Abstract
Vascular endothelial growth factor (VEGF) plays an important role in both physiologic and pathologic angiogenesis and contributes to increased permeability across both the blood-retinal and blood-brain barriers. After 2 decades of extensive research into the VEGF families and receptors, specific molecules have been targeted for drug development, and several medications have received US Food and Drug Administration approval. Bevacizumab, a full-length antibody against VEGF approved for the intravenous treatment of advanced carcinomas, has been used extensively in ophthalmology for exudative age-related macular degeneration, diabetic retinopathy, retinal vein occlusions, retinopathy of prematurity, and other chorioretinal vascular disorders. Pegaptanib and ranibizumab have been developed specifically for intraocular use, whereas the soon-to-be-introduced aflibercept (VEGF Trap-Eye) is moving through clinical trials for both intraocular and systemic use. Although these drugs exhibit excellent safety profiles, ocular and systemic complications, particularly thromboembolic events, remain a concern in patients receiving therapy. Patients experiencing adverse events that may be related to VEGF suppression should be carefully evaluated by both the ophthalmologist and the medical physician to reassess the need for intraocular therapy and explore the feasibility of changing medications. For this review a search of PubMed from January 1, 1985 through April 15, 2011, was performed using the following terms (or combination of terms): vascular endothelial growth factors, VEGF, age-related macular degeneration, diabetic retinopathy, retina vein occlusions, retinopathy of prematurity, intravitreal injections, bevacizumab, ranibizumab, and VEGF Trap. Studies were limited to those published in English. Other articles were identified from bibliographies of retrieved articles and archives of the author.
Angiogenesis consists of a series of highly complex biochemical and cellular processes requiring sequential receptor activation by several growth factors, such as acidic fibroblast growth factor (FGF), basic FGF, transforming growth factor (TGF)-α, TGF-β, hepatocyte growth factor, tumor necrosis factor-α, angiogenin, interleukin (IL)-8, and the angiopoietins.1,2 Whereas physiologic angiogenesis is necessary for human growth, development, maintenance, and repair, pathologic angiogenesis, in response to tissue hypoxia or inflammation, enables tumor growth and causes tissue destruction.
The idea that a soluble, inducible angiogenesis factor could be targeted to reduce tumor growth was first popularized by Folkman in 1971.3 By 1983, vascular permeability factor4 was discovered, and after DNA sequencing and recognition of its role in angiogenesis, it was renamed vascular endothelial growth factor (VEGF).5 Subsequently, several lines of investigation have identified VEGF as a critical, rate-limiting molecule in angiogenesis.6
Angiogenesis during embryologic growth and development requires functioning VEGF; without it, death ensues.7 Postnatal VEGF blockade results in stunted growth, increased mortality due to renal failure, and impaired organ development,8,9 while experimental blockade in juvenile primates causes ovarian failure and stunted growth due to chondrocyte failure within the epiphyseal growth plates.10
In adults, VEGF infusion stimulates microvascular perfusion in patients with severe coronary artery disease and ischemic limbs.11,12 Although new blood vessel growth in response to ischemic myocardium may be lifesaving,13 pathologic neovascularization and hyperpermeability in other organs, such as the eye, cause blindness from retinal detachment, uncontrollable glaucoma, and untreatable retinal edema.
This article discusses the importance of VEGF biochemistry and physiology as a principal cause of blinding chorioretinal vascular diseases, the newly developed drug therapies that target VEGF-mediated complications, and the adverse systemic effects resulting from their use.
A literature search of PubMed from January 1, 1985 through April 15, 2011, was performed using the following terms (or combination of terms): vascular endothelial growth factors, VEGF, age-related macular degeneration, diabetic retinopathy, retina vein occlusions, retinopathy of prematurity, intravitreal injections, bevacizumab, ranibizumab, and VEGF Trap. Studies were limited to those published in English. Other articles were identified from bibliographies of retrieved articles and archives of the author.
VEGF Characteristics
Three decades of intense research has uncovered the detailed biochemistry of VEGF and its receptors. More than just a single molecule, VEGF is actually several isomers that segregate into 5 distinct subgroups—VEGFA, VEGFB, VEGFC, VEGFD, and placental growth factor—with VEGFA emerging as the key regulator of both physiologic and pathologic angiogenesis.6 Variable splicing of the 8 exons of the VEGFA gene results in the synthesis of 6 different human isoforms—VEGF121, VEGF145, VEGF165, VEGF183, VEGF189, and VEGF20614—with VEGF165, the most common isoform (molecular weight of 30 kD), being the most important for angiogenesis.15 On the basis of these isoforms and their relative importance, distinct therapeutic strategies have developed: specific blockade of VEGF165, pan-VEGFA blockade, and pan-VEGF blockade.
Circulating VEGF initiates a biochemical cascade by activating 3 membrane-spanning tyrosine kinases: VEGFR-1, VEGFR-2, and VEGFR-3.16,17 Stimulation of VEGFR-1 releases tissue-specific growth factors, recruits endothelial progenitors, and induces matrix metalloproteinases, whereas VEGF-2 serves as the major mediator of the mitogenic, angiogenic, permeability-enhancing, and anti-apoptotic effects of VEGF.18 Soluble versions of these receptors have been found in the human cornea (critical for maintaining its avascularity) and the rat retina.19 Because VEGFR-1 possesses a higher affinity for VEGF than does VEGFR-2, its binding sequences have been used by drug developers (VEGF Trap-Eye).
VEGF Synthesis and Physiology
Vascular endothelial growth factor synthesis has been studied in numerous tissues under a myriad of conditions, and although several stimulating factors have been identified, common biochemical pathways lead to VEGF synthesis and emanate from VEGF production.20 Within the posterior segment of the eye, VEGF is produced by retinal pigment epithelial cells, neurons, glial cells, endothelial cells, ganglion cells, Müller cells, and smooth muscle cells.21 Although VEGF affects all cells within the retina, its primary targets are vascular endothelial cells.
