Abstract
Hairy cell leukemia (HCL) is a rare chronic lymphoproliferative disorder characterized by circulating B cells with cytoplasmic projections, pancytopenia, splenomegaly, and a typical flow cytometry pattern. Recently, the BRAF V600E mutation was uniformly identified in one HCL series, which may provide insights into the pathogenic mechanisms. The disease course is usually indolent but inexorably progressive. Patients require treatment when they have significant cytopenia or occasionally recurrent infections from immunocompromise. In the mid-1980s, interferon replaced splenectomy as the initial treatment. A few years later, 2 purine nucleoside analogs, cladribine and pentostatin, showed promising activity in HCL. Complete response rates approached 95% with cladribine given as a single 7-day intravenous infusion. Newer methods of cladribine administration and modified dosing schedules have since been studied. Pentostatin response rates are comparable. We generally prefer cladribine because of its ease of administration, single infusion schema, and favorable toxicity profile. Since the introduction of these drugs, which have never been randomly compared, long-term follow-up studies have confirmed impressive and durable response durations. However, roughly 40% of patients with HCL eventually relapse. In this setting, patients can be re-treated with purine analogs. Rituximab also has a reasonable response rate in relapsed HCL; it can be given as a single agent sequentially after purine nucleosides or concurrently. Immunotoxins have robust responses but remain in development. Targeting the BRAF pathway will be an exciting future area of research. Many patients have minimal residual disease after initial treatment, but the clinical significance of this remains unknown.
Hairy cell leukemia (HCL) is a rare adult B-cell lymphoid leukemia characterized by pancytopenia, splenomegaly, and absolute monocytopenia. Morphologically, HCL is characterized by circumferential cytoplasmic projections (Figure 1). Bone marrow biopsy will reveal hypercellularity in most cases, with hairy cells having nuclei widely separated by abundant cytoplasm, giving a “fried-egg” appearance (Figure 2). Classically, tartrate-resistant acid phosphatase activity confirmed the diagnosis of HCL.1 However, immunophenotyping by flow cytometry is now considered standard practice. Hairy cell leukemia is characterized by the B-cell antigens CD19, CD20, and CD22. In addition, they coexpress the surface antigens CD11c, CD25, and CD103. Hairy cells generally lack CD5, CD10, CD21, and CD23. Immunohistochemical stains for DBA44 and annexin A1 can also help confirm the diagnosis.
FIGURE 1.
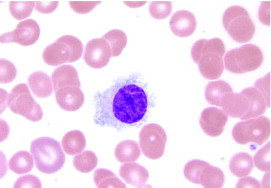
Peripheral blood smear specimen showing circumferential cytoplasmic projections characteristic of hairy cell leukemia (Wright-Giemsa, original magnification ×1000).
Photomicrograph courtesy of Robert W. Sharpe, MD, Department of Pathology, Scripps Clinic, La Jolla, CA.
FIGURE 2.
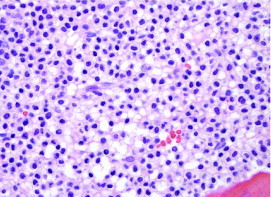
Bone marrow biopsy specimen from patient with hairy cell leukemia showing hypercellularity with hairy cells having nuclei widely separated by abundant cytoplasm, giving characteristic “fried egg” appearance (hematoxylin-eosin, original magnification ×600).
Photomicrograph courtesy of Robert W. Sharpe, MD, Department of Pathology, Scripps Clinic, La Jolla, CA.
Pathogenesis
For decades, the cellular event leading to HCL has evaded scientists and clinical researchers. Recently, 47 patients with HCL in Italy had whole-exome gene sequencing performed on leukemic cells and matched nonleukemic cells.2 In every patient, the well-known BRAF V600E mutation was identified. This mutation, more commonly known for its presence in melanoma, was a surprise finding. Implicated in the RAF-MEK-ERK kinase pathway, BRAF plays an important role in cell proliferation. Interestingly, the V600E mutation was not identified in other patients from the same institution with a variety of other B-cell malignant tumors. Further characterization of this pathway in HCL will no doubt lead to clinical studies of BRAF inhibitors in this disease, which have already made headlines in BRAF-mutated melanoma.3
When to Initiate Chemotherapy
Approximately one-quarter of patients are asymptomatic at presentation and are referred to the hematologist for evaluation of an abnormal complete blood cell count. Patients with HCL may also be symptomatic; they may have fatigue and dyspnea from anemia or show easy bruising and mucocutaneous bleeding from thrombocytopenia. Patients may have constitutional symptoms such as malaise or weight loss. Massive splenomegaly can lead to early satiety and increased abdominal girth. Although peripheral blood flow cytometry can frequently suggest the diagnosis, a bone marrow biopsy is essential for confirmation and to quantify the degree of hairy cell infiltration.
We generally initiate treatment for the symptomatic patient. In the asymptomatic patient, there are no absolute criteria for cytopenia, but we generally treat patients with a hemoglobin level less than 10 g/dL (to convert to g/L, multiply by 10), platelet counts less than 100 × 109/L (to convert to ×103/μL, divide by 1), and/or neutrophils less than 1.0 × 109/L (to convert to /μL, divide by 0.001). We evaluate patients every 3 to 6 months to closely monitor their disease with a history, physical examination, and complete blood cell count with differential.
Patients may also have active infections due to neutropenia, monocytopenia, or an associated immunocompromised state. One must also consider opportunistic infections, especially atypical Mycobacteria and other fungal causes such as Aspergillus. It may be best to treat the infection before initiating systemic chemotherapy because often chemotherapy will initially worsen cytopenia and cause further immunocompromise.
