Abstract
Gastrointestinal dysmotility and constipation are common problems in critical care patients. The majority of critical care patients are treated with opioids, which inhibit gastrointestinal (GI) motility and lead to adverse outcomes. We reasoned that methylnaltrexone (MNTX), a peripheral opioid antagonist approved for the treatment of opioid-induced constipation in patients with advanced illness receiving palliative care when response to laxative therapy has not been sufficient, could improve GI function in critically ill patients. The present study included all patients in our intensive care unit who required rescue medication for GI stasis during the 10-week period from September 1 to November 15, 2009. We compared conventional rescue therapy with subcutaneous MNTX. We performed a retrospective chart review of the 88 nonsurgical critical care patients receiving fentanyl infusions, 15 (17%) of whom met the criteria of absence of laxation within 72 hours of intensive care unit admission despite treatment with senna and sodium docusate. Eight of these 15 patients subsequently received conventional rescue therapy (combination of sodium picosulfate [5 mg] and 2 glycerin suppositories [4-g mold]), and 7 patients received MNTX (subcutaneous injection, 0.15 mg/kg). Laxation occurred within 24 hours in 6 of the 7 MNTX patients (86%) but in none of the 8 patients receiving conventional rescue therapy (P=.001). The median difference in time to laxation between the 2 groups was 3.5 days (P<.001). Although not statistically significant, all 7 patients treated with MNTX, but only 4 of 8 (50%) who received conventional rescue therapy, progressed to full target enteral feeding (P=.08). Intensive care unit mortality was 2 of 7 MNTX patients (29%) vs 4 of 8 (50%) in the standard therapy group (P=.61). We hypothesize that MNTX may play an important role in restoration of bowel function in critically ill patients.
Bowel dysfunction in the intensive care unit (ICU) represents an important problem in critical care, with up to 70% of patients suffering from constipation.1. There is increasing evidence from numerous sources that opioids contribute to perioperative and ICU bowel dysfunction.2 Other studies demonstrate that bowel dysfunction in critically ill patients is associated with adverse outcomes such as delayed gastric emptying leading to increased gastroesophageal reflux and aspiration, decreased enteral feeding, and later ICU discharge.3-5 Although bowel dysfunction in critically ill patients is multifactorial and some components are due to complex disease, there is increasing evidence that exogenous and endogenous opioids contribute to bowel dysmotility.6 Restoration of normal gastrointestinal (GI) function is essential for establishing enteral feeding, protects against bacterial translocation leading to sepsis, alleviates GI discomfort due to constipation, and shortens ICU stay. Methylnaltrexone (MNTX) is a recently approved peripheral opioid antagonist. It is a quaternary ammonium compound with a positive charge, which limits its ability to cross the blood-brain barrier. Unlike tertiary opioid antagonists such as naloxone or naltrexone, MNTX does not reverse centrally mediated analgesia or precipitate withdrawal. Methylnaltrexone is approved for treatment of opioid-induced constipation (OIC) in patients with advanced illness who are receiving palliative care when response to laxatives has not been sufficient.6 The success of MNTX in relieving OIC in palliative care patients7 prompted us to introduce this drug in a critical care setting in our ICU (Hammersmith Hospital, London), where opiate infusions are commonly used and conventional laxative therapy is often ineffective. Following its introduction in our ICU, we undertook a quality assurance efficacy analysis, comparing subcutaneous MNTX with our conventional rescue therapy. The present study represents our experience with MNTX in critically ill patients.
Patients and Methods
This study was a retrospective analysis that was part of a review of our current clinical practice using both MNTX and conventional rescue laxatives. Because this was a retrospective review of clinical practice using de-identified data, ethics board approval was not deemed necessary. All investigators abided by the Ethical Principles for Medical Research Involving Human Subjects outlined in the Declaration of Helsinki and adopted in October 2000 by the World Medical Association. Clinical medical and nursing staff uninvolved with this analysis anonymously extracted the data. All 88 nonsurgical critical care patients in our ICU during the 10-week period between September 1 and November 15, 2009, were retrospectively studied for GI function and management.
