Abstract
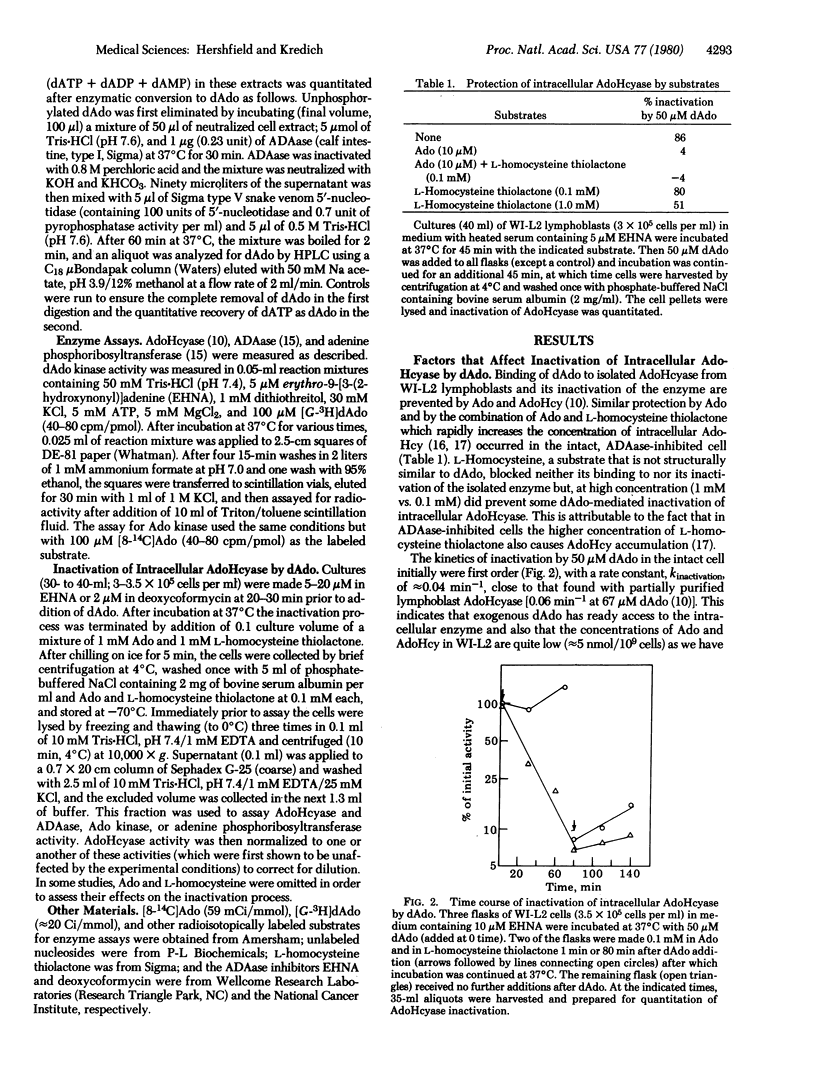
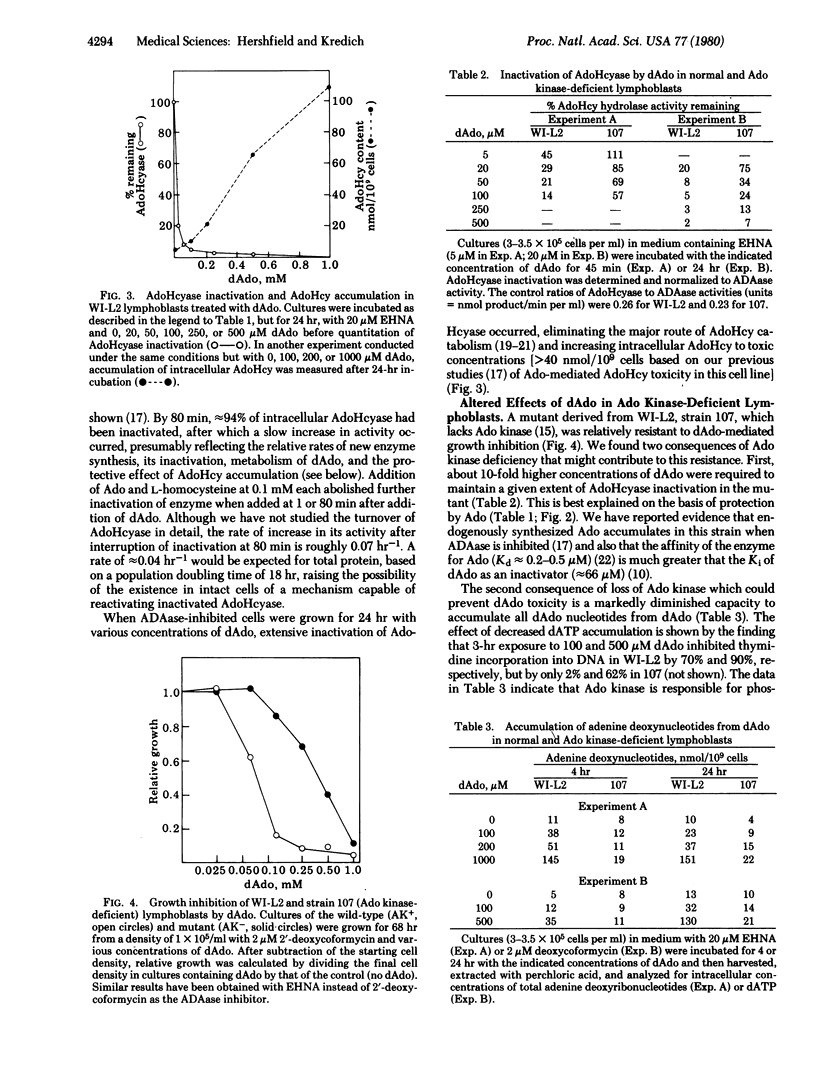
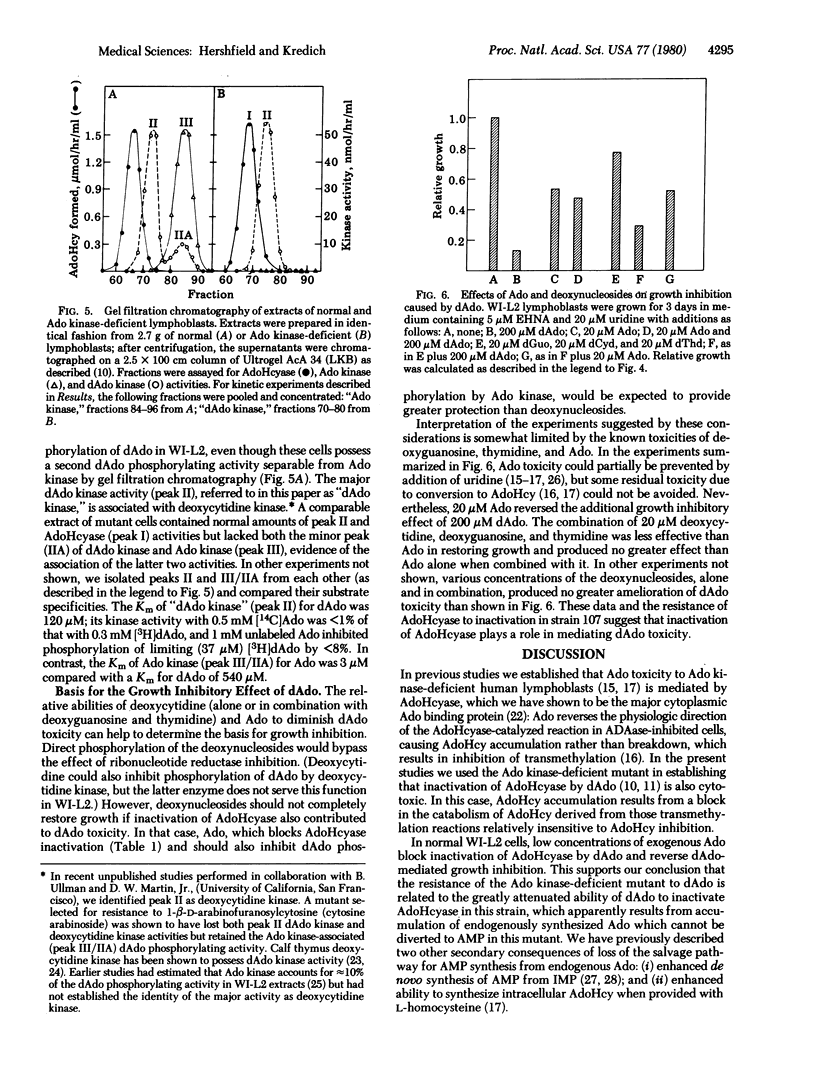
Accumulation of dATP derived from 2'-deoxyadenosine (dAdo), causing inhibition of ribonucleotide reductase and depletion of the other deoxynucleotide substrates required for DNA synthesis, has been suggested as the cause of the lymphopenia and immune defect in inheritable deficiency of adenosine deaminase (adenosine aminohydrolase, EC 3.5.4.4). dAdo also inactivates the enzyme S-adenosylhomocysteine hydrolase (AdoHcyase; S-adenosyl-L-homocystein hydrolase EC 3.3.1.1) which is involved in the catabolism of S-adenosyl-L-homocysteine (AdoHcy), both a product and a potent inhibitor of S-adenosylmethionine-dependent transmethylation. We have tried to determine whether inactivation of AdoHcyase might also contribute to dAdo toxicity to adenosine deaminase-inhibited cells. dAdo rapidly inactivates intracellular AdoHcyase and causes the accumulation of AdoHcy in WI-L2 human B lymphoblastoid cells. Low concentrations of adenosine (Ado), which block binding of dAdo to purified AdoHcyase, prevented inactivation of intracellular AdoHcyase and also lessened the growth-inhibitory effect of dAdo. A mutant of this cell line which lacks Ado kinase and accumulated endogenously synthesized Ado was resistant to the effects of dAdo on both growth and AdoHcyase activity. The mutant also accumulated far less dATP from dAdo than did its parent and was resistant to the inhibitory effect of dAdo on DNA synthesis, indicating the Ado kinase is involved in dAdo phosphorylation in these cells. Combinations of deoxycytidine, thymidine, and deoxyguanosine that could prevent