Abstract
Objective
To evaluate the rate of and potential risk factors for bloodstream infections (BSIs) using data from the REVEAL (Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension [PAH] Disease Management) REGISTRY®, which provides current information about patients with PAH.
Patients and Methods
Patients were enrolled from March 30, 2006, through December 8, 2009, and data on reported BSIs were collected through the third quarter of 2010. Bloodstream infection rates were calculated per 1000 patient-days of risk.
Results
Of 3518 patients enrolled, 1146 patients received intravenous (IV) prostanoid therapy for more than 1 day (no BSI, n=1023; ≥1 BSI, n=123; total BSI episodes, n=166). Bloodstream infections rates were significantly increased in patients receiving IV treprostinil vs IV epoprostenol (0.36 vs 0.12 per 1000 treatment days; P<.001), primarily due to gram-negative organisms (0.20 vs 0.03 per 1000 treatment days; P<.001). Multivariate analysis adjusting for age, causes of PAH, and year of BSI found that treatment with IV treprostinil was associated with a 3.08-fold increase (95% confidence interval, 2.05-4.62; P<.001) in BSIs of any type and a 6.86-fold increase (95% confidence interval, 3.60-13.07; P<.001) in gram-negative BSIs compared with treatment with IV epoprostenol.
Conclusion
Compared with IV epoprostenol therapy, treatment with IV treprostinil is associated with a significantly higher rate of gram-negative BSIs; observed differences in BSI rate did not seem to be due to any other analyzed factors.
Trial Registration
clinicaltrials.gov Identifier: NCT00370214
Abbreviations and Acronyms: BSI, bloodstream infection; CI, confidence interval; IV, intravenous; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; REVEAL, Registry to Evaluate Early and Long-term PAH Disease Management; RHC, right heart catheterization
Pulmonary arterial hypertension (PAH), classified as group 1 pulmonary hypertension, is a progressively fatal disease characterized by increased pulmonary vascular resistance (PVR) due to proliferation and remodeling of the pulmonary arterioles, leading to right ventricular failure and death.1,2 At present, PAH is defined as a resting mean pulmonary artery pressure of 25 mm Hg or higher, pulmonary capillary wedge pressure or left ventricular end-diastolic pressure of 15 mm Hg or lower, and PVR of 240 dyn/s per cm5 or more, based on right-sided heart catheterization (RHC), and in some cases, left-sided heart catheterization.2-4 The goals of therapy are to improve symptoms, quality of life, and survival.2,4,5
Parenteral prostanoids remain the most potent and effective therapies for PAH and have improved long-term outcomes and quality of life.2,4-6 Intravenous (IV) treprostinil and IV epoprostenol are administered via continuous infusion, with the associated risk of development of bloodstream infections (BSIs).7 Current guidelines emphasize the effect of BSIs on morbidity and mortality in patients with PAH and stress the importance of good clinical practice and patient education about catheter care.6
The risk of BSIs with long-term IV epoprostenol administration, most commonly caused by gram-positive pathogens, is well known.8,9 However, limited data are available on the rate and nature of BSIs in patients receiving IV treprostinil. Retrospective analyses comparing BSIs in patients receiving IV epoprostenol and IV treprostinil suggest higher overall BSI rates and an increased risk of gram-negative BSIs in patients receiving IV treprostinil.10,11
Using data from the REVEAL (Registry to Evaluate Early and Long-term PAH Disease Management) REGISTRY®, we calculated the rates and characteristics of BSIs and investigated potential risk factors for development of BSIs in patients with PAH receiving IV prostanoid therapy. We also compared BSI rates and the pathogens in patients receiving IV epoprostenol vs those receiving IV treprostinil.
Patients and Methods
Study Design
REVEAL is a 55-center, observational, US-based, longitudinal registry designed to provide current information on the demographic characteristics, course, and management of patients with PAH confirmed via RHC.12,13 Between March 30, 2006, and December 8, 2009, 3518 consecutive patients with PAH meeting enrollment criteria were enrolled in REVEAL. All patients were followed up for at least 5 years from enrollment. The enrollment date was the date of obtaining informed consent, and time of diagnosis was the date of diagnostic RHC. The design of REVEAL, baseline characteristics of enrolled patients, and survival after enrollment have been published previously.12-14 Data on BSIs were collected prospectively from enrollment in REVEAL through the last follow-up.
Study Participants
For the purposes of REVEAL, as previously reported, PAH was defined as mean pulmonary artery pressure of more than 25 mm Hg at rest or more than 30 mm Hg with exercise, pulmonary capillary wedge pressure or left ventricular end-diastolic pressure of 18 mm Hg or lower, and PVR of 240 dyn/s per cm5 or more.12,13
In the present analysis, 1146 patients with 1 or more IV prostanoid patient-days of risk from enrollment to September 20, 2010 (data collection end point), were included. Patients were either already receiving an IV prostanoid (including generic IV epoprostenol) at enrollment or IV prostanoid therapy was initiated during the study period. The IV prostanoid agents available during the observation period included IV epoprostenol (Flolan, epoprostenol sodium for injection; GlaxoSmithKline, Research Triangle Park, NC; and Veletri, epoprostenol for injection; Actelion Pharmaceuticals US, Inc, South San Francisco, CA, which was commercially available for the last 6 months of the study); and IV treprostinil (Remodulin; United Therapeutics Corp, Research Triangle Park, NC).