Long-Term Outlook
In deciding when to treat, the practitioner must recognize that HCL remains an incurable disease, as is true with other low-grade lymphoproliferative disorders. Although hematologic remissions can be maintained for extended periods, persistence of minimal residual disease (MRD) remains a controversial issue. In our experience at Scripps Clinic, Sigal et al4 showed that at 18 years after diagnosis, roughly half of all long-term complete responders to cladribine still have MRD. From this cohort of 19 patients followed up on average for nearly 2 decades, 7 patients had no evidence of disease even by flow cytometry. Very long-term remissions in HCL, therefore, may be possible.
Historical Perspective on Treatment Options
Before the development of modern chemotherapeutics, splenectomy was the treatment of choice for a disease in which chemotherapy was once considered contraindicated because of cytopenia. In one study of 65 patients undergoing splenectomy, the combined complete response (CR) and partial response (PR) rate was 100%; however, the 5-year survival rate was only 68%.5 In another series of 26 patients, the mean duration of response was only 5 months.6 Interpretation of these data is complex, though, because criteria defining response previously only included peripheral blood cell counts. In addition, these were retrospective series in which splenectomy indications and patient demographics varied widely. Currently, a CR is defined as eradication of hairy cells from the peripheral blood and bone marrow by morphologic analysis only. In 1984, interferon alfa was the first drug to change the HCL therapeutic landscape.7 Clinical relapse occurred, however, at a mean of 6 months after cessation of therapy.8 The CR rates with interferon are generally low; a large intergroup study published a decade later noted an 11% CR rate in 159 patients treated with interferon.9
Shortly after the advent of interferon therapy, Spiers et al10 showed promising activity of the purine nucleoside analog pentostatin (2′-deoxycoformycin) in HCL. As a weekly intravenous infusion, this drug produced a clinical and hematologic remission in 2 patients by 4 months. A group at Ohio State University documented a CR in 20 of 23 patients (87%) given a biweekly infusion. The mean duration of response was 12.6 months, and the drug was well tolerated.11
Cladribine
Soon after the Spiers et al report,10 investigation at Scripps Clinic showed promising activity for a newer synthetic purine nucleoside, 2-chlorodeoxyadenosine (cladribine). Like pentostatin, cladribine causes lymphocytotoxicity after intracellular phosphorylation. Unlike pentostatin, it is resistant to adenosine deaminase. Piro et al12 first reported the use of cladribine in 12 patients. Ranging in age from 36 to 61 years, these patients were all treated with a dose of 0.1 mg/kg per day for 7 days as a single continuous infusion. Seven patients had previously undergone splenectomy, and 5 had received interferon in the past. Eleven patients achieved a CR, as defined by a hemoglobin level greater than 12 g/dL, a platelet count greater than 130 × 109/L, and a neutrophil count greater than 1.5 × 109/L. The remaining patient had a PR. Normalization of peripheral blood cell counts occurred within 8 weeks, and the median duration of remission was 15.5 months.
Long-term Results
Using this approach of a single infusion of cladribine, we previously described the long-term follow-up in a cohort of 358 patients.13 One-quarter had previously undergone splenectomies, whereas nearly 40% had received prior systemic chemotherapy, including interferon. As portable outpatient infusion pumps became a reality, with the drug administered through a peripherally inserted central catheter, patients were no longer hospitalized. Independent of prior therapy, we observed a 91% CR rate and a 7% PR rate. For those who achieved CR, the median duration of response was 53 months, with a range of 1 to 134 months. One-quarter of patients, however, relapsed at a median of 29 months (range, 6-85 months). Of these 90 patients, 65 received another course of cladribine. There was still an 88% overall response rate (ORR), and most of these were CRs.
Cladribine was generally well tolerated. The main adverse event was febrile neutropenia, occurring in 42% of patients. Although a relatively high percentage, only 13% had documented infections, with herpesvirus and staphylococcal organisms being the main culprits. Grade 3 and 4 neutropenia occurred in 87%, whereas grade 3 and 4 thrombocytopenia and anemia were less common, at 20% and 22%, respectively. It took a median of 49 days before peripheral blood cell counts normalized (range, 9-378 days). The most common delayed infectious event was cutaneous herpes zoster. Secondary malignant neoplasms were also documented, and when compared with the Surveillance, Epidemiology, and End Results data, the excess observed-to-expected ratio was 1.88. This is a small yet statistically significant increase, as has been observed with other purine nucleoside analogs. In our group, these were mainly solid tumors. The most common were adenocarcinomas of the prostate, colon, and stomach followed by melanomas and various others in decreasing frequency. At 4 years, the time to treatment failure rate was 18.7%, whereas the overall survival (OS) rate was 96%. Even in the partial responders, the 4-year OS rate remained high at 92%.
Mostly from the same HCL cohort, we later reviewed data on 207 patients who were monitored for at least 7 years.14 Again, most (95%) achieved a CR, whereas 5% achieved a PR. In this group of patients, the mean first response duration was 98 months (range, 8-172 months). At a median of 42 months, however, 76 patients (37%) had relapsed. The range of relapse was wide, between 8 and 118 months. When we re-treated these patients with cladribine, a 92% ORR was achieved. A third of these patients had a second relapse; we administered cladribine to half of them and achieved a 60% CR rate. Duration of response decreased with each subsequent course. Even after a third cladribine course, a mean remission of 15 months was achieved (range, 10-37 months). Multivariate analysis showed that shorter disease duration, higher white blood cell count, and lower hemoglobin level were all risk factors for treatment failure. The second malignancy rate, mainly solid tumors, had an excess observed-to-expected ratio of 2.03 when compared with Surveillance, Epidemiology, and End Results data. In fact, having a preexisting malignant tumor led to a 3.7-fold increased risk of another malignant tumor. Nevertheless, at 108 months the OS rate was 97%, similar to the general population.
