Abstract
Background
The strength of each heart beat and the stiffness of large arteries contribute to blood pressure (BP). When the large arteries are stiff and their resistance greater, the afterload increases and this may change the function of the heart. However, the relation between common carotid artery stiffness and heart function in hypertensive patients has not been clarified.
Methods
Two hundred and twenty hypertensive patients underwent transthoracic and carotid echocardiography. Measurements of local arterial stiffness were taken at the right common carotid artery level and stiffness parameter (β), pressure-strain elasticity modulus and intima-media thickness were calculated. Brachial cuff BP was measured just before starting the carotid study. The patients with any cardiovascular disease, diabetes mellitus, stroke, transient ischemic attack, or carotid stenosis were excluded.
Results
Carotid artery stiffness parameter (β) was correlated with age and left ventricular mass index (p < 0.005). Even though β was not correlated with LV systolic function, it was inversely correlated with diastolic function as measured by early mitral annular velocity. When the artery was stiffer, early mitral annular velocity (e') decreased (p < 0.001) and the index of left atrial (LA) pressure (early diastolic mitral inflow E velocity/e') increased (p = 0.001). In logistic regression, diastolic dysfunction was affected by age (beta -0.385, p = 0.001), LA volume index (beta 0.175, p = 0.013) and β (beta -0.273, p = 0.019).
Conclusion
In hypertensive patients, changes in carotid artery stiffness can affect the diastolic function, independent of age and LA volume index. Therefore, measurements and control of carotid stiffness can play an important role in the prevention of diastolic heart failure.
Keywords: Carotid stiffness, Diastolic function, Female
Introduction
Carotid arterial stiffness is the vessel wall's tendency to resist deformation by systolic blood pressure (SBP) during the cardiac cycle.1) The consequences of arterial stiffening include increased systolic and pulse pressure (PP), left ventricular (LV) hypertrophy, impaired myocardial perfusion, and small vessel degeneration in the brain and kidneys.2) Accordingly, arterial stiffness is emerging as a key risk factor for atherosclerosis, myocardial infarction, stroke, dementia, renal disease, and mortality.3) Recently, the European guidelines for the diagnosis and treatment of hypertension recommended the assessment of arterial stiffness as an evidence of target organ damage.4) It seems logical to suggest that impaired arterial compliance would be associated with ventricular dysfunction via atherosclerosis. Arterial stiffness is correlated with the presence and severity of atherosclerosis, and subclinical atherosclerosis is also associated with myocardial dysfunction.5) However, the relation between common carotid artery stiffness and heart function in hypertensive patients has not been clarified. Therefore, in this study, we sought to assess the relationship between carotid artery stiffness and heart function (systolic & diastolic) in hypertensive patients.
Methods
Study subjects
We reviewed approximately 300 hypertensive patients who had transthoracic and carotid echocardiography on the same day. Hypertension was defined based on SBP ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg calculated as the means of 2 blood pressure measurements, patient's self-report of previous diagnosis of hypertension, or the undertaking of medical treatment thereof. The patients with diabetes, dyslipidemia, history of cardiovascular disease (coronary artery disease, congestive heart failure, stroke, transient ischemic attack or intermittent claudication) and carotid artery stenosis were excluded.
For the calculation of the carotid stiffness parameters, the blood pressure (BP) taken before the carotid examination was entered into the program. The BP measurements taken before echocardiography were used for the calculation of PP (systolic BP - diastolic BP). Weight (in kilograms) and height (in meters) were measured using standard techniques, and body mass index (BMI) was calculated as body weight divided by height squared. Body surface area was calculated using the DuBois formula (0.20247 × height0.725 × weight0.425). This study was approved by the institutional ethics board, and an informed consent was obtained from all participants.
Two-dimensional and Doppler echocardiography
Echocardiography was performed with an ultrasound system (Vivid 7 GE Vingmed, Horten, Norway) with a 2-4-MHz transducer. Standard 2-dimensional measurements [end-diastolic and end-systolic dimensions, ventricular septum and posterior wall thickness, left atrial volume index (LAVI), left ventricular mass index (LVMI), left ventricular (LV) outflow tract diameter] including LV ejection fraction were taken with the patient in the left lateral position.6)
Through the apical 4 chamber window, a 1- to 2-mm pulsed Doppler sample volume was placed at the mitral valve tip, and mitral flow velocities was recorded for the duration of 5 to 10 cardiac cycles. The following parameters were obtained: peak velocity of early filling (E) and late (A) filling, deceleration time of the E wave velocity, and ratio of E over A.
