Abstract
Recognition, management, and prevention of medical complications and comorbidities after liver transplant is the key to improved long-term outcomes. Beyond allograft-related complications, metabolic syndrome, cardiovascular disease, renal dysfunction, and malignancies are leading causes of morbidity and mortality in this patient population. Primary care physicians have an important role in improving outcomes of liver transplant recipients and are increasingly relied on for managing these complex patients. This review serves to assist the primary care physician in the long-term management issues of liver transplant recipients.
Abbreviations and Acronyms: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; CNI, calcineurin inhibitor; IBD, inflammatory bowel disease; LT, liver transplant; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin; NASH, nonalcoholic steatohepatitis-related cirrhosis; OLT, orthotopic liver transplant; PSC, primary sclerosing cholangitis
Outcomes after orthotopic liver transplant (OLT) have continued to improve over the years, with advances in surgical techniques, careful selection of donors and recipients, and improvements in medical management of the recipient. The current 1-year, 5-year, and 10-year survival rates of OLT recipients are 84%, 68%, and 54%, respectively.1 With more than 6000 OLTs performed annually in the Unites States, it can be estimated that with an increasing number of long-term survivors, primary care physicians will be seeing a larger number of liver transplant (LT) recipients in their practice. Beyond the first year, nontechnical, medical complications are the leading causes of long-term morbidity and mortality after OLT.2
Medical Complications After Liver Transplant
After 1 year posttransplant, nonhepatic-related causes of death include malignancy (22%), cardiovascular disease (11%), infection (9%), and renal failure (6%), whereas liver allograft failure accounts for less than one-third of deaths (Figure 1).2 Metabolic syndrome is uncommon in patients with end-stage liver disease before transplant (except in patients with nonalcoholic steatohepatitis [NASH]-related cirrhosis), but it increases dramatically after OLT, with 44% to 58% of patients affected (34% prevalence in nontransplant US adult population) and is associated with an important increase in cardiovascular morbidity.3-6 Many centers defer the management of metabolic syndrome and medical complications to primary care physicians.7 Table 1 summarizes the most common metabolic complications after OLT, and these complications are discussed in greater detail later in this article.
FIGURE 1.
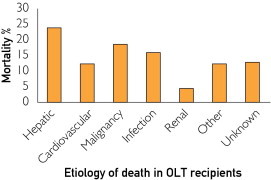
Causes of death at 1 year posttransplant among liver transplant recipients with a mean follow-up of 10 years. OLT = orthotopic liver transplant.
Data from Am J Transplant.2
TABLE 1.
Common Metabolic Complications in the Liver Transplant Recipient
| Complication | Incidence (%) | Risk factors |
|---|---|---|
| Hypertension | 60-70 | Chronic kidney disease, CNIs, corticosteroids, preexisting hypertension |
| Diabetes mellitus | 30-40 | Corticosteroids, CNIs, mTOR inhibitors, obesity, hepatitis C, preexisting insulin resistance |
| Hyperlipidemia | 45-69 | CNIs, mTOR inhibitors, corticosteroids, obesity, cholestatic liver disease, preexisting hyperlipidemia |
| Coronary artery disease | 9-25 | Hypertension, hyperlipidemia, diabetes, previous CAD, NAFLD, smoking, family history |
| Chronic kidney disease | 8-25 | Pretransplant kidney injury, hypertension, CNIs, nephrotoxins, diabetes |
CAD = coronary artery disease; CNI = calcineurin inhibitor (cyclosporine, tacrolimus); mTOR inhibitors = mammalian target of rapamycin inhibitors (like sirolimus); NAFLD = nonalcoholic fatty liver disease.
Hypertension
Hypertension, an uncommon feature in patients with chronic liver disease before transplant, develops in 60% to 70% of patients after OLT.4,8 This increase in prevalence is thought, in part, to be related to immunosuppressant medications, in particular calcineurin inhibitors (CNIs) by causing renal afferent vasoconstriction and chronic sympathetic overactivity and corticosteroids through mineralocorticoid effects. The effects of chronic kidney disease (CKD) and denervation relating to the surgery itself may also be contributors to the development of hypertension in these patients.9
The diagnosis of hypertension in OLT recipients is based on the recommendations of the Seventh Report of the Joint National Commission on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, for the general population.10 The management of hypertension in OLT recipients is similar to that in the general population, as no randomized controlled trials exist specifically for LT recipients. Target blood pressure of lower than 140/90 mm Hg is appropriate for most patients without other major cardiovascular risks; the target should be lower than 130/80 mm Hg in patients with diabetes, CKD, and/or a history of cardiovascular disease.10,11 Lifestyle modifications, including weight loss, physical activity, and dietary sodium restriction, are advised for all patients. Although no single antihypertensive agent has been shown to be superior to others, dihydropyridine calcium channel blockers (eg, amlodipine, nifedipine), which cause vasodilation of renal afferent arterioles, are the preferred first-line agents. The usual starting dose of amlodipine is 2.5 to 5.0 mg daily, and of nifedipine is 30 to 60 mg daily. Common adverse effects of these agents include headache, flushing, palpitations, and peripheral edema. Nondihydropyridine agents such as diltiazem or verapamil should be avoided as they increase the level of CNIs.12 After the early posttransplant period, angiotensin-converting enzyme inhibitors (ACEi's) and angiotensin receptor blockers (ARBs) have a nephroprotective effect (especially in diabetic patients with proteinuria), and they may also have an antifibrotic effect in patients at high risk for post-OLT hepatic fibrosis.13 Lisinopril (starting dose 10 mg daily) and enalapril (starting dose 5 mg daily) are commonly used ACEi's. Their adverse effects include hypotension, cough, and rarely angioedema. In patients who develop cough due to ACEi's, losartan (starting dose 25 mg daily) and valsartan (starting dose 80 mg daily) are the preferred ARBs. Close monitoring for hyperkalemia is recommended for both ACEi's and ARBs when used in association with CNIs. β-Blockers may be used as adjunctive treatment; however, it should be noted that carvedilol can increase the level of CNIs by inhibiting the P-glycoprotein pathway.14 In addition, nonselective β-blockers may reduce splanchnic pressure affecting portal inflow, which should be avoided in the early posttransplant setting. Thiazide or loop diuretics must be used with close follow-up owing to the risk for hyperuricemia and the potential for electrolyte abnormality and renal dysfunction. Antisympathetic antihypertensives such as clonidine and doxazosin may be used as second- or third-line agents for poorly controlled hypertension.15 Up to 30% of patients require 2 or more antihypertensive agents to achieve blood pressure goals.16 Table 2 summarizes key recommendations for management of metabolic complications in LT recipients.
TABLE 2.
