Abstract
Objective
To evaluate the relationship between the use of zolpidem and subsequent cancer risk in Taiwanese patients.
Methods
We used data from the National Health Insurance system of Taiwan to investigate whether use of zolpidem was related to cancer risk. For the study cohort, we identified 14,950 patients who had received a first prescription for zolpidem from January 1, 1998, through December 31, 2000. For each zolpidem user, we selected randomly 4 comparison patients without a history of using zolpidem who were frequency-matched by sex, age, and year of the index date. Incidence rates of all cancers and selected site-specific cancers were measured by the end of 2009, and related hazard ratios (HRs) and 95% confidence intervals (CIs) of the cancer were measured as well.
Results
The risk of developing any cancer was greater in patients using zolpidem than in nonusers (HR, 1.68; 95% CI, 1.55-1.82). The stratified analysis showed that the overall HR for high-dosage zolpidem (≥300 mg/y) was 2.38. The site-specific cancer risk was the highest for oral cancer (HR, 2.36; 95% CI, 1.57-3.56), followed by kidney cancer, esophageal cancer, breast cancer, liver cancer, lung cancer, and bladder cancer (HR, 1.60; 95% CI, 1.06-2.41). Men were at higher risk than women.
Conclusion
This population-based study revealed some unexpected findings, suggesting that the use of zolpidem may be associated with an increased risk of subsequent cancer. Further large-scale and in-depth investigations in this area are warranted.
Abbreviations and Acronyms: CI, confidence interval; CIN, cervical intraepithelial neoplasia; CIS, carcinoma in situ; HR, hazard ratio; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NHI, National Health Insurance; NHIRD, National Health Insurance Research Database; NHRI, National Health Research Institute
Sleep problems are not uncommon in the general population.1 Studies conducted in various countries have reported that approximately 30% of their adult samples report one or more of the symptoms of insomnia.2 These symptoms include difficulty initiating sleep, difficulty maintaining sleep, waking up too early, and, in certain cases, nonrestorative or poor quality of sleep.2 An investigation in Canada found that 25.3% of the total sample was dissatisfied with their sleep, 29.9% reported insomnia symptoms, and 9.5% met the criteria for insomnia syndrome.3 Similar results have been reported for Asians.4
Zolpidem (Ambien, Stilnox) is a nonbenzodiazepine hypnotic drug prescribed for the short-term treatment of insomnia. A single-blind trial published in 1991 reported on the nightly use of zolpidem for up to 6 months; the authors concluded that 10 mg/d is an appropriate starting dose and is effective and safe for the treatment of sleep disturbances of various origins.5 Generally, because of zolpidem-induced amnesia for sleep disturbances, this popular drug is thought to produce a patient's subjectively better quality of sleep without the evident adverse effects that usually accompany hypnotics. The risk of abuse and tolerance is low when zolpidem is used as directed.6-8 Common adverse effects include nausea or vomiting, amnesia, headache, hallucinations, and short-term memory loss. A number of users have reported sleepwalking.9
An early animal study found that rats that were administered high doses of zolpidem developed renal, thyroidal, and testicular cancers.10 However, information is scarce on the possible relationship between the use of zolpidem and the risk of developing certain types of human cancer, and no firm conclusions can be drawn from the limited literature.11,12 Researchers recognize that many adverse effects, drug interactions, and effectiveness are difficult to detect before drugs are approved and may become evident only after the drug has been used by millions of people for a long time. Because zolpidem remains the market leader,11 a small magnitude of hazard could have important clinical implications and would be of interest to the general public and the medical profession. A large, population-based study may help clarify this controversy. To that end, we evaluated the relationship between the use of zolpidem and subsequent cancer risk in Taiwanese patients.
Methods
National Health Insurance Research Database
Details of Taiwan's National Health Insurance (NHI) population-based cohort database, called the NHI Research Database (NHIRD), have been published previously.13 Briefly, the NHI program began in March 1995 and incorporated 13 insurance programs that provided health care to 99% of the Taiwanese population. The program contracted with 97% of the hospitals and clinics on the island, and it provides comprehensive medical services, including outpatient and inpatient care, dental care, physical therapy, preventive care, and prescriptions. The National Health Research Institute (NHRI) has been responsible for the administration of the NHIRD. The NHRI released the claims data of 1 million patients (approximately 5% of Taiwan's entire population), randomly selected from all insurants registered between 1996 and 2009, for inclusion in the database. The NHRI reported that no statistically significant differences were found in the distributions of age, sex, or health care expenditures between the subset of the NHIRD and all enrollees. The diagnoses recorded in the database were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). All data files were linked with scrambled identifications to secure insured privacy. This study was exempted from ethical review.