Pulse Doppler tissue imaging was carried out in the four-chamber view at the septal mitral annular level. The peak velocity of myocardial systolic wave (S'), early peak (e') and atrial (A') diastolic wave (in centimeters per second), and the E/e' ratio were recorded.7),8)
Carotid stiffness
High resolution B-mode ultrasound imaging of the carotid arteries was performed using a GE scanner (with a 12-MHz transducer; Vivid 7 GE Vingmed, Horten, Norway) with patient in the supine position.5) Every effort was made to examine the participants in the morning after an overnight fasting and smoking cessation. The best acoustic window was identified with the jugular vein above the common carotid artery and a series of images were acquired over a 20 second period. Five to six cardiac cycles on average were used for the estimation of carotid diameters. BP was determined by upper arm sphygmomanometry during carotid artery measurements. Strain represents a ratio of the amount of stress deformation relative to the unstressed state, and stiffness is a dimensionless quantity that expresses the tendency of an individual's arteries to deform under a given change in blood pressure. We therefore defined strain as (Ds - Dd) / Dd, stiffness as STIFF (β index) = ln (Ps / Pd) / Strain, and pressure-strain elasticity modulus (Ep) as (pressure - strain elasticity modulus) = (Ps - Pd) / (Ds - Dd) / Dd, where Ps is systolic BP, Pd is diastolic BP, Ds is arterial systolic diameter, and Dd is arterial diastolic diameter.9)
The interobserver and intraobserver variabilities for measuring have been compared in 40 consecutive measurements, and were 4.2 and 3.4%, respectively.
Statistical analysis