Summary Recommendations for the Primary Care of the Liver Transplant Recipient
| Hypertension |
| Target blood pressure is <140/90 or <130/80 mm Hg for OLT patients with diabetes, renal disease, or history of CAD |
| Dihydropyridine CCBs (amlodipine, nifedipine) and/or angiotensin-converting enzyme inhibitors (lisinopril, enalapril)/angiotensin receptor blockers (losartan, valsartan) are first-line agents for management of hypertension; the latter 2 may be preferred in patients with diabetes or proteinuria and may benefit patients with recurrent hepatitis C virus or nonalcoholic steatohepatitis |
| Avoid nondihydropyridine CCBs (diltiazem, verapamil) and use diuretics (hydrochlorothiazide, furosemide) with caution |
| Diabetes |
| Annual screening for diabetes with random or fasting blood glucose measurement is recommended; the diagnosis is based on American Diabetes Association guidelines |
| The management is similar to that of the nontransplant general population; insulin may be required in the early posttransplant period. Oral hypoglycemic agents are safe and effective at later stages |
| Dyslipidemia |
| Liver transplant is considered a risk factor for coronary heart disease; hence, the target low-density lipoprotein cholesterol is <130 mg/dL in the absence of any other associated risk factor, <100 mg/dL in the presence of any other associated coronary heart disease risk factor (smoking, hypertension, low high-density lipoprotein cholesterol, family history of early CAD, advanced age, and preexisting NAFLD), and <70 mg/dL if preexisting or current coronary heart disease |
| Statins are safe and effective; pravastatin and atorvastatin are preferred agents. Fish oil can be used for management of hypertriglyceridemia. Fibrates, niacin, and ezetimibe appear safe. All agents require close follow-up |
| Cardiovascular disease |
| Strict management of CAD risk factors is recommended |
| Aspirin prophylaxis is recommended (also prevents late hepatic artery thrombosis) |
| Chronic kidney disease |
| Optimal management of diabetes and hypertension can reduce the rate of renal damage |
| Careful monitoring for nephrotoxic medications and judicious use of contrast dye is advised |
| Follow serum trough levels of CNIs and monitor for drug interactions |
| Osteoporosis |
| Dual-energy x-ray absorptiometry is recommended every 2-3 y post OLT |
| Management of osteoporosis is similar to that for the nontransplant general population |
| Pregnancy |
| Pregnancies are considered high risk in OLT recipients |
| Conception should generally be delayed for 1 y post OLT; barrier contraceptives and low-dose oral contraceptives are safe and effective |
| CNIs should be continued and monitored during pregnancy |
| Breastfeeding is controversial, but benefit may outweigh the risk with low-dose CNI |
| Vaccinations15 |
| The ideal time to vaccinate OLT recipients is before immunosuppression, recognizing the probable need for booster immunizations post OLT |
| Vaccinations post OLT should be delayed until prednisone dose is lowered to less than 10 mg/d |
| Live-attenuated vaccines should be avoided after OLT |
| Prophylactic pneumococcal and influenza vaccine for all OLT patients and Haemophilus influenzae b vaccine for patients with splenectomy |
CAD = coronary artery disease; CCB = calcium channel blocker; CNI = calcineurin inhibitor; NAFLD = nonalcoholic fatty liver disease; OLT = orthotopic liver transplant.
In patients with poorly controlled hypertension despite the use of multiple agents, alterations in immunosuppression may be considered by the LT center. Options include reduction in corticosteroids,17 substituting tacrolimus for cyclosporine,18 reducing CNI doses by adding mycophenolate mofetil (MMF),19 or converting to sirolimus-based immunosuppression.20 These decisions should be made with the transplant hepatologist involved.
Diabetes
The prevalence of type 2 diabetes mellitus increases from 15% before OLT to 30% to 40% after transplant.3-5 Almost 80% of new-onset diabetes cases develop within the first month posttransplant, 12% after the first year of follow-up. In the long term, 20% to 37% of OLT recipients remain diabetic.21,22 Risk factors for post-OLT diabetes include pretransplant diabetes, obesity, hepatitis C infection, corticosteroids (by inducing insulin resistance, increasing gluconeogenesis, decreasing peripheral insulin utilization), CNIs (through pancreatic β-cell toxicity and inducing insulin resistance, commonly thought tacrolimus moreso than cyclosporine, but is controversial),23 and mammalian target of rapamycin (mTOR) inhibitor use (by inducing insulin resistance, increasing gluconeogenesis, and decreasing peripheral insulin utilization).21-25
Both pre- and post-OLT diabetes are risk factors associated with higher mortality and morbidity in OLT recipients.2,26 Post-OLT diabetes not only is associated with the usual microvascular and macrovascular complications but also has a significant impact on liver allograft survival, particularly in patients with hepatitis C. The 5-year likelihood of advanced fibrosis is increased in patients with diabetes when compared with patients who have normal insulin sensitivity (49% vs 20%, respectively; P=.01).27,28 Post-OLT diabetes has also been associated with late-onset hepatic artery thrombosis, acute and chronic rejection, and development of recurrent or de novo fatty liver disease.22 Per the 2003 International Consensus Guidelines for new-onset diabetes after transplant, weekly fasting plasma glucose screening is recommended for the first month after OLT, followed by screening at 3, 6, and 12 months and annually thereafter.29,30 Hemoglobin A1c may not be accurate in the early posttransplant period owing to anemia and high red blood cell turnover. The diagnosis of diabetes is the same as in the general population.31
There are no specific recommendations from the American Diabetes Association for the management of post-OLT diabetes; thus, management is similar to that for the general population. Lifestyle and dietary modifications should be recommended for all individuals. Insulin is often required in the perioperative and early postoperative period during high-dose corticosteroid use, but insulin can gradually be transitioned to oral hypoglycemic agents. All oral hypoglycemic agents, including metformin, sulfonylureas, and thiazolidinediones, can be used safely in the OLT population.32 Thiazolidinediones might have the additional benefit of improved liver biochemistry and histology in patients with NASH.33 In cases of diabetes that is poorly controlled despite aggressive medical management, the transplant hepatologist may consider withdrawing corticosteroids or possibly adding MMF to reduce CNI or mTOR inhibitor doses.20 Switching from tacrolimus to cyclosporine has not been reliably effective in reducing glucose levels and has the cost of worse hypertension and dyslipidemia.18,34
Dyslipidemia
Dyslipidemia is unusual in patients with cirrhosis, which usually results in marked decline in cholesterol levels due to impaired hepatic synthesis. After OLT, 45% to 69% of patients develop dyslipidemia, which is a risk factor for cardiovascular morbidity and mortality in long-term follow-up.5,16 Risk factors for dyslipidemia include pretransplant obesity, diabetes mellitus, and cholestatic liver disease, as well as immunosuppressant medications. Cyclosporine increases low-density lipoprotein and total cholesterol more than does tacrolimus.18 Sirolimus is strongly associated with dyslipidemia, even more so than cyclosporine, because it affects the insulin signaling pathway by increasing adipose tissue lipase activity and decreasing lipoprotein lipase.35
Based on this increased risk of dyslipidemia, monitoring of fasting lipid panel at 4 to 6 months after transplant and annually thereafter is recommended (Table 2). Liver transplant is considered a coronary heart disease risk equivalent and is considered high risk based on the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults.36 Risk stratification and management goals for hyperlipidemia are shown in Figure 2.11 Therapeutic lifestyle measures are recommended for all patients, although dietary modification alone is often inadequate, making pharmacotherapy necessary. As in the general population, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) are the first-line therapy for elevated cholesterol and triglyceride levels and are safe, well tolerated, and effective in OLT recipients.37 Calcineurin inhibitors as well as statins are metabolized by the CYP3A4 pathway, leading to a potentially increased risk of statin-related myopathy or toxicity; thus, careful clinical and laboratory follow-up is required. Pravastatin and fluvastatin are not metabolized by the CYP3A4 metabolic pathway and thus are preferred in some centers. Statin medications should be initiated at low doses and gradually titrated to desired management goals as tolerated.38 For example, pravastatin, 10 to 20 mg daily, or atorvastatin, 20 to 40 mg daily, can be initiated. Fibric acid derivatives (eg, fenofibrate, gemfibrozil) are generally well tolerated but when used in combination with statins are associated with an increased risk of myotoxicity requiring diligent follow-up. Data on transplant patients are limited for other lipid-lowering agents, including ezetimibe, but in a small study of OLT and other organ recipients, these agents appeared to be safe and effective.39 Niacin has not been systematically studied in OLT recipients, but it can be used for management of mixed hyperlipidemia, based on data from renal and cardiac transplant recipients. Caution must be observed to ensure that bile acid–binding agents such as cholestyramine, if used, are taken at least 2 hours separate from other medications, particularly immunosuppressants. Oral contraceptives, β-blockers, and thiazide diuretics may also potentially exacerbate dysplipidemia. In case of difficult to control hyperlipidemia, the LT center could consider switching from a sirolimus-based or cyclosporine-based regimen to alternative agents.