International Experience
Our long-term follow-up observations have been confirmed at several other institutions. Chadha et al15 at Northwestern University reported data on 86 patients with a mean follow-up of 9.7 years. A CR was seen in 79%, whereas all others achieved a PR. Most of these patients (70%) had no prior therapies. The relapse rate was 36%. For patients in CR, the CR occurred at a median of 35 months, much less than in our studies. A second course of cladribine was used to treat 23 patients who relapsed, and 12 of those achieved a CR. Partial responses were documented in 7 patients, which is in accordance with our results. Similarly, 17% of patients developed a second malignant neoplasm, all solid tumors. For all patients, the 12-year OS rate was 87%. These investigators believed that their slightly lower CR rates were due to more stringent criteria, including resolution of splenomegaly and adenopathy by computed tomography criteria.
At the University of Munich, extended results were reported by Jehn et al16 in 44 patients with a median follow-up of 8.5 years. All except 1 individual (98%) achieved a CR, defined as normalized peripheral blood cell counts, no morphologic evidence of HCL in the bone marrow, and lack of measurable disease. Seventeen patients (39%) have relapsed, with a median remission duration of 48 months (range, 8-131 months). Nine patients received a second course of cladribine, and half achieved a CR. At 12 years the OS was 79%. This study had strict monitoring criteria, with frequent bone marrow aspirations and biopsies performed. The authors noted that delayed clearance of HCL in the bone marrow was common, even 3 to 5 months after completing cladribine therapy. An 18% second malignancy rate was observed.
Summary
Cladribine has an excellent safety and efficacy profile in HCL that has been studied at multiple institutions internationally, with follow-up approaching a decade. We inform patients that they can expect a 95% ORR, an 85% CR rate, and that their chance of relapse at 4 years is approximately 30%.
Alternative Methods of Administration
Subcutaneous
A number of investigators have studied various routes of cladribine administration as clinical alternatives. In 1995, Juliusson et al17 from Sweden first reported on a 7-day daily subcutaneous injection of cladribine in 73 patients. An 81% CR rate was observed after 1 or 2 courses, and with a 20-month median follow-up, no relapses were indentified. Plasma levels of the drug were monitored and were similar to levels achieved after intravenous administration. The Swiss investigators reported results using 5 daily doses of subcutaneous cladribine at 0.14 mg/kg per day in 62 patients.18 Forty-seven patients (76%) achieved a CR, whereas 13 patients (21%) achieved a PR, for an ORR of 97%. The median time to treatment failure was 38 months. With follow-up of nearly 4 years, median OS has not yet been reached. Adverse effects were similar to those in our experience, with neutropenia observed in most patients. When fevers occurred, the infectious agent was not identified in most patients.
Weekly
Cladribine can also be given on a weekly schedule. First studied in 25 patients with HCL at a dose of 0.15 mg/kg intravenously once a week for 6 total weeks, cladribine produced a CR rate of 76%, whereas the rest of the cohort achieved a PR.19 Interestingly, only 4 of 25 patients developed a neutrophil count less than 0.5 × 109/L. In a separate group of 30 patients at the same Italian centers with a starting neutrophil count of less than 1.0 × 109/L, similar results were obtained.20 At 5 years of follow-up, the mean duration of response was 35 months (range, 6-58 months). In such an indolent disease, the authors concluded that delayed doses are safe and effective, especially in patients with severe neutropenia at diagnosis in whom infectious complications still remain.
With 10 years of follow-up, Zinzani et al21 reported on 2 nonrandomized trials comparing the weekly and daily intravenous routes. Thirty-seven patients received either 5 daily 2-hour doses of 0.14 mg/kg or 5 weekly doses. Patients receiving daily cladribine had a 72% rate of grade 3 to 4 neutropenia, with weekly patients having a significantly lower rate of 38%.
No significant differences were observed, however, in 2 prospective randomized trials. The Polish Adult Leukemia Group reported results from a randomization of 132 untreated HCL patients at 14 centers to receive 5 daily doses of cladribine at 0.12 mg/kg or 6 weekly doses.22 No differences were found in PR, ORR, OS, or progression-free survival (PFS) rates between the 2 arms. The CR rates were 76% and 72% in the daily and weekly arms, respectively, a finding that was also not statistically significant. Importantly, no differences were found in neutropenia or even progression to neutropenia in both arms. Infection rates were also similar. One of the unique aspects of this large study was that all patients were untreated. A second prospective randomized trial compared cladribine, 0.14 mg/kg, for 5 daily doses with 5 weekly doses.23 In this Swiss study of 100 patients, no significant differences were found in rates of grade 3 to 4 neutropenia (90% vs 80%), infection, or hospitalization within 10 weeks from treatment. The overall best response rate was 86% in both groups. The time to first infection or fever, however, was significantly longer in the weekly group. Also, in patients starting out with neutrophil counts greater than 1.0 × 109/L, those who received weekly cladribine developed significantly less grade 3 to 4 neutropenia. This finding may warrant further investigation in nonneutropenic patients.
Reduced Dose
The total cumulative dose of cladribine administered in these studies is approximately the same. With such excellent response rates, a lower dose has been considered. Forconi et al24 from the Italian Cooperative Group enrolled 148 patients with HCL into a prospective clinical trial comparing subcutaneous cladribine at 0.1 mg/kg per day for 5 vs 7 days. No differences were found in hematologic toxic effects between the 2 trial arms; however, infections and fevers were more common in the 7-day trial arm (23.9% vs 10.4%; P=.03). Hospitalization rates were also higher. However, the ORR was not significantly different between the 2 arms. At 3 years of follow-up, no differences in OS, treatment-free interval, or relapse-free survival have been observed between the 2 arms. This study demonstrates that a 25% reduction in the cladribine dose may be equally effective, although longer follow-up will be necessary to make firm conclusions.
Because there are no conclusive long-term follow-up randomized studies demonstrating a significant improvement with the standard dosing of cladribine, we give the drug as a single continuous intravenous infusion of 0.1 mg/kg per day for 7 days in our patients. Pharmacokinetic data suggest greater lymphotoxicity with prolonged exposure to cladribine than with brief exposure.25 However, intravenous dosing of 0.14 mg/kg for 2 hours for 5 consecutive days is also reasonable.