We performed Pearson's correlation analysis to assess the univariate correlations between arterial stiffness parameters, intima-media thickness (IMT), and LV structure and functional variables. Pearson's correlation analysis was also used to evaluate whether age, PP, sex, and BMI were associated with arterial stiffness, systolic and diastolic function, and LV structure. Covariate analysis was then applied to make an adjustment for the effect of age as well as for the combined effects of age, PP, sex, and BMI. The defining level of statistical significance (p value) was set at 0.05. Data analysis was performed using SPSS version 10.0 (SPSS Inc., Chicago, IL, USA).
Results
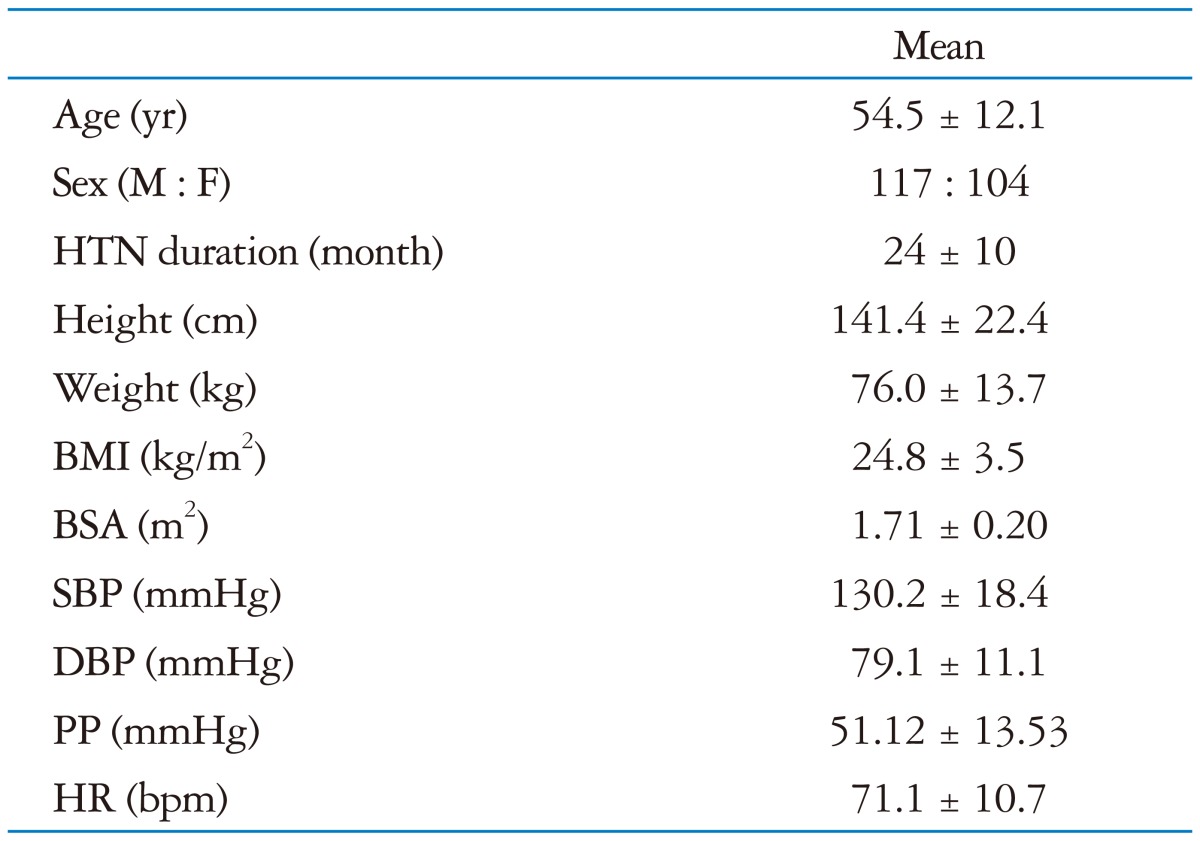
The main characteristics of the study population are reported in Table 1.
Table 1.
The main characteristics of the study population
The data are given as mean ± SD.
HTN: hypertension, BMI: body mass index, BSA: body surface area, SBP: systolic blood pressure, DBP: diastolic blood pressure, PP: pulse pressure, HR: heart rate
The mean age of the participants was 54.5 ± 12.1 years. Fifty three percent of the patients were males. All patients satisfied the hypertension criteria. Their BP was relatively well controlled, therefore the mean value of LV dimension, LVMI, LV ejection fraction and LAVI were not so deviated within normal range.
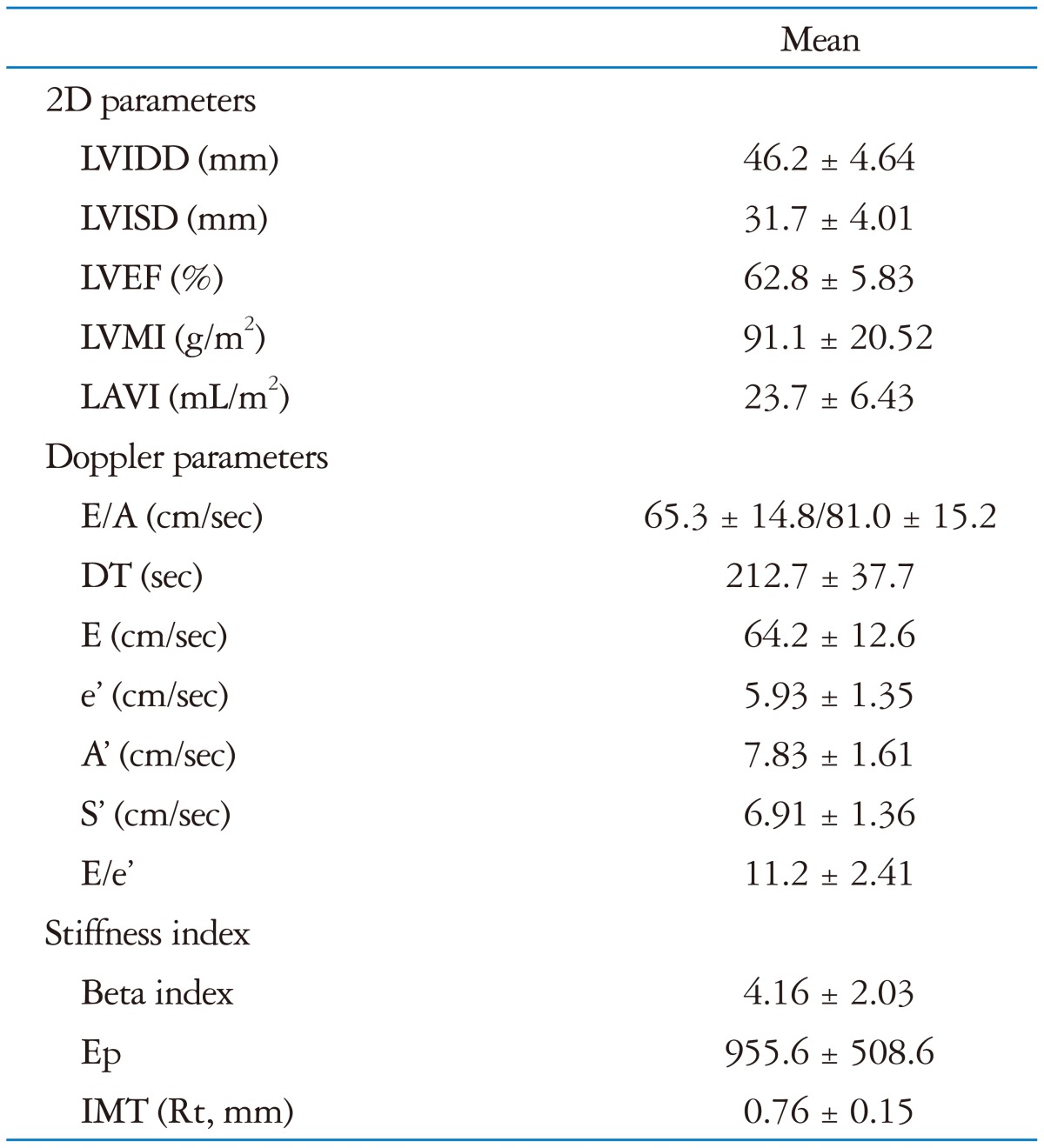
The echocardiographic parameters and arterial stiffness variables are shown in Table 2.
Table 2.
The echocardiographic parameters and arterial stiffness variables
The data are given as mean ± SD.
LVIDD: left ventricular internal diastolic dimension, LVISD: left ventricular internal systolic dimension, LVEF: left ventricular ejection fraction, LVMI: left ventricular mass index, LAVI: left atrial volume index, E: early diastolic mitral inflow velocity, A: late diastolic mitral inflow velocity, DT: deceleration time, e': early diastolic tissue velocity, A': late diastolic tissue velocity, S': systolic septal tissue velocity, Ep: pressure-strain elasticity modulus, IMT: intima media thickness