FIGURE 2.
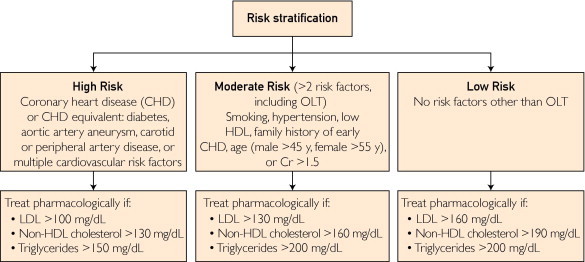
Risk stratification and management of hyperlipidemia in liver transplant recipients. Cr = creatinine, mg/dL; HDL = high-density lipoprotein cholesterol; LDL = low-density lipoprotein cholesterol; OLT = orthotopic liver transplant.
Hypertriglyceridemia, with normal cholesterol levels, is frequently noted in LT patients. Omega-3 fatty acids (fish oil), at a starting dose of 1000 mg twice daily and gradually titrated to a 4000-mg daily dose, may be used for managing isolated hypertriglyceridemia, although no controlled data exist in the transplant population.40 Fish oil may have other benefits, such as anti-inflammatory and/or antiproliferative properties and might improve hepatic steatosis, but more studies are needed in this immunosuppressed transplant population.41 It should be noted that fish oil may increase the low-density lipoprotein level, so a follow-up lipid panel should be performed.
Chronic Kidney Disease
Impairment of renal function is one of the most common complications in OLT recipients. The cumulative incidence of stage 4 CKD (estimated glomerular filtration rate, 15-29 mL/min per 1.73 m2) is 8%, 14%, 18%, and 25% at 1 year, 3 years, 5 years, and 10 years after OLT.42 It is associated with a 4.5-fold increased risk of mortality. After 5 years posttransplant, renal failure accounts for approximately 10% of the patient deaths.2
Risk factors for development of CKD include pretransplant factors, including preexisting CKD, advanced age, diabetes, hypertension, hepatitis C, and posttransplant factors, including early acute kidney injury, diabetes, and CNI use.2,42,43 Duration and etiology of pretransplant renal failure, in particular, are important predictors of posttransplant CKD. Patients with pretransplant hepatorenal syndrome are expected to recover renal function owing to an absence of parenchymal renal disease.44,45 Most other patients with stage 4 or 5 CKD who have end-stage liver disease often undergo combined liver-kidney transplant.43,45 Prolonged ischemic or toxic insults, such as hemodynamic instability, severe sepsis, and nephrotoxic drugs including iodinated contrast exposure, increase risk of renal injury before and after transplant. Calcineurin inhibitors nephrotoxicity is extremely common in patients taking these medications. Although acute CNI nephrotoxicity is due to renal afferent vasoconstriction and decreased glomerular filtration and is reversible with dose reduction and/or withdrawal, chronic CNI nephrotoxicity is characterized by tubulointerstitial fibrosis and is generally irreversible.46 Chronic CNI nephropathy is usually asymptomatic, with a gradual decline in renal function that includes a bland urine sediment and mild proteinuria.43
Steps to prevent the development and progression of renal failure include identifying patients with CKD at risk for posttransplant renal failure, avoiding renal hypoperfusion, maintaining optimal control of hypertension and diabetes and avoiding nephrotoxic medications, including nonsteroidal anti-inflammatory medications, and contrast exposure.43 Calcium channel blockers and ACEi's or angiotensin receptor blockers may be nephroprotective against CNI-induced injury.13,47 Discussion with the transplant center for reduction in the dose of CNIs, combining low-dose CNIs with renal-sparing MMF with or without concomitant glucocorticoids, could be considered.48 Complete withdrawal of CNI and using only MMF and glucocorticoids is associated with significant risk of graft dysfunction.19 Conversion to sirolimus-based immunosuppression may show a benefit to renal function if converted before 1 to 2 years of deteriorating renal function (as long as no proteinuria is noted)49; otherwise, it is not effective in improving renal function in LT recipients.50
Cardiovascular Disease
With the increased incidence of metabolic syndrome and CKD in OLT recipients, it is not surprising that cardiovascular morbidity and mortality are also increased. Over a median follow-up of approximately 4 years there is a 3-fold increased relative risk of ischemic cardiac events and 2.5-fold increased risk of cardiovascular deaths in LT recipients as compared with an age- and sex-matched nontransplant population.51 In long-term survivors after liver transplant, as many as 25% of patients have had a major cardiovascular event by 10 years posttransplant, despite careful cardiovascular evaluation before transplant (excluding patients with important preexisting cardiovascular disease).36 Cardiovascular disease was noted to be the third most common cause of late mortality in LT recipients, accounting for 12% to 16% of patient deaths.2 Risk-reduction strategies for all patients after OLT include healthy lifestyle changes and better control of hypertension, diabetes, and hyperlipidemia, as are also recommended for those in the general population; prophylactic low-dose aspirin may be beneficial, although adding aspirin has not been systematically studied in this population.
