Abstract
Objective
To determine the role of bone marrow biopsy (BMBX), performed in association with comprehensive blood and imaging tests, in the evaluation of patients with fever of unknown origin (FUO).
Patients and Methods
We reviewed the medical records of 475 hospitalized patients who underwent BMBX in our medical center from January 1, 2005, to April 30, 2010. We identified 75 patients who fulfilled the accepted classic Petersdorf criteria for FUO. All patients underwent in-hospital investigation for fever, including chest and abdominal computed tomography.
Results
In 20 patients (26.7%), BMBX established the final diagnosis. Sixteen patients had hematologic disorders, including 8 patients with non-Hodgkin lymphoma, 2 with acute leukemia, 1 with multiple myeloma, 1 with myelodysplastic syndrome, and 4 with myeloproliferative disorders. The remaining patients with diagnostic BMBX specimens had solid tumors (2 patients), granulomatous disease (1 patient), and hemophagocytic syndrome (1 patient). Multivariate analysis revealed the following as the significant positive predictive parameters for a diagnostic BMBX specimen: male sex (odds ratio [OR], 7.35; 95% confidence interval [CI], 1.19-45.45), clinical lymphadenopathy (OR, 21.98; 95% CI, 1.97-245.66), anemia (OR, 2.21; 95% CI, 1.28-3.80), and increased lactate dehydrogenase levels (OR, 1.003; 95% CI, 1.001-1.006).
Conclusion
Bone marrow biopsy is still a useful ancillary procedure for establishing the diagnosis of FUO, particularly if used in the appropriate clinical setting. Clinical and laboratory parameters associated with hematologic disease are predictive of a diagnostic BMBX specimen in patients with FUO.
Even in the current era of widespread use of advanced medical technologies, the investigation of fever of unknown origin (FUO) still remains a major diagnostic challenge for many physicians. Not much has changed in the past 5 decades since Petersdorf and Beeson1 established the working definition of FUO, namely, an illness of more than 3 weeks' duration, accompanied by a temperature greater than 38.3°C on several occasions, the cause of which was uncertain after 1 week of in-hospital investigation.
Diagnosing FUO is difficult for both patients and physicians because the spectrum of diseases causing FUO is wide and includes numerous conditions, entailing many noninvasive and invasive diagnostic procedures.2-10 The use of bone marrow biopsy (BMBX) has been traditionally considered a second-line procedure to achieve diagnosis because of the invasive nature of the procedure. Nevertheless, BMBX was shown in earlier studies to be a useful but limited adjunct to the clinical work-up of FUO. These reported studies were mostly undertaken in patients with human immunodeficiency virus (HIV) infection5,9 and in those suspected of having mycobacterial disease.11 A recent study conducted in hospitalized patients identified anemia and thrombocytopenia as positive predictors for a diagnostic BMBX specimen.12 However, the exact role for BMBX in the evaluation of patients who have already undergone extensive blood tests and computed tomography (CT) of the chest and abdomen has not yet been defined.
We present the diagnostic yield of BMBX in the evaluation of 75 patients with prolonged fever who were admitted to a tertiary medical center. Our results identify simple clinical and laboratory parameters as predictive indicators that increase the diagnostic yield of BMBX in the modern investigation of FUO. We believe that implementing these predictive laboratory findings will aid in optimal selection of patients best suited for BMBX as part of the clinical work-up of patients with FUO.
Patients and Methods
In this retrospective study we reviewed the medical records of 475 consecutive patients who underwent BMBX during their hospitalization in our medical center from January 1, 2005, to April 30, 2010. The Tel Aviv Sourasky Medical Center is a busy tertiary, 1100-bed university hospital in Tel Aviv, Israel. All the patients included in this study were admitted to 1 of the 9 general internal medicine wards. The study was approved by the local institutional review board.