Study Participants and Follow-up
For the study cohort, we identified 15,628 patients who had received a first prescription for zolpidem from January 1, 1998, through December 31, 2000. Patients who had received zolpidem before 1998 were excluded. We used the date on which the zolpidem treatment was first commenced as the index date. We excluded patients with a history of malignant cancer before receiving zolpidem treatment or those for whom the data on sex or age were missing (678 patients were excluded for these reasons). For each of the remaining 14,950 patients taking zolpidem, we randomly selected 4 insured people in the comparison cohort without zolpidem treatment, frequency-matched for sex, age (every 5 years), and year of the index date. The comparison group included 59,799 patients. Both cohorts were followed up until the end of 2009. The mean follow-up durations for the nonzolpidem and zolpidem groups were 8.93 (SD, 2.72) years and 8.62 (SD, 2.97) years, respectively (P<.0001). The NHIRD did not provide death data of insurants. The difference of follow-up duration is only 0.31 year, but it is statistically significant because of large sample sizes.
Patient comorbidities at baseline were identified, and we also noted whether they had been prescribed a benzodiazepine dose greater than the median dose (>5 mg). Comorbidities included diabetes mellitus (ICD-9-CM code 250), hypertension (ICD-9-CM code 401-405), hyperlipidemia (ICD-9-CM code 272), sleep disorder (ICD-9-CM codes 307.4 and 780.5), sleep apnea syndrome (ICD-9-CM codes 780.51, 780.53, and 780.57), anxiety (ICD-9-CM codes 300.0, 300.2, 300.3, 300.83, and 309.81), depression (ICD-9-CM codes 296.2, 296.3, 300.4, and 311), alcoholism (ICD-9-CM codes 303 and 305.0), and obesity (ICD-9-CM code 278.0).
Each study patient was tracked until one of the following conditions was met: a diagnosis of malignant cancer (ICD-9-CM codes 140-208), follow-up was censored at the time of loss to follow-up, death, the patient withdrew from the NHI, or the follow-up period elapsed (on December 31, 2009).
Statistical Analyses
Demographic factors, including sex, age, and baseline comorbidities, were compared between the zolpidem cohort and the comparison group with the χ2 test. Comorbidities and benzodiazepine use were defined for the period from 1998 to 2009. Multivariate Cox proportional hazards regression analysis was performed to measure the zolpidem use in association with the risk of cancer, controlling for demographic characteristics and comorbidities of diabetes, hypertension, hyperlipidemia, sleep disorder, anxiety, benzodiazepine use, obesity, alcoholism, and depression. We examined the risk of cancer in general and that of site-specific cancers, including oral, esophageal, stomach, colorectal, liver, lung, breast, cervix, prostate, endometrium, bladder, and kidney cancers. We further stratified zolpidem by annual dosage taken to estimate the cancer risk associated with taking zolpidem alone and with both zolpidem and benzodiazepine. Zolpidem dosage levels were categorized into none, 1 to 29 mg/y, 30 to 299 mg/y, and more than 300 mg/y. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated in the model. The cancer-free proportions of the 2 groups were estimated using the Kaplan-Meier method and compared by using a log-rank test. All analyses were performed using SAS statistical software, version 9.1 (SAS Institute Inc, Cary, NC); the statistical significance level was set at .05.
Results
Table 1 lists the characteristics of all 74,749 study participants in both the zolpidem cohort and the comparison cohort. Of the study participants, 59.5% were women, with a predominance (26.9%) of elderly patients. Compared with the comparison patients, zolpidem patients were more likely to have diabetes (14.9% vs 22.8%; P<.001), hypertension (38.2% vs 53.2%; P<.001), hyperlipidemia (21.3% vs 34.2%; P<.001), sleep disorder (12.8% vs 59.7%; P<.001), anxiety (7.80% vs 40.2%; P<.001), obesity (1.33% vs 3.16%; P<.001), alcoholism (0.87% vs 4.35%; P<.001), depression (2.46% vs 31.9%; P<.001), and benzodiazepine prescriptions (40.9% vs 88.0%; P<.001) (Table 1).