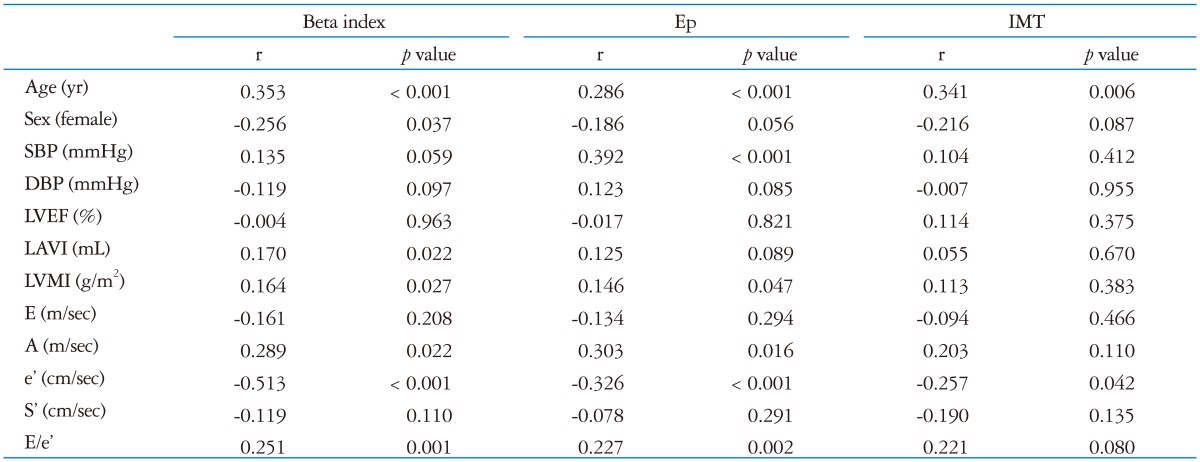
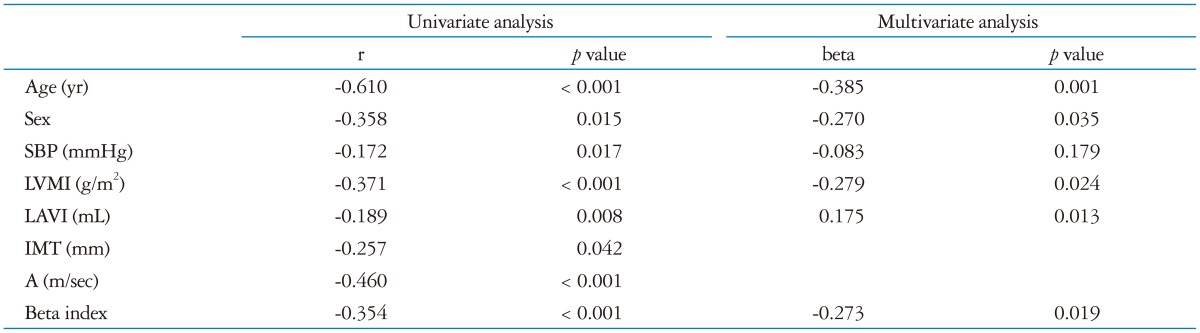
Correlations between beta index and diastolic function indices are presented in Table 3. Beta index, that is carotid stiffness, was found to have negative correlations with female sex and e' (early diastolic tissue velocity), while there was a positive correlation between beta index and age, LAVI, LVMI, the late portion of diastolic function (A wave) and E over e' (E/e'). Age, SBP, LVMI, A wave and E over e' (E/e') were also positively correlated with Ep and that was negatively correlated with e' wave. As expected, age was correlated significantly with arterial stiffness and diastolic functional variables. IMT was negatively correlated with age, but not with LV diastolic parameters. In logistic regression, diastolic dysfunction was affected by age (beta -0.385, p = 0.001), female sex (beta -0.270, p = 0.035), LAVI (beta 0.175, p = 0.013) and β (beta -0.273, p = 0.019) (Table 4).
Table 3.
The correlation of carotid stiffness and IMT with heart function
IMT: intima media thickness, Ep: pressure-strain elasticity modulus, SBP: systolic blood pressure, DBP: diastolic blood pressure, LVEF: left ventricular ejection fraction, LVMI: left ventricular mass index, LAVI: left atrial volume index, E: early diastolic mitral inflow velocity, A: late diastolic mitral inflow velocity, e': early diastolic tissue velocity, S': systolic septal tissue velocity
Table 4.
Univariate and multivariate analyses for diastolic dysfunction (e')