dATP-mediated depletion of deoxynucleotide pools but not AdoHcyase inactivation were less effective than Ado in preventing dAdo toxicity to normal lymphoblasts. Our results suggest that inactivation of AdoHcyase, as well as dATP accumulation, contributes to dAdo toxicity.
Full text
PDF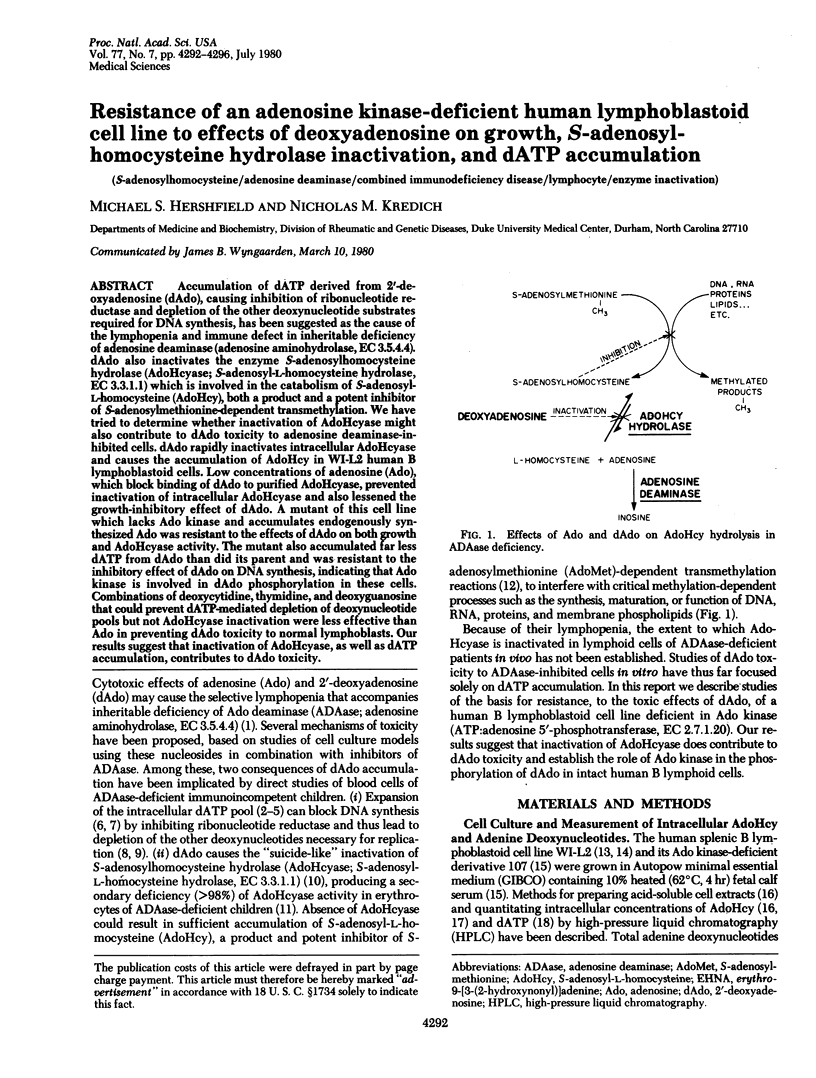

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson D. A., Kaye J., Matsumoto S., Seegmiller J. E., Thompson L. Biochemical basis for the enhanced toxicity of deoxyribonucleosides toward malignant human T cell lines. Proc Natl Acad Sci U S A. 1979 May;76(5):2430–2433. doi: 10.1073/pnas.76.5.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Differential sensitivity of human leukemic T cell lines and B cell lines to growth inhibition by deoxyadenosine. J Immunol. 1978 Nov;121(5):1726–1731. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- Donofrio J., Coleman M. S., Hutton J. J., Daoud A., Lampkin B., Dyminski J. Overproduction of adenine deoxynucleosides and deoxynucletides in adenosine deaminase deficiency with severe combined immunodeficiency disease. J Clin Invest. 1978 Oct;62(4):884–887. doi: 10.1172/JCI109201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham J. P., Ives D. H. Deoxycytidine kinase. I. Distribution in normal and neoplastic tissues and interrelationships of deoxycytidine and 1-beta-D-arabinofuranosylcytosine phosphorylation. Mol Pharmacol. 1969 Jul;5(4):358–375. [PubMed] [Google Scholar]
- Durham J. P., Ives D. H. Deoxycytidine kinase. II. Purification and general properties of the calf thymus enzyme. J Biol Chem. 1970 May 10;245(9):2276–2284. [PubMed] [Google Scholar]