Microbiologic Methods
Bloodstream infections were defined by a culture result that was gram-positive; gram-negative, with or without a gram-positive coinfection; or unknown. Unknown organisms were blood samples that were positive for an organism but did not grow in culture and were further unidentifiable by the laboratory.
Statistical Analyses
Most of the data were collected for all patients enrolled in REVEAL, and a subset of data was collected only for patients with BSIs. For data available for all patients, characteristics of patients with 1 or more BSIs were compared with characteristics of patients with no BSIs. The χ2 test was used for categorical variables, and the 2-sample t test was used for continuous variables. For characteristics that were collected only during a BSI, comparisons were made between gram-positive and gram-negative BSIs using χ2 tests and 2-sample t tests.
Rates of BSIs for all patients, patient subgroups, and at-risk periods of interest were reported per 1000 treatment days, calculated by dividing the number of events by the total exposure days; rates, rather than percentages, were reported because of variable durations of exposure. Poisson regression was used to estimate confidence intervals (CIs) for rates and rate ratios and to assess the significance of differences in BSI rates according to exposure and patient subgroups. All variables associated with BSIs in the univariate analysis were included in a multivariate Poisson regression generalized estimating equations model. The generalized estimating equations model was used because patients may have contributed multiple risk periods to the analysis that may not be independent.15 P values were obtained from the Wald χ2 statistic assessing model beta coefficients. All analyses were conducted using commercially available software (SAS version 9.1.3; SAS Institute, Inc, Cary, NC).
Results
Enrollment Characteristics
Of the 1146 patients who had 1 or more patient-days at risk and were included in the analysis, 1023 did not have a BSI, and 123 patients had 1 or more BSIs during follow-up (Figure 1). Ninety-four of 123 patients with a BSI (76.4%) had only 1 BSI after enrollment in REVEAL, and 29 of 123 patients (23.6%) had 2 or more BSIs during follow-up. In total, 166 BSI episodes were reported. Coinfections with both gram-negative and gram-positive organisms were reported in 6 patients; these patients were included in the gram-negative analyses. Of patients with 1 or more BSIs, most (105 of 123; 85.4%) were hospitalized because of the BSI. The mean age of patients in the REVEAL cohort with 1 or more patient-days at risk was 47.8 years at enrollment, and most patients (79.0%) were female (Table 1). The non-BSI group (mean ± SD age, 48.4±16.2 years) was significantly older than the group with 1 or more BSIs (mean ± SD age, 43.6±14.6 years) (P=.002). There were no other significant differences in patient demographic characteristics or clinical characteristics between those without a BSI and those with 1 or more BSIs, including New York Heart Association functional class or 6-minute walk test distance (Table 1). Of the 1146 patients included in the analysis, 83 (7.2%) were pediatric patients aged 18 years or younger at enrollment. No differences in BSI rates were found in the REVEAL pediatric patient population compared with the REVEAL adult patient population (data not shown). Approximately half of the patients (51.0%) had idiopathic PAH, 4.4% had familial PAH, and 44.1% had PAH associated with other conditions (Table 1).
FIGURE 1.
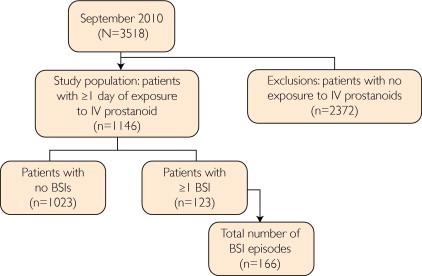
The REVEAL (Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management) study population comprising patients exposed to intravenous (IV) prostanoid therapy for ≥1 day. BSI = bloodstream infection.
TABLE 1.
Characteristics at REVEAL Enrollment in Patients Exposed to ≥1 Day of Intravenous Prostanoid Therapya
| Characteristic | All patients | No BSI | ≥1 BSI | P value |
|---|---|---|---|---|
| No. of patients | 1146 | 1023 | 123 | NA |
| Age at enrollment (y), mean ± SD | 48±16 | 48±16 | 44±15 | .002 |
| Pediatric patients (age ≤18 y) at diagnosis , No. (%) | 83 (7.2) | 75 (7.3) | 8 (6.5) | .74 |
| Sex, No. (%) | .98 | |||
| Male | 241 (21.0) | 215 (21.0) | 26 (21.1) | |
| Female | 905 (79.0) | 808 (79.0) | 97 (78.9) | |
| Time from diagnosis to enrollment (mo), mean ± SD | 38±42 | 38±42 | 38±39 | .94 |
| Group 1 diagnosis at enrollment, No. (%) | .07 | |||
| Idiopathic PAH | 585 (51.0) | 507 (49.6) | 78 (63.4) | |
| Familial PAH | 50 (4.4) | 46 (4.5) | 4 (3.3) | |
| APAH | ||||
| Congenital heart disease | 89 (7.8) | 81 (7.9) | 8 (6.5) | |
| Connective tissue disease | 258 (22.5) | 244 (23.9) | 14 (11.4) | |
| Drugs and toxins | 61 (5.3) | 54 (5.3) | 7 (5.7) | |
| HIV | 23 (2.0) | 22 (2.2) | 1 (0.8) | |
| Portal hypertension | 67 (5.8) | 58 (5.7) | 9 (7.3) | |
| Other APAHb | 8 (0.7) | 7 (0.7) | 1 (0.8) | |
| Pulmonary veno-occlusive disease | 2 (0.2) | 2 (0.2) | 0 (0.0) | |
| NYHA functional class at enrollment, No. (%) | .34 | |||
| I | 65 (6.2) | 59 (6.3) | 6 (5.1) | |
| II | 318 (30.3) | 274 (29.5) | 44 (37.3) | |
| III | 542 (51.7) | 485 (52.2) | 57 (48.3) | |
| IV | 123 (11.7) | 112 (12.0) | 11 (9.3) | |
| 6-Minute walk distance at enrollment (meters), No. (mean ± SD) | 898 (358.3±124.7) | 791 (356.0±126.5) | 107 (374.9±110.0) | .14 |
APAH = associated with PAH; BSI = bloodstream infection; HIV = human immunodeficiency virus; NA = not applicable; NYHA = New York Heart Association; PAH = pulmonary arterial hypertension.
