Abstract
Suspension-cultured tomato (Lycopersicon esculentum) cells react to stimulation by chitin fragments with a rapid, transient alkalinization of the growth medium, but behave refractory to a second treatment with the same stimulus (G. Felix, M. Regenass, T. Boller [1993] Plant J 4: 307–316). We analyzed this phenomenon and found that chitin fragments caused desensitization in a time- and concentration-dependent manner. Partially desensitized cells exhibited a clear shift toward lower sensitivity of the perception system. The ability of chitin oligomers to induce desensitization depended on the degree of polymerization (DP), with DP5 ≈ DP4 ≫ DP3 ≫ DP2 > DP1. This correlates with the ability of these oligomers to induce the alkalinization response and to compete for the high-affinity binding site on tomato cells and microsomal membranes, indicating that the alkalinization response and the desensitization process are mediated by the same receptor. The dose required for half-maximal desensitization was about 20 times lower than the dose required for half-maximal alkalinization; desensitization could therefore be used as a highly sensitive bioassay for chitin fragments and chitin-related stimuli such as lipochitooligosaccharides (nodulation factors) from Rhizobium leguminosarum. Desensitization was not associated with increased inactivation of the stimulus or with a disappearance of high-affinity binding sites from the cell surface, and thus appears to be caused by an intermediate step in signal transduction.
Plant cells have the ability to perceive a variety of microbial substances (Boller, 1995). Perception of some of these substances, such as elicitors, may initiate phytoalexin production and other responses associated with defense (Darvill and Albersheim, 1984; Ebel and Cosio, 1994). Perception of other microbial factors may initiate responses important for symbiosis, e.g. the initiation of nodule formation in legume roots by rhizobial Nod factors (Dénarié et al., 1992). Both types of chemoperception systems are highly sensitive and selective, indicating that they are mediated by specific receptors (Ebel and Cosio, 1994; Boller, 1995). Indeed, high-affinity binding sites with all of the characteristics of receptors have been identified and characterized for a number of microbial substances in various plants (Boller, 1995; Côté et al., 1995).
In previous studies with suspension-cultured tomato (Lycopersicon esculentum) cells, we have identified highly sensitive perception systems for glycopeptides with a fungal-specific N-linked glycan of 9 to 12 mannosyl units (Basse et al., 1992), for chitin fragments (Felix et al., 1993), for ergosterol (Granado et al., 1995), and for fungal xylanase (Felix et al., 1993, 1994). These compounds belong to very different classes of chemical structures but are highly typical for fungi and are not known to occur in plants. High-affinity binding sites specific for the glycopeptides (Basse et al., 1993) and for the chitin fragments (Baureithel et al., 1994) could be demonstrated on tomato cells and membranes.
Chemoperception systems in microbes and animals are often desensitized by the continuous presence of the stimulus, allowing an increase in the dynamic range of the sensory system (Dusenbery, 1992). We have observed a similar effect on some of the chemoperception systems in tomato cells. For example, cells reacted with a transient alkalinization of the growth medium when treated with chitin fragments but did not respond when treated with a second dose of chitin fragments, although they still reacted to xylanase (Felix et al., 1993) or ergosterol (Granado et al., 1995). Reciprocally, when cells were treated with ergosterol, they were refractory to further stimulation with ergosterol but still responded to chitin fragments and xylanase (Granado et al., 1995). These observations indicate that the different chemoperception systems are desensitized in an independent manner. Desensitization can therefore be used experimentally to distinguish different types (qualities) of stimuli. For example, tomato cells react to Nod factors (which contain a chitin oligomer as a backbone) with an alkalinization response and become refractory to subsequent stimulation by chitin. Cells pretreated with chitin fragments show no response to Nod factors, indicating that Nod factors and chitin fragments have the same sensory quality for the tomato cells (Staehelin et al., 1994).
In an attempt to study the processes underlying desensitization, we describe here the characteristics of the refractory behavior in tomato cells treated with chitin fragments. We present data on the time and dose dependence of desensitization, and show that this process is not associated with inactivation of the chitin fragments or with disappearance of the binding site for chitin fragments from the cells.
MATERIALS AND METHODS
Chitin Fragments and Nod Factors
Chintin fragments CH2, CH3, CH4, and CH5 were obtained from Seikagaku (Tokyo, Japan). The purified Nod factor Nod Rlv-V(Ac;C18:1) from Rhizobium leguminosarum bv viciae, CH5 modified at the nonreducing end by an O-acetyl group and an N-linked unsaturated fatty acid (18:1) (Spaink et al., 1991), was kindly provided by H.P. Spaink (Leiden State University, Leiden, The Netherlands).