Pentostatin
As previously mentioned, a second purine nucleoside analog, pentostatin, has also been rigorously studied in this disease. Given as a bolus intravenous dose of 4 mg/m2 every other week, in the initial reports it took a mean of 8 courses to achieve a CR, with a range of 4 to 15 courses.11 Doses were withheld in patients with worsening renal function or when the white blood cell count was less than 1.5 × 109/L (to convert to /μL, divide by 0.001). Main toxic effects included nausea, vomiting, myelosuppression, fever, erythematous pruritic papular rash, and mild conjunctivitis. Infections were unusual. In one of the first long-term follow-up studies of pentostatin, 23 of 24 patients who initially achieved a CR were alive at a median of 82 months (range, 54-104 months).26 However, 11 patients had relapsed at a median of 30 months (range, 7-80 months). No serious infections occurred in this group, and the second malignancy rate was small. From a French study of 50 HCL patients, with most having had prior treatments, an ORR of 96% was noted.27 However, the CR rate was only 44%. In this study, the median number of cycles given was 12. At a median of 38 months of follow-up, the OS rate was 86%. Of 7 deaths, 2 were due to pentostatin toxicity and 1 was due to infection. There was a 10% second malignancy rate. Interestingly, of the untreated patients who initially responded to pentostatin, none had disease progression at 21 months of follow-up.
An Intergroup National Cancer Institute study in 1986 randomized patients between pentostatin and interferon. Initial results showed significantly higher CR rates with pentostatin compared with interferon (76% vs 11%).9 Longer-term follow-up at a median of 9 years, including crossover from patients in whom interferon failed, confirmed the effectiveness of pentostatin.28 Inclusion criteria were similar to many of the cladribine studies, with one important difference: active infections were not considered an exclusion criterion. Mostly because of response failure, interferon patients eventually crossed over to the pentostatin arm. A total of 241 patients were treated with pentostatin. The 10-year OS rate was 81%. A confirmed CR was seen in 72%; these patients had a relapse-free survival rate of 67%.
A large, multicenter, retrospective French study of 238 patients showed similar results, but more information was collected on relapsed patients.29 In this group, nearly two-thirds of patients had received prior treatment with interferon. It took a median of 9 cycles of pentostatin to achieve a CR, which was 79%. The ORR was 96%. In those with an initial CR, the relapse rate was 14%. Ten of these patients who relapsed were re-treated with pentostatin, with 8 having a CR and 2 a PR. Nineteen different patients who relapsed were treated with cladribine, resulting in 11 CRs, 4 PRs, 2 deaths during treatment, and 1 nonresponder. On the other hand, 9 patients who did not respond to 6 initial courses of pentostatin received cladribine, with 7 achieving a CR or PR. A second malignancy rate of 7.5% in this group was not higher than in the general population.
Cladribine vs Pentostatin
To date, no randomized studies have compared the 2 purine analogs in this rare leukemia. The Royal Marsden Hospital compared the 2 drugs in a retrospective fashion.30 Between 1992 and 1997, 165 patients were treated with pentostatin, whereas a much smaller group of 45 received cladribine. Among all patients, a statistically significant difference was found in the disease-free interval, with pentostatin patients having a median time to relapse of 51.5 months (range, 14.2-105.4 months) vs 23.5 months (range, 7.2-58.6 months) in the cladribine group. However, 115 patients had received prior interferon, and when including only untreated patients, this apparent difference no longer persisted. The CR rates were similar in both groups. Longer follow-up of 16 years was reported in the same group and confirmed the high CR rates, but relapse rates were similar between both groups.31 Median PFS was 10.5 years and not significantly different between both groups. At 15 years, the relapse rate was 47% in the pentostatin group and 48% in the cladribine group. In re-treated patients, again no difference was found between the 2 agents. In this cohort, the second malignancy rate was similar to the general British population.
In view of these similar outcomes and lack of prospective randomized trials, we prefer cladribine as the first-line treatment for HCL. Cladribine is given as a single continuous infusion for 7 days as an outpatient regimen, rather than multiple bolus treatments of pentostatin during a 3- to 6-month period. The drug is well tolerated and has an excellent safety record. In patients who experience relapse, we readminister cladribine if the disease-free interval is longer than 18 months. Otherwise, because cross-reactivity has not been shown, it is reasonable to change patients to pentostatin therapy. We do not perform any further cancer screening tests other than those recommended by national agencies such as the US Preventative Services Task Force for normal matched population controls. The characteristics of cladribine and pentostatin are summarized in the Table.
TABLE.
Comparison of Cladribine and Pentostatin
| Variable | Cladribine | Pentostatin |
|---|---|---|
| Dose | 0.1 mg/kg per day for 7 days | 4 mg/m2 every other week for 8-12 doses |
| Route | IV (continuous or bolus), subcutaneous, or oral | IV bolus |
| CR rate, % | 79-98 | 72-79 |
| Notable toxic effects | Febrile neutropenia, myelosuppression, immunosuppression | Nausea, rash, myelosuppression, immunosuppression |
| PFS at 4 years, % | 50-88 | 72-76 |
CR = complete response; IV = intravenous; PFS = progression-free survival.
Purine Analog Relapse and Resistance
Rituximab
Hairy cells, like most B cells, express CD20. We performed a phase 2 study of the monoclonal CD20 antibody rituximab in patients relapsing after cladribine treatment, with a median of 73 months since their last infusion (range, 20-140 months).32 Patients were treated with rituximab, 375 mg/m2, intravenously every week for 4 weeks. None of the 24 patients treated had refractory disease, although 8 had received 2 prior courses of cladribine. Because of the delayed response of rituximab in indolent lymphomas, we evaluated patients at 6 months. Three patients achieved a CR, with no morphologic evidence of hairy cells in the bone marrow, although immunohistochemical analysis revealed MRD in all patients. Another 3 patients had a PR. Two of these 6 responders eventually relapsed, at 14 and 16 months. Half of all those treated experienced a febrile infusion reaction, and 1 elderly patient died of a ruptured diverticular abscess. A nonsignificant trend toward a better response to rituximab was seen in patients with a lower hairy cell burden in the marrow.