Other APAH included, but was not limited to, diagnoses of non–sickle cell hemoglobinopathy, sickle cell disease, thyroid disorder, Gaucher disease, hereditary hemorrhagic telangiectasia, and chronic myeloproliferative disorder.
BSI Rates
The overall BSI rate was 0.20 per 1000 treatment days (166 BSIs in 832,881 total days of drug exposure). The overall BSI rate was 3-fold greater in patients treated with IV treprostinil than in those treated with IV epoprostenol (P<.001; Figure 2, A). The difference in the overall rate of BSIs between the 2 IV prostanoid agents was primarily accounted for by differences in the rates of gram-negative BSIs, although the rates of both gram-negative and gram-positive BSIs were higher with IV treprostinil therapy than with IV epoprostenol therapy (P=.04 for gram-positive BSIs and P<.001 for gram-negative BSIs; Figure 2, A). For each full year of analysis, the rate of gram-negative BSIs was significantly greater in patients receiving IV treprostinil than in patients receiving IV epoprostenol (P≤.001; Figure 2, B); however, the rate of gram-negative BSIs decreased over time for each full year of analysis in patients receiving IV treprostinil.
FIGURE 2.
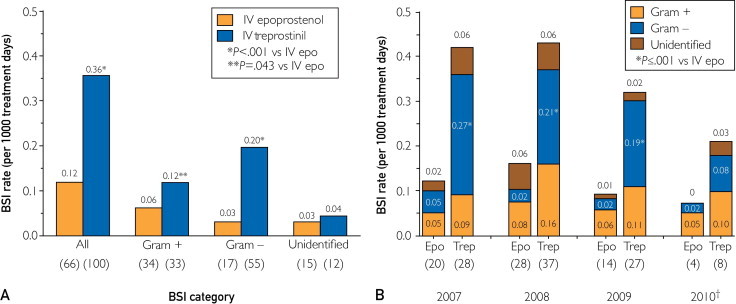
Bloodstream infection (BSI) rates in the study population per 1000 treatment days for all BSIs (A) and by year per 1000 treatment days for all prostanoids (B). Numbers in parentheses represent number of BSIs. †Data for 2010 extend to the end of the third quarter. Epo = epoprostenol; IV = intravenous; Trep = treprostinil; + = positive; − = negative.
Multivariate Analysis of Infection Rates
Adjusted for age, etiologic factors, and year of infection, compared with IV epoprostenol, IV treprostinil administration was associated with a 3.08-fold increase (95% CI, 2.05-4.62; P<.001) in BSIs of any type (Table 2). Younger patients were marginally less likely to have BSIs than were patients aged 18 to 40 years (P=.06), and patients older than 65 years were significantly less likely to have BSIs than were younger adults (P=.004). Patients with connective tissue disease associated with PAH were also less likely to have BSIs than were patients with other PAH subgroups (rate ratio, 0.52; 95% CI, 0.28-0.96; P=.04). Infections were marginally less likely in 2010 (P=.08); however, the differential rate of infection between IV epoprostenol and IV treprostinil did not differ across years (P=.87).
TABLE 2.
| Variable | All BSIs |
Gram-negative BSIs |
||
|---|---|---|---|---|
| Rate ratio (95% CI) | P valuec | Rate ratio (95% CI) | P valuec | |
| IV treprostinil (vs IV epoprostenol) | 3.08 (2.05-4.62) | <.001 | 6.86 (3.60-13.07) | <.001 |
| Age at enrollment (y) | ||||
| ≤18 (vs >18 to <41) | 0.46 (0.20-1.04) | .06 | 0.25 (0.06-1.07) | .06 |
| ≥41 to <65 (vs >18 to <41) | 0.62 (0.41-0.93) | .02 | 0.53 (0.28-1.00) | .05 |
| ≥65 (vs >18 to <41) | 0.28 (0.12-0.66) | .004 | 0.37 (0.14-1.02) | .06 |
| CTD-APAH (vs all other PAH subgroups) | 0.52 (0.28-0.96) | .04 | 0.54 (0.26-1.14) | .10 |
| Year of infection | ||||
| 2008 (vs 2007) | 1.17 (0.76-1.80) | .49 | 0.66 (0.33-1.32) | .24 |
| 2009 (vs 2007) | 0.78 (0.49-1.25) | .30 | 0.57 (0.29-1.14) | .12 |
| 2010 (vs 2007) | 0.56 (0.30-1.06) | .08 | 0.29 (0.11-0.76) | .01 |
| Year of infection by medication interaction | NA | .87d | NA | .86d |
BSI = bloodstream infection; CI = confidence interval; CTD-APAH = connective tissue disease–associated PAH; GEE = generalized estimating equations; IV = intravenous; NA = not available; PAH = pulmonary arterial hypertension.