SBP: systolic blood pressure, LVMI: left ventricular mass index, LAVI: left atrial volume index, IMT: intima-media thickness, A: late diastolic mitral inflow velocity
Therefore, we found that carotid stiffness was independently associated with LV diastolic dysfunction even after controlling for age, sex, LAVI and LVMI (Table 4).
Discussion
To the best of our knowledge, there are some data available on the association between arterial stiffness and diastolic function among subjects with cardiovascular risk factor, especially metabolic syndrome or obesity,10),11) and those with manifested arterial disease.12) However, little is known about this association in patients only with hypertension without any other diseases.
It has been shown that the local measurement of arterial stiffness may be useful for the detection of early arterial changes.13) Several plausible pathways exist whereby arterial compliance may contribute to the pathological changes in the LV that form the substrate for diastolic dysfunction.14) Increased stiffness of conduit arteries is associated with higher velocity of transmission of the pulse wave generated by LV ejection; early return of reflected waves that arrive back at the heart during LV systole may lead to augmentation of the central aortic pressure wave amplitude, thus increasing LV afterload and central PP.15) Increased afterload may promote myocyte hypertrophy and may also directly slow LV relaxation.16) The arterial stiffness leads to increased PP and to LV hypertrophy, which is one of the major determinants of cardiac diastolic dysfunction. Furthermore, in patients with presumed diastolic heart failure (HF) (clinical HF with preserved ejection fraction), Hundley et al.,17) observed reduced proximal aortic distensibility, which correlated strongly with exercise intolerance.