Enrollment Criteria
To be included in the study patients had to meet 3 criteria. First, they had to meet the 2 primary criteria of the FUO definition set by Petersdorf and Beeson1: (1) duration of illness of more than 3 weeks before diagnosis and (2) repeatedly documented increased body temperature of more 38.3°C. Second, BMBX had to have been performed as part of the evaluation for prolonged fever, preceded by CT. Third, the patient had to have no medical history associated with current immunosuppression. Patients were excluded from this study if they were known to have HIV infection or neutropenia (white blood cell count <1.0 × 109/L and/or granulocyte count <0.50 × 109/L [to convert white blood cells and granulocytes to /μL, divide by 0.001]), had undergone active immunosuppressive therapy or solid organ transplant, or had hypogammaglobulinemia (IgG <50%). All the patients were older than 18 years.
Diagnostic Work-Up
Diagnostic work-up included a standardized medical history, physical examination, routine blood tests, urinalysis, blood and urine cultures, chest radiography, and diagnostic contrast-enhanced chest, abdominal, and pelvic CT with submillimeter spatial resolution. The routine blood tests included complete blood cell count and differential; routine blood chemistry analysis; erythrocyte sedimentation rate or C-reactive protein; electrolytes and kidney function tests; liver enzymes and total bilirubin; alkaline phosphatase, γ-glutamyl transpeptidase, and lactate dehydrogenase (LDH); antinuclear antibodies, antineutrophil cytoplasmic antibodies, and rheumatoid factor; and serologic tests for cytomegalovirus, Epstein-Barr virus, and HIV. In some patients with appropriate clinical clues, the investigation was extended to include serologic tests, including for Q fever, Brucella, Bartonella, and others.
The CT results were initially categorized either as normal or abnormal. Abnormal results were further characterized as being either “suggestive of a malignant neoplasm,” namely, those studies that showed tumor masses suggestive of disease or prominent lymphadenopathy, splenomegaly, or hepatosplenomegaly. Alternatively, CT studies were categorized as “suggestive of infection” if they included one of the following: pulmonary infiltrates, serous collections, or signs of colitis or inflammation in other organs, such as bone or soft tissues. Studies that demonstrated ascites or nonspecific findings, such as slightly enlarged lymph nodes, spleen, or liver, were categorized as “others.”
Bone marrow biopsies were performed by puncture of the posterior iliac crest using a T-lock bone marrow biopsy needle (Angiotech, Gainesville, FL). Sections were immersed in formalin and processed in the pathology department. Specimens were fixed and decalcified, and slides were made and routinely stained with hematoxylin-eosin. When appropriate, immunoperoxidase stains, performed according to standard techniques, were performed to diagnose hematologic malignant neoplasms.
Statistical Analyses
The quantitative data were expressed as mean ± SD and the categorical data with their percentages. To compare the results of the 2 subgroups (diagnostic vs nondiagnostic), we used (1) the t test for independent groups and the nonparametric Mann-Whitney test in the case of quantitative data and (2) the χ2 test and Fisher exact test in the case of categorical data. The P values for the comparison between the 2 subgroups are reported. We also used the multivariate approach by applying the multivariate logistic regression model to examine the effect of several factors on the diagnostic vs nondiagnostic status. This model was used in a stepwise manner (the backward stepwise method) for all the potential predictors and with age and sex forced into the final model. Use of the multivariate logistic regression allows us to estimate the odds ratio (OR) of each of the predictors, which is the magnitude of the effect of the predictor variable on the outcome, controlled for the effect of the other predictors. The ORs for each predictor were reported with their P values and associated 95% confidence intervals (CIs). All analyses were performed using SPSS statistical software, version 18.0.0 (SPSS Inc, Chicago, IL).
Results
We reviewed the medical records of 475 patients who underwent BMBX at our medical center from January 1, 2005, to April 30, 2010. In 89 patients the indication for BMBX was prolonged fever. Of these patients, 75 were eligible for inclusion using the Petersdorf criteria. The patient selection algorithm is presented in Figure 1.