All patients were sedated with intravenous fentanyl and midazolam or propofol to facilitate mechanical ventilation, with regular documentation of the Richmond Agitation-Sedation Scale score and daily sedation holds according to departmental protocol. On admission to the ICU, nasogastric feeding was commenced in all patients via a fine-bore (10 F) tube placed in stomach not post pylorus. All patients were prescribed regular senna (15 mg once daily) and sodium docusate (250 mg twice daily) from day of admission as part of the departmental bowel care policy. If laxation did not occur after 72 hours, a rectal examination was performed and further rescue medications to encourage bowel movement were administered according to the attending clinician's choice. Rescue medications customarily used in the department for laxative-refractory constipation included a combination of sodium picosulfate (5 mg) and 2 glycerin suppositories (4-g mold) (described in this report as conventional rescue therapy) or MNTX (0.15 mg/kg by subcutaneous injection) at the discretion of the clinical staff. The success of rescue (time to laxation) was recorded by clinical and nursing staff who were not involved with this study as part of routine care. Data recorded included time to laxation following rescue medication, tolerance of nasogastric feeding, sedation score, and opiate requirements.
The data were retrospectively collected, de-identified, and analyzed. Normality distribution for the patient demographics and outcome parameters were assessed by the Kolmogorov-Smirnov test. Categorical variables were expressed as number (percentage) and continuous variables as mean ± SD. Comparisons between categorical variables were assessed using the Fisher exact test. Comparisons between continuous variables were assessed using the Wilcoxon rank sum test. All P values were 2-sided, and P=.05 was considered statistically significant.
Results
Opioid-induced constipation, defined for the purposes of this report as absence of laxation within 72 hours of ICU admission, occurred in 15 of the 88 patients studied (17%), despite all patients receiving regular senna and sodium docusate. Of the 15 patients with OIC, 7 were managed with MNTX (0.15 mg/kg subcutaneously) and 8 were managed with conventional rescue therapy consisting of sodium picosulfate (5 mg) and 2 glycerin suppositories (4-g mold). Rescue medication was continued every other day until the desired effect was achieved.
The patients' demographics are shown in Table 1. Admission diagnoses were similar between the 2 groups (MNTX and conventional rescue therapy) and included pneumonia, pancreatitis, and cardiogenic and septic shock. Both groups were receiving similar amounts of sedation. There were no statistically significant differences in age, gender, Acute Physiology and Chronic Health Evaluation II score, and agitation score between the 2 groups. Fentanyl dose was slightly higher in the MNTX group (178.6±85.9 vs 150.0±75.6 μg/h; P=.51). The MNTX group received more than twice the mean dose of norepinephrine (0.24±0.14 vs 0.11±0.08 μg/kg per min; P=.04).
TABLE 1.
Demographic Data From 15 ICU Patients Treated for Opioid-Induced Constipation, by Treatment Group
| Characteristic | Methylnaltrexone (n=7) | Conventional rescue therapy (n=8) | P value |
|---|---|---|---|
| Age (y) | 56.8±17.8 | 65.9±16.6 | .33 |
| Male:female ratio | 3:4 | 5:3 | .62 |
| APACHE II score | 18.8±6.0 | 19.6±7.3 | .83 |
| Fentanyl dose (μg/h) | 178.6±85.9 | 150±75.6 | .51 |
| Richmond Agitation-Sedation Scale score | −3.7±0.5 | −3.4±1.2 | .94 |
| Norepinephrine dose (μg/kg/min) | 0.24±0.14 | 0.11±0.08 | .04 |
Data are presented as mean ± SD unless indicated otherwise. APACHE = Acute Physiology and Chronic Health Evaluation; ICU = intensive care unit.