Malignancy
The risk of de novo extrahepatic malignancy after OLT is 2 to 4 times higher in LT recipients than in age- and sex-matched samples from the general population, affecting 2% to 16% of patients.52-54 In a large, multicenter, long-term database study of 798 adult LT recipients, the probability of developing any de novo malignancy within 1, 5, and 10 years was 3.5%, 11.9%, and 21.7%, respectively.52 One reason for this increased incidence is that the long-term use of immunosuppressive medications (particularly azathioprine and MMF) is thought to impair cancer surveillance mechanisms and create an environment for oncogenic viruses to thrive. In addition to immunosuppressant use, age, alcohol use before transplant, current or former cigarette smoking, and primary sclerosing cholangitis (PSC) are risk factors for de novo malignancy after OLT.52,53,55
Skin cancers are the most common de novo malignancies after OLT, accounting for almost half of all cancers, with an equal distribution between basal cell and squamous cell cancer, although the incidence of the latter is about 100 times more than that in the general population.56,57 The 5- and 10-year probability of skin cancer is estimated at 5.9% and 10.8%, respectively.52 These cancers tend to be much more aggressive in transplant recipients, with greater local invasion, higher tendency for multiple lesions and metastatic disease, and higher risk of recurrence. Annual thorough skin examination, sun protective strategies, and early treatment of actinic keratosis is recommended.57
Posttransplant lymphoproliferative disorders are the second most common malignancy in solid organ transplant, occurring in 0.9% to 2.6% of adult OLT recipients, with a higher than 10% incidence in pediatric OLT recipients.55,58 The cumulative incidence of posttransplant lymphoproliferative disorders in adults is estimated at 1.5%, 1.9%, and 3.2% at 1, 5, and 10 years after OLT, with a mean time to diagnosis between 26 and 32 months.52,55 Most cases (80%-90%) are associated with reactivation of or primary Epstein-Barr virus infection. Management options include reduction of immunosuppression, rituximab, combination chemotherapy, and adoptive immunotherapy through the aid of the oncology team.59
Liver transplant recipients are at increased risk for other cancers as well, with an estimated 10-year probability of developing a gastrointestinal, lung, female genitourinary, or oropharyngeal/laryngeal cancer at 3.6%, 2.0%, 1.8%, and 1.1%, respectively.52 This increased risk is seen largely in the transplant population with previous or current alcohol-related disease or PSC. In patients with alcoholic liver disease, annual surveillance is recommended, including mammography, Papanicolau smear, prostate specific antigen test, chest x-ray, and otolaryngology examination, in addition to strict adherence to colonoscopy guidelines for the general population.60 Patients with PSC, especially those with coexisting inflammatory bowel disease (IBD), are at higher risk for colon cancer. Hence, annual colonoscopy is recommended with random biopsies for dysplasia surveillance. Patients with PSC and no evidence of IBD may not require as frequent screening if their biopsies continue to show no active colitis. Optimal timing for screening in these individuals is unknown.61 Virally mediated malignancies including human herpesvirus 8–associated Kaposi sarcoma and human papilloma virus–associated anogenital lesion, albeit less common, are also increased in incidence compared with that in the general population and should be looked for in the appropriate clinical setting.57
American Cancer Society–recommended screening guidelines for the general population should be followed closely for all other OLT recipients (Table 3). In a study on the effects of an “intensified” surveillance protocol on early detection of de novo cancer and mortality in OLT recipients, Finkenstedt et al62 demonstrated that after introducing an intensified surveillance protocol (consisting of annual chest and abdominal computed tomographic scans, urologic evaluation [including measurement of prostate-specific antigen], gynecologic examination [including Papanicolau smear and mammography], dermatologic screening, and colonoscopy performed 3 years after OLT and every 5 years thereafter, except in patients with an adenoma before OLT or a history of IBD, in whom the first colonoscopy was performed 1 year after OLT), the detection rate of de novo cancers increased from 4.9% to 13% and were diagnosed in earlier stages; for nonskin cancers, the median tumor-related survival improved significantly from 1.2 to 3.3 years, and improvement in the median overall survival after OLT was noted as well.
TABLE 3.
Screening Recommendations for Malignancies in Liver Transplant Recipients
| Skin cancer | Annual dermatologic examination, especially in patients with history of skin cancer or actinic skin damage, patients who received a transplant for alcoholic liver disease, and white patients |
| Posttransplant lymphoproliferative disorders | High index of suspicion in patients presenting with category B symptoms, weight loss, or lymphadenopathy, especially in EBV-seronegative patients |
| Colon cancer | Strict adherence to guidelines for colorectal cancer screening in average-risk population; annual colonoscopy in patients who received a transplant for primary sclerosing cholangitis and/or inflammatory bowel disease with random surveillance biopsies |
| Cervical cancer | Strict adherence to pelvic examination and PAP smear recommendations for the general population, in accord with American Cancer Society recommendations |
| Lung cancer | Annual chest x-ray for high-risk OLT recipients, including smokers and patients who received a transplant for alcoholic liver disease |
| Oropharyngeal/laryngeal cancer | Annual otolaryngology evaluation for high-risk OLT recipients, including smokers and patients who received a transplant for alcoholic liver disease |
| Breast cancer | Annual mammogram starting at age 40, in accord with American Cancer Society recommendations |
| Prostate cancer | Annual PSA evaluation starting age 50, as for the general population in accord with the American Cancer Society recommendations |
EBV= Epstein-Barr virus; OLT = orthotopic liver transplant; PAP = Papanicolaou; PSA = prostate-specific antigen.
Other Issues in Liver Transplant Recipients
Osteoporosis
With the use of corticosteroids and CNIs along with prolonged postoperative convalescence, bone density decreases within the first 3 to 6 months after transplant and gradually returns to pretransplant levels. Two studies described several patients with pretransplant osteopenia/osteoporosis attributable to heavy alcohol use, smoking, poor nutrition, cholestatic liver disease, and hypogonadism. The risk of osteoporotic vertebral and nonaxial fractures was 14% and 21% at 1 and 2 years posttransplant, decreased with time, and was highest in patients with pretransplant osteopenia and cholestatic liver disease.63,64 All OLT recipients should be evaluated for osteoporosis with dual-energy x-ray absorptiometry.
The treatment of osteoporosis in OLT recipients is similar to that recommended for the general population. Nonpharmacologic measures include cessation of smoking, increased physical activity, and improvement in nutrition; 1500 mg of elemental calcium and 800 IU of vitamin D daily are recommended for all individuals. In a meta-analysis, bisphosphonate therapy during the first year in OLT recipients appeared to reduce accelerated bone loss and improve bone mineral density at the lumbar spine.65 As bone metabolism improves with time, it may be possible to discontinue bisphosphonate use. All patients with osteoporosis should undergo evaluation for vitamin D deficiency.
Hyperuricemia and Gout
Hyperuricemia is a common metabolic complication in transplant recipients owing to the use of CNIs (cyclosporine moreso than tacrolimus), which impair renal uric acid secretion. Gout has been reported in approximately 7% of OLT recipients.66 Management of these complications is similar to that for the general population, but nonsteroidal anti-inflammatory drugs should be used with caution. Allopurinol may interfere with the metabolism of azathioprine, and its use may result in supratherapeutic levels and increased risk of gastrointestinal and myelosuppressive adverse effects.
Mood Disorders
After OLT, approximately 22% of patients develop mood disorders, primarily depression and posttraumatic stress disorder, that are attributable in part to the patient's expectations of outcome after transplant and result in poor quality of life, poor medication adherence, and perceived disability.67 Selective serotonin reuptake inhibitors are the first line of therapy and are safe and effective in this population; citalopram and sertraline are used most commonly because they have minimal inhibitory effect on cytochrome P-450 pathway and do not alter CNI levels.