Tissue hypoxia, due to either primary vascular occlusive disease or anaerobic tumor metabolism, is the most common driver of VEGF synthesis.22 Under conditions with normal oxygen tension, the cell's oxygen sensor, hypoxia-inducible factor 1α, becomes hydroxylated,23 binds to the von Hippel-Lindau factor,24 and is degraded via the ubiquitin-proteasome system.25 Under hypoxic conditions, however, hydroxylation ceases, and stabilized hypoxia-inducible factor-1α binds to the hypoxia response element in the VEGF gene, thereby initiating VEGF synthesis. Although hypoxia is the most common inducer of VEGF synthesis, molecules associated with intraocular inflammatory conditions (epidermal growth factor, TGF-α, TGF-β, keratinocyte growth factor, insulin-like growth factor 1, FGF, IL-1α, IL-6, protein kinase C-β, and platelet-derived growth factor) can up-regulate VEGF messenger RNA synthesis (Figure 1).26
FIGURE 1.
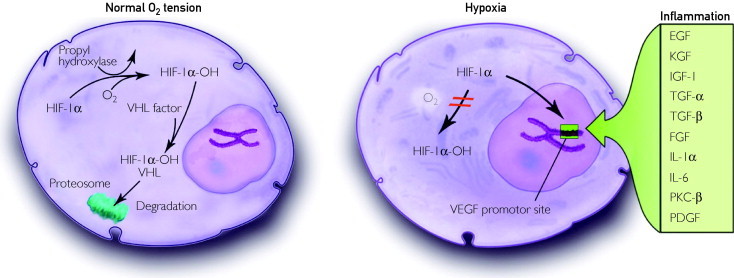
Under conditions of normal oxygen tension (left), HIF-1α undergoes hydroxylation, binds to the VHF, and undergoes degradation within proteosomes. When tissues experience localized hypoxia or inflammation (right), HIF-1α stabilizes and binds to the promoter site of the VEGF gene, thereby increasing VEGF synthesis. EGF = epidermal growth factor; FGF = fibroblast growth factor; HIF-1α = hypoxia-inducible factor-1α; IGF-1 = insulin-like growth factor 1; IL = interleukin; KGF = keratinocyte growth factor; O2 = oxygen; PDGF = platelet-derived growth factor; PKC-β = protein kinase C-β; TGF = transforming growth factor; VEGF = vascular endothelial growth factor; VHF = von Hippel-Lindau factor.
As both a growth factor and a survival factor, VEGF pursues several different tactics in target tissues (Figure 2). By stimulating the mitosis and swelling of arterial, venous, and lymphatic endothelial cells, VEGF initiates angiogenesis.27 Vascular endothelial growth factor causes vasodilation through dose-dependent nitric oxide release from endothelial cells.28 By increasing hydraulic conductivity,29 inducing fenestrations across cell bodies,30 and dissolving the tight junctions between endothelial cells by activating matrix metalloproteinases and phosphorylating both vascular endothelial cell cytoskeletal proteins31 and the junctional proteins occludin and ZO-1,32 VEGF breaks down the blood-retinal barrier, thereby increasing capillary leakage into the intercellular matrix. By modulating the phosphatidylinositol 3-kinase/Akt pathway, VEGF acts as a potent survival factor,33 and by synthesizing intercellular adhesion molecule 1,34 it induces monocyte chemotaxis and margination.
FIGURE 2.
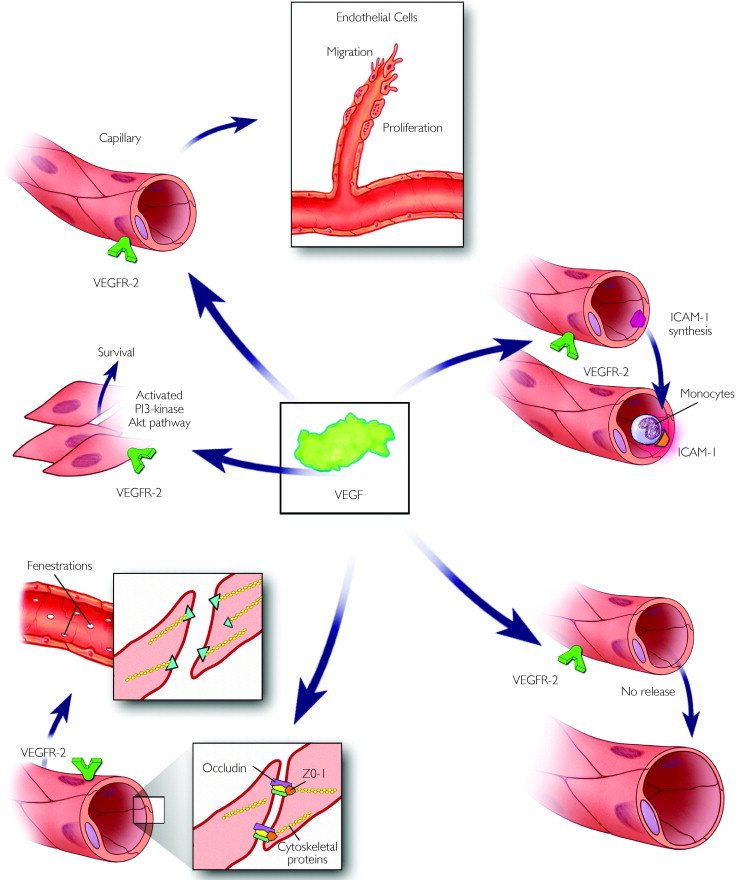
The primary site of VEGF action is vascular endothelium. In response to VEGF, endothelial cells proliferate and migrate; locally release nitric oxide, which causes vasodilation; and increase capillary permeability by creating cellular fenestrations and decreasing the integrity of tight junctions by phosphorylating intracellular and extracellular proteins. ICAM-1 = intercellular adhesion molecule 1; No = nitric oxide; Pl = phosphatidylinositol; VEGF = vascular endothelial growth factor; VEGFR-2 = vascular endothelial growth factor receptor 2; ZO = zonula occludins.
Ocular Conditions and Therapies
Although cataracts remain the most common cause of blindness worldwide, chorioretinal vascular diseases (age-related macular degeneration [AMD], diabetic retinopathy, and retinal vein occlusions) have become the major causes of blindness in developed nations (Figure 3).35
FIGURE 3.