The outcomes from intervention are shown in Table 2. The most striking result was the immediate effect of MNTX, with laxation occurring within 24 hours in 6 of the 7 patients (86%) vs none of the 8 patients in the conventional rescue group (P=.001). The time to laxation was 30.7±60.9 vs 96.0±18.2 hours (P=.02). The median difference in time to laxation between the 2 groups was 3.5 days (P<.001) (Figure). Methylnaltrexone was ineffective in one patient who had a large subcapsular liver hematoma and elevated intra-abdominal pressure (24 cm H2O), and who exhibited severe fecal impaction requiring manual evacuation. When this patient was excluded from analysis, the 6 other patients laxated within 7.8 hours vs 96.0 hours for patients who received conventional rescue treatment.
TABLE 2.
Intervention Outcomes in 15 ICU Patients Treated for Opioid-Induced Constipation, by Treatment Group
| Characteristic | Methylnaltrexone (n=7) | Conventional rescue therapy (n=8) | P value |
|---|---|---|---|
| No. of patients laxating within 24 h | 6 | 0 | .001 |
| Hours to laxation (mean ± SD) | 30.7±60.9 | 96.0±18.2 | .02 |
| No. of patients established on full enteral feed | 7 | 4 | .08 |
| Gastric residual volumes after 24 h (mL) | 50.0 (n=1) | 433±70 (n=4) | .17 |
| ICU mortality (No. of patients) | 2 | 4 | .61 |
| ICU length of stay (d) (mean ± SD) | 46.1±27.7 | 35.3±18.5 | .38 |
ICU = intensive care unit.
FIGURE.
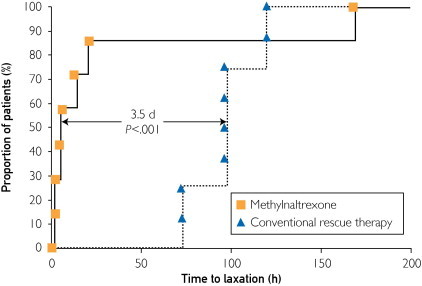
Time to laxation in 15 intensive care unit patients treated for opioid-induced constipation with methylnaltrexone or conventional rescue therapy.
All 7 patients treated with MNTX, but only 4 of 8 (50%) who received conventional rescue therapy, progressed to tolerating full target enteral feeding (P=.08). The residual gastric volume of the patients on conventional rescue therapy who did not progress to enteral feeding was 433±70 mL. In contrast, 6 of 7 patients treated with MNTX had no gastric residual volumes after treatment. The only patient with measurable residual gastric fluid in the MNTX group had a volume of 50 mL (P=.17). Consistent with improved feeding, ICU mortality was 2 of 7 MNTX patients (29%) vs 4 of 8 (50%) who received conventional rescue therapy (P=.61). Length of stay was not statistically significantly different between the 2 groups. The difference was potentially affected by a greater number of deaths (ICU mortality) in the conventional rescue therapy group. There were no serious adverse effects (one episode of vomiting in the patient with fecal impaction), no increase in fentanyl or analgesia requirements following administration of MNTX, and no signs of MNTX reversing the central effects of fentanyl.
Discussion
Opioids are a mainstay of care in the ICU, but OIC is a recognized complication in critically ill patients. Our most important observation is that MNTX can resolve OIC in many patients in this population. While MNTX is approved for treatment of OIC in palliative care patients with advanced illness, its use in the medical ICU has been limited and largely anecdotal. The findings in our series confirm the results in 3 previously published individual case reports of MNTX use in the postsurgical setting (2 from the University of Chicago) and extend the potential use of MNTX to medical ICUs. In each of the prior cases, there was an immediate effect of MNTX administration on bowel motility. In one report, MNTX was given intravenously to a critically ill burn patient.8 The purpose of that use was to facilitate feeding, although bowel motility was also restored. After 4 days of no appreciable bowel function, administration of MNTX produced immediate improvement in bowel sounds, flatus, gastric residual volumes, and subsequently feeding. In another case, a 54-year-old woman with a palliative ostomy and a long history of heroin use demonstrated no bowel function and severe abdominal distention 7 days after a palliative ostomy procedure.9 She responded with a brisk stool output of over 1 L via ostomy 15 minutes after administration of MNTX by subcutaneous injection. Both of these patients were receiving high doses of opioids, as were our critical care patients. In addition, a recent case report in a critically ill neonate with complex congenital heart disease complicated by 8 days of bowel dysmotility following iliosigmoid anastomosis demonstrated that MNTX (0.15 mg/kg subcutaneously) restored bowel function within 15 minutes of injection.10 The patient was receiving a fentanyl infusion of 2 μg/kg per hour. Our study confirms the observations in these isolated case reports in that 6 of our 7 MNTX-treated patients responded within the first day vs 0 of 8 patients treated with conventional rescue therapy (P=.001) (Figure). Similar to the results reported in surgical patients,8-10 3 of our 7 patients responded in less than 4 hours. It should be noted that our patients were critically ill medical patients, also receiving high doses of norepinephrine.