Other groups have reported higher response rates with rituximab, albeit in less heavily pretreated individuals. In the first report describing rituximab for treatment of HCL published more than a decade ago, Hagberg and Lundholm reported an ORR of 64%, including 6 CRs, in 11 patients.33 The higher rates could have been attributed to the inclusion of untreated patients in their study. The Swiss Group for Clinical Cancer Research also evaluated rituximab in 25 patients relapsing after prior cladribine treatment.34 Four weekly doses at 375 mg/m2 were administered to 24 patients, with 1 patient only receiving 1 infusion because of a dermal vasculitic reaction. The ORR was 80%, with a 32% CR rate. The median remission duration was just less than 3 years. Besides the expected infusional reactions seen with rituximab, more serious adverse events occurred in 7 patients. These events include one death from cardiac failure a day after the first infusion and another death from gastrointestinal bleeding 3 months after treatment.
At M.D. Anderson Cancer Center in Houston, TX, 15 patients, including those refractory to treatment with primary nucleoside analog, were given rituximab at the same dose for 8 weeks, with an additional 4 doses given to those showing an initial response.35 Eight patients (53%) achieved a CR, with an ORR of 80%. At a median of 32 months of follow-up, progression of disease occurred in 42%. Although these studies included small numbers of patients, their results suggest that 8 to 12 doses may be superior to previously used regimens.
Sequential Therapy
The question then arises, as it has with other indolent B-cell malignant neoplasms, should rituximab be used as the front-line treatment? Should it be combined with cladribine, or should it be given sequentially? Ravandi et al36 treated 13 patients with HCL, 11 whose conditions were newly diagnosed, with cladribine at a dose of 5.6 mg/m2 intravenously daily for 5 days followed by 8 weekly doses of rituximab. One patient had received cladribine 9 years earlier and subsequently relapsed, whereas the other had a variant HCL treated with chlorambucil. One month after cladribine therapy and again after rituximab, an additional bone marrow biopsy was performed to evaluate for MRD using flow cytometry and polymerase chain reaction. All patients achieved a CR. One month after cladribine therapy, MRD was detected in 5 of 11 evaluable patients. One month after rituximab therapy, however, MRD was detected in only 1 of 12 evaluable patients. No patients have relapsed, although follow-up at the time of the report was only 14 months. No unexpected toxic effects occurred.
These researchers updated their experience in 45 HCL patients, 38 with untreated disease.37 In 24% of patients, MRD was detected at the completion of cladribine and rituximab treatment. These studies demonstrate the effectiveness of rituximab in reducing or eradicating MRD. However, the clinical value of MRD eradication remains to be demonstrated. As noted in the Scripps Clinic study, many asymptomatic patients with normal blood cell counts nearly 20 years after cladribine therapy still demonstrate MRD.4
Cervetti et al38 treated patients with rituximab sequentially after cladribine treatment but only administered the monoclonal antibody when MRD was detectable. With this protocol, rituximab was administered anytime from 1.5 to an astounding 113 months after cladribine (mean, 4.3 months). Twenty-seven patients were treated at a single Italian institution with cladribine induction. Patients younger than 60 years were treated with 5 mg/m2 intravenously daily for 5 days, whereas older patients received 5 mg/m2 intravenously weekly for 6 weeks, akin to some of the data presented earlier. The ORR was 89% after cladribine. When MRD was detected, rituximab was given at 375 mg/m2 every week for 4 doses. Two months after rituximab treatment, the ORR was 100%, and at a median follow-up of 3 years, a so-called molecular response was seen in 70%. Five-year PFS was 83%. The quality of hematologic response to rituximab significantly affected PFS, with a 2-year PFS of 94% in those with an initial CR and only 50% in those with a PR (P<.001). Interestingly, MRD status also influenced PFS rates. Two-year PFS was 30% in MRD-positive patients and 100% in MRD-negative patients (P<.001). Although this is a small study without OS information, the controversy regarding MRD eradication in HCL remains unresolved.
Concurrent Therapy
In many other B-cell malignant neoplasms, rituximab is combined with conventional chemotherapy without major adverse effects. Else et al39 from the Royal Marsden group in Britain have shown combination therapy with purine analogs to be safe and effective in relapsed HCL patients. Fourteen patients were treated concurrently, whereas 4 patients were treated sequentially. Patients were not uniformly treated; 12 received pentostatin and 6 received cladribine. Patients were also given 4 to 8 weekly doses of rituximab. The ORR was 100%, with 16 of 18 achieving a CR. At a median follow-up of 3 years, all 16 of these relapsed patients maintained their CR status. In 13 patients, MRD was evaluated, and all tested negative for MRD after treatment. When compared with their original disease-free survival after first-line treatment, rituximab combination treatment resulted in significantly longer remission rates.
This study did not compare concurrent rituximab with sequential therapy. It demonstrates, however, that combination therapy is safe, as has been shown in other indolent B-cell disorders. With HCL being such a rare chronic leukemia, prospective randomized data evaluating first-line rituximab will be hard to come by. Furthermore, the high CR rates seen with cladribine and the economics of monoclonal antibodies make the use of combination therapy of questionable value. However, the possibility of combination treatments leading to deeper, longer-lasting remissions warrants further investigation.