All numbers were reported from the main effects–only multivariate Poisson regression GEE model. The GEE model was used because patients may contribute multiple risk periods to the analysis, which may not be independent.15
P values were obtained using the Wald χ2 statistic assessing model beta coefficients.
P values were obtained from a supplementary GEE model that was fit including an interaction term. The interaction was not included in the final model because there was no evidence that year was associated with the size of the medication effect.
For gram-negative infections, IV treprostinil therapy was associated with a 6.86-fold increase (95% CI, 3.60-13.07; P<.001) in BSIs compared with IV epoprostenol therapy (Table 2). Significantly fewer gram-negative infections occurred in 2010 than in 2007 (rate ratio, 0.29; 95% CI, 0.11-0.76; P=.01); however, the rate ratio for the medication difference remained constant over time (P=.86).
BSI Organisms Reported
The most frequently reported gram-positive organism was Staphylococcus aureus. Other gram-positive organisms included, but were not limited to, Staphylococcus epidermidis, Enterococcus, and Corynebacterium jeikeium (Table 3). Although the percentage of patients who had a gram-positive BSI treated with IV epoprostenol was significantly greater than the percentage of patients treated with IV treprostinil (P=.001), the number of patients infected with each specific gram-positive organism was similar in the IV epoprostenol and IV treprostinil groups. The strain of gram-positive organisms was not reported for 4 BSIs in IV epoprostenol recipients and for 2 BSIs in IV treprostinil recipients.
TABLE 3.
| Characteristic | All BSI episodes (N=166) | IV epoprostenol (n=66) | IV treprostinil (n=100) | P value |
|---|---|---|---|---|
| Gram-positive organism, No. (%) | 73 (44.0) | 39 (59.1) | 34 (34.0) | .001 |
| Staphylococcus aureus | 46 | 23 | 23 | |
| Staphylococcus epidermidis | 5 | 3 | 2 | |
| Enterococcus species | 5 | 4 | 1 | |
| Corynebacterium jeikeium | 1 | 1 | 0 | |
| Unknown | 6 | 4 | 2 | |
| Otherc | 15 | 7 | 8 | |
| Gram-negative organism, No. (%) | 72 (43.4) | 17 (25.8) | 55 (55.0) | <.001 |
| Pseudomonas aeruginosa | 20 | 2 | 18 | |
| Moraxella catarrhalis | 0 | 0 | 0 | |
| Escherichia coli | 3 | 0 | 3 | |
| Stenotrophomonas maltophilia | 2 | 0 | 2 | |
| Klebsiella oxytoca | 9 | 1 | 8 | |
| Enterobacter cloacae | 10 | 1 | 9 | |
| Unknown | 2 | 1 | 1 | |
| Otherd | 32 | 12 | 20 |
BSI = bloodstream infection; IV = intravenous.
BSIs are not mutually exclusive; >1 infection can be counted per patient, and patients may have >1 organism per BSI episode; thus, the number of organisms may sum to more than the number of total infections.
Other gram-positive organisms included coagulase-negative Staphylococcus such as Staphylococcus epidermidis, Staphylococcus hominis, and Staphylococcus capitis; gram-positive rods such as diphtheroid; Dietzia species; and non anthracis Bacillus species; methicillin-resistant Staphylococcus aureus; Micrococcus species; Candida albicans; and nonhemolytic Streptococcus organisms.
Other gram-negative organisms included Acinetobacter such as Acinetobacter baumannii; serratia such as Serratia marcescens; Brevundimonas vesicularis; Stenotrophomonas species; Roseomonas species; Pseudomonas species such as Pseudomonas fluorescens, Pseudomonas putida, and Pseudomonas luteola; Aeromonas hydrophila; Pantoea species such as Pantoea agglomerans; Enterobacter aerogenes; Klebsiella pneumoniae; Neisseria species; Herbaspirillum species; Salmonella species; Citrobacter freundii; mixed gram-negative flora; and gram-negative rods.
The most frequently reported gram-negative organism was Pseudomonas aeruginosa (Table 3). Other reported gram-negative organisms included, but were not limited to, Escherichia coli, Stenotrophomonas maltophilia, Klebsiella oxytoca, and Enterobacter cloacae. A significantly greater percentage of patients treated with IV treprostinil experienced gram-negative BSIs than did patients treated with IV epoprostenol (P<.001). The number of patients in the IV treprostinil group was greater for each specific gram-negative organism compared with those in the IV epoprostenol group; the strain of gram-negative organism was unknown in 1 patient in each group.