TABLE 1.
Baseline Characteristics of the Zolpidem and Nonzolpidem Cohorts
| Characteristic | Nonzolpidem cohort, No. (%) (n=59,799) | Zolpidem cohort, No. (%) (n=14,950) | P value |
|---|---|---|---|
| Sex | >.99 | ||
| Female | 35,595 (59.5) | 8899 (59.5) | |
| Male | 24,204 (40.5) | 6051 (40.5) | |
| Age (y) | >.99 | ||
| <20 | 1391 (2.3) | 348 (2.3) | |
| 20-34 | 10,149 (17.0) | 2537 (17.0) | |
| 35-44 | 11,052 (18.5) | 2763 (18.5) | |
| 45-54 | 11,136 (18.6) | 2784 (18.6) | |
| 55-64 | 10,012 (16.7) | 2503 (16.7) | |
| ≥65 | 16,059 (26.9) | 4015 (26.9) | |
| Mean (SD) | 51.7 (17.5) | 51.8 (17.4) | .32 |
| Medical history | |||
| Diabetes mellitus | 8883 (14.9) | 3413 (22.8) | <.001 |
| Hypertension | 22,863 (38.2) | 7952 (53.2) | <.001 |
| Hyperlipidemia | 12,711 (21.3) | 5110 (34.2) | <.001 |
| Sleep disorder | 7663 (12.8) | 8929 (59.7) | <.001 |
| Anxiety | 4667 (7.8) | 6004 (40.2) | <.001 |
| Benzodiazepine treated | 24,463 (40.9) | 13,159 (88.0) | <.001 |
| Obesity | 798 (1.3) | 473 (3.2) | <.001 |
| Alcoholism | 522 (0.9) | 650 (4.4) | <.001 |
| Depression | 1471 (2.5) | 4763 (31.9) | <.001 |
Table 2 presents the adjusted HRs of overall cancer risk and site-specific cancer risk associated with zolpidem use for each sex. The HR of overall cancer for patients using zolpidem was 1.68 (95% CI, 1.55-1.82), compared with patients not using zolpidem. A Kaplan-Meier analysis showed that the cancer-free rate was significantly lower in the zolpidem cohort than in the nonzolpidem cohort (log-rank P<.0001; Figure).
TABLE 2.
HRs (95% CIs) for the Association Between Specific Cancers and Zolpidem Use: Results of Cox Proportional Hazards Regression Analysisa
| Variable | Allb |
Womenc |
Menc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Zolpidem cohort | Nonzolpidem cohort | HR (95% CI) | Zolpidem cohort | Nonzolpidem cohort | HR (95% CI) | Zolpidem cohort | Nonzolpidem cohort | HR (95% CI) | |
| Overall | 1047 | 2924 | 1.68 (1.55-1.82)d | 512 | 1473 | 1.67 (1.49-1.87)d | 535 | 1451 | 1.70 (1.52-1.91)d |
| Oral cancer | 47 | 94 | 2.36 (1.57-3.56)d | 7 | 16 | 1.89 (0.70-5.07) | 40 | 78 | 2.48 (1.58-3.89)d |
| Esophagus cancer | 21 | 46 | 1.95 (1.07-3.55)e | 2 | 4 | 2.11 (0.35-12.7) | 19 | 42 | 1.91 (1.02-3.61)e |
| Stomach cancer | 53 | 207 | 1.28 (0.92-1.79) | 21 | 87 | 1.22 (0.71-2.09) | 32 | 120 | 1.32 (0.86-2.03) |
| Colorectal cancer | 109 | 490 | 1.04 (0.83-1.32) | 63 | 251 | 1.13 (0.83-1.53) | 46 | 239 | 0.96 (0.67-1.37) |
| Liver cancer | 177 | 408 | 1.81 (1.48-2.22)d | 62 | 168 | 1.42 (1.03-1.96)e | 115 | 240 | 2.12 (1.64-2.75)d |
| Lung cancer | 142 | 386 | 1.64 (1.32-2.03)d | 49 | 156 | 1.31 (0.91-1.89) | 93 | 230 | 1.85 (1.40-2.45)d |