Cell Culture and Alkalinization Response
The tomato (Lycopersicon esculentum) cell line Msk8 (Koornneef et al., 1987) was maintained as a suspension culture (Felix et al., 1991a) and used 4 to 10 d after subculture for experiments. To measure alkalinization of the growth medium (the alkalinization response), 2.5-mL aliquots of the suspension were placed in open, 20-mL vials on a rotary shaker at 120 cycles min−1. The pH in the medium was continuously measured using a small, combined-glass electrode (Metrohm, Herisau, Switzerland) and registered on a pen recorder. The ΔpHmax, which occurred 3 to 5 min after application of chitin fragments, was derived from the recordings (Felix et al., 1993). The maximal pH increase obtained after stimulation with saturating doses of CH4 (>1 nm) varied little within one experiment using one batch of cells (± approximately 0.03 pH unit), but varied between 0.5 and 0.8 in different experiments using different batches of cells.
To use desensitization as a bioassay for chitin-related stimuli, aliquots of cell suspension were pretreated for 60 min with chitin fragments or the compounds to be tested, and the alkalinization response (ΔpHmax) to subsequent treatment with 10 nm CH4 was recorded.
Binding Assay for Chitin Fragments
Binding of chitin fragments to whole cells was studied with a 35S-labeled derivative of CH5 as described previously (Baureithel et al., 1994). One-milliliter aliquots of cell suspension containing approximately 0.3 g fresh weight of cells were incubated with 100 nCi of CH5-Gly-[35S]Met-Boc (specific activity approximately 1000 Ci mmol−1) and 1 or 10 nm CH5 for 20 min on ice. Cells were collected on a paper filter and washed with fresh, ice-cold medium. Radioactivity bound to the cells was measured by scintillation counting.
Reproducibility
Data shown in figures are from single experiments representative of at least three independent repetitions.
RESULTS
Dependence of Desensitization on Dose and Time
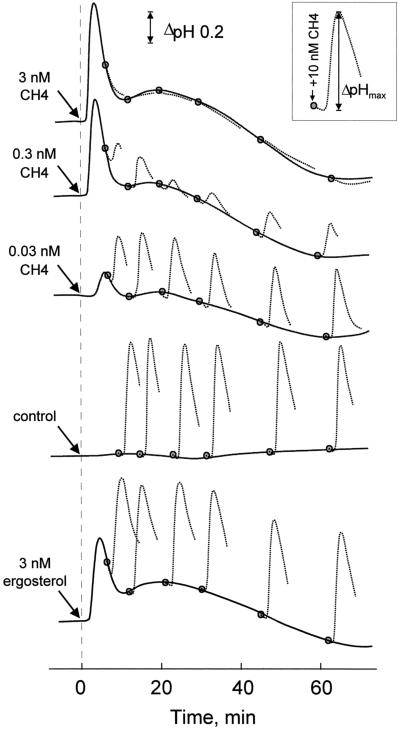
Suspension-cultured tomato cells react to treatment with subnanomolar concentrations of chitin fragments or ergosterol with rapid alkalinization of their extracellular medium (Felix et al., 1993; Granado et al., 1995). Examples of the alkalinization response are shown in Figure 1. In cell cultures treated with doses of 0.03, 0.3, or 3 nm N,N′,N“,N“′-tetraacetylchitotetraose (CH4) or 3 nm ergosterol, the pH in the medium started to increase after a short lag, reached a maximum after approximately 5 min, and then decreased rapidly. ΔpHmax was 0.76 and 0.54 in cells treated with 3 nm CH4 and 3 nm ergosterol, respectively. After this transient alkalinization the pH did not reach a constant, stable value but slowly oscillated below and above the baseline observed in untreated control cells (values for later time points not shown). Increasing the concentration of CH4 to 1 μm resulted in a pH profile indistinguishable from that observed with 3 nm CH4 (data not shown), indicating that the response of tomato cells was saturated at concentrations ≥3 nm.
Figure 1.
Induction of extracellular alkalinization in suspension-cultured tomato cells in response to two consecutive stimuli. Solid lines, Extracellular pH in untreated cells (control) or cells treated with CH4 or ergosterol at the concentrations indicated. Shaded circles and dotted lines, Extracellular pH after a second stimulation with 10 nm CH4 (shown in the inset). The pH of the growth medium was 5.1 at the start of the experiment.