FIGURE 1.
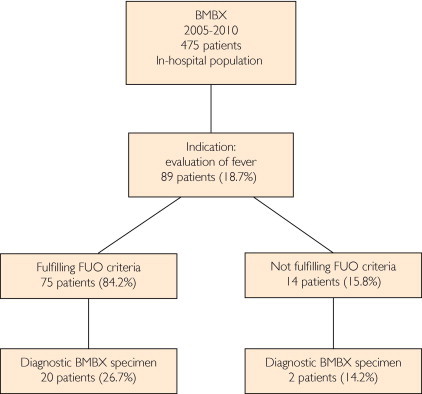
Patient selection algorithm. BMBX = bone marrow biopsy; FUO = fever of unknown origin.
Baseline characteristics of the study population are summarized in Table 1. Our study population showed an almost equal male-female distribution (37 men and 38 women), with a mean age of 59.6 years (range, 21-85 years). The median duration of fever was 66.5 days (range, 21-548 days). Notably, the B symptoms of night sweats and weight loss (excluding fever) were common in the study population (54.7%).
TABLE 1.
Baseline Characteristics of the Study Patients
| Characteristic | Finding (N=75) |
|---|---|
| Age, mean ± SD (y) | 59.6±17.9 |
| Sex | |
| Male | 37 (49.3) |
| Female | 38 (50.7) |
| Fever duration, mean ± SD (d) | 66.5±88.6 |
| B symptoms (exclusive of fever) | 41 (54.7) |
| Pruritus | 6 (8.0) |
| Rash | 7 (9.3) |
| Clinical lymphadenopathy | 12 (16.0) |
| Clinical splenomegaly | 14 (18.7) |
| Clinical hepatosplenomegaly | 17 (22.7) |
| Abnormal chest or abdomen CT | 53 (70.7) |
| Suspected malignant neoplasm on chest or abdomen CT | 38 (50.7) |
| Diagnostic BMBX specimen | 20 (26.7) |
Data are presented as No. (percentage) of patients unless otherwise indicated. Splenomegaly indicates hepatosplenomegaly or splenomegaly alone. Hepatosplenomegaly indicates hepatomegaly, splenomegaly, or both. BMBX = bone marrow biopsy; CT = computed tomography.
Table 2 lists the conditions diagnosed as causing the FUO as determined during the entire inpatient work-up. Hematologic disorders and particularly malignant neoplasms were the most common diagnoses (41.3%), followed by inflammatory (25.3%) and infectious (14.7%) diseases, which together comprised more than 80% of the total patient population whose conditions were diagnosed by BMBX. Notably, solid tumors were an uncommon cause of FUO (5.3%) and were mostly metastatic. Only 5 patients (6.7%) had undiagnosed conditions after this extensive work-up.
TABLE 2.