| Breast cancerf | 99 | 259 | 1.84 (1.40-2.42)d | 99 | 259 | 1.84 (1.40-2.42)d | |||
| Cervical cancerf | 28 | 110 | 1.62 (1.00-2.62) | 28 | 110 | 1.62 (1.00-2.62) | |||
| Prostate cancerg | 59 | 160 | 1.39 (0.99-1.95) | 59 | 160 | 1.39 (0.99-1.95) | |||
| Endometrial cancerf | 7 | 40 | 1.20 (0.47-3.03) | 7 | 40 | 1.20 (0.47-3.03) | |||
| Bladder cancer | 40 | 110 | 1.60 (1.06-2.41)e | 12 | 34 | 1.66 (0.79-3.50) | 28 | 76 | 1.54 (0.94-2.52) |
| Kidney cancer | 39 | 76 | 2.18 (1.41-3.36)d | 27 | 41 | 2.54 (1.47-4.40)d | 12 | 35 | 1.68 (0.81-3.49) |
| Other cancers | 226 | 538 | 2.16 (1.81-2.58)d | 135 | 307 | 2.31 (1.83-2.91)d | 91 | 231 | 1.98 (1.50-2.62)d |
International Classification of Diseases, Ninth Revision, Clinical Modification codes are as follows: oral cancer, 140.xx, 141.xx, 143.xx-146.xx, and 148.xx-149.xx; esophagus cancer, 151.xx; stomach cancer, 151.xx; colorectal cancer, 153.xx and 154.xx; liver cancer, 155.xx; lung cancer, 162.xx; breast cancer, 174.xx; cervical cancer, 180.xx; endometrial cancer, 182.xx; prostate cancer, 185.xx; bladder cancer, 188.xx; kidney cancer, 189.xx. CI = confidence interval; HR = hazard ratio.
Adjusted for sex, age, benzodiazepine use, anxiety, hypertension, diabetes mellitus, obesity, alcoholism, and depression.
Adjusted for age, benzodiazepine use, anxiety, hypertension, diabetes mellitus, obesity, alcoholism, and depression.
P<.001.
P<.05.
For women.
For men.
FIGURE.
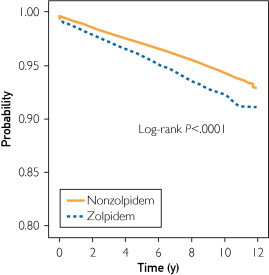
Kaplan-Meier model for estimating the cancer-free proportions of patients treated with or without zolpidem (log-rank P<.001).
The specific analyses by cancer type showed that patients using zolpidem were at the highest risk of developing oral cancer (HR, 2.36; 95% CI, 1.57-3.56), followed by kidney cancer (HR, 2.18; 95% CI, 1.41-3.36), and esophageal cancer (HR, 1.95; 95% CI, 1.07-3.55) (Table 2). The stratified analyses by sex are also given in Table 2.
The interactions between zolpidem use and other risk factors in Cox proportional hazards regression after controlling for age and sex by each risk factor are presented in Table 3. Compared with the nonzolpidem, nonbenzodiazepine cohort, the zolpidem cohort (both with and without benzodiazepine use) had an increased HR for cancer; the risk was highest for patients using both zolpidem and benzodiazepine (HR, 1.73; 95% CI, 1.59-1.88) (Table 3). When accounting for the interactions between zolpidem and other risk factors, the zolpidem cohort had a significantly higher HR (P<.01) for cancer than the nonzolpidem cohort for all the risk factors except obesity (P=.18) (Table 3).
TABLE 3.