The etiology of critical care bowel dysfunction is unclear and likely multifactorial. The lack of suitable therapy has hampered study of bowel function in the ICU, although the problem has been increasingly recognized as clinically important. Studies of critically ill patients using various laxative regimens have demonstrated that morphine administration is associated with decreased laxation and that late defecation is associated with an increased length of hospital stay and higher risk of infection in ICU patients.2,5,11 There have been previous attempts to utilize high doses of the tertiary antagonist naloxone, which has limited bioavailability, via enteral administration in ICU patients. One study showed a decrease in aspiration, improved gastric emptying, and reduced ventilator pneumonic rates but no improvement in bowel function.12 In clinical practice, many patients with gastric stasis are unable to tolerate this enteral regimen. Aside from tolerability, the ability to utilize a peripheral opioid antagonist, which does not precipitate opioid withdrawal, represents a major clinical advantage over strategies using tertiary opioid antagonists.
Opioid-induced constipation in critically ill patients appears to differ from postoperative ileus (POI), in which peripheral opioid antagonists have shown mixed results,13 perhaps because of differences in opioid dosing. In the ICU setting, patients receive high-dose opioid infusions for days. The patients in our study received high doses of fentanyl (>150 μg/h) over a prolonged period. In addition, in the ICU setting, we and others utilized MNTX to treat OIC rather than to prevent POI. The selective peripheral opioid antagonist alvimopan was developed to facilitate recovery of postoperative gut function in patients undergoing laparotomy and bowel resection.6,14 Alvimopan, which is orally administered, is approved in the United States for hospitalized patients, but no more than 15 doses can be administered. Because MNTX could be given parenterally, it was proposed as a potentially useful agent to prevent POI.15 A subsequent prospective, randomized trial of 65 patients undergoing colectomy demonstrated improvements in recovery of gastric function,16 but 2 large multinational trials failed to confirm this finding.17 Recent data from a double-blind randomized study of 33 postoperative patients with 2 days of OIC during rehabilitation from complex orthopedic procedures support our observations of a therapeutic effect of MNTX.18 In that study, MNTX caused first laxation in a median of 15 hours vs 55 hours with conventional therapy (P=.02), and 33% of patients treated with MNTX laxated within 2 hours vs none of the control patients. Similar to our ICU patients, these orthopedic patients had received sustained high doses of opioids. Although our patients received fentanyl exclusively (as part of the departmental sedation policy), the therapeutic effects of MNTX appear to occur with all μ-receptor opioids.7,18
Aside from the effect of opioids on bowel motility, emerging literature suggests that parenteral MNTX may be useful in the nonsurgical ICU setting both to facilitate feeding and to accelerate gastric emptying in patients receiving opioids. Two volunteer studies showed that low-dose opioids can significantly impede gastric emptying via a peripheral mechanism.19,20 We observed a clinically significant improvement in feeding and decreased gastric residual volumes in our patients with MNTX treatment. Although our study was too small to demonstrate statistical significance, the improvement in enteral feeding that we observed suggests that further study is merited. The reduction in residual volumes following MNTX was dramatic and facilitated early target enteral feeding. There is a trend toward improved outcome, but our study was too small to document this statistically.