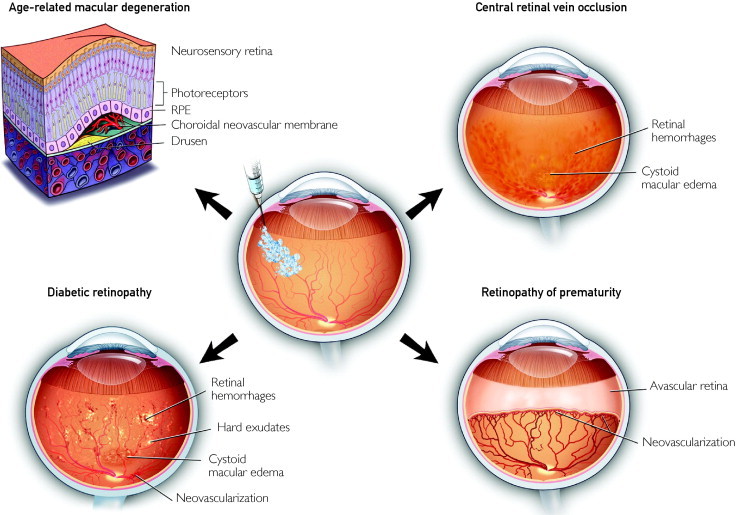
The most common indications for intraocular anti-VEGF injections include exudative age-related macular degeneration, background and proliferative diabetic retinopathy, branch and central (shown in diagram) retinal vein occlusions, and retinopathy of prematurity. Intraocular anti-VEGF drugs generally work through 2 mechanisms: decreasing vascular permeability, thereby allowing the absorption of edema, and decreasing neovascularization, thereby preventing hemorrhages and tissue distortion by fibrous proliferation. RPE = retinal pigment epithelium; VEGF = vascular endothelial growth factor.
Age-Related Macular Degeneration
Age-related macular degeneration is the most common cause of blindness in patients older than 65 years, and although only 10% of patients with AMD have exudative, or wet, AMD, this subtype affects more than 200,000 new patients each year in the United States and accounts for 90% of the severe loss of vision.36 Age-related macular degeneration risk factors, like those of coronary artery disease, include smoking, systemic arterial hypertension, and hyperlipidemia, as well as mutations in several immunomodulatory proteins, including complement factor H.37 Focal ischemia of the outer retina along with inflammation induces the growth of neovascular membranes from the choriocapillaris into either the sub-retinal pigment epithelium or sub-photoreceptor space, which when complicated by transudation and bleeding, causes moderate to severe dysfunction of the photoreceptor-retinal pigment epithelium-choriocapillaris complex.38,39
Several factors, including VEGF detection within neovascular membranes,40 the high incidence of blindness due to exudative AMD, and aging of the population, have made AMD the primary driver of ocular anti-VEGF drug development. Pegaptanib, an aptamer to VEGF165, became the first available ophthalmic anti-VEGF agent in 2004. When compared with sham injections, intravitreal injections of pegaptanib every 6 weeks decrease the average 1-year vision loss due to AMD (loss of 7 letters vs loss of 15 letters).41 In a postapproval study, pegaptanib maintained vision gains in patients previously treated with the newer, more potent, antibody-based anti-VEGF agents (bevacizumab or ranibizumab).42 Since the introduction of bevacizumab and ranibizumab, however, pegaptanib use has decreased substantially.
Bevacizumab, a full-length, murine-derived antibody with 2 VEGF binding sites, received US Food and Drug Administration (FDA) approval in 2004 for the treatment of advanced adenocarcinoma of the colon. A small prospective study with intravenous infusions demonstrated its effectiveness against AMD,43 but concern over adverse systemic events halted further systemic administration for ocular disease. Other systemic anti-VEGF drug use for AMD—aflibercept in a phase 1 trial (all subsequent ocular trials have involved intravitreal injections) and sorafenib, an oral tyrosine kinase inhibitor, administered with ranibizumab as salvage treatment in 3 patients with unresponsive AMD44—has been limited.
For several reasons, bevacizumab was not originally considered an appropriate choice for intravitreal injections. Animal studies suggested that antibody fragments penetrated the retina better than full-length antibodies. Because the murine-derived Fc segment of the full-length antibody might promote an immune response, the safety of intraocular injections was questioned. In 2005, however, successful intravitreal injections of bevacizumab were performed for patients with AMD45 and central retinal vein occlusion.46 Off-label intravitreal use of bevacizumab then spread quickly among retina surgeons around the world. A retrospective study demonstrated the effectiveness of bevacizumab in the treatment of AMD, with average vision improving from 20/235 to 20/172.47
To circumvent the theoretical concerns associated with the intravitreal injection of full-length antibodies, bevacizumab was cleaved into a smaller Fab fragment (48 kDa compared with the original 148 kDa)48 and was affinity-enhanced by 5- to 20-fold49 to create ranibizumab. Ranibizumab received FDA approval in 2006 after the results of 2 multicenter AMD trials. Patients in the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration (MARINA) study gained more letters of vision than sham injected controls (improvement of 7.2 letters vs loss of 10.4 letters),50 whereas patients in the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) study improved more than patients treated with photodynamic therapy (10.7 letters vs loss of 9.8 letters).51
Despite the ranibizumab trials, however, bevacizumab quickly became the drug of first choice among the majority (58%) of retina surgeons52 for 2 reasons: low cost (approximately $50/dose vs $2029/dose for ranibizumab) and “first to market” availability. Because a rigorously controlled trial of bevacizumab vs ranibizumab had never been performed, the National Institutes of Health funded the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT). The drugs performed comparably in both the monthly (ranibizumab: 8.5 letters gained; bevacizumab: 8.0 letters gained) and as needed (ranibizumab: 6.8 letters gained; bevacizumab: 5.9 letters gained) treatment arms.53
Anti-VEGF treatment options are about to increase again as aflibercept (VEGF Trap-Eye) will likely receive FDA approval some time soon. This engineered protein consists of VEGFR-1 and VEGFR-2 binding sequences fused to the Fc segment of an antibody backbone,54 creating a molecule with a structure similar to that of etanercept. The VEGF Trap-Eye's greater VEGF binding affinity (140 times that of ranibizumab) coupled with larger molecular size (twice that of ranibizumab) may allow for a less-frequent dosing schedule than with either bevacizumab or ranibizumab.55 In phase 3 AMD trials, monthly injections of the VEGF Trap-Eye improved vision compared with ranibizumab (10.8 letters vs 8.1 letters), and the 2-month dosing regimen (after 3 monthly loading doses) produced results similar to those with monthly ranibizumab (J. Heier, MD, oral communication, February 2011).