Effects of Drug Characteristics and Drug Delivery System on BSI Type
In the 123 patients with a BSI, other than the specific prostanoid agent administered, there were no significant differences between the gram-positive and gram-negative BSIs insofar as prostanoid dosage (P=.17), pump rate (P=.65), or concentration (P=.18). Bloodstream infections led to discontinuation of IV therapy in 6.4% of all episodes (10 of 156), 4.7% of gram-positive BSIs (3 of 64), and 7.5% of gram-negative BSIs (5 of 67) (gram-positive vs gram-negative, P=.51). Most patients were using the CADD-Legacy infusion pump (Smiths Medical, St Paul, MN). Likewise, there were no differences in catheter type or number of lumens between gram-positive and gram-negative BSIs at the time of infection (data not shown). The most common type of catheter was a Hickman line, most of the lines had a single lumen, and most patients used a closed-hub system and kept their medication refrigerated (data not shown).
Of the BSIs in which the type of catheter system was known, most occurred in patients using a closed, split-septum hub system (91.4% overall [64 of 70], 93.8% of gram-positive BSIs [30 of 32], and 88.0% of gram-negative BSIs [22 of 25]; gram-positive vs gram-negative, P=.45).
Survival in Patients With BSIs
Patient mortality from the time of first on-study BSI was determined (Figure 3). In patients with gram-negative infections, an elevated risk of death was observed during the 1- to 3-month period after a BSI. At 3 months after infection, both gram-positive and gram-negative infections were associated with more than 10% mortality (Figure 3); however, an insufficient number of BSIs precluded detection of differences in survival at later time points.
FIGURE 3.
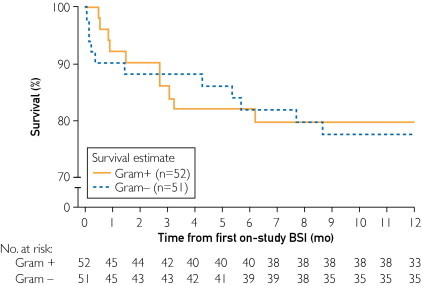
Percentage of survival in REVEAL (Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management) patients receiving intravenous prostanoid therapy for ≥1 day with gram-positive (+) or gram-negative (−) bloodstream infection (BSI) episodes from time of first on-study BSI.
Discussion
In the present study, the overall BSI rate was 3.08-fold higher in patients with PAH receiving IV treprostinil compared with those receiving IV epoprostenol. The rates of gram-positive and gram-negative BSIs were also higher in patients receiving IV treprostinil when compared with IV epoprostenol (1.84-fold and 6.86-fold, respectively).
The Centers for Disease Control and Prevention conducted a retrospective pooled data analysis from 2003 to 2006 of patients with PAH treated with either IV treprostinil or IV epoprostenol using data available from 7 pulmonary hypertension centers for IV treprostinil and 5 centers for both drugs.11 Their initial report suggested that BSI rates, particularly gram-negative infections, were higher in patients treated with IV treprostinil than in those receiving IV epoprostenol (all BSI episodes, 1.11 vs 0.43 per 1000 treatment days, respectively; and gram-negative BSIs, 0.76 vs 0.06 per 1000 treatment days, respectively).11 The authors speculated that the disparity could be due to differences in (1) preparation and storage of the 2 prostanoid agents; (2) type of diluent used, including differences in pH; (3) anti-inflammatory activity of the 2 agents; or (4) catheter care practices. At multivariate analysis, IV treprostinil was the only significant predictor of developing a BSI, and more specifically, a gram-negative BSI.10 In a separate pediatric population, the overall rates of BSI episodes were 2.7-fold higher in patients receiving IV treprostinil than in those receiving IV epoprostenol (1.38 vs 0.51 BSIs per 1000 catheter days, respectively; P<.01);16 the difference between gram-negative pathogens was even higher (0.91 vs 0.08 gram-negative BSIs per 1000 catheter days, respectively; P<.01).16
The potential complication of BSIs associated with continuous IV administration of prostanoids may be a significant cause of morbidity and mortality in patients with PAH.6 The present retrospective REVEAL analysis indicated that there is a higher rate of BSIs in patients receiving IV treprostinil vs IV epoprostenol. However, since the initiation of REVEAL,10,11 2 changes have occurred in IV administration of prostanoids: (1) on September 20, 2008, the package insert for Remodulin (IV treprostinil; United Therapeutics Corp) was changed to include the recommended use of Flolan Sterile Diluent for Injection (GlaxoSmithKline; used with brand and generic IV epoprostenol); and (2) the use of a closed-hub catheter system was recommended.6 The data in this REVEAL analysis were collected before and after implementation of these 2 changes. A more recent prospective study of patients receiving IV treprostinil has reported that use of Flolan Sterile Diluent for Injection seems to decrease the overall rate of BSIs in patients receiving IV treprostinil from 0.90 to 0.32 per 1000 treatment days (P=.06) and significantly decreased the number of gram-negative BSIs from 0.71 to 0.08 per 1000 treatment days in the same cohort of patients receiving IV treprostinil (P=.01).17 Evaluation of the effect of diluent type on BSI rate could be useful. However, in addition to not collecting diluent type, catheter care was also changed during the study,6 both of which could have contributed to the overall decrease in BSIs and the decrease in gram-negative BSIs. Catheter system recommendations now include the use of a closed-hub system (split-septum clave) and waterproofing of catheter hub connections. These current recommendations have resulted in a significant decrease in the risk of catheter-related BSIs in patients with PAH, from 0.89 per 1000 catheter days with a non–closed-hub system to 0.10 per 1000 catheter days with a closed-hub system.16,18
Recent reports of this decrease in the number of BSI episodes16,18 may also be related to ongoing improvement in management of all patients with indwelling catheters but may also result from changes in clinical practice insofar as the diluent used with IV treprostinil. However, according to data obtained from REVEAL, most of the BSI episodes occurred in patients using a closed-hub system (64 of 70 BSIs; 91.4%), and the percentage of patients using a closed-hub system was similar regardless of whether the organism responsible was gram-positive or gram-negative (93.8% and 88.0%, respectively). For BSIs recorded in patients who were not using a closed-hub system, the rate of gram-positive BSIs was approximately half that of gram-negative BSIs (6.3% vs 12.0%). Further analysis is required to determine whether the overall risk of BSIs and the risks related to treatment with IV epoprostenol and/or IV treprostinil therapy are influenced by the use of a closed-hub system.