Final Diagnoses of the Study Patients
| Diagnosis | No. (%) of patients (N=75) |
|---|---|
| Hematologic diseases | |
| Leukemia | |
| Acute lymphoblastic leukemia | 1 |
| Acute myeloid leukemia | 1 |
| Non-Hodgkin lymphoma | |
| Diffuse large B-cell lymphoma | 7 |
| Peripheral T-cell lymphoma | 3 |
| Small lymphocytic lymphoma | 4 |
| Low-grade lymphoma, others | 3 |
| Hodgkin disease | 5 |
| Myeloproliferative disease | |
| Primary myelofibrosis | 3 |
| Chronic myeloid leukemia | 1 |
| Myelodysplastic syndrome | 1 |
| Multiple myeloma | 1 |
| Lymphoproliferative disorder, unspecified | 1 |
| Total | 31 (41.3) |
| Inflammatory disorders | |
| Vasculitis | |
| Temporal arteritis | 1 |
| Polymyalgia rheumatica | 1 |
| Polyarteritis nodosa | 1 |
| pANCA-associated vasculitis | 1 |
| Behçet syndrome | 1 |
| Still disease | 2 |
| Seronegative arthritis | 1 |
| Relapsing perichondritis | 1 |
| Sjögren syndrome | 1 |
| Granulomatous disease | 1 |
| Steroid responsive disorders, unspecified | 8 |
| Total | 19 (25.3) |
| Infectious disorders | |
| Viral infection | |
| Cytomegalovirus infection | 1 |
| Viral disease, unspecified | 1 |
| Bacterial infection | |
| Tuberculosis | 1 |
| Q fever | 1 |
| Nocardiosis | 1 |
| Methicillin-resistant Staphylococcus aureus infection | 1 |
| Neisseria sicca bacteremia | 1 |
| Fungal infection | |
| Pneumocistis carinii pneumonia in HIV-positive patient | 1 |
| Others | |
| Pneumonia | 1 |
| Sinusitis | 1 |
| Oral cavity infection | 1 |
| Total | 11 (14.7) |
| Solid malignant neoplasms | |
| Metastatic prostate cancer | 1 |
| Metastatic pancreatic cancer | 1 |
| Metastatic renal cell carcinoma | 1 |
| Metastatic lung or upper gastrointestinal carcinoma | 1 |
| Total | 4 (5.3) |
| Miscellaneous | |
| Histiocytosis X | 2 |
| Littoral cell angioma of spleen | 1 |
| Hemophagocytic syndrome | 1 |
| Total | 4 (5.3) |
| No diagnosis | 5 (6.7) |
| Lost to follow-up | 1 (1.3) |
HIV = human immunodeficiency virus; pANCA = perinuclear antineutrophil cytoplasmic antibody.
In 20 patients (26.7%) a final diagnosis was established after a BMBX. Table 3 details the specific diagnoses gained from diagnostic BMBX specimens. Hematologic disorders were the predominant group, comprising 16 (80.0%) of 20 patients with definitive histopathologic findings. Non-Hodgkin lymphomas and myelofibrosis constituted most of the patient group. Remarkably, we did not identify Hodgkin lymphoma or infectious diseases in patients undergoing BMBX.
TABLE 3.
Final Diagnoses Determined by Diagnostic Bone Marrow Biopsy Specimens
| Diagnosis | No. of patients |
|---|---|
| Hematologic disorders | |
| Leukemia | |
| ALL | 1 |
| AML M0 | 1 |
| Non-Hodgkin lymphoma | |
| SLL | 4 |
| DLBCL | 3 |
| T-cell lymphoma | 1 |
| Hodgkin lymphoma | 0 |
| Multiple myeloma | 1 |
| MDS | 1 |
| MPD | |
| Myelofibrosis | 3 |
| CML | 1 |
| Total | 16 |
| Solid malignant neoplasm | |
| Metastatic lung or upper gastrointestinal carcinoma | 1 |
| Metastatic prostate carcinoma | 1 |
| Total | 2 |
| Other diagnoses | |
| Granuloma | 1 |
| Hemophagocytic syndrome | 1 |
| Total | 2 |
ALL = acute lymphocytic leukemia; AML = acute myeloid leukemia; CML = chronic myeloid leukemia; DLBCL = diffuse large B-cell lymphoma; MDS = myelodysplastic syndrome; MPD = myeloproliferative disease; SLL = small lymphocytic lymphoma.
To ascertain whether certain clinical or laboratory parameters could predict whether a BMBX specimen could be diagnostic, we performed a univariate analysis of clinical and laboratory parameters comparing the group of patients with a diagnostic BMBX specimen with the group of patients with a nondiagnostic BMBX specimen (Table 4). A marginally significant difference was noted regarding B symptoms, which were surprisingly less prominent in the diagnostic group (P=.07), whereas clinical splenomegaly was significantly more frequently present in the diagnostic group (P=.04). Notably, clinical lymphadenopathy was also more frequent in the diagnostic group, although only marginally significant (P=.07).