Hazard Ratios (95% Confidence Intervals) for Overall Cancer Risk Associated With Zolpidem Use, Accounting for the Interactions Between Zolpidem and Other Risk Factorsa
| Risk factor | Nonzolpidem cohort | Zolpidem cohort | P valueb |
|---|---|---|---|
| Benzodiazepine treated | |||
| No | 1.00 (Reference) | 1.72 (1.39-2.13)c | <.001 |
| Yes | 1.26 (1.17-1.36)c | 1.73 (1.59-1.88)c | <.001 |
| Sleep disorder | |||
| No | 1.00 (Reference) | 1.96 (1.78-2.16)c | <.001 |
| Yes | 0.69 (0.62-0.78)c | 1.20 (1.10-1.32)c | <.001 |
| Anxiety | |||
| No | 1.00 (Reference) | 1.80 (1.66-1.96)c | <.001 |
| Yes | 0.71 (0.61-0.81)c | 1.06 (0.95-1.19) | <.001 |
| Diabetes mellitus | |||
| No | 1.00 (Reference) | 1.58 (1.46-1.72)c | <.001 |
| Yes | 0.94 (0.85-1.03) | 1.33 (1.17-1.51)c | <.001 |
| Hypertension | |||
| No | 1.00 (Reference) | 1.85 (1.65-2.07)c | <.001 |
| Yes | 0.77 (0.72-0.84)c | 1.15 (1.05-1.26)d | <.001 |
| Hyperlipidemia | |||
| No | 1.00 (Reference) | 1.65 (1.51-1.79)c | <.001 |
| Yes | 0.70 (0.64-0.76)c | 1.07 (0.95-1.20) | <.001 |
| Obesity | |||
| No | 1.00 (Reference) | 1.54 (1.43-1.65)c | <.001 |
| Yes | 0.67 (0.44-1.03) | 1.03 (0.65-1.64) | .18 |
| Alcoholism | |||
| No | 1.00 (Reference) | 1.50 (1.40-1.61)c | <.001 |
| Yes | 1.21 (0.83-1.77) | 2.70 (2.04-3.57)c | .002 |
| Depression | |||
| No | 1.00 (Reference) | 1.64 (1.51-1.77)c | <.001 |
| Yes | 0.68 (0.53-0.88)d | 1.24 (1.10-1.41)c | <.001 |
Adjusted for sex and age.
Compared with the nonzolpidem group.
P<.001.
P<.05.
The interaction among different dosage levels of zolpidem and combined use of benzodiazepine was examined. Table 4 indicates that, compared with the subgroup without benzodiazepine combined use, the HRs of cancer increased with zolpidem dosage (HR, 3.15; 95% CI, 2.25-4.41; for 30-299 mg/y; HR, 6.24; 95% CI, 4.13-9.43; for ≥300 mg/y) but decreased to 2.64-fold (95% CI, 2.34-2.99) and 3.30-fold (95% CI, 2.91-3.75) risks for 30 to 299 mg/y and 300 mg/y or more, respectively, when with combined benzodiazepine use.
TABLE 4.
Cox Proportional Hazards Regression Analysis Measured HRs (95% CIs) of Cancers by Zolpidem Dosage in Association With Using Zolpidem Alone and Using Both Zolpidem and Benzodiazepinea
| Zolpidem, mg/y | Overall |
Zolpidem only |
Zolpidem and benzodiazepine |
P value | |||
|---|---|---|---|---|---|---|---|
| No. of events/No. of patients | HR (95% CI) | No. of events/No. of patients | HR (95% CI) | No. of events/No. of patients | HR (95% CI) | ||
| 0 | 2924/59,800 | 1.00 (Reference) | 1316/35,336 | 1.00 (Reference) | 1608/24,464 | 1.47 (1.36-1.59)b | <.001 |
| 1-29 | 188/4578 | 0.99 (0.85-1.15) | 32/1211 | 0.92 (0.65-1.31) | 156/3367 | 1.45 (1.23-1.72)b | .04 |
| 30-299 | 413/5381 | 1.90 (1.70-2.13)b | 35/419 | 3.15 (2.25-4.41)b | 378/4962 | 2.64 (2.34-2.99)b | .50 |
| ≥300 | 446/4990 | 2.38 (2.12-2.67)b | 23/161 | 6.24 (4.13-9.43)b | 423/4829 | 3.30 (2.91-3.75)b | .049 |
CI = confidence interval; HR = hazard ratio.
P<.0001.
Discussion
The results of this population-based cohort study demonstrate significant associations between the use of zolpidem and the increased risk for overall cancer, as well as oral, liver, lung, breast, esophageal, bladder, and kidney cancers. These unexpected findings may arouse public interest regarding safety issues in zolpidem use.