At different times after the application of the first stimulus, cells were treated with 10 nm CH4 as a second stimulus (Fig. 1). Cells initially treated with 3 nm CH4 did not react to the second stimulus, confirming earlier findings in cells treated with an initial dose of 10 nm CH4 (Felix et al., 1993). Cells treated with an initial dose of 0.3 or 0.03 nm CH4 exhibited an alkalinization response when stimulated a second time (Fig. 1). However, ΔpHmax was considerably lower than in control cells that had not received a first stimulus or that had been stimulated with 3 nm ergosterol (Fig. 1). In these cultures, the ΔpHmax after stimulation with 10 nm CH4 remained at approximately 0.7 to 0.8 throughout the experiment.
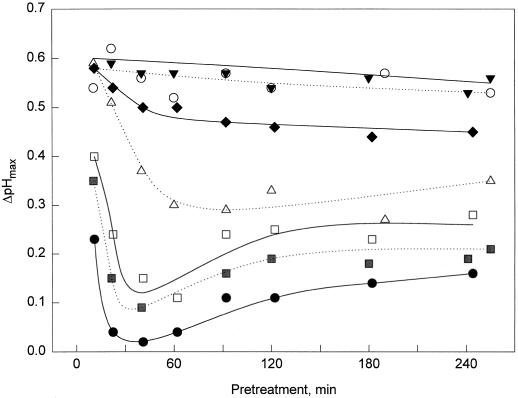
The responsiveness of cells after a first stimulation was assayed for a broader range of CH4 concentrations and over a prolonged period of time (Fig. 2). While treatment with 1 pm CH4 did not cause a decrease in the ΔpHmax reached in response to the second stimulation with 10 nm CH4, a significant decrease in response occurred in cells treated with 3 and 10 pm CH4. In cells pretreated with doses of 30 to 300 pm, the responsiveness was minimal after 30 to 60 min of treatment and then slowly recovered, but did not reach the responsiveness of untreated cultures within the 4 h of the experiment. Thus, desensitization is a gradual process that depends on the duration of the pretreatment as well as on the initial dose of the first stimulus (Figs. 1 and 2).
Figure 2.
Time dependence of desensitization induced by different doses of CH4. ΔpHmax elicited by 10 nm CH4 in cells pretreated for different times with the concentrations of CH4 indicated. ○, Control (no CH4); ▾, 1 pm; ♦, 3 pm; ▵, 10 pm; □, 30 pm; ░⃞, 100 pm; and •, 300 pm.
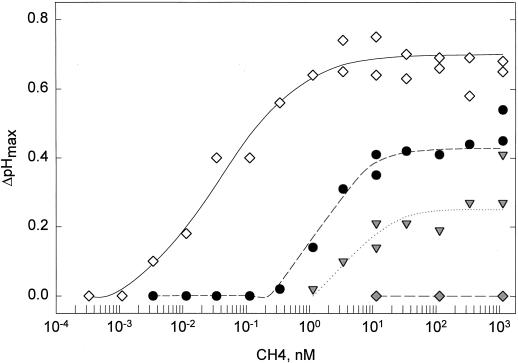
To study the sensitivity of the perception system after a first stimulation, the dose-response relationship for CH4-induced alkalinization was measured with untreated control cells and with cells pretreated for 60 min with 0.03, 0.3, and 3 nm CH4 (Fig. 3). In control cells the EC50 value was approximately 40 pm CH4. In contrast, EC50 values were 2 nm and about 10 nm in cells pretreated with 0.03 and 0.3 nm CH4, respectively. Cells pretreated with 3 nm CH4 did not exhibit measurable alkalinization when treated with CH4 up to concentrations of 1 μm (Fig. 3). This decrease in the sensitivity of the cells was specific for the response to chitin fragments, and no change in dose dependency for ergosterol was observed after pretreatment with 3 nm CH4 (data not shown).
Figure 3.
Dose-response curves for induction of the alkalinization response (ΔpHmax) by CH4 in cells pretreated for 60 min with different concentrations of CH4. ⋄, Control (no CH4); •, 0.03 nm; ▾, 0.3 nm; and ♦, 3 nm.