Previous studies on hypertensive patients have also reported an association between arterial stiffness and LV structural changes,18) including concentric remodelling and hypertrophy,19) which are themselves associated with diastolic dysfunction.20),21) However, in this study, even in subjects with hypertension with normal LV mass, carotid stiffness was increased with diastolic dysfunction. Therefore the measurement of carotid stiffness may be a good tool for early detection of decreased carotid dispensability.
The relationship between elastic properties of the arteries and LV diastolic function has been demonstrated in different clinical scenarios. Sakane et al.,22) found in 119 patients that cardio-ankle vascular index was independently related to E/A ratio in patients with preserved LV ejection fraction. Abhayaratna et al.,23) demonstrated that in a cohort of 188 patients (aged ≥ 65 years), increasing arterial stiffness detected with applanation tonometry was associated with the severity of LV diastolic dysfunction. Vinereanu et al.,24) showed that arterial stiffness was inversely related to long axis LV function (systolic and early diastolic mitral annular velocities) and LV flow propagation velocity. In a study on a hypertension patient group, Mottram et al.,14) showed that arterial compliance, measured using applanation tonometry, is an independent predictor of diastolic dysfunction. These authors also showed that arterial stiffness was independently related to early diastolic tissue velocity (=e') and suggested a possible causal link through the promotion of subendocardial ischaemia.
The relation between arterial compliance and diastolic dysfunction may be particularly important with respect to hypertensive women, who have a higher prevalence of diastolic HF than do men.25-27) Experimental and clinical studies suggest that, in response to increased afterload, women exhibit a greater degree of concentric remodelling,28),29) which as discussed is associated with both arterial stiffness and diastolic dysfunction. In addition, several studies have reported increased values of arterial stiffness in women.30) In the present study, sex was associated with reduced E' velocity and independently related to arterial stiffness in hypertensive patients (Table 3). After adjustment for other factors, female sex was independently predictive of diastolic dysfunction. In addition, the results indicate that an interaction between sex and arterial compliance may be important, such that arterial dysfunction may contribute to diastolic dysfunction in hypertensive women to a greater extent than in hypertensive men who also have diastolic dysfunction.
In this article, we calculated carotid artery stiffness using distensibility (strain) and pressure-strain elasticity modulus. Since this procedure enables relatively accurate data to be acquired easily and other cardiovascular events to be predicted, it would be important to pay more attention to performing the assessment of arterial stiffness in patients with early-stage hypertension or normal LV mass to prevent LV diastolic dysfunction and maintain normal blood pressure especially in women.
References
- 1.Hoeks AP, Brands PJ, Smeets FA, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16:121–128. doi: 10.1016/0301-5629(90)90139-4. [DOI] [PubMed] [Google Scholar]
- 2.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 3.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL The task force for the management of arterial hypertension of the European Society of Hypertension; The task force for the management of arterial hypertension of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O'Leary DH, Bluemke DA, Lima JA. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201. doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Vriz O, Bossone E, Bettio M, Pavan D, Carerj S, Antonini-Canterin F. Carotid artery stiffness and diastolic function in subjects without known cardiovascular disease. J Am Soc Echocardiogr. 2011;24:915–921. doi: 10.1016/j.echo.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Fahs CA, Smith DL, Horn GP, Agiovlasitis S, Rossow LM, Echols G, Heffernan KS, Fernhall B. Impact of excess body weight on arterial structure, function, and blood pressure in firefighters. Am J Cardiol. 2009;104:1441–1445. doi: 10.1016/j.amjcard.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Kosmala W, O'Moore-Sullivan T, Plaksej R, Przewlocka-Kosmala M, Marwick TH. Improvement of left ventricular function by lifestyle intervention in obesity: contributions of weight loss and reduced insulin resistance. Diabetologia. 2009;52:2306–2316. doi: 10.1007/s00125-009-1507-4. [DOI] [PubMed] [Google Scholar]
