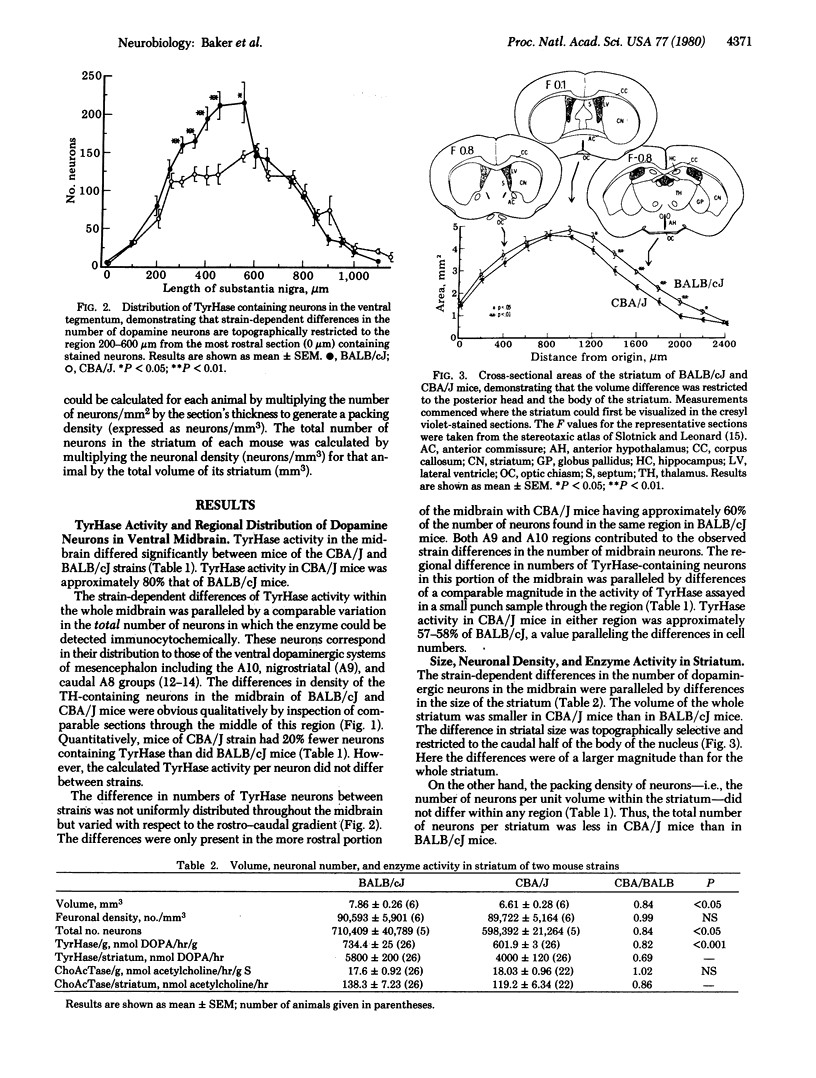
Abstract
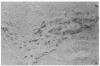
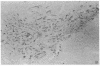
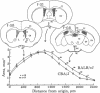
The activity of tyrosine hydroxylase [TyrHase; tyrosine-3-monooxygenase; L-tyrosine, tetrahydropteridine: oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2] is 20% less in whole midbrain of CBA/J mice than BALB/cJ mice and is paralleled by a comparable difference in the number of dopaminergic neurons in which the enzyme can be detected immunocytochemically. The strain-dependent difference in numbers of TyrHase-containing neurons and of TyrHase activity is not homogeneous in the midbrain but is restricted (along the rostral-caudal axis) to the medial one-third, where almost 2-fold variations are found. The volume of the striatum, a major projection field of midbrain dopamine neurons, is 20% smaller in CBA/J than in BALB/cJ mice; the difference is regional and is concentrated in the caudal half. Because the packing density of intrinsic neurons of the striatum is similar in both strains, CBA/J mice contain 20% fewer neurons than do BALB/cJ mice. The activities of TryHase and of choline acetyltransferase (ChoAcTase; acetyl-CoA:choline-O-acetyltransferase, EC 2.3.1.6) in the whole striatum of CBA/J mice are less than in BALB/cJ. The strain-dependent differences in midbrain TyrHase activity are due to variations in the number of dopamine neurons and directly correlate with differences in the number of striatal cholinergic neurons. There is genetic control of the number of neurons of a neurochemically specific class in the mammalian brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher S. G., Butcher L. L. Origin and modulation of acetylcholine activity in the neostriatum. Brain Res. 1974 May 10;71(1):167–171. doi: 10.1016/0006-8993(74)90202-9. [DOI] [PubMed] [Google Scholar]
- Ciaranello R. D., Barchas R., Kessler S., Barchas J. D. Catecholamines: strain differences in biosynthetic enzyme activity in mice. Life Sci I. 1972 Jun 15;11(12):565–572. doi: 10.1016/0024-3205(72)90191-9. [DOI] [PubMed] [Google Scholar]
- Coyle J. T. Tyrosine hydroxylase in rat brain--cofactor requirements, regional and subcellular distribution. Biochem Pharmacol. 1972 Jul 15;21(14):1935–1944. doi: 10.1016/0006-2952(72)90006-8. [DOI] [PubMed] [Google Scholar]
- Eleftheriou B. E. A gene influencing hypothalamic norepinephrine levels in mice. Brain Res. 1974 Apr 26;70(3):538–540. doi: 10.1016/0006-8993(74)90265-0. [DOI] [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf E., Greilsamer J., Mack G., Mandel P. Correlation of behavioural differences in three strains of mice with differences in brain amines. Nature. 