According to the Taiwan Cancer Registry, since 1982 cancer has been the leading cause of death among the general population in Taiwan. The age-adjusted incidence rate has increased steadily, and in 2007 it reached 270 new cases per 100,000 people.14 However, data from Surveillance Epidemiology and End Results indicate a different trend, with overall cancer incidence rates reportedly decreasing by 0.7% per year between 1999 and 2006 for all racial and ethnic groups combined.15 Because this issue continues to be a challenge for public health in Taiwan, it has gained the attention of the government; the result has been more population-based investigations regarding cancer preventive epidemiology. The NHI program provides adequate data for such studies because of its comprehensive health coverage. The NHIRD contains ambulatory service records, hospital service records, and prescription claim data. It allows researchers to select specific study groups and matched comparison groups, with ensured representation of the underlying population groups. We recently used NHIRD data to evaluate the risk of malignant tumors in patients with end-stage renal disease and found some positive risk. A report on this study has been previously published.16 The current study used a similar design to determine whether zolpidem use relates to the risk of cancer.
To the best of our knowledge, the current study was the first population-based study of zolpidem users in Taiwan (N=14,950 patients). To create a comparison group, we randomly matched each zolpidem user with 4 people from the general population who did not use zolpidem.
As is the case with benzodiazepine use, the probability of a patient using zolpidem increased with age, female sex, and concomitant use of other psychotropics17,18; our results confirmed this pattern. Kripke11,19,20 has extensively studied the association between hypnotics and cancer risk, and his published work addresses this issue. On the basis of epidemiological data and laboratory studies, Kripke suggests that new hypnotics may increase cancer risk.
Zolpidem use appeared to promote viral infections, which may have indicated suppression of the immune function. Either immunosuppression or viral infections might increase cancer development.11,20 Cancers known to be related to viral infections include oral, liver, and cervical cancers.21-23 Our results demonstrate that zolpidem users have a significantly higher rate of subsequent oral and liver cancer development but not cervical cancer. One possible reason to explain this finding is the effect of national cancer screening. Cervical, oral, breast, prostate, and colorectal cancers are the main targets of Taiwan's free cancer screening program. We assumed that zolpidem users visit physicians relatively frequently and would be screened for cancer whenever necessary. Regarding the carcinogenesis of cervical cancer, cervical intraepithelial neoplasia (CIN) and carcinoma in situ (CIS) lesions are known to precede the development of invasive cancer. Frequent Papanicolaou smear examinations would detect cervical CIS, CIN, and dysplasia, enabling the patient to receive treatment before the disease progresses into invasive cancer. We did not include CIN and CIS as end points for cervical cancer, and this omission may have decreased the number of cases of invasive cervical cancer that we extracted from the database.
The cancer screening effect also partially applies for oral and breast cancers, which showed significantly higher rates in zolpidem users. More cancers can be detected if more frequent cancer screening is conducted among users of zolpidem. Prostate and colorectal cancers, however, did not show any significant difference between zolpidem users and nonusers.
Zolpidem can reduce the arousal response to nocturnal acid exposure and can also increase the duration of each esophageal acid reflux event. Patients with gastroesophageal reflux disease who take zolpidem thus experience significantly higher esophageal exposure to gastric acid, which increases the likelihood of developing esophageal cancer.12 Our data support this theory, and analysis revealed a significantly higher rate of esophageal cancer among zolpidem users. Other cancers that had significantly higher rates in zolpidem users than nonusers were lung, bladder, and kidney cancers. To date, we do not have any biological hypotheses to explain these associations.
Zolpidem users may also take benzodiazepine; thus, benzodiazepine may also be associated with cancer risk. In addition, sleep disorder may be associated with cancer risk. We wanted to investigate whether the relationship between zolpidem use and cancer risk may actually arise from an interaction between zolpidem and other risk factors. Table 3 clarifies this concern and shows that zolpidem independently increases the overall cancer risk. The dose level of zolpidem also plays an important role. As indicated in Table 4, higher doses of zolpidem were associated with greater cancer risk, especially for patients who took zolpidem without taking benzodiazepine. Table 4 indicates that the group receiving benzodiazepine plus zolpidem had a lower HR than the group receiving zolpidem alone at higher zolpidem dosages (30-299 and ≥300 mg/y but not for the lower zolpidem dosage [1-39 mg/y]). Therefore, the data in Table 4 should be considered cautiously because the zolpidem-only groups are too small and their HRs are implausible. In addition, complex drug interaction is a possible explanation.
Some readers may suspect reverse causality, which would imply that patients who are already at risk for cancer may have a greater tendency to start using zolpidem than those not at risk for cancer. To clarify this concern, we used the Kaplan-Meier method without adjusting for sex, age, and comorbidity to evaluate it. There was no time lag between the 2 groups (zolpidem vs nonzolpidem). We found that the cancer-free proportion of patients treated with and without zolpidem differed significantly over time (Figure). This finding indicates that cancer risk increases in conjunction with the length of time of zolpidem use; thus, reverse causality seems unlikely.
