Abstract
OBJECTIVE
To assess the efficacy of exercise and antidepressant medication in reducing depressive symptoms and improving cardiovascular biomarkers in depressed patients with coronary heart disease (CHD).
BACKGROUND
Although there is good evidence that clinical depression is associated with poor prognosis, optimal therapeutic strategies are currently not well-defined.
METHODS
101 outpatients with CHD and elevated depressive symptoms underwent assessment of depression including a psychiatric interview and the Hamilton Rating Scale for Depression (HAM-D). Participants were randomized to 4 months of aerobic exercise (3 times/week), sertraline (50-200 mg/day), or placebo. Additional assessments of cardiovascular biomarkers included measures of heart rate variability (HRV), endothelial function, baroreflex sensitivity, inflammation, and platelet function.
RESULTS
After 16 weeks, all groups showed improvement on HAM-D scores. Participants in both aerobic exercise (M= −7.5 [95% CI = −9.8, −5.0]) and sertraline (M= −6.1 [95% CI = −8.4, −3.9] achieved larger reductions in depressive symptoms compared to placebo (M= −4.5 [95% CI = −7.6, −1.5]; p = .034); exercise and sertraline were equally effective in reducing depressive symptoms (p = .607). Exercise and medication tended to result in greater improvements in HRV compared to placebo (p = .052); exercise tended to result in greater improvements in HRV compared to sertraline (p =.093)
CONCLUSIONS
Both exercise and sertraline resulted in greater reductions in depressive symptoms compared to placebo in CHD patients. Evidence that active treatments may also improve cardiovascular biomarkers suggests that they may have a beneficial effect on clinical outcomes as well as quality of life.
Keywords: Depression, Exercise, Sertraline, Heart rate variability, Inflammation, Biomarkers, antidepressant medication, SSRI
The term “cardiovascular vulnerable patient” has been used to describe patients susceptible to acute coronary events based upon plaque, blood markers, or myocardial characteristics (1). There now is growing evidence that psychological depression is associated with increased vulnerability, as it is a significant and independent risk factor for adverse outcomes in patients with coronary heart disease (CHD) (2-4).
Clinical depression is relatively common in CHD patients, with estimates of 15-20% of cardiac patients meeting criteria for major depressive disorder (MDD) and an additional 20% reporting elevated depressive symptoms (5-7). A number of studies have reported that the presence of clinical depression is associated with more than a doubling of risk for mortality and non-fatal cardiac events (8,9), and that even subclinical elevations in depressive symptoms are associated with worse prognosis in patients with established CHD (6,7).
The high prevalence of elevated depressive symptoms in CHD populations and the increased risk for untoward cardiac events associated with depression led the American Heart Association to issue an advisory statement recommending that CHD patients should be screened for depressive symptoms (4). However, the value of screening patients for depression has been controversial (10,11), due in part to a paucity of evidence supporting the value of treating depression in cardiac patients. While antidepressant medication is widely considered to be the treatment of choice (12), for a number of patients, medication fails to adequately relieve depressive symptoms or does so at the cost of unwanted side effects (13). Moreover, there have been few randomized clinical trials (RCTs) of established treatments for depression in cardiac patients, and results have been negative or equivocal (14-18).
There is now good reason to believe that exercise may be a viable alternative to traditional mental health interventions in cardiac patients. An increasing number of studies have shown that exercise may reduce depressive symptoms in patients with MDD (19) and that exercise may be comparable to established psychological (20) or pharmacological therapies (21,22). However, to our knowledge, no RCT has examined the effects of exercise on depression in CHD patients. Because depression also is related to biomarkers of cardiovascular risk, including reduced heart rate variability (HRV) (23), low baroreflex sensitivity (BRS) (24), impaired vascular endothelial function (25), increased platelet activation and heightened inflammation (26), as well as health behaviors such as physical inactivity (27), a secondary goal of the study was to examine the effects of exercise and antidepressant medication on these key cardiovascular biomarkers.
METHODS
Trial Overview
The “Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy” (UPBEAT) study sought to (a) evaluate the effectiveness of exercise training in reducing depression in CHD patients; and (b) examine changes in physiologic biomarkers of cardiac vulnerability, including measures of HRV, BRS, vascular endothelial function, inflammation, and platelet aggregation. Enrollment began in June 2006 and ended in September 2010. Participants were recruited from physician referrals, community-based screenings, and mass media advertisements. Following completion of baseline assessments (see below), participants were randomized to Exercise, Sertraline, or to a Placebo pill. At the conclusion of the 4-month treatment period, assessments were repeated.