TABLE 4.
Univariate Analysis of Patients With a Diagnostic Bone Marrow Biopsy Specimena
| Variableb | Diagnostic (n=20) | Nondiagnostic (n=55) | P value |
|---|---|---|---|
| Age, mean ± SD (y) | 64.8±15.5 | 57.6±18.3 | .13 |
| Sex | .31 | ||
| Male | 12 (60.0) | 25 (45.5) | |
| Female | 8 (40.0) | 30 (54.6) | |
| Symptoms | |||
| Fever duration, mean ± SD (d) | 54.3±51.0 | 70.6±98.3 | .49 |
| B symptoms (exclusive of fever) | 7 (35.0) | 34 (61.8) | .07 |
| Pruritus | 0 | 6 (10.9) | .18 |
| Signs and imaging | |||
| Clinical lymphadenopathy | 6 (30.0) | 6 (10.9) | .07 |
| Clinical splenomegaly | 7 (35.0) | 7 (12.7) | .04 |
| Clinical hepatosplenomegaly | 7 (35.0) | 9 (16.4) | .11 |
| Abnormal chest radiography findings | 2 (10.0) | 10 (18.2) | .50 |
| Chest or abdomen CT “suggestive of malignancy” | 13 (65.0) | 28 (50.9) | .31 |
| Laboratory parameters, mean ± SD | |||
| C-reactive protein, mg/L | 129.1±77.5 | 110.2±83.4 | .38 |
| White blood cells, /μL | 8.7±5.6 | 10.1±8.0 | .49 |
| Hemoglobin, g/dL | 9.6±1.8 | 11.0±1.5 | .001 |
| Platelets, ×109/μL | 233.5±150 | 342.0±214 | .04 |
| Serum urea nitrogen, mg/dL | 16.2±6 | 16.8±7.5 | .46 |
| Creatinine, mg/dL | 1.0±0.2 | 1.0±0.2 | .60 |
| Total protein, g/dL | 6.2±2.3 | 6.6±0.9 | .20 |
| Globulins, g/dL | 3.2±1.4 | 3.1±0.7 | .98 |
| Albumin, g/dL | 3.3±0.8 | 3.5±0.6 | .17 |
| LDH, U/L | 725.9±508 | 542.1±320 | .07 |
| Total bilirubin, mg/dL | 0.7±0.6 | 0.5±0.5 | .09 |
| ALT, U/L | 32.1±21.7 | 37.2±33.9 | .54 |
| Alkaline phosphatase, U/L | 185.5±174.5 | 137.9±170.8 | .29 |
Data are presented as No. (percentage) of patients unless otherwise indicated. ALT = alanine aminotransferase; BUN = blood urea nitrogen; CT = computed tomography; LDH = lactate dehydrogenase.
To convert C-reactive protein to nmol/L, multiply by 9.524; to convert white blood cells to ×109/L, multiply by 0.001; to convert hemoglobin values to g/L, multiply by 10; to convert platelets to ×109/L, multiply by 1; to convert BUN to mmol/L, multiply by 0.357; to convert creatinine values to μmol/L, multiply by 88.4; to convert protein to g/L, multiply by 10; to convert globulins to g/L, multiply by 10; to convert albumin to g/L, multiply by 10; to convert LDH to μkat/L, multiply by 0.0167; to convert bilirubin to μmol/L, multiply by 17.104; to convert ALT to μkat/L, multiply by 0.0167; to convert alkaline phosphatase to μkat/L, multiply by 0.0167.
Laboratory parameters that were significantly different between the groups were the hemoglobin and platelet levels, which were lower in the diagnostic group (P=.001 and P=.04, respectively). The 2 groups did not differ significantly with respect to age, sex, duration of fever, presence of pruritus, clinical lymphadenopathy, and hepatosplenomegaly. Importantly, CT scans with positive findings, including those with findings “suggestive of malignancy,” did not differ significantly between the 2 groups.
