Abstract
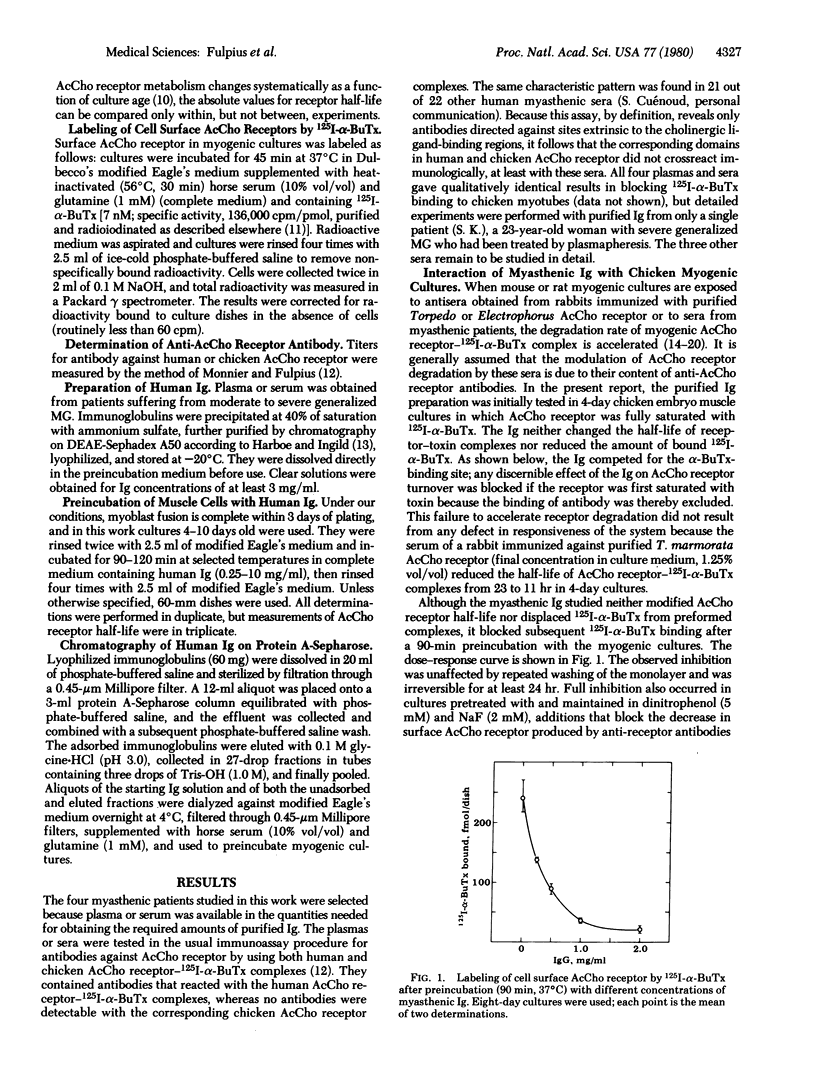
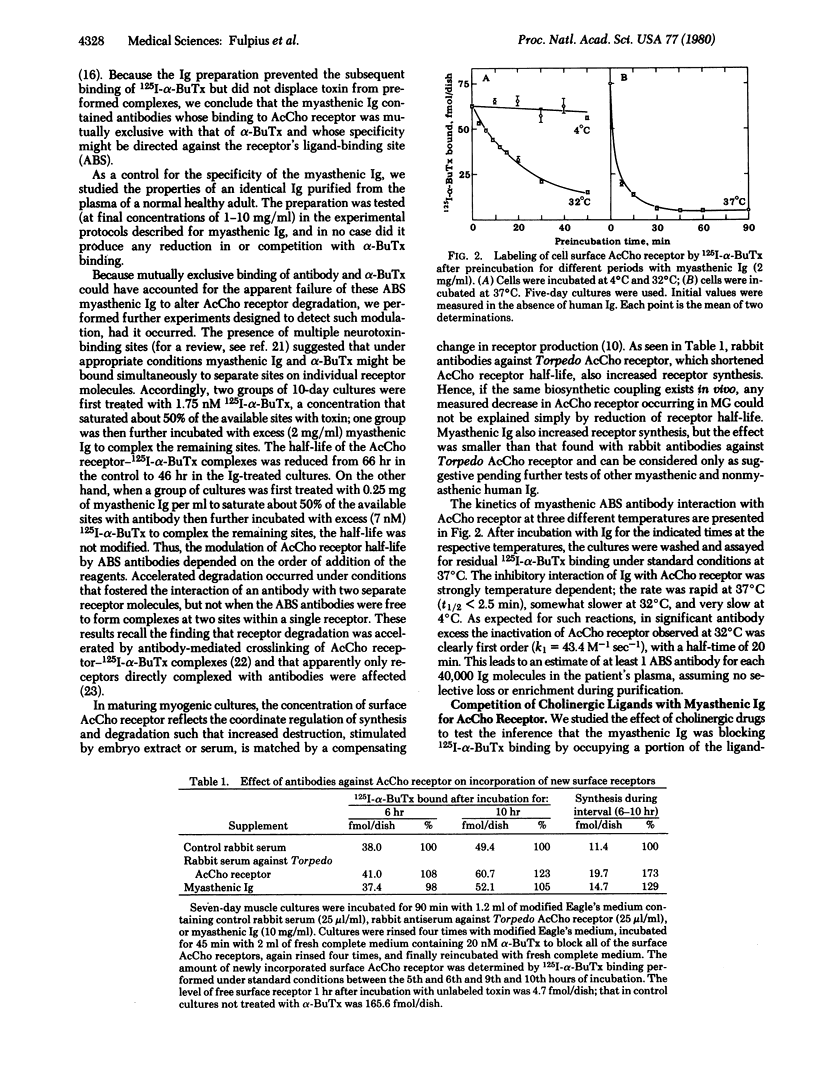
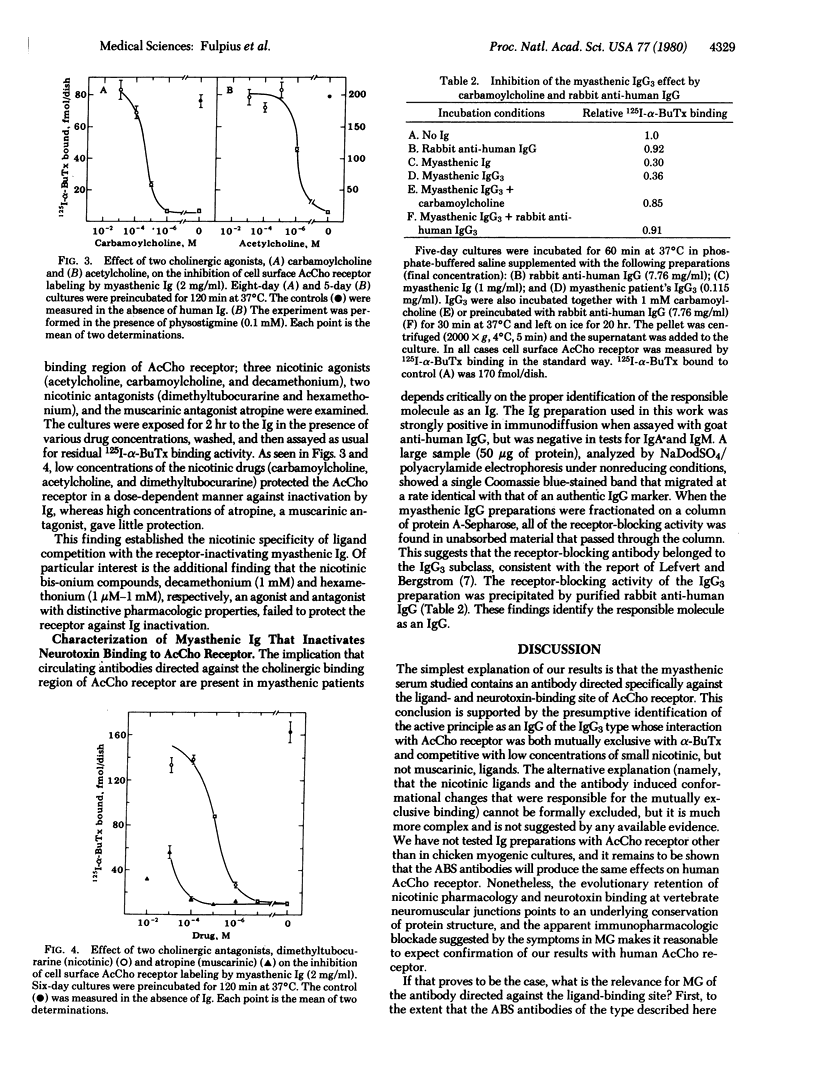
We have purified immunoglobulins from sera of myasthenic patients and have identified antibodies directed against the cholinergic ligand-binding site of the nicotinic acetylcholine receptor. In the serum of one patient analyzed in detail these antibodies belonged to the IgG3 class, and their effects were as follows: (i) In chicken embryo myogenic cultures, antibody binding was both competitive with 125I-labeled alpha-bungarotoxin and irreversible on a time scale of hours. (ii) 125I-Labeled alpha-bungarotoxin was not displaced by antibody from preformed complexes and, conversely, antibody was not displaced by toxin. (iii) Antibody binding was competitive with some, but not all, nicotinic agents. Thus, acetylcholine, carbamoylcholine, and dimethyltubocurarine competed effectively whereas decamethonium and hexamethonium did not, suggesting that the two classes of nicotinic ligands probably interact at different, nonoverlapping receptor subsites. (iv) There was no competitive binding between these antibodies and the muscarinic antagonist atropine. (v) Both this class of myasthenic immunoglobulins and rabbit antibodies raised against Torpedo acetylcholine receptors increased the rate of receptor degradation. However, synthesis and degradation remained coupled and there was a compensating increase in receptor synthesis. We propose that immunoglobulins directed against the ligand-binding site of acetylcholine receptors may account for the characteristic curare-like symptoms of early myasthenia and their response to cholinesterase inhibition, for the apparent decrease in receptors measurable by 125I-labeled alpha-bungarotoxin binding, and for initiating localized complement activation at the postsynaptic membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almon R. R., Appel S. H. Interaction of myasthenic serum globulin with the acetylcholine receptor. Biochim Biophys Acta. 1975 May 30;393(1):66–77. doi: 10.1016/0005-2795(75)90217-2. [DOI] [PubMed] [Google Scholar]