- Garrett C., Santi D. V. A rapid and sensitive high pressure liquid chromatography assay for deoxyribonucleoside triphosphates in cell extracts. Anal Biochem. 1979 Nov 1;99(2):268–273. doi: 10.1016/s0003-2697(79)80005-6. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Green H., Chan T. Pyrimidine starvation induced by adenosine in fibroblasts and lymphoid cells: role of adenosine deaminase. Science. 1973 Nov 23;182(4114):836–837. doi: 10.1126/science.182.4114.836. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S. Apparent suicide inactivation of human lymphoblast S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine and adenine arabinoside. A basis for direct toxic effects of analogs of adenosine. J Biol Chem. 1979 Jan 10;254(1):22–25. [PubMed] [Google Scholar]
- Hershfield M. S., Kredich N. M., Ownby D. R., Ownby H., Buckley R. In vivo inactivation of erythrocyte S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine in adenosine deaminase-deficient patients. J Clin Invest. 1979 Apr;63(4):807–811. doi: 10.1172/JCI109367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield M. S., Krodich N. M. S-adenosylhomocysteine hydrolase is an adenosine-binding protein: a target for adenosine toxicity. Science. 1978 Nov 17;202(4369):757–760. doi: 10.1126/science.715439. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Seegmiller J. E. Regulation of de novo purine biosynthesis in human lymphoblasts. Coordinate control of proximal (rate-determining) steps and the inosinic acid branch point. J Biol Chem. 1976 Dec 10;251(23):7348–7354. [PubMed] [Google Scholar]