Pregnancy
It is recommended to wait 1 year after LT before conception to minimize the effects of immunosuppressant requirements and the risk of acute rejection and opportunistic infections (in particular, cytomegalovirus).68 Barrier contraceptives and low-dose oral contraceptives are safe and effective in OLT recipients.69 Seventy to eighty percent of pregnancies in LT recipients result in viable births, but these pregnancies are associated with a slight increase in the risk of maternal (pregnancy-induced hypertension and preeclamsia) and fetal (prematurity and low birth weight) complications. Pregnancy does not change graft and patient survival, however. There is a small increase in the incidence of congenital malformations among children born to transplant patients (4%-5% compared with 3% in the general population), especially those taking MMF and azathioprine.70,71 During pregnancy, careful monitoring of immunosuppressant drug levels is necessary because the volume of drug distribution changes.
Breastfeeding
Breastfeeding remains a controversial issue. Manufacturers warn against breastfeeding, as both CNIs and azathioprine have been found in breast milk. Case reports have suggested that the neonate receives only limited exposure, with no major adverse effects noted, particularly with tacrolimus.72 No data exist on the presence of MMF or sirolimus in breast milk. Certainly the benefits of breastfeeding may very well outweigh the risks.
Immunosuppressive Medications
The commonly used immunosuppressive medications include CNIs such as cyclosporine and tacrolimus, antimetabolite agents including azathiopurine or MMF, and corticosteroids and mTOR inhibitors such as sirolimus or everolimus. Early after OLT, most transplant centers use a combination of 2 to 4 agents, eventually tapering to monotherapy with CNIs. Patients with a renal transplant generally continue taking 2 to 3 drug regimens long-term.
CNIs suppress interleukin 2–dependent T-cell proliferation by inhibiting calcineurin. Ideal trough levels (obtained 1 hour before next dose) decrease over the posttransplant follow-up and are guided by the transplant center. Typical long-term trough levels range from approximately 50 to 100 ng/mL for cyclosporine and approximately 4 to 6 ng/mL for tacrolimus. Antimetabolite agents selectively inhibit T- and B-cell proliferation by interfering with purine synthesis. Mycophenolate mofetil is often used within the first few months after OLT and is frequently discontinued within the first year after OLT. In some circumstances, MMF may be used on a long-term basis to enable lowering the dose of CNIs (for renal protection). Sirolimus inhibits T-cell proliferation by cell cycle inhibition and is sometimes used in OLT as a CNI-sparing strategy (for renal dysfunction) or in patients receiving a transplant for hepatocellular carcinoma or other malignancies (for antiproliferative effects).73 Corticosteroids inhibit multiple cytokines, including interleukin 1, 2, and 6, tumor necrosis factor, and interferon γ, and are most often used early after transplant and at times of acute cellular rejection.15,74 The common adverse effects of these medications are listed in Table 4.
TABLE 4.
Common Adverse Effects of Immunosuppressive Medicationsa
| Adverse effect | CNI |
Antimetabolite |
mTOR | GS | ||
|---|---|---|---|---|---|---|
| CYA | Tac | MMF | AZA | |||
| Diabetes | + | ++ | − | − | + | +++ |
| Hypertension | +++ | ++ | − | − | ++ | +++ |
| Hyperlipidemia | ++ | + | − | − | +++ | ++ |
| Chronic kidney disease | +++ | +++ | − | − | ++ | − |
| Osteoporosis | + | + | − | − | − | +++ |
| Bone marrow suppression | − | − | ++ | ++ | + | − |
| Dermatologic | ||||||
| Alopecia | − | ++ | − | +/− | − | − |
| Dermatitis | − | + | − | + | ++ | + |
| Hirsuitism | ++ | − | − | − | − | + |
| Gingival hyperplasia | + | − | − | − | − | − |
| Neurotoxicityb | ||||||
| Headache | ++ | ++ | ++ | + | ++ | + |
| Tremor | ++ | ++ | ++ | − | − | − |
| Seizure | + | + | − | − | − | − |
| Gastrointestinal toxicity | + | + | +++ | + | ++ | + |
AZA = azathioprine; CNI = calcineurin inhibitor; CYA = cyclosporine; GS = glucocorticoid; MMF = mycophenolate mofetil; mTOR = mammalian target of rapamycin (sirolimus or everolimus); Tac = tacrolimus; + = infrequent occurrence (3%-20%); ++ = frequent occurrence (20%-49%); +++ = very frequently reported (>50%); − = none reported.
Leukoencephalopathy is reported with tacrolimus but at <0.1%.
Drugs that inhibit or induce cytochrome P-450 3A metabolism can result in slow or rapid metabolism of CNIs and mTOR inhibitors, increasing the likelihood of drug-induced toxicity or allograft rejection, respectively. Immunosuppressant levels should be checked 48 to 72 hours after initiation of any new medication expected to affect CNI levels. Several routinely prescribed antibiotics may have an effect on CNI levels and should be used judiciously. Care should be taken to review medications that can enhance CNI-induced nephrotoxicity and hyperkalemia, including nonsteroidal anti-inflammatory medications, ACEi's, and spironolactone. Acetaminophen, up to 4 g per day, and narcotics are safe in OLT recipients. Any changes in the immunosuppressive medications should be made in close consultation with the transplant center to avoid the risk of organ rejection and medication toxicity.15,74 Common drug interactions are listed in Table 5.
TABLE 5.
Common Drug Interactions of CNIs and mTOR Inhibitors
| Drugs that may increase level of CNIs or mTOR inhibitors |
| Antibiotics: macrolides (azithromycin, erythromycin, clarithromycin) |
| Antifungals: caspofungin, azoles (fluconazole, itraconazole, voriconazole) |
| Calcium channel inhibitors: nondihydropyridine (diltiazem, verapamil) |
| Statins: simvastatin, atorvastatin |
| Others: protease inhibitors, amiodarone, omeprazole, rabeprazole, cimetidine, metoclopramide, allopurinol, colchicine, bromocriptine, grapefruit juice |
| Drugs that may decrease level of CNIs or mTOR inhibitors |
| Antibiotics: rifampin, rifabutin, nafcillin |
| Anticonvulsants: carbamazepine, phenytoin, phenobarbital |
| Others: St John's wort, orlistat, ticlopidine, octreotide |
CNI = calcineurin inhibitor; mTOR = mammalian target of rapamycin.
Liver Allograft Dysfunction
If liver enzymes (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase) or function tests (bilirubin, international normalized ratio) are elevated 1.5 or more times the upper limits of normal, further evaluation is warranted. The LT center should be contacted. Many patients have long-term mild increases in some of the liver enzymes, and thus a change above baseline should trigger further investigation. The differential diagnosis of elevated liver enzymes in recipients of OLT is shown in Table 6. If the patient is experiencing a community-acquired viral infection, simply rechecking tests in 1 to 2 weeks is appropriate. Evaluation includes liver ultrasonography and Doppler scan of the hepatic artery and venous structures, cytomegalovirus polymerase chain reaction test, and symptom-driven work-up. Liver biopsy and magnetic or endoscopic retrograde cholangiopancreatography may be necessary depending on the liver enzyme pattern, previous biliary complications, and symptom complex.15 Hepatitis C recurrence is universal, and approximately 20% to 30% of patients develop cirrhosis within 5 to 10 years after OLT.75 Histologic recurrence can only be diagnosed by liver biopsy because all patients have serologic recurrence immediately posttransplant. Acute cellular rejection (which requires an expert hepatopathologist to assess) must be ruled out. With the increasing burden of metabolic syndrome posttransplant, there is a risk of recurrent or de novo NASH in transplanted livers.76,77 Late biliary and vascular complications, including hepatic artery thrombosis and stenosis, cause progressive ischemic biliary destruction and manifest as jaundice, intrahepatic biliary strictures, recurrent cholangitis, and intrahepatic abscesses. Because the allograft is not innervated, patients do not always develop right upper quadrant abdominal pain, nor do bile ducts necessarily dilate; thus, biliary imaging is needed if clinical suspicion is present. The transplant center should be notified in the case of allograft dysfunction, as many of these situations can be life-threatening if not managed promptly. The use of low-dose aspirin can decrease the likelihood of late hepatic artery thrombosis.78
TABLE 6.