To further investigate which parameters could be independently predictive of a diagnostic BMBX specimen, we performed a multivariate logistic regression analysis. In this analysis we included age, sex, and all parameters that in the univariate analysis reached a P<.10. Table 5 gives the results of the multivariate analysis identifying clinical lymphadenopathy (OR, 21.98; 95% CI, 1.97-245.66; P=.01) and male sex (OR, 7.35; 95% CI, 1.19-45.45; P=.03) to be associated independently with a diagnostic BMBX specimen. Lower hemoglobin values (OR, 2.21; 95% CI, 1.28-3.80; P=.004) and increased LDH values (OR, 1.003; 95% CI, 1.001-1.006; P=.01) emerged as the laboratory parameters indicative of a diagnostic BMBX specimen. Notably, the absence of B symptoms (exclusive of fever) was associated with the diagnostic group (OR, 0.10; 95% CI, 0.02-0.59; P=.01). Age was not found to be significant in the multivariate analysis (OR, 1.04; 95% CI, 1.00-1.09; P=.08). Figure 2 illustrates the sequential decreased yield of BMBX as it relates to increased hemoglobin levels. There was a significant trend of a decreased diagnostic yield of BMBX when hemoglobin levels were higher (P=.04).
TABLE 5.
Multivariate Analysis for Prognostic Determinants of a Diagnostic Bone Marrow Biopsy Specimen in Work-up for Fever of Unknown Origin
| Determinant | Odds ratio (95% CI) | P value |
|---|---|---|
| Clinical lymphadenopathy | 21.98 (1.97-245.66) | .01 |
| Male sex | 7.35 (1.19-45.45) | .03 |
| Low hemoglobin level | 2.21 (1.28-3.80) | .004 |
| Lactate dehydrogenase | 1.003 (1.001-1.006) | .01 |
| B symptoms (excluding fever) | 0.10 (0.02-0.59) | .01 |
| Age | 1.04 (1.00-1.09) | .08 |
In this analysis the following parameters were included: age, sex, B symptoms, clinical lymphadenopathy, splenomegaly, hemoglobin level, platelet count, lactate dehydrogenase level, and bilirubin level. CI = confidence interval.
FIGURE 2.
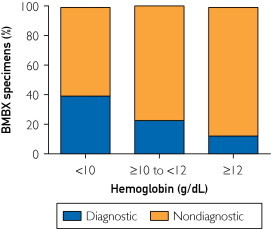
The rate of diagnostic and nondiagnostic bone marrow biopsy (BMBX) specimens in patients with hemoglobin levels of 12 g/dL or higher (to convert to g/L, multiply by 10) (n=16), between 10 and 12 g/dL (n=31), and less than 10 g/dL (n=28).
Discussion
In this study we attempted to determine the role of BMBX in the setting of a population of hospitalized, immunocompetent patients using all the routine diagnostic means available in most medical centers, including extensive blood tests combined with CT of the chest and abdomen.
Because of its invasive nature, BMBX is not always used as a first-line procedure in most cases of FUO, and accordingly only a few reports have addressed the utility of this readily available tool for diagnosis. Indeed, some authors do not even consider BMBX to be part of the standard routine algorithm for the diagnosis of FUO.13 In the present study, the rate of diagnostic biopsy specimens compares favorably with that reported in previous publications, which was in the range of 14% to 20%.9,14,15 We cautiously believe that we may attribute our modest increment in diagnostic yield of BMBX to a rate of 26% in our patient population to the fact that these patients had already undergone extensive work-up, including imaging studies. Performing CT and other imaging techniques as part of the early work-up of FUO can guide the treating physician to additional diagnostic procedures other than BMBX,2,13 and as a consequence the patient population undergoing BMBX may differ from that in the past. These figures are also complemented by a marked decrease in the rate of patients with undiagnosed conditions in our cohort of 6.6%, compared with the older and other more recent studies, which had higher proportions of patients with undiagnosed conditions.