- 12.Dijk JM, Algra A, van der Graaf Y, Grobbee DE, Bots ML SMART study group. Carotid stiffness and the risk of new vascular events in patients with manifest cardiovascular disease. The SMART study. Eur Heart J. 2005;26:1213–1220. doi: 10.1093/eurheartj/ehi254. [DOI] [PubMed] [Google Scholar]
- 13.Antonini-Canterin F, Carerj S, Di Bello V, Di Salvo G, La Carrubba S, Vriz O, Pavan D, Balbarini A, Nicolosi GL Research Group of the Italian Society of Cardiovascular Echography (SIEC) Arterial stiffness and ventricular stiffness: a couple of diseases or a coupling disease? A review from the cardiologist's point of view. Eur J Echocardiogr. 2009;10:36–43. doi: 10.1093/ejechocard/jen236. [DOI] [PubMed] [Google Scholar]
- 14.Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Rourke MF. Diastolic heart failure, diastolic left ventricular dysfunction and exercise intolerance. J Am Coll Cardiol. 2001;38:803–805. doi: 10.1016/s0735-1097(01)01452-8. [DOI] [PubMed] [Google Scholar]
- 16.Leite-Moreira AF, Correia-Pinto J, Gillebert TC. Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction. Cardiovasc Res. 1999;43:344–353. doi: 10.1016/s0008-6363(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 17.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 18.Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–494. doi: 10.1161/01.hyp.36.4.489. [DOI] [PubMed] [Google Scholar]
- 19.Palmieri V, Bella JN, Roman MJ, Gerdts E, Papademetriou V, Wachtell K, Nieminen MS, Dahlöf B, Devereux RB. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003;21:781–787. doi: 10.1097/00004872-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Ren JF, Pancholy SB, Iskandrian AS, Lighty GW, Jr, Mallavarapu C, Segal BL. Doppler echocardiographic evaluation of the spectrum of left ventricular diastolic dysfunction in essential hypertension. Am Heart J. 1994;127(4 Pt 1):906–913. doi: 10.1016/0002-8703(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 21.Qu P, Ding Y, Xia D, Wang H, Tian X. Variations in cardiac diastolic function in hypertensive patients with different left ventricular geometric patterns. Hypertens Res. 2001;24:601–604. doi: 10.1291/hypres.24.601. [DOI] [PubMed] [Google Scholar]
- 22.Sakane K, Miyoshi T, Doi M, Hirohata S, Kaji Y, Kamikawa S, Ogawa H, Hatanaka K, Kitawaki T, Kusachi S, Yamamoto K. Association of new arterial stiffness parameter, the cardio-ankle vascular index, with left ventricular diastolic function. J Atheroscler Thromb. 2008;15:261–268. doi: 10.5551/jat.e576. [DOI] [PubMed] [Google Scholar]
- 23.Abhayaratna WP, Barnes ME, O'Rourke MF, Gersh BJ, Seward JB, Miyasaka Y, Bailey KR, Tsang TS. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients > or =65 years of age. Am J Cardiol. 2006;98:1387–1392. doi: 10.1016/j.amjcard.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Vinereanu D, Nicolaides E, Boden L, Payne N, Jones CJ, Fraser AG. Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart. 2003;89:449–450. doi: 10.1136/heart.89.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL Cardiovascular Health Study Research Group. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 26.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 27.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 28.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 29.Aurigemma GP, Gaasch WH. Gender differences in older patients with pressure-overload hypertrophy of the left ventricle. Cardiology. 1995;86:310–317. doi: 10.1159/000176895. [DOI] [PubMed] [Google Scholar]
- 30.Gatzka CD, Kingwell BA, Cameron JD, Berry KL, Liang YL, Dewar EM, Reid CM, Jennings GL, Dart AM ANBO2 investigators. Australian Comparative Outcome Trial of Angiotensin-Converting Enzyme Inhibitor- and Diuretic-Based Treatment of Hypertension in the Elderly. Gender differences in the timing of arterial wave reflection beyond differences in body height. J Hypertens. 2001;19:2197–2203. doi: 10.1097/00004872-200112000-00013. [DOI] [PubMed] [Google Scholar]