- Hershfield M. S., Snyder F. F., Seegmiller J. E. Adenine and adenosine are toxic to human lymphoblast mutants defective in purine salvage enzymes. Science. 1977 Sep 23;197(4310):1284–1287. doi: 10.1126/science.197600. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Spector E. B., Seegmiller J. E. Purine synthesis and excretion in mutants of the WI-L2 human lymphoblastoid line deficient in adenosine kinase (AK) and adenine phosphoribosyltransferase (APRT). Adv Exp Med Biol. 1977;76A:303–313. doi: 10.1007/978-1-4613-4223-6_38. [DOI] [PubMed] [Google Scholar]
- KLENOW H. Further studies on the effect of deoxyadenosine on the accumulation of deoxyadenosine triphosphate and inhibition of deoxyribonucleic acid synthesis in Ehrlich ascites tumor cells in vitro. Biochim Biophys Acta. 1962 Dec 31;61:885–896. doi: 10.1016/0926-6550(62)90005-1. [DOI] [PubMed] [Google Scholar]
- KLENOW H. On the effect of some adenine derivatives on the incorporation in vitro of isotopically labelled compounds into the nucleic acids of Ehrlich ascites tumor cells. Biochim Biophys Acta. 1959 Oct;35:412–421. doi: 10.1016/0006-3002(59)90391-9. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Hershfield M. S. S-adenosylhomocysteine toxicity in normal and adenosine kinase-deficient lymphoblasts of human origin. Proc Natl Acad Sci U S A. 1979 May;76(5):2450–2454. doi: 10.1073/pnas.76.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Martin D. V., Jr Role of S-adenosylhomocysteine in adenosinemediated toxicity in cultured mouse T lymphoma cells. Cell. 1977 Dec;12(4):931–938. doi: 10.1016/0092-8674(77)90157-x. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Hodge L. D. Gene expression in synchronized lymphocytes: studies on the control of synthesis of immunoglobulin polypeptides. J Cell Physiol. 1971 Apr;77(2):265–276. doi: 10.1002/jcp.1040770215. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Buell D. N., Creech C., Hirshaut Y., Silverberg H. Further characterization of the WI-L1 and WI-L2 lymphoblastoid lines. J Natl Cancer Inst. 1971 Mar;46(3):647–654. [PubMed] [Google Scholar]