Desensitization as a Bioassay for Chitin Fragments
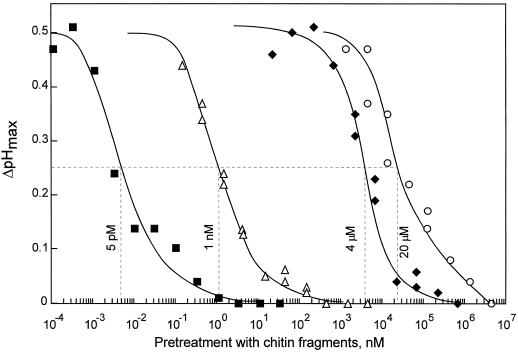
Desensitization of cells could be used as a sensitive bioassay for chitin-related stimuli. The EC50 for desensitization (i.e. the concentration that leads to 50% reduction of the ΔpHmax in response to the second stimulation by 10 nm CH4) was determined for chitin oligomers with a DP between 1 and 5. The EC50 of CH4 and CH5 for desensitization was approximately 5 pm (Fig. 4; data not shown for CH5). The smaller chitin fragments, CH3, CH2, and CH1, were much less effective inducers of desensitization, with EC50 values of 1 nm, 4 μm, and 20 μm, respectively (Fig. 4). The relative effectiveness of chitin oligomers in the desensitization assay was CH5 ≈ CH4 ≫ CH3 ≫ CH2 > CH1, reflecting their relative effectiveness at stimulating the alkalinization response (Felix et al., 1993). However, the bioassay involving desensitization was much more sensitive, since the EC50 values for desensitization were about 20 times lower than the EC50 values for the alkalinization response.
Figure 4.
Dose-response curves for induction of desensitization by different chitin oligomers. Alkalinization (ΔpHmax) in response to 10 nm CH4 was measured in cells pretreated with different amounts of chitin oligomers for 60 min. ▪, CH4; ▵, CH3; ♦, CH2; and ○, CH1. Hatched lines indicate concentrations of the prestimuli that reduce ΔpHmax in response to 10 nm CH4 by 50% (EC50 values for desensitization).
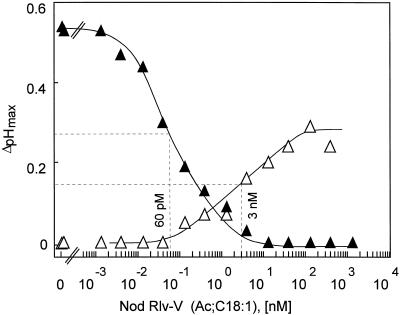
A similar difference in sensitivity of the two bioassays was also observed with Nod factors from R. leguminosarum. As described previously for other Nod factors (Staehelin et al., 1994), Nod Rlv-V(Ac;C18:1), a Nod factor that contains a CH5 backbone (Spaink et al., 1991), was found to induce alkalinization and desensitization to subsequent stimulation with (underivatized) chitin fragments. Cells reacted with measurable alkalinization to concentrations of this Nod factor greater than 0.1 nm, and their response was half-maximal at approximately 3 nm (Fig. 5). In the desensitization bioassay, 60 pm Nod factor was sufficient to reduce the subsequent stimulation by CH4 by 50% (Fig. 5). As observed previously (Staehelin et al., 1994), the maximal pH increase reached with saturating concentrations was lower with the Nod factor than with CH4: 0.30 pH units with >30 nm Nod Rlv-V compared with 0.55 pH units with 10 nm CH4 (Fig. 5). On the other hand, Nod Rlv-V at concentrations greater than 3 nm induced complete desensitization (Fig. 5).
Figure 5.
Dose-response curves for induction of an alkalinization response and for induction of desensitization by a purified Nod factor of R. leguminosarum, Nod Rlv-V(Ac;C18:1). ▵, Alkalinization (ΔpHmax) in response to different concentrations of Nod factor. ▴, Effect of 60 min of pretreatment with different concentrations of the Nod factor on alkalinization (ΔpHmax) induced by 10 nm CH4. Hatched lines indicate the EC50 values.