Supportive Care
Hematopoietic Growth Factors
Neutrophil growth factors have been applied to reduce complications from cladribine-induced severe neutropenia because it occurs in 30% to 40% of patients. At Scripps Clinic, we performed a phase 2 trial investigating filgrastim at a dose of 5 μg/kg per day subcutaneously for 3 consecutive days before cladribine administration, then daily after cladribine was finished until resolution of neutropenia.40 We treated 35 patients and compared them with historical controls. The median nadir neutrophil count was roughly 0.5 × 109/L compared with 0.3 × 109/L in patients not receiving filgrastim (P=.04). After a median of 10 days of filgrastim, the time for neutrophils to recover to greater than 1.0 × 109/L was shortened from 22 days in the historical control arm to 9 days. However, we noted no differences with regard to the number of febrile days or hospitalization rates. Therefore, routine use of filgrastim is not recommended. Because the rates of severe anemia from cladribine are low, we similarly do not recommend prophylactic erythropoietin-stimulating agents, although this has never been prospectively studied.
Antimicrobials
Prophylactic antimicrobial drugs should be used judiciously. In general, we do not prescribe antibiotics, antifungals, or antivirals in the otherwise healthy patient with HCL undergoing treatment. However, for patients with prior life-threatening infections or those at high risk of developing one, we generally administer these antimicrobial and granulocyte colony-stimulating factor drugs to support them through their neutropenic phase.
Experimental Therapies
Inevitably, a rare patient will be refractory to all approved treatments. Immunotoxin conjugates were first studied at the National Cancer Institute. These drugs combine a toxin with a monoclonal antibody. A decade ago, the drug BL22, which contains an anti-CD22 variable domain conjugated with the Pseudomonas exotoxin, produced a CR in 11 of 16 patients treated.41 Two patients developed a reversible, severe hemolytic uremic syndrome. A phase 2 trial in 36 patients, though, produced a CR rate of 47% after 2 cycles of the drug, with 8% developing hemolytic uremic syndrome.42 A recent phase 1 trial of moxetumomab, a National Cancer Institute drug with higher CD22 affinity, demonstrated a response rate of 47% in 32 heavily pretreated patients.43 Conjugated immunotoxins have an increasing role in HCL management, as with other hematologic malignant neoplasms. Other treatments have also been reported in HCL, including alemtuzumab and bendamustine.44,45 Novel BRAF inhibitors such as vemurafenib, which has shown improved survival in metastatic melanoma,3 warrant investigation as well. As mentioned previously, the distinctive V600E mutation may be ubiquitous in HCL.2 Our general treatment algorithm for HCL is depicted in Figure 3.
FIGURE 3.
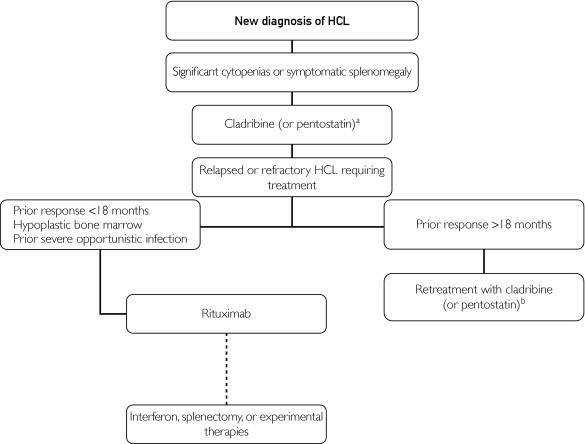
Treatment algorithm. aCladribine preferred because of its ease of administration, favorable toxicity profile, and higher complete response rates. bIndicates consider switching to other purine analog given lack of cross-resistance. HCL = hairy cell leukemia.
Recommendations
First described by Bouroncle et al46 in 1958, HCL used to be a difficult-to-treat malignant neoplasm with a generally poor prognosis. With purine analogs, patients with HCL can now achieve high CR rates and lead normal lives, with survival approximating that of the general population. This is an astonishing achievement. Because of ease of administration, good tolerability, and long-term safety data, we recommend cladribine as first-line therapy for HCL. Only patients with significant cytopenia, symptoms, or recurrent infections should be treated. Cladribine should be given on an outpatient basis as a single, 7-day, continuous, intravenous infusion at a dose of 0.1 mg/kg per day. The main adverse event to be aware of is febrile neutropenia. Because no prospective randomized studies have been performed, we reserve pentostatin for patients who have experienced early relapse with cladribine therapy, typically at less than 6 months.
For late relapses, we administer a second course of cladribine, with further courses to follow if subsequent relapse-free intervals are longer than 12 to 18 months. In the appropriate setting, sequential rituximab therapy should be considered, especially for patients believed to be at high risk of relapse. Rituximab monotherapy is also useful for patients unable to tolerate the myelosuppression or immunosuppression after purine analogs. A rare patient may still respond to interferon or splenectomy. Patients refractory to treatment should be considered for clinical trials, with new conjugated immunotoxins representing an exciting area of drug development.
The MRD status in patients with HCL remains controversial. To date, we remain unconvinced that routinely administering monoclonal antibodies to eradicate MRD achieves clinically meaningful results. Likewise, the cause of the increased incidence of secondary malignant neoplasms remains controversial. Whether purine analogs or the disease itself contributes to this is unknown. Nevertheless, the outlook of HCL has changed significantly during the past 2 decades from a disease treated with supportive care and splenectomies to one that responds completely to a single infusion of cladribine chemotherapy.