- Almon R. R., Appel S. H. Serum acetylcholine-receptor antibodies in myasthenia gravis. Ann N Y Acad Sci. 1976;274:235–243. doi: 10.1111/j.1749-6632.1976.tb47689.x. [DOI] [PubMed] [Google Scholar]
- Appel S. H., Anwyl R., McAdams M. W., Elias S. Accelerated degradation of acetylcholine receptor from cultured rat myotubes with myasthenia gravis sera and globulins. Proc Natl Acad Sci U S A. 1977 May;74(5):2130–2134. doi: 10.1073/pnas.74.5.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Kullberg R. W., Heinemann S. F. Human myasthenic sera reduce acetylcholine sensitivity of human muscle cells in tissue culture. Nature. 1977 May 19;267(5608):263–265. doi: 10.1038/267263a0. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Angus C. W., Adams R. N., Kao I. Effect of myasthenic patients' immunoglobulin on acetylcholine receptor turnover: selectivity of degradation process. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3422–3426. doi: 10.1073/pnas.75.7.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Angus C. W., Adams R. N., Kao I. Effect of myasthenic patients' immunoglobulin on acetylcholine receptor turnover: selectivity of degradation process. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3422–3426. doi: 10.1073/pnas.75.7.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D. B., Angus C. W., Adams R. N., Michelson J. D., Hoffman G. J. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978 May 18;298(20):1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- Drachman D. B. Myasthenia gravis (first of two parts). N Engl J Med. 1978 Jan 19;298(3):136–142. doi: 10.1056/NEJM197801192980305. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Lambert E. H., Howard F. M. Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc. 1977 May;52(5):267–280. [PubMed] [Google Scholar]
- Fambrough D. M., Drachman D. B., Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973 Oct 19;182(4109):293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- Heinemann S., Bevan S., Kullberg R., Lindstrom J., Rice J. Modulation of acetylcholine receptor by antibody against the receptor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3090–3094. doi: 10.1073/pnas.74.7.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S., Merlie J., Lindstrom J. Modulation of acetylcholine receptor in rat diaphragm by anti-receptor sera. Nature. 1978 Jul 6;274(5666):65–68. doi: 10.1038/274065a0. [DOI] [PubMed] [Google Scholar]