UPBEAT was an investigator-initiated trial that was sponsored by the National Heart, Lung, and Blood Institute. Sertraline (Zoloft, Pfizer) and matching placebo pills were supplied by Pfizer, Inc (New York, NY), which had no role in the oversight or design of the study or in the analysis or interpretation of the data. The trial was approved by the Institutional Review Board at Duke University Medical Center and written informed consent was obtained from all participants. An independent data and safety monitoring board oversaw the conduct of the study and reviewed the safety and efficacy data.
Participants
Eligibility criteria included age 35 years or greater, documented coronary heart disease (CHD) (e.g., prior myocardial infarction, revascularization procedure, or significant [>70% stenosis] coronary atherosclerosis) and elevated scores (> 7) on the Beck Depression Inventory (BDI-II)(28). Exclusion criteria included the presence of another primary psychiatric diagnosis, such as a history of bipolar disorder or psychosis; medical comorbidities that would preclude participation in the trial (e.g., significant musculoskeletal disease, cancer); current psychotherapy or use of anti-depressants or other psychotropic medications; history of inability to tolerate or benefit from sertraline; use of dietary supplements or herbal therapies with purported psychoactive indications; current active alcohol or drug abuse or dependence; active suicidal intent; and participation in regular exercise >1 day/week.
Assessment procedures
Medical screening
Prior to entry into the study, patients underwent a physical examination by a study physician. Blood pressure was measured by standard sphygmomanometry in sitting and standing positions. Blood specimens following an overnight fast were acquired for routine electrolytes, pregnancy and liver function tests, blood count, and thyroid stimulating hormone. If a patient was found to have any significant medical condition that would contraindicate safe participation in the study, s/he was excluded from participation.
Depression assessment
All potential participants were evaluated using the Structured Clinical Interview for Depression (29) to determine the presence of MDD, and the 17-item Hamilton Depression Rating Scale (HAM-D) (30) to assess the severity of depressive symptoms, both at baseline and after 4 months. Assessments were performed by clinical psychologists blinded to treatment condition. To ensure patient safety, psychologists blinded to treatment group administered the HAM-D by telephone weekly for the first four weeks and biweekly for the subsequent 12 weeks to monitor symptom severity and presence of suicidality.
Exercise testing
Graded treadmill exercise testing was conducted before and after treatment to document patients’ aerobic capacity. Patients exercised to exhaustion or other standard endpoints under continuous electrocardiographic monitoring in which workloads were increased at a rate of 1 metabolic equivalent per minute (31). Expired gases were analyzed continuously by a Parvo Medics True One measurement system (Parvo Medics, Sandy, Utah).
Heart Rate Variability (HRV)
For our primary cardiovascular biomarker, the ECG was recorded for 24 hours using the Lifecard CF, a 3-channel digital compact flash Holter recorder (DelMar Reynolds, Irvine California). During the recording period, patients engaged in their normal patterns of activity. ECG data were downloaded and edited using the Pathfinder digital ambulatory ECG analyzer (DelMar Reynolds, Irvine, California) and HRV was estimated from the standard deviation of all normal R-R intervals (SDNN).
Endothelial function assessed by flow mediated dilation (FMD)
Brachial artery FMD was assessed following overnight fasting (32). Longitudinal B-mode ultrasound images of the brachial artery, 4-6 cm proximal to the antecubital crease, were obtained using an Acuson (Mountain View, California) Aspen ultrasound platform with an 11 MHz linear array transducer. Images were obtained after 10 min of supine relaxation and during reactive hyperemia, induced following inflation of a forearm pneumatic occlusion cuff to supra-systolic pressure (~200 mm Hg) for 5 minutes. FMD was defined as the maximum percent change in arterial diameter relative to resting baseline from 10-120 sec post-deflation of the occlusion cuff.
Baroreflex sensitivity (BRS)
Beat-by-beat systolic blood pressure (SBP) and heart rate were collected using the Finapres noninvasive BP monitor (model 2300; Ohmeda, Madison, WI). Recordings of beat-by-beat BP and R-R interval (derived as 60,000/HR) were edited for artifacts, linearly interpolated, and resampled at a frequency of 4 Hz, in order to generate an equally spaced time series. A fast Fourier transformation was then applied to the interpolated data after detrending and application of a Hanning filtering window. BRS was estimated from the magnitude of the transfer function relating R-R interval oscillations to SBP oscillations across the 0.07 to 0.1299 Hz, or low frequency band.
Inflammation and platelet function
Plasma inflammatory markers were measured by enzyme-linked immunosorbent assay (ELISA). Platelet factor 4 (PF4) and beta thromboglobulin (βTG) were measured by ELISA using commercially available kits manufactured by American Diagnostica (Stamford, CT) and Diagnostica Stago (Parsippany, NJ), respectively.
Treatment
Participants were randomly assigned to Exercise, Sertraline, or Placebo in a pre-determined 2:2:1 ratio. Randomization was performed centrally by computer with conditional randomization (stratified by age [35-59; >60], CHD status [prior MI/no MI], and depression severity [HAM-D > 18; HAM-D ≤18); patients were provided with sealed envelopes containing their group assignments.