A few caveats should be considered regarding our observations. First, this is a retrospective observational study. Second, data on the safety of MNTX in the ICU population are limited. Although MNTX has been utilized widely in palliative care patients, rare incidences of perforation have been reported21; however, we did not observe evidence of perforation in our own critically ill patients. Safety studies in the immediate postoperative period after colectomy have shown it to be safe in that setting in substantially higher doses.17 However, it is important to rule out obstruction or serious damage to the bowel wall before proceeding with use of MNTX in the critical care setting.
Conclusion
Opioid-induced constipation despite laxative therapy is a common problem in critically ill patients. We found MNTX to be very effective in producing laxation when compared with conventional rescue laxatives in our critically ill patients. In this retrospective study, MNTX was well tolerated and did not demonstrate any signs of reversing the central effects of fentanyl. In addition to its benefit in reversing gut dysmotility, MNTX may be of further benefit in improving enteral feeding. Our observations demonstrate a potential role for MNTX in managing OIC in critically ill patients and suggest that a larger controlled study in the ICU environment is merited.22
Acknowledgements
We thank Dr Lorin Johnson for his assistance in reviewing the submitted manuscript and statistical data and acknowledge the support of the UK NIHR Biomedical Research Centre.
Footnotes
Potential Competing Interests: Dr Moss serves as a paid consultant to Salix Pharmaceuticals, which has acquired the license for the development of MNTX. He receives royalties from the University of Chicago.
References
- 1.Nassar A.P., Jr, da Silva F.M., de Cleva R. Constipation in intensive care unit: incidence and risk factors. J Crit Care. 2009;24(4):630.e9–630.e12. doi: 10.1016/j.jcrc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Van der Spoel J.I., Oudemans-van Straaten H.M., Kuiper M.A., van Roon E.N., Zandstra D.F., van der Voort P.H. Laxation of critically ill patients with lactulose or polyethylene glycol: a two-center randomized, double-blind, placebo-controlled trial. Crit Care Med. 2007;35(12):2726–2731. doi: 10.1097/01.CCM.0000287526.08794.29. [DOI] [PubMed] [Google Scholar]
- 3.Wilmer A., Tack J., Frans E. Duodenogastroesophageal reflux and esophageal mucosal injury in mechanically ventilated patients. Gastroenterology. 1999;116(6):1293–1299. doi: 10.1016/s0016-5085(99)70492-0. [DOI] [PubMed] [Google Scholar]
- 4.Mostafa S.M., Bhandari S., Ritchie G., Gratton N., Wenstone R. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91(6):815–819. doi: 10.1093/bja/aeg275. [DOI] [PubMed] [Google Scholar]
- 5.Gacouin A., Camus C., Gros A. Constipation in long-term ventilated patients: associated factors and impact on intensive care unit outcomes. Crit Care Med. 2010;38(10):1933–1938. doi: 10.1097/CCM.0b013e3181eb9236. [DOI] [PubMed] [Google Scholar]
- 6.Moss J., Rosow C.E. Development of peripheral opioid antagonists: new insights into opioid effects. Mayo Clin Proc. 2008;83(10):1116–1130. doi: 10.4065/83.10.1116. [DOI] [PubMed] [Google Scholar]
- 7.Thomas J., Karver S., Cooney G.A., Chamberlain B.H. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358(22):2332–2343. doi: 10.1056/NEJMoa0707377. [DOI] [PubMed] [Google Scholar]
- 8.Woo M., O'Connor M., Yuan C.-S., Moss J. Reversal of opioid-induced gastric dysfunction in a critically ill burn patient after methylnaltrexone. Anesth Analg. 2008;107(6):1965–1967. doi: 10.1213/ane.0b013e31818556d3. [DOI] [PubMed] [Google Scholar]