References
- 1.Yam L.T., Li C.Y., Lam K.W. Tartrate-resistant acid phosphatase isoenzyme in the reticulum cells of leukemic reticuloendotheliosis. N Engl J Med. 1971;284(7):357–360. doi: 10.1056/NEJM197102182840704. [DOI] [PubMed] [Google Scholar]
- 2.Tiacci E., Trifonov V., Schiavoni G. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364(24):2305–2315. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman P.B., Hauschild A., Robert C. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigal D.S., Sharpe R., Burian C., Saven A. Very long-term eradication of minimal residual disease in patients with hairy cell leukemia after a single course of cladribine. Blood. 2010;115(10):1893–1896. doi: 10.1182/blood-2009-10-251645. [DOI] [PubMed] [Google Scholar]
- 5.Golomb H.M., Vardiman J.W. Response to splenectomy in 65 patients with hairy cell leukemia: an evaluation of spleen weight and bone marrow involvement. Blood. 1983;61(2):349–352. [PubMed] [Google Scholar]
- 6.Magee M.J., McKenzie S., Filippa D.A., Arlin Z.A., Gee T.S., Clarkson B.D. Hairy cell leukemia: durability of response to splenectomy in 26 patients and treatment of relapse with androgens in six patients. Cancer. 1985;56(11):2557–2562. doi: 10.1002/1097-0142(19851201)56:11<2557::aid-cncr2820561103>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Quesada J.R., Reuben J., Manning J.T., Hersh E.M., Gutterman J.U. Alpha interferon for induction of remission in hairy-cell leukemia. N Engl J Med. 1984;310(1):15–18. doi: 10.1056/NEJM198401053100104. [DOI] [PubMed] [Google Scholar]
- 8.Quesada J.R., Hersh E.M., Manning J. Treatment of hairy cell leukemia with recombinant alpha-interferon. Blood. 1986;68(2):493–497. [PubMed] [Google Scholar]
- 9.Grever M., Kopecky K., Foucar M.K. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an Intergroup study. J Clin Oncol. 1995;13(4):974–982. doi: 10.1200/JCO.1995.13.4.974. [DOI] [PubMed] [Google Scholar]
- 10.Spiers A.S., Parekh S.J., Bishop M.B. Hairy-cell leukemia: induction of complete remission with pentostatin (2'-deoxycoformycin) J Clin Oncol. 1984;2(12):1336–1342. doi: 10.1200/JCO.1984.2.12.1336. [DOI] [PubMed] [Google Scholar]
- 11.Kraut E.H., Bouroncle B.A., Grever M.R. Pentostatin in the treatment of advanced hairy cell leukemia. J Clin Oncol. 1989;7(2):168–172. doi: 10.1200/JCO.1989.7.2.168. [DOI] [PubMed] [Google Scholar]
- 12.Piro L.D., Carrera C.J., Carson D.A., Beutler E. Lasting remissions in hairy-cell leukemia induced by a single infusion of 2-chlorodeoxyadenosine. N Engl J Med. 1990;322(16):1117–1121. doi: 10.1056/NEJM199004193221605. [DOI] [PubMed] [Google Scholar]
- 13.Saven A., Burian C., Koziol J.A., Piro L.D. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;92(6):1918–1926. [PubMed] [Google Scholar]
- 14.Goodman G.R., Burian C., Koziol J.A., Saven A. Extended follow-up of patients with hairy cell leukemia after treatment with cladribine. J Clin Oncol. 2003;21(5):891–896. doi: 10.1200/JCO.2003.05.093. [DOI] [PubMed] [Google Scholar]
- 15.Chadha P., Rademaker A.W., Mendiratta P. Treatment of hairy cell leukemia with 2-chlorodeoxyadenosine (2-CdA): long-term follow-up of the Northwestern University experience. Blood. 2005;106(1):241–246. doi: 10.1182/blood-2005-01-0173. [DOI] [PubMed] [Google Scholar]
- 16.Jehn U., Bartl R., Dietzfelbinger H., Haferlach T., Heinemann V. An update: 12-year follow-up of patients with hairy cell leukemia following treatment with 2-chlorodeoxyadenosine. Leukemia. 2004;18(9):1476–1481. doi: 10.1038/sj.leu.2403418. [DOI] [PubMed] [Google Scholar]
- 17.Juliusson G., Heldal D., Hippe E. Subcutaneous injections of 2-chlorodeoxyadenosine for symptomatic hairy cell leukemia. J Clin Oncol. 1995;13(4):989–995. doi: 10.1200/JCO.1995.13.4.989. [DOI] [PubMed] [Google Scholar]
- 18.von Rohr A., Schmitz S.F., Tichelli A. Treatment of hairy cell leukemia with cladribine (2-chlorodeoxyadenosine) by subcutaneous bolus injection: a phase II study. Ann Oncol. 2002;13(10):1641–1649. doi: 10.1093/annonc/mdf272. [DOI] [PubMed] [Google Scholar]
- 19.Lauria F., Bocchia M., Marotta G., Raspadori D., Zinzani P.L., Rondelli D. Weekly administration of 2-chlorodeoxyadenosine in patients with hairy-cell leukemia: a new treatment schedule effective and safer in preventing infectious complications. Blood. 1997;89(5):1838–1839. [PubMed] [Google Scholar]
- 20.Lauria F., Bocchia M., Marotta G., Raspadori D., Zinzani P.L., Rondelli D. Weekly administration of 2-chlorodeoxyadenosine in patients with hairy-cell leukemia is effective and reduces infectious complications. Haematologica. 1999;84(1):22–25. [PubMed] [Google Scholar]
- 21.Zinzani P.L., Tani M., Marchi E. Long-term follow-up of front-line treatment of hairy cell leukemia with 2-chlorodeoxyadenosine. Haematologica. 2004;89(3):309–313. [PubMed] [Google Scholar]
- 22.Robak T., Jamroziak K., Gora-Tybor J. Cladribine in a weekly versus daily schedule for untreated active hairy cell leukemia: final report from the Polish Adult Leukemia Group (PALG) of a prospective, randomized, multicenter trial. Blood. 2007;109(9):3672–3675. doi: 10.1182/blood-2006-08-042929. [DOI] [PubMed] [Google Scholar]