Diabetic Retinopathy
Diabetic retinopathy is the most common cause of blindness in patients between the ages of 20 and 65 years. Prolonged elevation of blood glucose level causes oxidative stress (formation of free radicals) via several pathways: increased polyol pathway flux, advanced glycation end products, activation of protein kinase C, and increased hexosamine pathway flux. These contribute to swelling and death of endothelial cells, basement membrane thickening, loss of vascular pericytes,56 and increased VEGF synthesis. Vascular endothelial growth factor–mediated breakdown of the blood-retinal barrier allows for diffusion of serum proteins into the interstitial space, resulting in diabetic macular edema (DME). Higher VEGF concentrations, associated with more severe ischemia, promote preretinal neovascularization with fibrous proliferation (proliferative diabetic retinopathy), which can cause vitreous hemorrhages and traction retinal detachments.
For 3 decades, laser photocoagulation of the macula and peripheral retina has been the standard treatment for both DME and proliferative diabetic retinopathy. Two recently completed randomized studies for the treatment of DME demonstrated that ranibizumab injections produced more letters of visual improvement than either laser photocoagulation alone or ranibizumab with laser (7.7 vs 5.1 vs 6.8; 6.1 vs 0.8 vs 5.9).57,58 A large prospective trial comparing bevacizumab injections with laser produced similar results (8.0 letters vs loss of 0.5 letter).59 In a phase 3 trial, oral ruboxistaurin, a protein kinase C inhibitor, decreased the probability of diabetic macular edema progression.60
Retinal Vein Occlusions
Branch retinal vein occlusions (BRVOs) and central retinal vein occlusions (CRVOs) afflict 1.8% and 0.5%, respectively, of the population aged 50 years or older.61 The pathophysiologic mechanisms leading to intravascular obstruction—occurring at arteriovenous crossings in BRVO and the lamina cribosa in CRVO—can be explained by the Virchow triad: hypercoagulability, hemodynamic changes (stasis and turbulence), and endothelial injury.62 Impaired venous outflow causes retinal ischemia and VEGF synthesis with subsequent macular edema and retinal or iris neovascularization.
Anti-VEGF drugs are particularly effective for vein occlusions because these eyes contain some of the highest vitreous VEGF concentrations.63 When compared with sham injections for BRVO, ranibizumab injections result in greater improvement in visual acuity (18.3 letters vs 7.3 letters).64 Similar improvements have been found for ranibizumab compared with observation for CRVO (14.9 letters vs 0.8 letter).65 Intravitreal bevacizumab significantly improves visual acuity in both BRVO (20/125 baseline vs 20/55 at 6 months) and CRVO (20/270 baseline vs 20/135 at 6 months).66 In another study, 68% of bevacizumab-treated eyes gained at least 15 letters of vision.67 Bevacizumab also causes rapid regression of iris neovascularization in patients with neovascular glaucoma.68
Retinopathy of Prematurity
Because the retina is not fully vascularized until 36 weeks of gestational age, premature infants may develop severe retinal ischemia, leading to VEGF synthesis, fibrovascular proliferation, traction retinal detachments, and blindness. The recent Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) trial determined that intravitreal bevacizumab was superior to laser photoablation (adverse outcomes: 4% vs 22%; P=.003) for eyes with zone 1 disease, but not for eyes with zone 2 disease (P=.27).69 Although these results appear promising, the precise role of bevacizumab in the management of retinopathy of prematurity is evolving.
Several of the previously referenced pivotal studies have been summarized in the Table.
TABLE.
Pivotal Studies of Vascular Endothelial Growth Factor Inhibitors in Ophthalmology
| Reference, year (condition) | Phase | Trial arms | No. of patients | Outcomes | Significance |
|---|---|---|---|---|---|
| MARINA50 (AMD) | 3 | Ranibizumab (0.3 mg)Ranibizumab (0.5 mg)Observation | 238240238 | +6.5 letters+7.2 letters−10.4 letters | P<.001P<.001 |
| ANCHOR51 (AMD) | 3 | Ranibizumab (0.3 mg)Ranibizumab (0.5 mg)Photodynamic therapy | 140140143 | +8.5 letters+11.3 letters−9.5 letters | P<.001P<.001 |
| CATT53 (AMD) | 4 | Ranibizumab monthlyBevacizumab monthlyRanibizumab as neededBevacizumab as needed | 301286298300 | +8.5 letters+8.0 letters+6.8 letters+5.9 letters | EquivalentEquivalentInconclusive |
| READ-257 (BDR) | 3 | Laser photocoagulationRanibizumabRanibizumab + laser | 343334 | +5.1 letters+7.7 letters+6.8 letters | NSNS |
| RESTORE58 (BDR) | 3 | Laser photocoagulationRanibizumabRanibizumab + laser | 111116118 | +0.8 letters+6.1 letters+5.8 letters | P<.0001P<.0001 |
| CRUISE65 (CRVO) | 3 | ObservationRanibizumab (0.3 mg)Ranibizumab (0.5 mg) | 130132130 | +0.8 letter+12.7 letters+14.9 letters | P<.0001P<.0001 |
| BRAVO64 (BRVO) | 3 | Laser photocoagulationRanibizumab (0.3 mg)Ranibizumab (0.5 mg) | 132134131 | +7.3 letters+16.6 letters+18.3 letters | P<.0001P<.0001 |
| BEAT-ROP69 (ROP) | 4 | ||||
| Zone 1 | Laser photocoagulation | 34 | 42% recurrence | ||
| Bevacizumab | 33 | 6% recurrence | P=.003 | ||
| Zone 2 | Laser photocoagulation | 41 | 12% recurrence | ||
| Bevacizumab | 42 | 5% recurrence | P=.27 |
AMD = age-related macular degeneration; BDR = background diabetic retinopathy; BRVO = branch retinal vein occlusion; CRVO = central retinal vein occlusion; NS = not significant; ROP = retinopathy of prematurity.