Inactivation of Chitin Fragments by Cell Suspensions
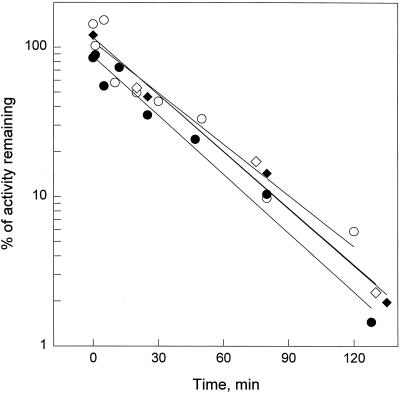
A simple explanation for the refractory behavior of cells would be a strongly accelerated inactivation of the chitin fragments in cells pretreated with the stimulus, e.g. by a chitinase activity induced by the pretreatment. As observed previously, the activity of chitin fragments added to tomato cell suspensions disappeared rapidly (Felix et al., 1993) (Fig. 6). This was tested by adding 10 nm CH4 to suspensions and assaying samples at intervals for their capacity to induce alkalinization (in fresh aliquots of cells). However, the rate of inactivation in cells pretreated with 1 nm CH4 for 60 min was indistinguishable from that in cells without pretreatment (Fig. 6). In both suspensions, activity disappeared with first-order kinetics, leading to an apparent half-life of approximately 25 min for CH4 (Fig. 6). The same rate of inactivation was observed when CH4 was incubated in the cell-free medium obtained from the two cultures by filtration (Fig. 6). Because no inactivation was observed after heat treatment of the medium (95°C for 5 min; data not shown), the inactivation of CH4 is best ascribed to the presence of an enzyme activity, most likely a chitinase activity, in the culture medium. The data described above show that desensitization is not associated with enhanced degradation or inactivation of the chitin fragments in prestimulated cells.
Figure 6.
Inactivation of chitin fragments in cell suspensions before and after desensitization. Inactivation was tested in suspensions without pretreatment (○, ⋄) or 60 min after pretreatment with 1 nm CH4 (•, ♦). At time 0, CH4 (10 nm) was added to cell suspensions or the corresponding culture medium freed of cells by filtration. Samples were taken at intervals, and serial dilutions were assayed for the induction of the alkalinization response. Equivalents of CH4 in the samples were determined from a standard curve obtained with untreated CH4. ○, Cell suspension without pretreatment; ⋄, cell-free medium of control cells; •, cell suspension pretreated with 1 nm CH4; ♦, cell-free medium from pretreated cells.
Binding of Chitin Fragments by Desensitized Cells
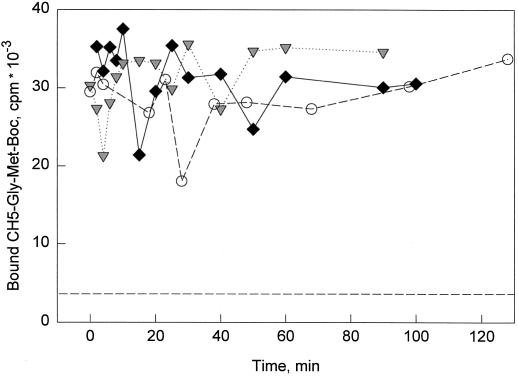
We have previously developed an assay to study the high-affinity binding sites for chitin fragments on intact tomato cells (Baureithel et al., 1994). Using the radioligand CH5-Gly-[35S]Met-Boc in the presence of 10 nm unlabeled CH5, conditions that saturate the binding sites present on intact cells to approximately 80% (Kd 1.4 nm; Baureithel et al., 1994), we measured binding sites present on the cell surface in the course of desensitization (Fig. 7). We observed no significant change in binding to cells after pretreatment with 0.03 or 0.3 nm CH4 (Fig. 7), indicating that the number of binding sites did not change during the desensitization process. Similarly, no change in binding could be observed during the desensitization process when binding was studied under less-saturating conditions (with only 1 nm unlabeled CH5, approximately 30% saturation; data not shown). These results suggest that desensitization is not caused by changes in the number or the affinity of the binding sites present on the cells.
Figure 7.
Binding of chitin fragments to intact tomato cells. Cell suspensions were treated with 0.03 nm (♦) or 0.3 nm (▾) CH4 at time 0 as indicated. ○, Control (no CH4). At intervals, 1-mL samples were taken and assayed for binding of the radioligand CH5-Gly-[35S]Met-Boc in the presence of 10 nm unlabeled CH5. The dotted line without symbols indicates binding of radioligand in the presence of 10 μm CH5 (nonspecific binding).