Common Causes of Liver Allograft Dysfunction
| Allograft rejection (acute or chronic) |
| Cytomegalovirus infection or reactivation |
| Recurrence of primary liver disease |
| Hepatitis C |
| Hepatitis B |
| Autoimmune liver diseases (autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis) |
| Alcoholism |
| Hepatocellular cancer |
| Nonalcoholic steatohepatitis |
| Vascular complications (hepatic artery thrombosis or stenosis, venous outflow impairment) |
| Biliary complications (bile leak, biliary strictures, stones/casts) |
| Drug-induced liver injury |
| Sepsis or systemic infection |
| Development of new unrelated liver disease in allograft |
When to Notify the Transplant Center
A close collaboration among primary care physicians and the transplant center is required for optimal care of OLT recipients. The transplant center should be notified for further work-up if there is (1) difficulty controlling metabolic complications (diabetes, hypertension, hyperlipidemia, chronic kidney disease) so immunosuppressant medication regimen changes can be considered; (2) development of new malignancy, including skin cancers, in LT recipients; (3) introduction of new long-term medications with potential to interact with CNIs; (4) pregnancy after LT (for close monitoring of medication levels); and (5) new elevation (or change from baseline mild elevations) in liver enzymes (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase) or function test results (bilirubin, international normalized ratio) 1.5 times or more than the upper limits of normal. In addition, if there are any other issues of concern, the transplant center should be contacted.
Conclusion
Increased awareness and regular assessment for metabolic syndrome, renal function, and malignancies by primary care physicians can improve long-term morbidity and mortality after OLT. Close collaboration among primary care physicians, transplant physicians, and surgeons is required for optimal care of these complex patients.
Article Highlights.
-
■
Liver transplant recipients frequently have multiple comorbidities after transplant that require careful long-term management and close follow-up.
-
■
The approach to management of many metabolic comorbidities is similar to that in the general population, but refractory disease requiring combination therapy is more common.
-
■
Cholesterol-lowering agents are not contraindicated in patients with liver abnormalities after liver transplant (or before in most settings) and can be used with close follow-up and monitoring.
-
■
Chronic kidney disease is common after liver transplant and relates not only to immunosuppressive medications but also to management of medical comorbidities such as diabetes and hypertension. Care must be taken not to use potential nephrotoxins in treating these patients (eg, avoid NSAIDs, unnecessary contrast agents).
-
■
Long-term immunosuppression increases the risk of infection but also increases malignancy risk, and screening guidelines should be adhered to.
-
■
Close collaboration with the transplant program is beneficial.
Acknowledgments
The authors thank Laura J. Myrhe, PharmD, for her assistance in preparing Table 4.
References
- 1.2009 OPTN/SRTR Annual Report. http://optn.transplant.hrsa.gov/ar2009/Chapter_IV_AR_CD.htm?cp=5#3 Accessed October 7, 2011.
- 2.Watt K.D., Pedersen R.A., Kremers W.K., Heimbach J.K., Charlton M.R. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10(6):1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laish I., Braun M., Mor E., Sulkes J., Harif Y., Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17(1):15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 4.Laryea M., Watt K.D., Molinari M. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13(8):1109–1114. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi G., Marchesini G., Marzocchi R., Pinna A.D., Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14(11):1648–1654. doi: 10.1002/lt.21588. [DOI] [PubMed] [Google Scholar]
- 6.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults; findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 7.Heller J.C., Prochazka A.V., Everson G.T., Forman L.M. Long-term management after liver transplantation: primary care physician versus hepatologist. Liver Transpl. 2009;15(10):1330–1335. doi: 10.1002/lt.21786. [DOI] [PubMed] [Google Scholar]
- 8.Canzanello V.J., Schwartz L., Taler S.J. Evolution of cardiovascular risk after liver transplantation: a comparison of cyclosporine A and tacrolimus (FK506) Liver Transpl Surg. 1997;3(1):1–9. doi: 10.1002/lt.500030101. [DOI] [PubMed] [Google Scholar]
- 9.Carlson S.H., Osborn J.W., Wyss J.M. Hepatic denervation chronically elevates arterial pressure in Wistar-Kyoto rats. Hypertension. 1998;32(1):46–51. doi: 10.1161/01.hyp.32.1.46. [DOI] [PubMed] [Google Scholar]
- 10.Chobanian A.V., Bakris G.L., Black H.R., National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 11.Mells G., Neuberger J. Reducing the risks of cardiovascular disease in liver allograft recipients. Transplantation. 2007;83(9):1141–1150. doi: 10.1097/01.tp.0000262706.28513.6a. [DOI] [PubMed] [Google Scholar]
- 12.Neal D.A., Brown M.J., Wilkinson I.B., Byrne C.D., Alexander G.J. Hemodynamic effects of amlodipine, bisoprolol, and lisinopril in hypertensive patients after liver transplantation. Transplantation. 2004;77(5):748–750. doi: 10.1097/01.tp.0000116418.78963.dc. [DOI] [PubMed] [Google Scholar]
- 13.Lubel J.S., Herath C.B., Burrell L.M., Angus P.W. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23(9):1327–1338. doi: 10.1111/j.1440-1746.2008.05461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galioto A., Semplicini A., Zanus G., Fasolato S., Sticca A., Boccagni P. Nifedipine versus carvedilol in the treatment of de novo arterial hypertension after liver transplantation: results of a controlled clinical trial. Liver Transpl. 2008;14(7):1020–1028. doi: 10.1002/lt.21442. [DOI] [PubMed] [Google Scholar]
- 15.McGuire B.M., Rosenthal P., Brown C.C., American Society of Transplantation Long-term management of the liver transplant patient: recommendations for the primary care doctor. Am J Transplant. 2009;9(9):1988–2003. doi: 10.1111/j.1600-6143.2009.02733.x. [DOI] [PubMed] [Google Scholar]
- 16.Neal D.A., Tom B.D., Luan J. Is there disparity between risk and incidence of cardiovascular disease after liver transplant? Transplantation. 2004;77(1):93–99. doi: 10.1097/01.TP.0000100685.70064.90. [DOI] [PubMed] [Google Scholar]