Aerobic exercise
Patients in the Exercise condition attended three supervised group aerobic exercise sessions per week for 16 weeks. Based on peak heart rate achieved during the initial treadmill test, patients were assigned training ranges equivalent to 70-85% maximum heart rate reserve. Each aerobic session consisted of 30 minutes of walking or jogging on a treadmill at an intensity that would maintain heart rate within the assigned training range.
Sertraline/Placebo pill
Participants in the two “pill” conditions were given the selective serotonin reuptake inhibitor, sertraline, or matching placebo. Medications were taken once daily; the dosage depended on the clinical response, but usually each patient started at 50 mg (1 pill) of drug or placebo and progressed up to 200 mg (4 pills) contingent upon therapeutic response and presence of side effects. The treating psychiatrist was blinded to pill condition, and used supportive measures to help manage medication side effects.
Outcome assessors were unaware of patients’ treatment assignments, and only the research pharmacist was aware of which patients were assigned to sertraline or to placebo.
The primary endpoint was the HAM-D score at the end of 4 months. Secondary endpoints included remission of depression, defined as no DSM-IV diagnosis of MDD (33) and a HAM-D score < 8 (34), and cardiovascular biomarkers.
Statistical analysis
The effect of treatment on the primary and secondary outcomes was evaluated using the general linear model (GLM) for continuous variables and unadjusted chi-square tests for the categorical depression diagnoses and side effect outcomes, using the SAS 9.1 software (SAS Institute, Cary, NC). Separate models were estimated for each outcome, with the following predictors: a treatment group indicator variable, the corresponding pre-treatment value of the outcome, age, gender, and ethnicity (Caucasian vs. non-Caucasian). For the HAM-D outcome, we tested two orthogonal contrasts: 1) active treatments (i.e., Exercise and Sertraline) versus Placebo and 2) Exercise versus Sertraline. In cases where model residuals did not meet assumptions, we transformed the outcome and corresponding baseline level of the outcome to ranks, again using GLM. This occurred for SDNN, BRS, CRP, IL-6, βTG, and PF4. This approach reduces potential bias due to outlying values, allows the data to better meet model assumptions with respect to the distribution of residuals, and has been shown to be equivalent to conventional nonparametric tests (35). Unless otherwise indicated, treatment effects were analyzed following the intent-to-treat principle, with post-treatment missing data managed using the multiple imputation method available in SAS PROC MI. We estimated that our sample size would yield about 80% power to detect a 0.66 standard deviation difference between the active treatments and placebo control, and a 0.65 standard deviation difference between the two active treatments.
RESULTS
Participant flow
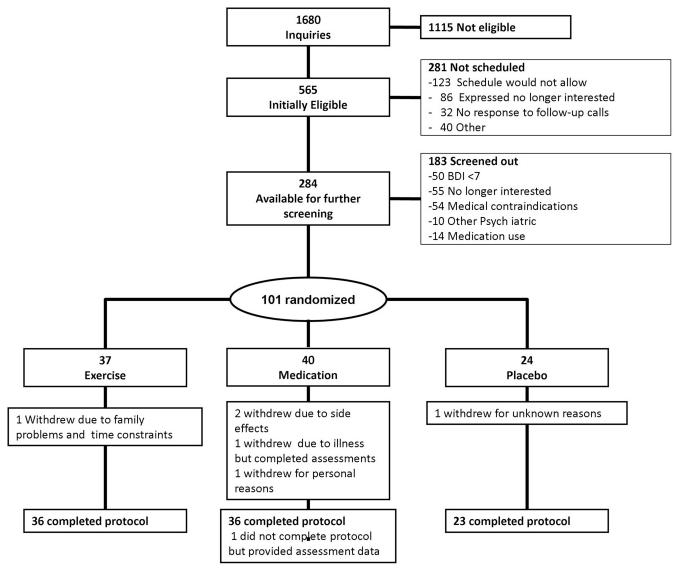
Figure 1 displays the flow of participants through the course of the study. Of the 1680 participants who initially inquired about the study, 284 met our initial inclusion criteria. After screening, 101 participants were randomized: 37 to the Exercise condition, 40 to Sertraline, and 24 to Placebo.
Figure 1.
Participant Flow in the UPBEAT Randomized Clinical Trial
Participant characteristics
The sample was generally middle-aged and older (mean age = 63.9 years), primarily white (73%), and male (68%). The majority of participants were married, relatively affluent, and had at least some college education (Table 1). Forty-seven participants (47%) met Diagnostic Statistical Manual-IV criteria for MDD at baseline, 46% (N = 17) in Exercise; 50% (N= 20) in Sertraline, and 42% (N = 10) in Placebo.