DISCUSSION
Extracellular alkalinization has been observed in cell cultures of many different plant species treated with a variety of elicitor preparations and wound-related stimuli. This decrease of H+ in the culture medium has been found to coincide with an increase in K+ in the medium and a depolarization of the plasma membrane (Mathieu et al., 1991; Kuchitsu et al., 1993; Nürnberger et al., 1994; Felix and Boller, 1995). However, the cellular mechanisms regulating these ion fluxes are not known, nor have the ion channels and/or ion pumps involved been identified. In this study we made use of the alkalinization response as an indicator of the plant's reaction, much like the change in membrane potential in neurophysiology, to analyze perception of chitin fragments by tomato cells. Treatment of these cells with chitin fragments or related molecules such as Nod factors led to rapid adaptation or desensitization of the perception system. Desensitization of chitin perception does not affect the response to unrelated stimuli such as ergosterol or xylanase; reciprocally, cells stimulated by these unrelated stimuli show no desensitization of the perception for chitin fragments (Felix et al., 1993; Granado et al., 1995) (Fig. 1).
The transient nature of alkalinization induced by chitin fragments is probably connected to desensitization. In cells treated with saturating doses of chitin fragments (e.g. ≥1 nm CH4) ΔpHmax was reached after approximately 3 to 4 min. Thereafter, the pH decreased, irrespective of chitin fragments still present (or added as a second dose) and even though cells still had the capacity to respond with further alkalinization when treated with unrelated stimuli. Therefore, the decrease in pH that follows the peak of alkalinization is probably caused by a readjustment mechanism that prevails as soon as the perception system becomes desensitized. Indeed, cells rapidly readjust the pH of the growth medium when alkalinization is mimicked by the addition of small amounts of base (data not shown). Although response to chitin fragments lasts for only a few minutes, desensitization persists for several hours, even in the absence of the stimulus (Felix et al., 1993).
The alkalinization response elicited by ergosterol is also transient (Fig. 1), indicating that desensitization takes place in a similar manner. In contrast to chitin fragments and ergosterol, xylanase elicits an alkalinization lasting for several hours (Felix et al., 1993), and the refractory behavior toward further additions of xylanase could reflect a continuous, saturated response rather than desensitization. In tobacco, the elicitor activity of xylanase has been attributed to the xylanase protein itself rather than to plant cell wall fragments released by its enzyme activity (Sharon et al., 1993). We have similar evidence for direct elicitor activity of xylanase in tomato (M. Bürgin, G. Felix, and T. Boller, unpublished results).
The relative ability of chitin fragments of different lengths to induce desensitization paralleled their ability to induce alkalinization and their affinity for the chitin-binding site on intact cells and microsomal membranes (Baureithel et al., 1994). Similarly, the relative effectiveness of the Nod factors in all of these assays was between that of CH4 and CH3 (Baureithel et al., 1994) (Fig. 5). These data strongly indicate that induction of the alkalinization response and desensitization proceed by binding to the same receptor. Desensitization can be observed at approximately 20-fold lower concentrations than induction of alkalinization. Measurable alkalinization probably requires the coherent, synchronous response of many cells. The response to low concentrations might be impeded by the kinetics of diffusion, leading to a broad, nonmeasurable peak of alkalinization, or faint alkalinization might be masked by nonstimulated (or desensitized) cells that readjust the pH in the medium. Desensitization, in contrast, appears to proceed in a cumulative manner, and low concentrations of chitin fragments can cause progressive desensitization (Fig. 2).
Desensitization of the chitin-perception system is not unique to the tomato cells used in this study; it was also observed in a cell culture of tobacco (data not shown). However, desensitization was not observed in a cell culture derived from a wild species of tomato, Lycopersicon peruvianum. In these cells, consecutive treatments with chitin fragments stimulated repeated alkalinization (data not shown), as was also observed for stimulation of these cells with systemin (Felix and Boller, 1995). The transient character of medium alkalinization appears to be caused by inactivation/degradation of the chitin fragments rather than desensitization. In rice cells, chitin fragments with a DP > 7 have been reported to stimulate a more permanent alkalinization response (Kuchitsu et al., 1993). Apparently, no rapid desensitization comparable with the one described in this study takes place.
We tested two simple hypotheses to account for the phenomenon of desensitization, but had to reject both of them. The first hypothesis was that desensitization might be connected to an increased ability of the cells to modify or inactivate the ligand, for example, by increased degradation or uptake or by the production of a specific inhibitor of binding. However, our data show that the rate of disappearance of biologically active chitin fragments was the same in control cells as in prestimulated, desensitized cells. In both cases chitin fragments disappear with apparent first-order kinetics and a half-life of approximately 25 min (Fig. 6). The same rate of inactivation was observed in the corresponding culture medium freed of cells by filtration. Inactivation is thus best explained by chitinase activity that is released by the cells and acts at a substrate concentration far below its Km.