Other Conditions and Drugs
Anti-VEGF agents have been administered for other retinal conditions (juxtafoveal telangiectasia, radiation retinopathy), glaucoma (trabeculectomy bleb failure, neovascular glaucoma), and ocular surface disorders (corneal vascularization). They have been combined with currently available (photodynamic therapy) as well as experimental (radiation therapy, complement and integrin inhibitors) therapies. New anti-VEGF agents are in varying stages of development (topical pazopanib, small molecule interfering RNA).
Systemic Concerns
Normal physiologic function and maintenance of tissue integrity require low levels of systemic VEGF activity. Suppression of this baseline activity, seen with intravenous bevacizumab, causes hypertension, nephrotic syndrome, thromboembolic events, bowel perforation, and delayed wound healing. The treatment of advanced cancer with bevacizumab, compared with chemotherapy alone, increases the risk of both arterial thrombotic events (3.8% vs 1.7%)70 and mortality, the most common causes being neutropenia, gastrointestinal perforation, and hemorrhage, by a factor of 1.46.71 Because of concerns over these serious adverse events, the FDA voted in December 2010 to remove breast cancer as an indication for bevacizumab. The FDA cited multistudy evidence that bevacizumab-treated patients did not live longer and were at higher risk of gastrointestinal perforations, stroke, delayed wound healing, organ failure, and reversible posterior leukoencephalopathy syndrome (hypertension, headaches, confusion, seizures, vision loss).
Anti-VEGF–mediated systemic arterial hypertension is believed to result from down-regulation of nitric oxide synthase, whereas several pathophysiologic mechanisms may explain the association between anti-VEGF agents and thromboembolic events. Because VEGF mediates fibrinolytic processes, drug-mediated VEGF suppression may inactivate enzymes in the plasminogen activator pathway and decrease the synthesis of matrix metalloproteinases,72,73 thereby leading to the oversynthesis of matrix proteins. Other possible mechanisms include the down-regulation of nitric oxide (a natural antithrombotic agent),74 increased endothelial cell apoptosis,75 increased erythropoietin synthesis (which increases blood viscosity),76 and destabilization of cholesterol plaques.77 Adverse events that have been attributed to VEGF suppression are represented in Figure 4.
FIGURE 4.
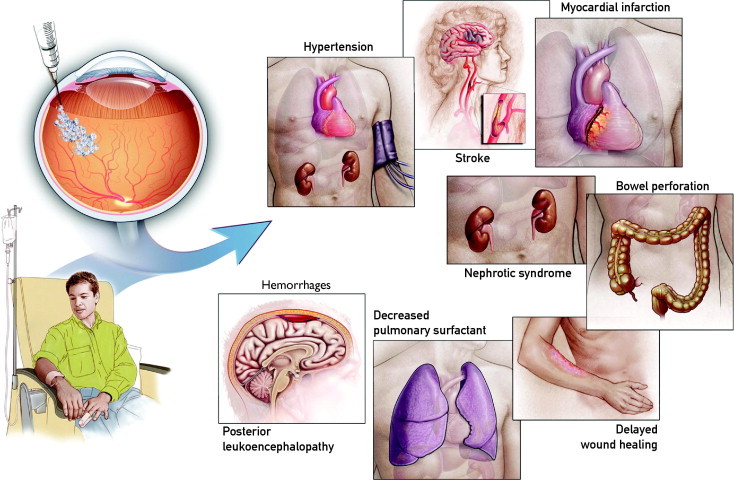
Possible systemic complications of anti-VEGF drug therapy. VEGF = vascular endothelial growth factor.
Of potential importance to ophthalmologists is that these systemic complications might occur despite the far lower doses of bevacizumab used for the treatment of ocular conditions (1.25 mg per injection vs 2.5 mg/kg/per week intravenously for tumors). Because of its short serum half-life (6 hours), ranibizumab has not been detected in contralateral eyes, and levels in the systemic circulation remain relatively low. Bevacizumab, with a much longer serum half-life (20 days), can reach concentrations of 20 ng/mL to 687 ng/mL, dwarfing those of serum VEGF (100 pg/mL). Therefore, intraocular injections of bevacizumab can lower blood VEGF levels by up to 117-fold within 1 day and by 4-fold up to 1 month later, changes comparable to those achieved with intravenous therapy.78,79 These findings raise the possibility that intravitreally administered bevacizumab may suppress baseline physiologic VEGF activity. Bevacizumab has also been detected in contralateral eyes after injections in rabbits and has been associated with the regression of contralateral proliferative diabetic retinopathy and iris neovascularization.80 Fortunately, patients who receive simultaneous bilateral anti-VEGF injections every 30 days do not have a higher risk of adverse systemic events than those receiving single doses.81
Data regarding systemic complications of intravitreally administered anti-VEGF drugs is confounded by the fact that most treated patients have several coexisting risk factors for vascular events (advanced age, hypertension, and hyperlipidemia). Although current data do not conclusively associate intravitreal anti-VEGF use with adverse systemic events, several trends are worrisome. The phase 3 ranibizumab trials (MARINA50 and ANCHOR51) did not show a significantly elevated risk of thromboembolic events, but the Safety and Efficacy of a Flexible Dosing Regimen of Ranibizumab in Neovascular Age-Related Macular Degeneration (SUSTAIN) trial found a 10% incidence of stroke in treated patients with a history of preexisting cerebrovascular disease.82 A meta-analysis of several ranibizumab trials showed a higher incidence of stroke in treated patients compared with controls (2.2% vs 0.7%; P=.045), and an analysis using combined data showed an elevated risk (7.8% in the ranibizumab groups vs 4.2% in the controls) of nonocular hemorrhage (ecchymosis, gastrointestinal hemorrhages, hematomas, vaginal hemorrhages, and subdural hematomas).83 Fortunately, no similar association was seen with myocardial infarction.84