In addition to specific drug therapy, other factors that may be related to the risk of BSI are prostanoid dosage, pump rate, infusion concentration, pump type, catheter type, and number of lumens. However, the data from REVEAL do not demonstrate relationships between any of these factors and the rates of either gram-positive or gram-negative BSIs.
Several limitations of this analysis, in addition to the general limitations inherent in an uncontrolled observational study, should be noted. First, the overall BSI rate was low, resulting in insufficient power in the subgroup analyses. Second, whether the BSI was the cause of hospitalization or occurred during hospitalization from another cause remains unknown. Third, neither the diluent type at the time of the BSI nor drug administration interruptions were collected in REVEAL, precluding evaluation of their potential effect(s) on BSI rates in patients treated with IV prostanoids. Fourth, REVEAL did not capture BSI types or rates for patients who were not receiving an IV prostanoid. Fifth, whether the responsible causative organisms were specifically gram-positive or gram-negative could not be determined in 27 of 166 BSIs (16.3%).
A correlation between prostanoid and BSI type in patients with multiple BSIs remains to be established. Such analyses could provide important information about the prevalence of gram-negative vs gram-positive infections, as well as the antimicrobial and anti-inflammatory properties of the specific drugs, which could facilitate implementation of individualized treatment plans in patients at greater risk of development of a BSI. However, the power required to generate such an analysis would necessitate a substantially larger study.
Conclusion
Overall BSI rates have decreased from previous reports. However, the present data suggest that the risk of a gram-negative BSI is greater with IV treprostinil therapy than with IV epoprostenol therapy. In addition, the data suggest that initial antibiotic therapy in a patient receiving IV treprostinil suspected of having a BSI should be initiated with a broad-spectrum antibiotic(s) with coverage for both gram-negative and gram-positive organisms, at least until speciation and sensitivities of the organism(s) have been identified. Furthermore, we emphasize the importance of following recommended best practice guidelines in patients receiving IV prostanoids, in particular, use of Flolan Sterile Diluent, a closed-hub catheter system, and implementation of infection control measures.
Further investigation of the clinical effects of these findings is warranted, in particular in those patients with PAH with a history of BSI who continue treatment with an IV prostanoid. Corroboration of the present results is paramount to improving patient morbidity, mortality, and overall quality of life.
Acknowledgments
Manuscript preparation and editing support were provided by Mary Hines, BSc (Hons), Scarlett Geunes-Boyer, PhD, Latoya M. Mitchell, PhD, and Jennifer M. Kulak, PhD, of in Science Communications, Springer Healthcare. SAS programming support was provided by Ginny Lai, MPH, of ICON Late Phase & Outcomes Research.
Ms Kitterman thanks Dr Lynette Brown for her guidance and support of this project.
Participating Investigators: We thank the Principal Investigators and their Study Coordinators for their participation in REVEAL: David Badesch, MD, and Deb McCollister, RN, University of Colorado Health Sciences Center, Aurora; Erika Berman-Rosenzweig, MD, and Daniela Brady, RN, Columbia University, New York, NY; Charles Burger, MD, and Pamela Long, RN, CCRP, Mayo Clinic, Jacksonville, FL; Sif Hansdottir, MD, and Page Scovel, RN, BSN University of Iowa Hospitals & Clinics, Iowa City; Murali Chakinala, MD, FCCP, and Ellen Lovato, RN, BSN, Washington University, St Louis, MO; Monica Colvin-Adams, MD, and Nonyelum Harcourt Fairview, University of Minnesota Medical Center, and Nonyelum Harcourt Fairview, Minneapolis; Maria Rosa Costanzo, MD, Midwest Heart Foundation, and Debbie Heidenreich, RN, BSN Naperville, IL; Curt Daniels, MD, and Julianne Williamson-Mueller, RN, BSN, Children's Research Institute at Ohio State, Columbus; Curt Daniels, MD, and Jami Maccombs, Ohio State University, Columbus; Raed Dweik, MD, and Jennie Newman, Cleveland Clinic Foundation, Cleveland, OH; Greg Elliott, MD, and Natalie Kitterman, RN, BSN, CCRP, Intermountain Medical Center and the University