The second hypothesis was that the receptor itself might be altered in its affinity to the ligand, or that it might be inactivated or internalized. However, our binding data show that neither the number nor the affinity of the binding sites for chitin fragments were noticeably altered in desensitized cells compared with control cells (Fig. 7). We could not detect alterations in the medium or at the cell surface that could explain the phenomenon of desensitization. Therefore, we propose that desensitization occurs at an intracellular step in the signal transduction pathway leading to induction of alkalinization. Assuming that the alkalinization response induced by different stimuli is based on the regulation of common channels or pumps, signaling must converge at a certain point beforehand, and, because desensitization is specific to the stimulus, a step occurring before this convergence must be the target of desensitization.
A known mechanism for rapid desensitization of perception systems in animal cells involves ligand-induced phosphorylation of the receptor, as exemplified in the well-studied case of adrenergic receptors (Lefkowitz et al., 1993). Induction of alkalinization in tomato cells is correlated with specific changes in protein phosphorylation and can be inhibited by the protein kinase inhibitor K-252a (Felix et al., 1991b, 1994). If phosphorylation at the receptor or a step farther downstream in the signaling pathway is involved in desensitization, then the relevant phosphorylations are expected to be added quickly, within 5 min of stimulation, and to persist throughout the period in which desensitization is observed.
In microbial organisms and animals, adaptation and desensitization are common characteristics of signal perception. These processes are important to detect changes in signal intensities and gradients of stimulus concentrations, e.g. in bacterial chemotaxis (Armitage, 1992) or in the orientation of insects toward odorous sources (Stengl et al., 1992). In both cases, removal of the stimulus usually leads to a rapid reversal of desensitization (Armitage, 1992; Stengl et al., 1992). We can only speculate about the biological role of desensitization in tomato cells. Plants, as nonmotile organisms, might not be confronted with the rapid increases and decreases in stimulus concentration that are characteristic of chemotaxis. Nevertheless, they might need information about increases in stimulus concentration. Desensitization might allow an increase in the dynamic range of chitin-fragment perception (Dusenbery, 1992), as indicated by the shift in sensitivity of the perception system after a first stimulation. Therefore, a cell that has not been exposed to chitin fragments previously will be maximally sensitive and react in a dynamic range between 0.01 and 1 nm. After a response to these small doses of stimulus, however, it will react in a higher dynamic range between 0.5 and 50 nm.
In conclusion, the desensitization of the perception system for chitin fragments occurs rapidly in a time- and concentration-dependent manner, and appears to be based on intermediate steps in signaling rather than on the interaction of the stimulus with its binding site (receptor) on the cell surface. A deeper understanding of this phenomenon must await characterization of the receptor involved and the identification of the elements downstream in the signal chain.
ACKNOWLEDGMENTS
We thank M. Regenass for his excellent technical assistance, Dr. H.P. Spaink (Leiden State University) for the gift of Nod factors, and Drs. M. Collinge and T. Meindl for helpful comments on the manuscript.