- Kao I., Drachman D. B. Myasthenic immunoglobulin accelerates acetylcholine receptor degradation. Science. 1977 Apr 29;196(4289):527–529. doi: 10.1126/science.850793. [DOI] [PubMed] [Google Scholar]
- Lefvert A. K., Bergström K. Immunoglobulins in myasthenia gravis: effect of human lymph IgG 3 and F (ab')2 fragments on a cholinergic receptor preparation from Torpedo marmorata. Eur J Clin Invest. 1977 Apr;7(2):115–119. doi: 10.1111/j.1365-2362.1977.tb01582.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Maelicke A., Fulpius B. W., Klett R. P., Reich E. Acetylcholine receptor. Responses to drug binding. J Biol Chem. 1977 Jul 25;252(14):4811–4830. [PubMed] [Google Scholar]
- Miskin R., Easton T. G., Maelicke A., Reich E. Metabolism of acetylcholine receptor in chick embryo muscle cells: effects of RSV and PMA. Cell. 1978 Dec;15(4):1287–1300. doi: 10.1016/0092-8674(78)90054-5. [DOI] [PubMed] [Google Scholar]
- Miskin R., Easton T. G., Reich E. Plasminogen activator in chick embryo muscle cells: induction of enzyme by RSV, PMA and retinoic acid. Cell. 1978 Dec;15(4):1301–1312. doi: 10.1016/0092-8674(78)90055-7. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Fulpius B. W. A radioimmunoassay for the quantitative evaluation of anti-human acetylcholine receptor antibodies in myasthenia gravis. Clin Exp Immunol. 1977 Jul;29(1):16–22. [PMC free article] [PubMed] [Google Scholar]
- Reiness C. G., Weinberg C. B., Hall Z. W. Antibody to acetylcholine receptor increases degradation of junctional and extrajunctional receptors in adult muscle. Nature. 1978 Jul 6;274(5666):68–70. doi: 10.1038/274068a0. [DOI] [PubMed] [Google Scholar]
- Zurn A. D., Fulpius B. W. Accessibility to antibodies of acetylcholine receptors in the neuromuscular junction. Clin Exp Immunol. 1976 Apr;24(1):9–17. [PMC free article] [PubMed] [Google Scholar]


