. J Sex Med. 2012;9(4):1122–1123. doi: 10.1111/j.1743-6109.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 29.Rosen R.C., Riley A., Wagner G., Osterloh I.H., Kirkpatrick J., Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 30.Porst H., Padma-Nathan H., Giuliano F., Anglin G., Varanese L., Rosen R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology. 2003;62(1):121–125. doi: 10.1016/s0090-4295(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 31.Althof S.E., Berner M.M., Goldstein I. Interrelationship of sildenafil treatment effects on the physiological and psychosocial aspects of erectile dysfunction of mixed or organic etiology. J Sex Med. 2010;7(9):3170–3178. doi: 10.1111/j.1743-6109.2010.01882.x. [DOI] [PubMed] [Google Scholar]
- 32.Eardley I., Donatucci C., Corbin J. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7(1, pt 2):524–540. doi: 10.1111/j.1743-6109.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosen R.C., Kostis J.B. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am J Cardiol. 2003;92(9A):9M–18M. doi: 10.1016/s0002-9149(03)00824-5. [DOI] [PubMed] [Google Scholar]
- 34.Shindel A.W. 2009 Update on phosphodiesterase type 5 inhibitor therapy, part 2: updates on optimal utilization for sexual concerns and rare toxicities in this class. J Sex Med. 2009;6(9):2352–2364. doi: 10.1111/j.1743-6109.2009.01447.x. [DOI] [PubMed] [Google Scholar]
- 35.Kloner R.A. Cardiovascular effects of the 3 phosphodiesterase-5 inhibitors approved for the treatment of erectile dysfunction. Circulation. 2004;110(19):3149–3155. doi: 10.1161/01.CIR.0000146906.42375.D3. [DOI] [PubMed] [Google Scholar]
- 36.Kloner R.A., Jackson G., Emmick J.T. Interaction between the phosphodiesterase 5 inhibitor, tadalafil and 2 alpha-blockers, doxazosin and tamsulosin in healthy normotensive men. J Urol. 2004;172(5, pt 1):1935–1940. doi: 10.1097/01.ju.0000142687.75577.e4. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton S.J., Chew G.T., Watts G.F. Therapeutic regulation of endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res. 2007;4(2):89–102. doi: 10.3132/dvdr.2007.026. [DOI] [PubMed] [Google Scholar]
- 38.Orabi H., Albersen M., Lue T.F. Association of lower urinary tract symptoms and erectile dysfunction: pathophysiological aspects and implications for clinical management. Int J Impot Res. 2011;23(3):99–108. doi: 10.1038/ijir.2011.14. [DOI] [PubMed] [Google Scholar]
- 39.Parsons J.K. Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010;5(4):212–218. doi: 10.1007/s11884-010-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