Data regarding adverse events due to bevacizumab must be evaluated carefully because, unlike ranibizumab, which has had numerous such trials, bevacizumab has not been subjected to randomized sham-treated control groups. During the first few years of intravitreal bevacizumab use, a voluntary reporting site documented only rare incidences of systemic adverse events.85 A large, prospective pan-American study showed acute blood pressure elevation in 0.59% of patients after intravitreal injections,86 but other studies showed no definite elevation. A Medicare analysis of 146,942 patients discovered no difference in the rates of myocardial infarction, stroke, bleeding, or mortality between bevacizumab and other AMD therapies.87 Although not powered to identify small differences in adverse events among the treatment groups, the CATT trial reported a higher mortality rate among patients receiving bevacizumab, compared with ranibizumab, for reasons not previously associated with VEGF suppression.53
The BEAT-ROP69 study found a slightly higher mortality rate in bevacizumab-treated babies compared to those treated with laser (6.6% vs 2.6%; P=.44); most babies died of respiratory failure. The study was underpowered to detect differences in adverse events, but concerns over systemic safety should be considered because VEGF plays a role in the synthesis of pulmonary surfactant, a key compound for neonatal survival.88
The management of intravitreally treated patients with VEGF-sensitive diseases has not been standardized. The phase 3 AMD studies with pegaptanib excluded patients with myocardial infarctions within 6 months of entry and stroke within 12 months of entry,41 and the phase 3 DME trial with the VEGF Trap-Eye will stratify patients according to previous myocardial infarction or cerebrovascular accident; however, neither the ranibizumab trials50,51 nor the CATT trial53 excluded these patients. This indicates that even clinical investigators cannot agree on exclusionary systemic criteria for the use of intraocular anti-VEGF agents. Because the evidence for systemic complications has thus far been suggestive but inconclusive, most surgeons do not view recent surgery or thromboembolic events as exclusionary for intraocular anti-VEGF treatments.
Because many patients receiving intravitreal injections have elevated risk for thromboembolic diseases, some will experience myocardial infarctions or strokes during the course of treatment. No consensus exists regarding how to proceed with ocular therapy under these circumstances. Many physicians continue anti-VEGF injections because the alternative therapies are considerably less effective. However, given the possible association between anti-VEGF therapy and stroke, it is reasonable for the ophthalmologist and general physician to confer and reassess the need for further injections or, in response to an adverse event, consider changing drugs to theoretically minimize the risk of additional adverse events. If vision in the treated eye cannot be improved and the patient has good vision in the contralateral eye, then discontinuing therapy may be considered. If the patient has been receiving bevacizumab, then switching to ranibizumab, with a more robust set of safety data and shorter systemic half-life, may be appropriate. Finally, if the ocular disease has responded well to bevacizumab or ranibizumab, then switching to pegaptanib, which some investigators claim to be theoretically safer and has been shown to maintain previous visual gains after pan-VEGFA blockade, may be a reasonable alternative.
Conclusion
Our deepening understanding of the pathophysiology of blood-retinal barrier breakdown and neovascularization has emphasized the important role of VEGF in both processes. In addition to its central role in propagating tumor growth, VEGF has emerged as a critical molecule in the pathogenesis of our most common blinding diseases. The introduction of anti-VEGF drugs has revolutionized the treatment of these diseases by stabilizing and, in many cases, reversing vision loss. Although these drugs have low ocular and systemic complication rates, therapy needs to be reassessed if complications possibly related to VEGF suppression are seen.
Article Highlights.
-
■
In addition to causing breakdown of the blood-retinal barrier, vascular endothelial growth factor (VEGF) plays an important role in both physiologic and pathologic angiogenesis.
-
■
VEGF production is stimulated by ischemia, which stabilizes hypoxia-inducible factor-1α, as well as several inflammatory cytokines.
-
■
Several of the most important chorioretinal vascular diseases, including age-related macular degeneration, diabetic retinopathy, retinal vein occlusions, and retinopathy of prematurity, cause loss of vision through VEGF-associated neovascularization or macular edema.
-
■
Current treatment of exudative age-related macular degeneration, the most common cause of blindness in industrialized nations, consists of intravitreal injections of bevacizumab or ranibizumab (a humanized, affinity-enhanced antibody fragment).
-
■
Intraocular anti-VEGF injections are becoming increasingly important for the treatment of macular edema due to background diabetic retinopathy and retinal vein occlusions, as well as pathologic neovascularization due to retinopathy of prematurity.
-
■
After intraocular injections, anti-VEGF drugs reach the systemic circulation in concentrations sufficient to decrease baseline VEGF levels.
-
■
Concerns regarding the ability of intravitreally administered anti-VEGF drugs to cause thromboembolic adverse events have not been resolved.
Footnotes
Potential Competing Interests: Dr Stewart has received research support from Regeneron and Bayer. He has served on an Advisory Board for Regeneron.