- 17.Stegall M.D., Everson G.T., Schroter G. Prednisone withdrawal late after adult liver transplantation reduces diabetes, hypertension, and hypercholesterolemia without causing graft loss. Hepatology. 1997;25(1):173–177. doi: 10.1002/hep.510250132. [DOI] [PubMed] [Google Scholar]
- 18.Neal D.A., Gimson A.E., Gibbs P., Alexander G.J. Beneficial effects of converting liver transplant recipients from cyclosporine to tacrolimus on blood pressure, serum lipids, and weight. Liver Transpl. 2001;7(6):533–539. doi: 10.1053/jlts.2001.24637. [DOI] [PubMed] [Google Scholar]
- 19.Schlitt H.J., Barkmann A., Böker K.H. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet. 2001;357(9256):587–591. doi: 10.1016/s0140-6736(00)04055-1. [DOI] [PubMed] [Google Scholar]
- 20.Kreis H., Oberbauer R., Campistol J.M. Rapamune Maintenance Regimen Trial: Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15(3):809–817. doi: 10.1097/01.asn.0000113248.59077.76. [DOI] [PubMed] [Google Scholar]
- 21.Oufroukhi L., Kamar N., Muscari F. Predictive factors for posttransplant diabetes mellitus within one-year of liver transplantation. Transplantation. 2008;85(10):1436–1442. doi: 10.1097/TP.0b013e31816f1b7c. [DOI] [PubMed] [Google Scholar]
- 22.Moon J.I., Barbeito R., Faradji R.N., Gaynor J.J., Tzakis A.G. Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: long-term follow up. Transplantation. 2006;82(12):1625–1628. doi: 10.1097/01.tp.0000250361.60415.96. [DOI] [PubMed] [Google Scholar]
- 23.Kuo H.T., Sampaio M.S., Ye X., Reddy P., Martin P., Bunnapradist S. Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing database. Transplantation. 2010;89(9):1134–1140. doi: 10.1097/TP.0b013e3181d2fec1. [DOI] [PubMed] [Google Scholar]
- 24.Navasa M., Bustamante J., Marroni C. Diabetes mellitus after liver transplantation: prevalence and predictive factors. J Hepatol. 1996;25(1):64–71. doi: 10.1016/s0168-8278(96)80329-1. [DOI] [PubMed] [Google Scholar]
- 25.Vodenik B., Rovira J., Campistol J.M. Mammalian target of rapamycin and diabetes: what does the current evidence tell us? Transplant Proc. 2009;41(6, suppl):S31–S38. doi: 10.1016/j.transproceed.2009.06.159. [DOI] [PubMed] [Google Scholar]
- 26.John P.R., Thuluvath P.J. Outcome of liver transplantation in patients with diabetes mellitus: a case-control study. Hepatology. 2001;34(5):889–895. doi: 10.1053/jhep.2001.29134. [DOI] [PubMed] [Google Scholar]
- 27.Veldt B.J., Poterucha J.J., Watt K.D. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2009;9(6):1406–1413. doi: 10.1111/j.1600-6143.2009.02642.x. [DOI] [PubMed] [Google Scholar]
- 28.Hanouneh I.A., Feldstein A.E., McCullough A.J. The significance of metabolic syndrome in the setting of recurrent hepatitis C after liver transplantation. Liver Transpl. 2008;14(9):1287–1293. doi: 10.1002/lt.21524. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson A., Davidson J., Dotta F. Guidelines for the treatment and management of new-onset diabetes after transplantation. Clin Transplant. 2005;19(3):291–298. doi: 10.1111/j.1399-0012.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 30.Lane J.T., Dagogo-Jack S. Approach to the patient with new-onset diabetes after transplant (NODAT) J Clin Endocrinol Metab. 2011;96(11):3289–3297. doi: 10.1210/jc.2011-0657. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watt K.D., Charlton M.R. Metabolic syndrome and liver transplantation: a review and guide to management. J Hepatol. 2010;53(1):199–206. doi: 10.1016/j.jhep.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Boettcher E., Csako G., Pucino F., Wesley R., Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emre S., Genyk Y., Schluger L.K. Treatment of tacrolimus-related adverse effects by conversion to cyclosporine in liver transplant recipients. Transpl Int. 2000;13(1):73–78. doi: 10.1007/s001470050012. [DOI] [PubMed] [Google Scholar]
- 35.Morard I., Dumortier J., Spahr L. Conversion to sirolimus-based immunosuppression in maintenance liver transplantation patients. Liver Transpl. 2007;13(5):658–664. doi: 10.1002/lt.21116. [DOI] [PubMed] [Google Scholar]
- 36.Ciccarelli O., Kaczmarek B., Roggen F. Long-term medical complications and quality of life in adult recipients surviving 10 years or more after liver transplantation. Acta Gastroenterol Belg. 2005;68(3):323–330. [PubMed] [Google Scholar]
- 37.Martin J.E., Cavanaugh T.M., Trumbull L. Incidence of adverse events with HMG-CoA reductase inhibitors in liver transplant patients. Clin Transplant. 2008;22(1):113–119. doi: 10.1111/j.1399-0012.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 38.Asberg A. Interactions between cyclosporin and lipid-lowering drugs: implications for organ transplant recipients. Drugs. 2003;63(4):367–378. doi: 10.2165/00003495-200363040-00003. [DOI] [PubMed] [Google Scholar]
- 39.Almutairi F., Peterson T.C., Molinari M. Safety and effectiveness of ezetimibe in liver transplant recipients with hypercholesterolemia. Liver Transpl. 2009;15(5):504–508. doi: 10.1002/lt.21710. [DOI] [PubMed] [Google Scholar]
- 40.McKenney J.M., Sica D. Prescription omega-3 fatty acids for the treatment of hypertriglyceridemia. Am J Health Syst Pharm. 2007;64(6):595–605. doi: 10.2146/ajhp060164. [DOI] [PubMed] [Google Scholar]
- 41.Lee S., Gura K.M., Puder M. Omega-3 fatty acids and liver disease. Hepatology. 2007;45(4):841–845. doi: 10.1002/hep.21645. [DOI] [PubMed] [Google Scholar]
- 42.Ojo A.O., Held P.J., Port F.K. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 43.Bloom R.D., Reese P.P. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol. 2007;18(12):3031–3041. doi: 10.1681/ASN.2007040394. [DOI] [PubMed] [Google Scholar]
- 44.Campbell M.S., Kotlyar D.S., Brensinger C.M. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005;11(9):1048–1055. doi: 10.1002/lt.20445. [DOI] [PubMed] [Google Scholar]
- 45.Davis C.L. Impact of pretransplant renal failure: when is listing for kidney-liver indicated? Liver Transpl. 2005;11(suppl 2):S35–S44. doi: 10.1002/lt.20617. [DOI] [PubMed] [Google Scholar]
- 46.Nankivell B.J., Borrows R.J., Fung C.L., O'Connell P.J., Allen R.D., Chapman J.R. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349(24):2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 47.Artz M.A., Hilbrands L.B., Borm G., Assmann K.J., Wetzels J.F. Blockade of the renin-angiotensin system increases graft survival in patients with chronic allograft nephropathy. Nephrol Dial Transplant. 2004;19(11):2852–2857. doi: 10.1093/ndt/gfh462. [DOI] [PubMed] [Google Scholar]
- 48.Orlando G., Baiocchi L., Cardillo A. Switch to 1.5 grams MMF monotherapy for CNI-related toxicity in liver transplantation is safe and improves renal function, dyslipidemia, and hypertension. Liver Transpl. 2007;13(1):46–54. doi: 10.1002/lt.20926. [DOI] [PubMed] [Google Scholar]