- 9.Ladanyi A., Temkin S.M., Moss J. Subcutaneous methylnaltrexone to restore postoperative bowel function in a long-term opiate user. Int J Gynecol Cancer. 2010;20(2):308–310. doi: 10.1111/IGC.0b013e3181cd1828. [DOI] [PubMed] [Google Scholar]
- 10.Garten L., Degenhardt P., Bührer C. Resolution of opioid-induced postoperative ileus in a newborn infant after methylnaltrexone. J Pediatr Surg. 2011;46(3):e13–e15. doi: 10.1016/j.jpedsurg.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Hadfield R.J., Sinclair D.G., Houldsworth P.E., Evans T.W. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995;152(5, pt 1):1545–1548. doi: 10.1164/ajrccm.152.5.7582291. [DOI] [PubMed] [Google Scholar]
- 12.Meissner W., Dohrn B., Reinhart K. Enteral naloxone reduces gastric tube reflux and frequency of pneumonia in critical care patients during opioid analgesia. Crit Care Med. 2003;31(3):776–780. doi: 10.1097/01.CCM.0000053652.80849.9F. [DOI] [PubMed] [Google Scholar]
- 13.Viscusi E.R., Gan T.J., Leslie J.B. Peripherally acting μ-opioid receptor antagonists and postoperative ileus: mechanisms of action and clinical applicability. Anesth Analg. 2009;108(6):1811–1822. doi: 10.1213/ane.0b013e31819e0d3a. [DOI] [PubMed] [Google Scholar]
- 14.Taguchi A., Sharma N., Saleem R.M. Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med. 2001;345(13):935–940. doi: 10.1056/NEJMoa010564. [DOI] [PubMed] [Google Scholar]
- 15.Moss J., Yuan C.S. Selective postoperative inhibition of gastrointestinal opioid receptors [comment] N Engl J Med. 2002;346(6):455. doi: 10.1056/NEJM200202073460617. [DOI] [PubMed] [Google Scholar]
- 16.Viscusi E., Rathmell J., Fichera A., Gan T.J., Israel R.J. A double-blind, randomized, placebo-controlled trial of methylnaltrexone (MNTX) for post-operative bowel dysfunction in segmental colectomy patients [abstract A893] Anesthesiology. 2005;103:A893. http://www.asaabstracts.com/strands/asaabstracts/abstract.htm;jsessionid=894AF59C8421CA3C93777E9DA2D5CA5A?year=2005&index=11&absnum=1630 Accessed January 13, 2012. [Google Scholar]
- 17.Yu C.S., Chun H.K., Stambler N. Safety and efficacy of methylnaltrexone in shortening the duration of postoperative ileus following segmental colectomy: results of two randomized, placebo-controlled phase 3 trials. Dis Colon Rectum. 2011;54(5):570–578. doi: 10.1007/DCR.0b013e3182092bde. [DOI] [PubMed] [Google Scholar]
- 18.Anissian L, Schwartz HW, Vincent K, et al. Subcutaneous methylnaltrexone for treatment of acute opioid-induced constipation: Phase 2 study in rehabilitation after orthopedic surgery [published online ahead of print Oct 13, 2011]. J Hosp Med. doi: 10.1002/jhm.943 [DOI] [PubMed]
- 19.Yuan C.S., Foss J.F., O'Connor M., Roizen M.F., Moss J. Effects of low-dose morphine on gastric emptying in healthy volunteers. J Clin Pharmacol. 1998;38(11):1017–1020. doi: 10.1177/009127009803801105. [DOI] [PubMed] [Google Scholar]
- 20.Murphy D.B., Sutton J.A., Prescott L.F., Murphy M.B. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology. 1997;87(4):765–770. doi: 10.1097/00000542-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Mackey A.C., Green L., Greene P., Avigan M. Methylnaltrexone and gastrointestinal perforation. J Pain Symptom Manage. 2010;40(1):e1–e3. doi: 10.1016/j.jpainsymman.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Hall J.B. Creating the animated intensive care unit. Crit Care Med. 2010;38(10, suppl):S668–S675. doi: 10.1097/CCM.0b013e3181f203aa. [DOI] [PubMed] [Google Scholar]