References
- 1.Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992;267(16):10931–10934. [PubMed] [Google Scholar]
- 2.Yancopoulos G.D., Davis S., Gale N.W., Rudge J.S., Wiegand S.J., Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Senger D.R., Galli S.J., Dvorak A.M., Perruzzi C.A., Harvey V.S., Dvorak H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N., Henzel W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N., Carver-Moore K., Chen H. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 8.Gerber H.P., Hillan K.J., Ryan A.M. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 9.Kitamoto Y., Tokunaga H., Tomita K. Vascular endothelial growth factor is an essential molecule for mouse kidney development: glomerulogenesis and nephrogenesis. J Clin Invest. 1997;99(10):2351–2357. doi: 10.1172/JCI119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan A.M., Eppler D.B., Hagler K.E. Preclinical safety evaluation of rhuMAbVEGF, an antiangiogenic humanized monoclonal antibody. Toxicol Pathol. 1999;27(1):78–86. doi: 10.1177/019262339902700115. [DOI] [PubMed] [Google Scholar]
- 11.Henry T.D., Annex B.H., McKendall G.R. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 12.Makinen K., Manninen H., Hedman M. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol Ther. 2002;6(1):127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 13.Kalil R.A., Salles F.B., Giusti I.I. VEGF gene therapy for angiogenesis in refractory angina: phase I/II clinical trial. Rev Bras Cir Cardiovasc. 2010;25(3):311–321. doi: 10.1590/s0102-76382010000300006. [DOI] [PubMed] [Google Scholar]
- 14.Bhagat N., Grigorian R.A., Tutela A., Zarbin M.A. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1–32. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Houck K.A., Leung D.W., Rowland A.M., Winer J., Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267(36):26031–26037. [PubMed] [Google Scholar]
- 16.Shibuya M., Yamaguchi S., Yamane A. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase (flt) closely related to the fms family. Oncogene. 1990;5(4):519–524. [PubMed] [Google Scholar]
- 17.Terman B.I., Carrion M.E., Kovacs E., Rasmussen B.A., Eddy R.L., Shows T.B. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6(9):1677–1683. [PubMed] [Google Scholar]
- 18.Chung A.S., Lee J., Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10(7):505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 19.Wen Y., Edelman J.L., Kang T., Zeng N., Sachs G. Two functional forms of vascular endothelial growth factor receptor-2/Flk-1 mRNA are expressed in normal rat retina. J Biol Chem. 1998;273(4):2090–2097. doi: 10.1074/jbc.273.4.2090. [DOI] [PubMed] [Google Scholar]
- 20.Dor Y., Porat R., Keshet E. Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol. 2001;280(6):C1367–C1374. doi: 10.1152/ajpcell.2001.280.6.C1367. [DOI] [PubMed] [Google Scholar]
- 21.Famiglietti E.V., Stopa E.G., McGookin E.D., Song P., LeBlanc V., Streeter B.W. Immunocytochemical localization of vascular endothelial growth factor in neurons and glial cells of human retina. Brain Res. 2003;969(1-2):195–204. doi: 10.1016/s0006-8993(02)03766-6. [DOI] [PubMed] [Google Scholar]
- 22.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64(5-6):993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 23.Epstein A.C., Gleadle J.M., McNeill L.A. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell P.H., Wiesener M.S., Chang G.W. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell P.H. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16(4-5):523–530. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 27.Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 28.Ku D.D., Zaleski J.K., Liu S., Brock T.A. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am J Physiol. 1993;265(2 pt 2):H586–H592. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 29.Bates D.O., Curry F.E. Vascular endothelial growth factor increases microvascular permeability via a Ca(2+)-dependent pathway. Am J Physiol. 1997;273(2 pt 2):H687–H694. doi: 10.1152/ajpheart.1997.273.2.H687. [DOI] [PubMed] [Google Scholar]
- 30.Roberts W.G., Palade G.E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108(pt 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 31.Dejana E., Corada M., Lampugnani M.G. Endothelial cell-to-cell junctions. FASEB J. 1995;9(10):910–918. [PubMed] [Google Scholar]
- 32.Antonetti D.A., Lieth E., Barber A.J., Gardner T.W. Molecular mechanisms of vascular permeability in diabetic retinopathy. Sem Ophthalmol. 1999;14(4):240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 33.Gerber H.P., Dixit V., Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273(21):13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto K., Khosrof S., Bursell S.E. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA. 1999;96(19):10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Congdon N., O'Colmain B., Klaver C.C., Eye Diseases Prevalence Research Group Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 36.Ferris F.L., III, Fine S.L., Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 37.Jager R.D., Mieler W.F., Miller J.W. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 38.Stefansson E., Geirsdottir A., Sigurdsson H. Metabolic physiology in age related macular degeneration. Prog Retin Eye Res. 2011;30(1):72–80. doi: 10.1016/j.preteyeres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Buschini E., Piras A., Nuzzi R., Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol. 2011;95(1):14–25. doi: 10.1016/j.pneurobio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Lopez P.F., Sippy B.D., Lambert H.M., Thach A.B., Hinton D.R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37(5):855–868. [PubMed] [Google Scholar]
- 41.Gragoudas E.S., Adamis A.P., Cunningham E.T., Jr, Feinsod M., Guver D.R., VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 42.Friberg T.R., Tolentino M., LEVEL Study Group Pegaptanib sodium as maintenance therapy in neovascular age-related macular degeneration: the LEVEL study. Br J Ophthalmol. 2010;94(12):1611–1617. doi: 10.1136/bjo.2009.174946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michels S., Rosenfeld P.J., Puliafito C.A., Marcus E.N., Venkatraman A.S. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112(6):1035–1047. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Diago T., Pulido J.S., Molina J.R., Collett L.C., Link T.P., Ryan E.H., Jr. Ranibizumab combined with low-dose sorafenib for exudative age-related macular degeneration. Mayo Clin Proc. 2008;83(2):231–234. doi: 10.1111/j.1600-0420.2007.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenfeld P.J., Moshfeghi A.A., Puliafito C.A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 46.Rosenfeld P.J., Fung A.E., Puliafito C.A. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36(4):336–339. [PubMed] [Google Scholar]