Abbreviations:
- Boc
4-butoxycarbonyl
- CH5
CH4, CH3, CH2, and CH1, chitin fragments with DP 5, 4, 3, 2, and 1, respectively (CH1 = Glc-NAc)
- ΔpHmax
maximal increase in pH above baseline
- DP
degree of polymerization
- EC50
dose to induce a half-maximal pH increase
- Nod
nodulation
LITERATURE CITED
- Armitage JP. Behavioral responses in bacteria. Annu Rev Physiol. 1992;54:683–714. doi: 10.1146/annurev.ph.54.030192.003343. [DOI] [PubMed] [Google Scholar]
- Basse CW, Bock K, Boller T. Elicitors and suppressors of the defense response in tomato cells: purification and characterization of glycopeptide elicitors and glycan suppressors generated by enzymatic cleavage of yeast invertase. J Biol Chem. 1992;267:10258–10265. [PubMed] [Google Scholar]
- Basse CW, Fath A, Boller T. High affinity binding of glycopeptide elicitor to tomato cells and microsomal membranes and displacement by specific glycan suppressors. J Biol Chem. 1993;268:14724–14731. [PubMed] [Google Scholar]
- Baureithel K, Felix G, Boller T. Specific, high affinity binding of chitin fragments to tomato cells and membranes: competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium. J Biol Chem. 1994;269:17931–17938. [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Côté F, Cheong J-J, Alba R, Hahn MG. Characterization of binding proteins that recognize oligoglucoside elicitors of phytoalexin synthesis in soybean. Physiol Plant. 1995;93:401–410. [Google Scholar]
- Darvill AG, Albersheim P. Phytoalexins and their elicitors: a defense against microbial infection of plants. Annu Rev Plant Physiol. 1984;35:243–275. [Google Scholar]
- Dénarié J, Debellé F, Rosenberg C. Signalling and host range variation in nodulation. Annu Rev Microbiol. 1992;46:494–531. doi: 10.1146/annurev.mi.46.100192.002433. [DOI] [PubMed] [Google Scholar]
- Dusenbery DB (1992) Sensory Ecology: How Organisms Acquire and Respond to Information. Freeman, New York
- Ebel J, Cosio EG. Elicitors of plant defense responses. Int Rev Cytol. 1994;148:1–36. [Google Scholar]
- Felix G, Boller T. Systemin induces rapid ion fluxes and ethylene biosynthesis in Lycopersicon peruvianumcells. Plant J. 1995;7:381–389. [Google Scholar]
- Felix G, Grosskopf DG, Regenass M, Basse CW, Boller T. Elicitor-induced ethylene biosynthesis in tomato cells. Characterization and use as a bioassay for elicitor action. Plant Physiol. 1991a;97:19–25. doi: 10.1104/pp.97.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Grosskopf DG, Regenass M, Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci USA. 1991b;88:8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Regenass M, Boller T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993;4:307–316. [Google Scholar]
- Felix G, Regenass M, Spanu P, Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse-labeling with [33P]phosphate. Proc Natl Acad Sci USA. 1994;91:952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado J, Felix G, Boller T. Perception of fungal sterols in plants: subnanomolar concentrations of ergosterol elicit extracellular alkalinization in tomato cells. Plant Physiol. 1995;107:486–490. doi: 10.1104/pp.107.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, Martinelli L. A genetic analysis of cell culture traits in tomato. Theor Appl Genet. 1987;74:633–641. doi: 10.1007/BF00288863. [DOI] [PubMed] [Google Scholar]
- Kuchitsu K, Kikuyama M, Shibuya N. NAcetylchitooligosaccharides, biotic elicitors for phytoalexin production, induce transient membrane depolarization in suspension-cultured rice cells. Protoplasma. 1993;174:79–81. [Google Scholar]
- Lefkowitz RJ, Cotecchia S, Kjelsberg MA, Pitcher J, Koch WJ, Inglese J, Caron MG. Adrenergic receptors: recent insights into their mechanism of activation and desensitization. Adv Second Messenger Phosphoprotein Res. 1993;28:1–9. [PubMed] [Google Scholar]
- Mathieu Y, Kurkdjian A, Xia H, Guern J, Koller A, Spiro MD, O' Neill M, Albersheim P, Darvill A. Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J. 1991;1:333–343. doi: 10.1046/j.1365-313X.1991.t01-10-00999.x. [DOI] [PubMed] [Google Scholar]
- Nürnberger T, Jabs D, Nennstiel D, Sacks WR, Hahlbrock K, Scheel D. Specific recognition of a fungal oligopeptide elicitor by parsley cells. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Sharon A, Fuchs Y, Anderson JD. The elicitation of ethylene biosynthesis by a Trichodermaxylanase is not related to the cell wall degradation activity of the enzyme. Plant Physiol. 1993;102:1325–1329. doi: 10.1104/pp.102.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink HP, Sheeley DM, Van Brussel AAN, Glushka J, York WS, Tak T, Geiger O, Kennedy EP, Reinhold VN, Lugtenberg BJJ. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991;354:125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Staehelin C, Granado J, Müller J, Wiemken A, Mellor RB, Felix G, Regenass M, Broughton WJ, Boller T. Perception of Rhizobiumnodulation factors by tomato cells and inactivation by root chitinases. Proc Natl Acad Sci USA. 1994;91:2196–2200. doi: 10.1073/pnas.91.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengl M, Hatt H, Breer H. Peripheral processes in insect olfaction. Annu Rev Physiol. 1992;54:665–681. doi: 10.1146/annurev.ph.54.030192.003313. [DOI] [PubMed] [Google Scholar]