We found by univariate analysis that clinical splenomegaly, lower hemoglobin level, and platelet count were associated with a positive diagnostic BMBX specimen; however, in the multivariate analysis, our findings extended to include clinical lymphadenopathy, male sex, lower hemoglobin levels, increased LDH levels, and the absence of B symptoms (excluding fever) as independent positive predictive parameters. Our results are compatible with those of a recent study by Hot et al,12 who also showed lower hemoglobin and platelets levels to be predictive of a diagnostic and “beneficial” BMBX specimen. In the latter study, the inclusion criteria included a minimal diagnostic work-up for FUO, and CT was not obligatory. Because CT is currently one of the basic first investigations used for FUO, the yield of BMBX in the context of the modern work-up algorithm of FUO had to be addressed. Despite the methodologic differences between our study and that of Hot et al, the conclusions were similar, probably because in cases in which BMBX is still indicated after CT, this imaging modality does not really add to the yield of diagnostic BMBX, irrespective of whether the findings were normal or pathologic.
Considering that most of our diagnostic BMBXs detected a diagnosis of hematologic disorders, it stands to reason that laboratory and clinical parameters associated with bone marrow involvement, namely, anemia, lower platelet count, increased LDH values, and clinical lymphadenopathy, should be considered as markers associated with a beneficial BMBX specimen. Interestingly, our study indicates that BMBX is mostly effective in establishing the diagnosis of malignant neoplasms, which constituted 14 of the 20 diagnostic BMBX specimens. It may also be inferred that BMBX has almost no role in cases of FUO in which clinical data suggest an infectious or inflammatory origin. Counterintuitively, our group of diagnostic BMBX findings, which as posited herein consisted mainly of hematologic malignant neoplasms, was marked by the absence of B symptoms. A possible explanation for this finding may lie in the fact that unlike most of the other parameters in this study, this variable is subjective because it is reported by the patients themselves. Variability in the patients' reports regarding night sweats and fever may account for this finding.
Our results confirm the assertion made by Hot and colleagues that BMBX should be considered early in the evaluation of patients with thrombocytopenia or anemia in whom a primary hematologic disease is suspected.12 We strongly believe that BMBX should not be delayed in these patients because some of the origins are of an aggressive neoplastic nature, requiring early therapeutic intervention. This finding is indeed important because 52% to 100% of patients with malignant tumor–related FUO will die within 5 years after diagnosis is made.14,16 A possible drawback to the routine use of BMBX is that the procedure is for the most part performed in central medical centers. Decidedly, because of its invasive nature and limited yield, BMBX should not be used indiscriminately in the work-up of FUO, yet our data suggest certain clinical and laboratory indexes that should alert the physician to an increased probability for a diagnostic BMBX specimen.
Conclusion
At present, the cause of FUO remains difficult to determine and evades diagnosis in many patients despite considerable advances in medical diagnostics. The current study shows that clinical and laboratory parameters indicative of hematologic disease predict for a diagnostic BMBX specimen in the work-up of FUO. Bone marrow biopsy is an inexpensive tool at the disposal of most medical centers, and in the appropriate clinical setting it should continue to constitute part of the modern diagnostic work-up of FUO. Future research is warranted to better define the routine implementation of BMBX into the work-up of FUO.
Acknowledgment
Drs Ben-Baruch and Canaani contributed equally to this work.
Footnotes
Dr. Braunstein is an independent statistical consultant for biomedical research, Tel Aviv, Israel.