- 23.Zenhausern R., Schmitz S.F., Solenthaler M. Randomized trial of daily versus weekly administration of 2-chlorodeoxyadenosine in patients with hairy cell leukemia: a multicenter phase III trial (SAKK 32/98) Leuk Lymphoma. 2009;50(9):1501–1511. doi: 10.1080/10428190903131755. [DOI] [PubMed] [Google Scholar]
- 24.Forconi F., Cencini E., Zaja F. Analysis of toxicity and efficacy of subcutaneous cladribine at reduced or standard doses (five versus seven consecutive days) in patients with hairy cell leukemia (HCL) in the ICGHCL2004 Protocol by the Italian Cooperative Group on HCL [ASH abstract 701] Blood. 2010;116 [Google Scholar]
- 25.Beutler E. Cladribine (2-chlorodeoxyadenosine) Lancet. 1992;340(8825):952–956. doi: 10.1016/0140-6736(92)92826-2. [DOI] [PubMed] [Google Scholar]
- 26.Kraut E.H., Grever M.R., Bouroncle B.A. Long-term follow-up of patients with hairy cell leukemia after treatment with 2'-deoxycoformycin. Blood. 1994;84(12):4061–4063. [PubMed] [Google Scholar]
- 27.Ribeiro P., Bouaffia F., Peaud P.Y. Long term outcome of patients with hairy cell leukemia treated with pentostatin. Cancer. 1999;85(1):65–71. doi: 10.1002/(sici)1097-0142(19990101)85:1<65::aid-cncr9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Flinn I.W., Kopecky K.J., Foucar M.K. Long-term follow-up of remission duration, mortality, and second malignancies in hairy cell leukemia patients treated with pentostatin. Blood. 2000;96(9):2981–2986. [PubMed] [Google Scholar]
- 29.Maloisel F., Benboubker L., Gardembas M. Long-term outcome with pentostatin treatment in hairy cell leukemia patients: a French retrospective study of 238 patients. Leukemia. 2003;17(1):45–51. doi: 10.1038/sj.leu.2402784. [DOI] [PubMed] [Google Scholar]
- 30.Dearden C.E., Matutes E., Hilditch B.L., Swansbury G.J., Catovsky D. Long-term follow-up of patients with hairy cell leukaemia after treatment with pentostatin or cladribine. Br J Haematol. 1999;106(2):515–519. doi: 10.1046/j.1365-2141.1999.01546.x. [DOI] [PubMed] [Google Scholar]
- 31.Else M., Dearden C.E., Matutes E. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145(6):733–740. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 32.Nieva J., Bethel K., Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102(3):810–813. doi: 10.1182/blood-2003-01-0014. [DOI] [PubMed] [Google Scholar]
- 33.Hagberg H., Lundholm L. Rituximab, a chimaeric anti-CD20 monoclonal antibody, in the treatment of hairy cell leukaemia. Br J Haematol. 2001;115(3):609–611. doi: 10.1046/j.1365-2141.2001.03143.x. [DOI] [PubMed] [Google Scholar]
- 34.Zenhausern R., Simcock M., Gratwohl A., Hess U., Bargetzi M., Tobler A. Rituximab in patients with hairy cell leukemia relapsing after treatment with 2-chlorodeoxyadenosine (SAKK 31/98) Haematologica. 2008;93(9):1426–1428. doi: 10.3324/haematol.11564. [DOI] [PubMed] [Google Scholar]
- 35.Thomas D.A., O'Brien S., Bueso-Ramos C. Rituximab in relapsed or refractory hairy cell leukemia. Blood. 2003;102(12):3906–3911. doi: 10.1182/blood-2003-02-0630. [DOI] [PubMed] [Google Scholar]
- 36.Ravandi F., Jorgensen J.L., O'Brien S.M. Eradication of minimal residual disease in hairy cell leukemia. Blood. 2006;107(12):4658–4662. doi: 10.1182/blood-2005-11-4590. [DOI] [PubMed] [Google Scholar]
- 37.Ravandi F., Jorgensen J., O'Brien S. Phase II study of sequential therapy with cladribine followed by an extended course rituximab in patients with hairy cell leukemia [ASH abstract 2458] Blood. 2010;116 [Google Scholar]
- 38.Cervetti G., Galimberti S., Andreazzoli F. Rituximab as treatment for minimal residual disease in hairy cell leukaemia: extended follow-up. Br J Haematol. 2008;143(2):296–298. doi: 10.1111/j.1365-2141.2008.07333.x. [DOI] [PubMed] [Google Scholar]
- 39.Else M., Dearden C.E., Matutes E. Rituximab with pentostatin or cladribine: an effective combination treatment for hairy cell leukemia after disease recurrence. Leuk Lymphoma. 2011;52(suppl 2):75–78. doi: 10.3109/10428194.2011.568650. [DOI] [PubMed] [Google Scholar]
- 40.Saven A., Burian C., Adusumalli J., Koziol J.A. Filgrastim for cladribine-induced neutropenic fever in patients with hairy cell leukemia. Blood. 1999;93(8):2471–2477. [PubMed] [Google Scholar]
- 41.Kreitman R.J., Wilson W.H., Bergeron K. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345(4):241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 42.Kreitman R.J., Stetler-Stevenson M., Margulies I. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27(18):2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreitman R.J., Tallman M.S., Coutre S. A phase 1 study of moxetumomab pasudotox, an anti-CD22 recombinant immunotoxin, in relapsed/refractory hairy cell leukemia (HCL): updated results [ASH abstract 2516] Blood. 2010;116 [Google Scholar]
- 44.Fietz T., Rieger K., Schmittel A., Thiel E., Knauf W. Alemtuzumab (Campath 1H) in hairy cell leukaemia relapsing after rituximab treatment. Hematol J. 2004;5(5):451–452. doi: 10.1038/sj.thj.6200373. [DOI] [PubMed] [Google Scholar]
- 45.Kreitman R.J., Arons E., Stetler-Stevenson M., Miller K.B. Response of hairy cell leukemia to bendamustine. Leuk Lymphoma. 2011;52(6):1153–1156. doi: 10.3109/10428194.2011.562575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouroncle B.A., Wiseman B.K., Doan C.A. Leukemic reticuloendotheliosis. Blood. 1958;13(7):609–630. [PubMed] [Google Scholar]