- Lowe J. K., Gowans B., Brox L. Deoxyadenosine metabolism and toxicity in cultured L5178Y cells. Cancer Res. 1977 Sep;37(9):3013–3017. [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966 Oct 25;241(20):4802–4809. [PubMed] [Google Scholar]
- REICHARD P., CANELLAKIS Z. N., CANELLAKIS E. S. Studies on a possible regulatory mechanism for the biosynthesis of deoxyribonucleic acid. J Biol Chem. 1961 Sep;236:2514–2519. [PubMed] [Google Scholar]
- Uberti J., Lightbody J. J., Johnson R. M. The effect of nucleosides and deoxycoformycin on adenosine and deoxyadenosine inhibition of human lymphocyte activation. J Immunol. 1979 Jul;123(1):189–193. [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Walker R. D., Duerre J. A. S-adenosylhomocysteine metabolism in various species. Can J Biochem. 1975 Mar;53(3):312–319. doi: 10.1139/o75-044. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Carson D. A., Seegmiller J. E. Adenosine kinase initiates the major route of ribavirin activation in a cultured human cell line. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3042–3044. doi: 10.1073/pnas.75.7.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann R. L., Mitchell B. S., Edwards N. L., Fox I. H. Biochemical basis for differential deoxyadenosine toxicity to T and B lymphoblasts: role for 5'-nucleotidase. Proc Natl Acad Sci U S A. 1979 May;76(5):2434–2437. doi: 10.1073/pnas.76.5.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]