References
- 1.Petersdorf R.G., Beeson P.B. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 1961;40:1–30. doi: 10.1097/00005792-196102000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Quinn M.J., Sheedy P.F., II, Stephens D.H., Hattery R.R. Computed tomography of the abdomen in evaluation of patients with fever of unknown origin. Radiology. 1980;136(2):407–411. doi: 10.1148/radiology.136.2.7403516. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T., Herrera M.F., Onuma L. Diagnostic laparotomy in fever of unknown origin. Rev Invest Clin. 1991;43(1):25–30. [PubMed] [Google Scholar]
- 4.Holtz T., Moseley R.H., Scheiman J.M. Liver biopsy in fever of unknown origin: a reappraisal. J Clin Gastroenterol. 1993;17(1):29–32. doi: 10.1097/00004836-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Benito N., Nunez A., de Gorgolas M. Bone marrow biopsy in the diagnosis of fever of unknown origin in patients with acquired immunodeficiency syndrome. Arch Intern Med. 1997;157(14):1577–1580. [PubMed] [Google Scholar]
- 6.Meller J., Ivancevic V., Conrad M., Gratz S., Munz D.L., Becker W. Clinical value of immunoscintigraphy in patients with fever of unknown origin. J Nucl Med. 1998;39(7):1248–1253. [PubMed] [Google Scholar]
- 7.Volk E.E., Miller M.L., Kirkley B.A., Washington J.A. The diagnostic usefulness of bone marrow cultures in patients with fever of unknown origin. Am J Clin Pathol. 1998;110(2):150–153. doi: 10.1093/ajcp/110.2.150. [DOI] [PubMed] [Google Scholar]
- 8.Meller J., Altenvoerde G., Munzel U. Fever of unknown origin: prospective comparison of [18F]FDG imaging with a double-head coincidence camera and gallium-67 citrate SPET. Eur J Nucl Med. 2000;27(11):1617–1625. doi: 10.1007/s002590000341. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed S., Siddiqui A.K., Mehrotra B. Diagnostic yield of bone marrow examination in fever of unknown origin. Am J Med. 2003;115(7):591–592. doi: 10.1016/s0002-9343(03)00450-9. [DOI] [PubMed] [Google Scholar]
- 10.Keidar Z., Gurman-Balbir A., Gaitini D., Israel O. Fever of unknown origin: the role of 18F-FDG PET/CT. J Nucl Med. 2008;49(12):1980–1985. doi: 10.2967/jnumed.108.054692. [DOI] [PubMed] [Google Scholar]
- 11.Riley U.B., Crawford S., Barrett S.P., Abdalla S.H. Detection of mycobacteria in bone marrow biopsy specimens taken to investigate pyrexia of unknown origin. J Clin Pathol. 1995;48(8):706–709. doi: 10.1136/jcp.48.8.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hot A., Jaisson I., Girard C. Yield of bone marrow examination in diagnosing the source of fever of unknown origin. Arch Intern Med. 2009;169(21):2018–2023. doi: 10.1001/archinternmed.2009.401. [DOI] [PubMed] [Google Scholar]
- 13.Mourad O., Palda V., Detsky A.S. A comprehensive evidence-based approach to fever of unknown origin. Arch Intern Med. 2003;163(5):545–551. doi: 10.1001/archinte.163.5.545. [DOI] [PubMed] [Google Scholar]
- 14.Larson E.B., Featherstone H.J., Petersdorf R.G. Fever of undetermined origin: diagnosis and follow-up of 105 cases, 1970-1980. Medicine (Baltimore) 1982;61(5):269–292. [PubMed] [Google Scholar]
- 15.de Kleijn E.M., Vandenbroucke J.P., van der Meer J.W., Netherlands FUO Study Group Fever of unknown origin (FUO), I: a prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. Medicine (Baltimore) 1997;76(6):392–400. doi: 10.1097/00005792-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Kazanjian P.H. Fever of unknown origin: review of 86 patients treated in community hospitals. Clin Infect Dis. 1992;15(6):968–973. doi: 10.1093/clind/15.6.968. [DOI] [PubMed] [Google Scholar]