- 47.Arevalo J.F., Sanchez J.G., Wu L., Pan-American Collaborative Retinal Study Group Intravitreal bevacizumab for subfoveal choroidal neovascularization in age-related macular degeneration at twenty-four months: the Pan-American Collaborative Retina Study. Ophthalmology. 2010;117(10):1974–1981. doi: 10.1016/j.ophtha.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y., Wiesmann C., Fuh G. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293(4):865–881. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara N., Damico L., Shams N., Lowman H., Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 50.Rosenfeld P.J., Brown D.M., Heier J.S., MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 51.Brown D.M., Kaiser P.K., Michels M., ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 52.Brechner R.J., Rosenfeld P.J., Babish J.D., Caplan S. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 Medicare fee-for-service Part B claims file. Am J Ophthalmol. 2011;151(5):887–895. doi: 10.1016/j.ajo.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Martin D.F., Maguire M.G., Ying G.S., Grunwald J.E., Fine S.L., Jaffe G.J., CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holash J., Davis S., Papadopoulos N. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart M.W., Rosenfeld P.J. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92(5):667–668. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- 56.Li W., Yanoff M., Liu X., Ye X. Retinal capillary pericyte apoptosis in early human diabetic retinopathy. Chin Med J (Engl) 1997;110(9):659–663. [PubMed] [Google Scholar]
- 57.Nguyen Q.D., Shah S.M., Khwaja A.A. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell P., Bandello F., Schmidt-Erfurth U. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 59.Michaelides M., Kaines A., Hamilton R.D. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–1086. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 60.Aiello L.P., Davis M.D., Girach A., PKC-DRS2 Group Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113(12):2221–2230. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 61.Klein R., Moss S.E., Meuer S.M., Klein B.E. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(4):513–518. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 62.Rehak M., Wiedemann Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010;8(9):1886–1894. doi: 10.1111/j.1538-7836.2010.03909.x. [DOI] [PubMed] [Google Scholar]
- 63.Aiello L.P., Avery R.L., Arrigg P.G. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 64.Campochiaro P.A., Heier J.S., Feiner L., BRAVO investigators Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102–1112. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 65.Brown D.M., Campochiaro P.A., Singh R.P., CRUISE investigators Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124–1133. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 66.Figueroa M.S., Contreras I., Noval S., Arruabarrena G. Results of bevacizumab as the primary treatment for retinal vein occlusions. Br J Ophthalmol. 2010;94(8):1052–1056. doi: 10.1136/bjo.2009.173732. [DOI] [PubMed] [Google Scholar]
- 67.Wu L., Arevalo J.F., Berrocal M.H. Comparison of two doses of intravitreal bevacizumab as the primary treatment for macular edema secondary to branch retinal vein occlusions: results of the Pan American Collaborative Retina Study Group at 24 months. Retina. 2009;29(10):1396–1403. doi: 10.1097/IAE.0b013e3181bcef53. [DOI] [PubMed] [Google Scholar]
- 68.Yazdani S., Hendi K., Pakravan M., Mahdavi M., Yaseri M. Intravitreal bevacizumab for neovascular glaucoma: a randomized controlled trial. J Glaucoma. 2009;18(8):632–637. doi: 10.1097/IJG.0b013e3181997211. [DOI] [PubMed] [Google Scholar]
- 69.Mintz-Hittner H.A., Kennedy K.A., Chuang A.Z., BEAT-ROP Cooperative Group Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scappaticci F.A., Skillings J.R., Holden S.N. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99(16):1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 71.Ranpura V., Hapani S., Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305(5):487–494. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 72.Pepper M.S., Ferrara N., Orci L., Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181(2):902–906. doi: 10.1016/0006-291x(91)91276-i. [DOI] [PubMed] [Google Scholar]
- 73.Hiratsuka S., Nakamura K., Iwai S. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2(4):289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 74.Tziros C., Freedman J.E. The many antithrombotic actions of nitric oxide. Curr Drug Targets. 2006;7(10):1243–1251. doi: 10.2174/138945006778559111. [DOI] [PubMed] [Google Scholar]
- 75.Bombeli T., Karsan A., Tait J.F., Harlan J.M. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89(7):2429–2442. [PubMed] [Google Scholar]
- 76.Tam B.Y., Wei K., Rudge J.S. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12(7):793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 77.Russell D.A., Abbott C.R., Gough M.J. Vascular endothelial growth factor is associated with histological instability of carotid plaques. Br J Surg. 2008;95(5):576–581. doi: 10.1002/bjs.6100. [DOI] [PubMed] [Google Scholar]
- 78.Qian J., Lu Q., Tao Y., Jiang Y.R. Vitreous and plasma concentrations of apelin and vascular endothelial growth factor after intravitreal bevacizumab in eyes with proliferative diabetic retinopathy. Retina. 2011;31(1):161–168. doi: 10.1097/IAE.0b013e3181e46ad8. [DOI] [PubMed] [Google Scholar]
- 79.Matsuyama K., Ogata N., Matsuoka M., Wada M., Takahashi K., Nishimura T. Plasma levels of vascular endothelial growth factor and pigment epithelium-derived factor before and after intravitreal injection of bevacizumab. Br J Ophthalmol. 2010;94(9):1215–1218. doi: 10.1136/bjo.2008.156810. [DOI] [PubMed] [Google Scholar]
- 80.Avery R.L., Pearlman J., Pieramici D.J. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695–1715. doi: 10.1016/j.ophtha.2006.05.064. e1–15. [DOI] [PubMed] [Google Scholar]
- 81.Mahajan V.B., Elkins K.A., Russell S.R. Bilateral intravitreal injection of antivascular endothelial growth factor therapy. Retina. 2011;31(1):31–35. doi: 10.1097/IAE.0b013e3181ed8c80. e1-15. [DOI] [PubMed] [Google Scholar]
- 82.Holz F.G., Amoaku W., Donate J. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118(4):663–671. doi: 10.1016/j.ophtha.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 83.Gillies M.C., Wong T.Y. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2007;356(7):748–749. author reply 749-750. [PubMed] [Google Scholar]
- 84.Ueta T., Yanagi Y., Tamaki Y., Yamaguchi T. Cerebrovascular accidents in ranibizumab. Ophthalmology. 2009;116(2):362. doi: 10.1016/j.ophtha.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 85.Fung A.E., Rosenfeld P.J., Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90(11):1344–1349. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu L., Martinez-Castellanos M.A., Quiroz-Mercado H., Pan American Collaborative Retina Group (PACORES) Twelve-month safely of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES) Graefes Arch Clin Exp Ophthalmol. 2008;246(1):81–87. doi: 10.1007/s00417-007-0660-z. [DOI] [PubMed] [Google Scholar]
- 87.Curtis L.H., Hammill B.G., Schulman K.A., Cousins S.W. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128(10):1273–1279. doi: 10.1001/archophthalmol.2010.223. [DOI] [PubMed] [Google Scholar]
- 88.Compernolle V., Brusselmans K., Acker T. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8(7):702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]