of Utah, Salt Lake City; Harrison Farber, MD, and Kim Tobin Finch, Boston University School of Medicine, Boston, MA; Robert Frantz, MD, and Louise Durst, RN, Mayo Clinic College of Medicine, Rochester, MN; Adaani Frost, MD, and Helena Purl, RN, BSN, Baylor College of Medicine, Houston, TX; Mardi Gomberg, MD, and Sandra Coslet, RN, MSN, MBA, University of Chicago Hospitals, Chicago, IL; James Gossage, MD, and Melissa James, RN, Medical College of Georgia, Augusta; Dan Grinnan, MD, and Amy Frayser, Virginia Commonwealth University, Richmond; Paul Hassoun, MD, and Jude Aidam, MB, ChB, MPH, CM, CCRP, Johns Hopkins Medical Center, Baltimore, MD; Kristin Highland, MD, and Laura Bailey, Medical University of South Carolina, Charleston; Nicholas Hill, MD, and Karen Visnaw, RN, Tufts-New England Medical Center, Boston, MA; Dunbar Ivy, MD, and Kathleen Miller-Reed, RN, Children's Hospital Department of Cardiology, Aurora, CO; James Klinger, MD, and Barbara Smithson, RN, BSN, Rhode Island Hospital, Providence; Steve Knoper, MD, University of Arizona, Tucson; Deborah Jo Levine, MD, and Regina Whitener, RN, BSN, University of Texas Health Science Center, San Antonio; George Mallory, MD, and Ann Bogran, RN, BSN, Texas Children's Hospital, Houston; Catherine Markin, MD, and Lisa Roessel, RN, FNP, ARNP-BC, Legacy Clinic Northwest, Portland, OR; Michael Mathier, MD, and Mary Pollera, RN, University of Pittsburgh School of Medicine, Pittsburgh, PA; Wesley McConnell, MD, and Kim Hobbs, MSN, ARNP, Kentuckiana Pulmonary Associates, Louisville, KY; Dana McGlothlin, MD, and Erin Kobashigawa, BA, UCSF Medical Center, San Francisco, CA; Donald Moore, MD, and Mary Beth Boyd, RN, BSN, Children's Hospital at Vanderbilt, Nashville, TN; Hassan Alnuaimat, MD, and Christina Eagan, University of Florida, Gainesville; Srinivas Murali, MD, and Pranav Ravi, MBBS, Allegheny General Hospital, Pittsburgh, PA; Steven Nathan, MD, and Lori Schlegel, RN, BSN, Inova Heart and Vascular Institute, Falls Church, VA; Ronald Oudiz, MD, and Joy Beckman, RN, MSN, LA Biomedical Research Institute at Harbor-UCLA, Torrance, CA; Myung Park, MD, and Faith E. Pa’ahana-Janowick, RN, BSN, University of Maryland School of Medicine, Baltimore; Ivan Robbins, MD, and Tracy Oyler, RN, Vanderbilt University Medical Center, Nashville, TN; David Ross, MD, and Michaela Dyke, UCLA Medical Center, Los Angeles, CA; Ghulam Saydain, MD, FCCP, and Muhammad Usman, MD, Wayne State University, Detroit, MI; Robert Schilz, DO, PhD, and Dave Haney, RRT, University Hospital of Cleveland, Cleveland, OH; Shelley Shapiro, MD, PhD, and Wendy Hill, RN, NP, MSN, VA Greater Los Angeles Health System, Los Angeles, CA; Roxana Sulica, MD, and Rebecca Fenton, RN, Beth Israel Medical Center, New York, NY; John Swisher, MD, and Laura Karasick, Suncoast Lung Center, Sarasota, FL; Darren Taichman, MD, PhD, and Mamta J. Patel, RN, BSN, University of Pennsylvania Medical Center, Philadelphia; Jose Tallaj, MD, and Terri Tahmaseb, RN, University of Alabama at Birmingham; Arunabh Talwar, MD, and Rebecca Miller, North Shore University-Long Island Jewish Medical Center, New Hyde Park, NY; Victor Tapson, MD, And Sandy McNeilly, Duke University Medical Center, Durham, NC; Victor Test, MD, and Luis Santana, CCRC, UCSD Medical Center, La Jolla, CA; Ramagopal Tumuluri, MD, and Susan Oxborough, RN, CVN, St Luke's Medical Center-Aurora, Milwaukee, WI; Hector Ventura, MD, and Bobbett Harris, Ochsner Clinic Foundation, New Orleans, LA; Aaron Waxman, MD, PhD, and Laurie Lawler, RN, Massachusetts General Hospital, Boston; Sheila Weaver, MD, and Ellen Bedenko, RN, Temple Lung Center, Philadelphia, PA; James White, MD, PhD, and Karen Frutiger, RN, BSN, University of Rochester Medical Center, Rochester, NY; Jeffrey Wilt, MD, and Beth VanOver, RN, BSN, Spectrum Health Hospitals, Grand Rapids, MI; Delphine Yung, MD, and Anne Davis, RN, Seattle Children's, Seattle, WA; and Roham Zamanian, MD, and Val Scott, RN, Stanford University Medical Center, Palo Alto, CA.
Footnotes
Grant Support: This work and the REVEAL REGISTRY® were supported by Actelion Pharmaceutical US, Inc.
Potential Competing Interests: Ms Kitterman has served on the advisory boards of Actelion, United Therapeutics, and Gilead; has participated in the Simply Speaking PAH program through Rush University; and has received honoraria for speaker's bureau participation from Actelion, Gilead, and United Therapeutics.