1974 Feb 15;247(5441):483–485. doi: 10.1038/247483a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G. Enzymes associated with the metabolism of catecholamines, acetylcholine and gaba in human controls and patients with Parkinson's disease and Huntington's chorea. J Neurochem. 1976 Jan;26(1):65–76. [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G., Fibiger H. C., Wickson V. Neostriatal choline acetylase and cholinesterase following selective brain lesions. Brain Res. 1971 Dec 10;35(1):308–314. doi: 10.1016/0006-8993(71)90625-1. [DOI] [PubMed] [Google Scholar]
- Moisset B. Factors contributing to the modulation of norepinephrine uptake by synaptosomes from mouse brain cortex. Brain Res. 1977 Jan 31;121(1):113–120. doi: 10.1016/0006-8993(77)90441-3. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973 Sep 14;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Reis D. J. Monoamine-synthesizing enzymes in central dopaminergic, noradrenergic and serotonergic neurons. Immunocytochemical localization by light and electron microscopy. J Histochem Cytochem. 1976 Jul;24(7):792–306. doi: 10.1177/24.7.8567. [DOI] [PubMed] [Google Scholar]
- Ross R. A., Judd A. B., Pickel V. M., Joh T. H., Reis D. J. Strain-dependent variations in number of midbrain dopaminergic neurones. Nature. 1976 Dec 16;264(5587):654–656. doi: 10.1038/264654a0. [DOI] [PubMed] [Google Scholar]
- Schrier B. K., Shuster L. A simplified radiochemical assay for choline acetyltransferase. J Neurochem. 1967 Oct;14(10):977–985. doi: 10.1111/j.1471-4159.1967.tb09509.x. [DOI] [PubMed] [Google Scholar]
- Segal D. S., Kuczenski R. T., Mandell A. J. Strain differences in behavior and brain tyrosine hydroxylase activity. Behav Biol. 1972 Feb;7(7):75–81. doi: 10.1016/s0091-6773(72)80190-1. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Teitelman G., Baker H., Joh T. H., Reis D. J. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: possible role of tissue environment. Proc Natl Acad Sci U S A. 1979 Jan;76(1):509–513. doi: 10.1073/pnas.76.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiplady B., Killian J. J., Mandel P. Tyrosine hydroxylase in various brain regions of three strains of mice differing in spontaneous activity, learning ability, and emotionality. Life Sci. 1976 May 15;18(10):1065–1070. doi: 10.1016/0024-3205(76)90139-9. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G., Wimer C. C., Wimer R. E. Relationships between neurotransmitter metabolism and behaviour in seven inbred strains of mice. Brain Res. 1973 Oct 26;61:428–434. doi: 10.1016/0006-8993(73)90551-9. [DOI] [PubMed] [Google Scholar]
- Will B. E. Neurochemical correlates of individual differences in animal learning capacity. Behav Biol. 1977 Feb;19(2):143–171. doi: 10.1016/s0091-6773(77)91458-4. [DOI] [PubMed] [Google Scholar]