- 49.McKenna G.J., Ruiz R., Onaca N. The impact of timing in sirolimus conversion for renal insufficiency in liver transplant recipients [abstract] Liver Transpl. 2011;17(suppl 3):S84. [Google Scholar]
- 50.Asrani S.K., Leise M.D., West C.P. Use of sirolimus in liver transplant recipients with renal insufficiency: a systematic review and meta-analysis. Hepatology. 2010;52(4):1360–1370. doi: 10.1002/hep.23835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnston S.D., Morris J.K., Cramb R., Gunson B.K., Neuberger J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73(6):901–906. doi: 10.1097/00007890-200203270-00012. [DOI] [PubMed] [Google Scholar]
- 52.Watt K.D., Pedersen R.A., Kremers W.K., Heimbach J.K., Sanchez W., Gores G.J. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137(6):2010–2017. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fung J.J., Jain A., Kwak E.J., Kusne S., Dvorchik I.B.E. De novo malignancies after liver transplantation: a major cause of late death. Liver Transpl. 2001;7(11, suppl 1):S109–S118. doi: 10.1053/jlts.2001.28645. [DOI] [PubMed] [Google Scholar]
- 54.Aberg F., Pukkala E., Höckerstedt K., Sankila R., Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14(10):1428–1436. doi: 10.1002/lt.21475. [DOI] [PubMed] [Google Scholar]
- 55.Chak E., Saab S. Risk factors and incidence of de novo malignancy in liver transplant recipients: a systematic review. Liver Int. 2010;30(9):1247–1258. doi: 10.1111/j.1478-3231.2010.02303.x. [DOI] [PubMed] [Google Scholar]
- 56.Jiménez-Romero C., Manrique Municio A., Marqués Medina E. Incidence of de novo nonmelanoma skin tumors after liver transplantation for alcoholic and nonalcoholic liver diseases. Transplant Proc. 2006;38(8):2505–2507. doi: 10.1016/j.transproceed.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 57.Euvrard S., Kanitakis J., Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 58.Aucejo F., Rofaiel G., Miller C. Who is at risk for post-transplant lymphoproliferative disorders (PTLD) after liver transplantation? J Hepatol. 2006;44(1):19–23. doi: 10.1016/j.jhep.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Kamdar K.Y., Rooney C.M., Heslop H.E. Posttransplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant. 2011;16(3):274–280. doi: 10.1097/MOT.0b013e3283465715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varma V., Webb K., Mirza D.F. Liver transplantation for alcoholic liver disease. World J Gastroenterol. 2010;16(35):4377–4393. doi: 10.3748/wjg.v16.i35.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanouneh I.A., Macaron C., Lopez R., Zein N.N., Lashner B.A. Risk of colonic neoplasia after liver transplantation for primary sclerosing cholangitis. Inflamm Bowel Dis. 2012;18(2):269–274. doi: 10.1002/ibd.21692. [DOI] [PubMed] [Google Scholar]
- 62.Finkenstedt A., Graziadei I.W., Oberaigner W. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am J Transplant. 2009;9(10):2355–2361. doi: 10.1111/j.1600-6143.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 63.Leidig-Bruckner G., Hosch S., Dodidou P. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet. 2001;357(9253):342–347. doi: 10.1016/S0140-6736(00)03641-2. [DOI] [PubMed] [Google Scholar]
- 64.Guichelaar M.M., Schmoll J., Malinchoc M., Hay J.E. Fractures and avascular necrosis before and after orthotopic liver transplantation: long-term follow-up and predictive factors. Hepatology. 2007;46(4):1198–1207. doi: 10.1002/hep.21805. [DOI] [PubMed] [Google Scholar]
- 65.Kasturi K.S., Chennareddygari S., Mummadi R.R. Effect of bisphosphonates on bone mineral density in liver transplant patients: a meta-analysis and systematic review of randomized controlled trials. Transpl Int. 2010;23(2):200–207. doi: 10.1111/j.1432-2277.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- 66.Neal D.A., Tom B.D., Gimson A.E., Gibbs P., Alexander G.J. Hyperuricemia, gout, and renal function after liver transplantation. Transplantation. 2001;72(10):1689–1691. doi: 10.1097/00007890-200111270-00021. [DOI] [PubMed] [Google Scholar]
- 67.Rothenhäusler H.B., Ehrentraut S., Kapfhammer H.P. Psychiatric and psychosocial outcome of orthotopic liver transplantation. Psychother Psychosom. 2002;71(5):285–297. doi: 10.1159/000064811. [DOI] [PubMed] [Google Scholar]
- 68.McKay D.B., Josephson M.A. Reproduction and transplantation: report on the AST consensus conference on reproductive issues and transplantation. Am J Transpl. 2005;5(7):1592–1599. doi: 10.1111/j.1600-6143.2005.00969.x. [DOI] [PubMed] [Google Scholar]
- 69.Surti B., Tan J., Saab S. Pregnancy and liver transplantation. Liver Int. 2008;28(9):1200–1206. doi: 10.1111/j.1478-3231.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 70.Nagy S., Bush M.C., Berkowitz R., Fishbein T.M., Gomez-Lobo V. Pregnancy outcome in liver transplant recipients. Obstet Gynecol. 2003;102(1):121–128. doi: 10.1016/s0029-7844(03)00369-7. [DOI] [PubMed] [Google Scholar]
- 71.Coscia L.A., Constantinescu S., Moritz M.J. Report from the National Transplantation Pregnancy Registry (NTPR): outcomes of pregnancy after transplantation. Clin Transpl. 2007:29–42. [PubMed] [Google Scholar]
- 72.Grimer M., Caring for Australians with Renal Impairment (CARI) The CARI guidelines: calcineurin inhibitors in renal transplantation; pregnancy, lactation and calcineurin inhibitors. Nephrology (Carlton) 2007;12(suppl 1):S98–S105. doi: 10.1111/j.1440-1797.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 73.Liang W., Wang D., Ling X. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18(1):62–69. doi: 10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 74.Benten D., Staufer K., Sterneck M. Orthotopic liver transplantation and what to do during follow-up: recommendations for the practitioner. Nat Clin Pract Gastroenterol Hepatol. 2009;6(1):23–36. doi: 10.1038/ncpgasthep1312. [DOI] [PubMed] [Google Scholar]
- 75.Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl. 2002;8(10, suppl 1):S14–S18. doi: 10.1053/jlts.2002.35781. [DOI] [PubMed] [Google Scholar]
- 76.Contos M.J., Cales W., Sterling R.K. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7(4):363–373. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]
- 77.Charlton M., Kasparova P., Weston S. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7(7):608–614. doi: 10.1053/jlts.2001.25453. [DOI] [PubMed] [Google Scholar]
- 78.Vivarelli M., La Barba G., Cucchetti A. Can antiplatelet prophylaxis reduce the incidence of hepatic artery thrombosis after liver transplantation? Liver Transpl. 2007;13(5):651–654. doi: 10.1002/lt.21028. [DOI] [PubMed] [Google Scholar]