The current study had some limitations that need to be addressed. First, information on smoking habit, alcohol consumption, body mass index, and family history of cancer was unavailable from the NHIRD. All of these are risk factors for multiple cancers and could plausibly also be associated with zolpidem use. The current study cannot control these possible confounders. However, we have included obesity and alcoholism as risk factors in the models and adjusted for them in the analyses. Second, the organ-specific pattern of cancer occurrence does not appear to correspond to any biological hypothesis of zolpidem action. We think it likely that our findings in part reflect a healthy nonuser effect (ie, those with healthier lifestyles and behaviors may be less likely to require zolpidem treatment or may be more likely to address such problems with nonpharmacological approaches). Third, evidence derived from any cohort study is generally less than that from randomized trials because a cohort study design is subject to many biases related to confounding adjustment. Despite our meticulous study design with adequate control of confounding factors, a key limitation is that bias could still remain if there are unmeasured or unknown confounders. Fourth, the diagnoses in NHI claims primarily serve the purpose of administrative billing and do not undergo verification for scientific purposes. We were unable to contact the patients directly regarding their use of zolpidem because of the anonymity of the identification numbers. In addition, prescriptions issued before 1996 for the drugs under study were not reflected in our analysis. This omission could have underestimated the cumulative dosage and may have weakened the observed association. Fifth, comorbidities were detected between 1998 and 2009. Because the comorbidities do not necessarily precede the use of zolpidem or benzodiazepine, they may be caused by zolpidem and benzodiazepine. This raises a question of whether control for comorbidities incident after zolpidem use is commenced may produce overcontrol and thus underestimation of causal associations. Overcontrol may be a conservative statistical choice. Finally, it is difficult to explain why the HR appears lower when any additional risk factor, except for alcoholism and benzodiazepine, is combined with the risk of zolpidem. Some undetermined mechanisms or intrinsic limitation of this study may lead to the results. Apart from these potential problems, the data on zolpidem prescription and cancer diagnosis were highly reliable.
Conclusion
This population-based, retrospective cohort study found that the use of zolpidem is significantly associated with an increased risk for overall cancer, as well as some individual cancers. The general population may be surprised by these unexpected findings, the reasons for which remain unclear. Our findings would require confirmation by further large, population-based, unbiased studies before any firm conclusions can be drawn.
Acknowledgments
Chia-Hung Kao and Li-Min Sun contributed equally to this work.
Footnotes
For editorial comment, see page 417
Grant Support: This work was supported by the study projects DMR-100-076 and DMR-100-077 in our hospital, the Taiwan Department of Health Clinical Trial and Research Center and for Excellence grant DOH101-TD-B-111-004, and the Taiwan Department of Health Cancer Research Center for Excellence grant DOH101-TD-C-111-005.
Contributor Information
Chia-Hung Kao, Email: d10040@mail.cmuh.org.tw.
Chih-Hsin Muo, Email: A17776@mail.cmuh.org.tw.
References
- 1.Wallander M.A., Johansson S., Ruigómez A., García Rodríguez L.A., Jones R. Morbidity associated with sleep disorders in primary care: a longitudinal cohort study. Prim Care Companion J Clin Psychiatry. 2007;9(5):338–345. doi: 10.4088/pcc.v09n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S., Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey I. Sleep. 1999;22(suppl 2):S347–S353. [PubMed] [Google Scholar]
- 3.Morin C.M., LeBlanc M., Daley M., Gregoire J.P., Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Cho Y.W., Shin W.C., Yun C.H., Hong S.B., Kim J., Earley C.J. Epidemiology of insomnia in Korean adults: prevalence and associated factors. J Clin Neurol. 2009;5(1):20–23. doi: 10.3988/jcn.2009.5.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlich D., L'Heritier C., Coquelin J.P., Attali P., Kryrein H.J. Long-term treatment of insomnia with zolpidem: a multicentre general practitioner study of 107 patients. J Int Med Res. 1991;19(3):271–279. doi: 10.1177/030006059101900313. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman J.A. Update on the safety considerations in the management of insomnia with hypnotics: incorporating modified-release formulations into primary care. Prim Care Companion J Clin Psychiatry. 2007;9(1):25–31. doi: 10.4088/pcc.v09n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajak G., Bandelow B. Safety and tolerance of zolpidem in the treatment of disturbed sleep: a post-marketing surveillance of 16944 cases. Int Clin Psychopharmacol. 1998;13(4):157–167. doi: 10.1097/00004850-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Monti J.M., Monti D., Estevez F., Giuati M. Sleep in patients with chronic primary insomnia during long-term zolpidem administration and after its withdrawal. Int Clin Psychopharmacol. 1996;11(4):255–263. doi: 10.1097/00004850-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki T., Miyaoka T., Tsuji S., Inami Y., Nishida A., Horiguchi J. Adverse reactions to zolpidem: case reports and a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6) doi: 10.4088/PCC.09r00849bro. pii: PCC.09r00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissinger J. NDA 19-908 Ambien Pharmacology Memos & Exclusivity Summary. http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/019908_S000_PHARM_MEMOS&EXCLUSIVITY_SUMMARY.pdf 1992:3. Accessed July 21, 2011.