Ms Poms serves as a consultant for clinical research studies and advisory boards for Actelion, Gilead, United Therapeutics, and GlaxoSmithKline; has received honoraria for speaker's bureau participation from Actelion, Gilead, and United Therapeutics; and has received grants from United Therapeutics and GlaxoSmithKline.
Mr Miller is employed by ICON Late Phase & Outcomes Research, a company that receives support from Actelion and other pharmaceutical companies.
Ms Lombardi has served on the advisory boards of Actelion, United Therapeutics, and Gilead, and has received honoraria for speaker's bureau participation from Actelion, United Therapeutics, and Gilead.
Dr Farber serves as a consultant for Actelion, Gilead, Novartis, Bayer, and United Therapeutics; is on the speaker's bureau for Actelion and Gilead; has received a research grant from United Therapeutics; and has received honoraria for his service on the REVEAL Steering Committee, which is supported by Actelion.
Dr Barst serves as a consultant for and has received honoraria from Actelion, Bayer, Corridor Pharmaceuticals, Eli Lilly & Company, GlaxoSmithKline, Gilead, Ikaria, Merck, Novartis, Pfizer, and VentriPoint; has provided expert testimony on diet pill litigation for the plaintiffs; has received grants from Actelion, Eli Lilly, Gilead, National Heart, Lung, and Blood Institute (National Institutes of Health), Novartis, Pfizer, and United Therapeutics; and has received honoraria for her service on the REVEAL Steering Committee, which is supported by Actelion.
References
- 1.McGoon M.D., Kane G.C. Pulmonary hypertension: diagnosis and management. Mayo Clin Proc. 2009;84(2):191–207. doi: 10.4065/84.2.191. [published correction appears in Mayo Clin Proc. 2009;84(4):386] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin V.V., Archer S.L., Badesch D.B., ACCF/AHA ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc, and the Pulmonary Hypertension Association [published correction appears in Circulation. 2009; 120(2):e13] Circulation. 2009;119(16):2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 3.Badesch D.B., Champion H.C., Sanchez M.A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1, suppl):S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N., Hoeper M.M., Humbert M., ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) [published correction appears in Eur Heart J. 2011;32(8):926] Eur Heart J. 2009;30(20):2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 5.Barst R.J., Gibbs J.S., Ghofrani H.A. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1, suppl):S78–S84. doi: 10.1016/j.jacc.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doran A.K., Ivy D.D., Barst R.J., Hill N., Murali S., Benza R.L., Scientific Leadership Council of the Pulmonary Hypertension Association Guidelines for the prevention of central venous catheter-related blood stream infections with prostanoid therapy for pulmonary arterial hypertension. Int J Clin Pract Suppl. 2008;160:5–9. doi: 10.1111/j.1742-1241.2008.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galiè N., Manes A., Branzi A. Prostanoids for pulmonary arterial hypertension. Am J Respir Med. 2003;2(2):123–137. doi: 10.1007/BF03256644. [DOI] [PubMed] [Google Scholar]
- 8.Oudiz R.J., Widlitz A., Beckmann X.J. Micrococcus-associated central venous catheter infection in patients with pulmonary arterial hypertension. Chest. 2004;126(1):90–94. doi: 10.1378/chest.126.1.90. [DOI] [PubMed] [Google Scholar]
- 9.Yap R.L., Mermel L.A. Micrococcus infection in patients receiving epoprostenol by continuous infusion. Eur J Clin Microbiol Infect Dis. 2003;22(11):704–705. doi: 10.1007/s10096-003-1024-1. [DOI] [PubMed] [Google Scholar]
- 10.Kallen A.J., Lederman E., Balaji A. Bloodstream infections in patients given treatment with intravenous prostanoids. Infect Control Hosp Epidemiol. 2008;29(4):342–349. doi: 10.1086/529552. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Bloodstream infections among patients treated with intravenous epoprostenol or intravenous treprostinil for pulmonary arterial hypertension: seven sites, United States, 2003-2006. MMWR Morb Mortal Wkly Rep. 2007;56(8):170–172. [PubMed] [Google Scholar]
- 12.Badesch D.B., Raskob G.E., Elliott C.G. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 13.McGoon M.D., Krichman A., Farber H.W. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc. 2008;83(8):923–931. doi: 10.4065/83.8.923. [DOI] [PubMed] [Google Scholar]
- 14.Benza R.L., Miller D.P., Gomberg-Maitland M. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 15.Liang K.-Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 16.Ivy D.D., Calderbank M., Wagner B.D. Closed-hub systems with protected connections and the reduction of risk of catheter-related bloodstream infection in pediatric patients receiving intravenous prostanoid therapy for pulmonary hypertension. Infect Control Hosp Epidemiol. 2009;30(9):823–829. doi: 10.1086/605320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich J.D., Glassner C., Wade M. The effect of diluent pH on bloodstream infection rates in patients receiving IV treprostinil for pulmonary arterial hypertension. Chest. 2012;141(1):36–42. doi: 10.1378/chest.11-0245. [DOI] [PubMed] [Google Scholar]
- 18.Akagi S., Matsubara H., Ogawa A. Prevention of catheter-related infections using a closed hub system in patients with pulmonary arterial hypertension. Circ J. 2007;71(4):559–564. doi: 10.1253/circj.71.559. [DOI] [PubMed] [Google Scholar]