- 11.Kripke D.F. Possibility that certain hypnotics might cause cancer in skin. J Sleep Res. 2008;17(3):245–250. doi: 10.1111/j.1365-2869.2008.00685.x. [DOI] [PubMed] [Google Scholar]
- 12.Gagliardi G.S., Shah A.P., Goldstein M. Effect of zolpidem on the sleep arousal response to nocturnal esophageal acid exposure. Clin Gastroenterol Hepatol. 2009;7(9):948–952. doi: 10.1016/j.cgh.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Liang J.A., Sun L.M., Muo C.H., Sung F.C., Chang S.N., Kao C.H. The analysis of depression and subsequent cancer risk in Taiwan. Cancer Epidemiol Biomarkers Prev. 2011;20(3):473–475. doi: 10.1158/1055-9965.EPI-10-1280. [DOI] [PubMed] [Google Scholar]
- 14.Taiwan Cancer Registry Cancer Statistics Annual Report. http://tcr.cph.ntu.edu.tw/main.php?Page=N2 Accessed July 21, 2011.
- 15.Edwards B.K., Ward E., Kohler B.A. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang J.A., Sun L.M., Yeh J.J., Sung F.C., Chang S.N., Kao C.H. the association between malignancy and end-stage renal disease in Taiwan. Jpn J Clin Oncol. 2011;41(6):752–757. doi: 10.1093/jjco/hyr051. [DOI] [PubMed] [Google Scholar]
- 17.Andersen A.B., Frydenberg M. Long-term use of zopiclone, zolpidem and zaleplon among Danish elderly and the association with sociodemographic factors and use of other drugs. Pharmacoepidemiol Drug Saf. 2011;20(4):378–385. doi: 10.1002/pds.2104. [DOI] [PubMed] [Google Scholar]
- 18.Johnell K., Fastbom J. The use of benzodiazepines and related drugs amongst older people in Sweden: associated factors and concomitant use of other psychotropics. Int J Geriatr Psychiatry. 2009;24(7):731–738. doi: 10.1002/gps.2189. [DOI] [PubMed] [Google Scholar]
- 19.Kripke D.F. Mortality associated with prescription hypnotics: National Center for Biotechnology Information. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=eurekah.section.28844 Accessed July 21, 2011.
- 20.Kripke D.F. Evidence That New Hypnotics Cause Cancer. http://escholarship.org/uc/item/12r2f32g Accessed July 21, 2011.
- 21.Chocolatewala N.M., Chaturvedi P. Role of human papilloma virus in the oral carcinogenesis: an Indian perspective. J Cancer Res Ther. 2009;5(2):71–77. doi: 10.4103/0973-1482.52788. [DOI] [PubMed] [Google Scholar]
- 22.Pecic V., Stankovic-Djordjevic D., Nestorovic M. Hepatitis C virus-related hepatocellular carcinoma and liver cirrhosis. J BUON. 2011;16(2):277–281. [PubMed] [Google Scholar]
- 23.Bosch F.X. Human papillomavirus: science and technologies for the elimination of cervical cancer. Expert Opin Pharmacother. 2011;12(14):2189–2204. doi: 10.1517/14656566.2011.596527. [DOI] [PubMed] [Google Scholar]