Table 1.
Background Characteristics of Sample
| Exercise N=37 |
Sertraline N = 40 |
Placebo N = 24 |
P-value* | |
|---|---|---|---|---|
| Age (years), m (SD) |
64.7 (11.0) | 63.4 (10.2) | 63.5 (11.4) | .911 |
| Gender: Male % (N) | 65% (24) | 63% (25) | 83% (20) | .189 |
| Ethnicity % (N) | .403 | |||
| Caucasian | 81% (30) | 70% (28) | 67% (16) | |
| African American | 8% (3) | 23% (9) | 13% (3) | |
| Hispanic | 3% (1) | 0 | 4% (1) | |
| Asian/Pacific | 5% (2) | 3% (1) | 8% (2) | |
| Islander | ||||
| Native American | 0 | 3% (1) | 8% (2) | |
| Other | 3% (1) | 3% (1) | 0 | |
| Level of Education | .792 | |||
| % (N) < High | 8% (3) | 8% (3) | 0 | |
| School | ||||
| High School | 11% (4) | 28% (11) | 21% (5) | |
| Some College | 14% (5) | 28% (11) | 29% (7) | |
| Completed College | 11% (4) | 15% (6) | 8% (2) | |
| Some Post-Graduate | 16% (6) | 13% (5) | 13% (3) | |
| Complete Post- Graduate |
11% (4) | 5% (2) | 25% (6) | |
| Annual Household Income |
.276 | |||
| < $45K | 43% (16) | 53% (21) | 33% (8) | |
| $45-50K | 19% (7) | 15% (6) | 13% (3) | |
| $50-75K | 14% (5) | 3% (1) | 13% (3) | |
| > $75K | 22% (8) | 25% (10) | 33% (8) | |
| Married | 73% (27) | 70% (28) | 67% (16) | .869 |
| Employed Full Time | 32% (12) | 25% (10) | 38% (9) | .553 |
| Medication | ||||
| Beta blockers | 92% (34) | 83% (33) | 96% (23) | .201 |
| ACE Inhibitors or | 51 %(19) | 60% (24) | 75% (18) | .182 |
| ARBs | ||||
| Aspirin | 92% (34) | 83% (33) | 96% (23) | .201 |
| Other Antiplatelets | 49%(18) | 53% (21) | 58% (14) | .761 |
| Cholesterol- Lowering |
86% (32) | 78% (31) | 79% (19) | .567 |
| Nitrates | 22% (8) | 40% (16) | 29% (7) | .214 |
| Prior MI | 35% (13) | 28% (11) | 25% (6) | .647 |
| Prior percutaneous revascularization |
68% (25) | 51% (20) | 75% (18) | .097 |
| Prior CABG | 22% (8) | 33% (13) | 29%(7) | .558 |
| Total Cholesterol (mg/dl) |
152.8 (35.1) | 165.8 (49.0) | 158.6 (27.9) | .602 |
| LDL-cholesterol (mg/dl) |
83.5 (30.5) | 92.9 (39.7) | 82.1 (15.1) | .743 |
| HDL-cholesterol (mg/dl) |
44.0 (11.3) | 42.6 (10.1) | 46.6 (12.8) | .620 |
| Serum Triglycerides (mg/dl) |
126.4 (62.5) | 144.2 (80.9) | 157.0 (140.0) | .559 |
| Baseline HAM-D | 13.4 (5.9) | 14.3 (5.8) | 14.5 (5.8) | .764 |
| BDI at Baseline | 18.5 (7.0) | 17.7 (9.3) | 17.5 (9.5) | .550 |
| DSM Diagnosis of Major Depression |
46% (17) | 50% (20) | 42% (10) | .549 |
| Systolic Blood Pressure (mmHg) |
130 (22) | 132 (20) | 126 (14) | .604 |
| Diastolic Blood Pressure (mmHg) |
80 (11) | 72 (11) | 71 (10) | .670 |
| Peak VO2, ml/kg/minute) |
19.5 (4.8) | 18.5 (6.0) | 20.1 (7.4) | .564 |
| Body Mass Index (kg/m2) |
31.0 (5.7) | 31.5 (4.4) | 30.8 (5.1) | .947 |
Note. Values are % (N) for categorical variables and mean (standard deviation) for continuous variables.
p values provided at the journal’s request are based on Pearson chi-square test for categorical variables and Kruskal-Wallis or general linear model test for continuous variables.
Adherence to exercise and medication
Of the 48 expected exercise sessions , the median number attended was 45 sessions (94%). Pill adherence was defined as the number of pills taken divided by the number prescribed. The median adherence to the pill conditions was 100% (interquartile range [IQR], 97-101%), ranging from 83 to 112%. The median sertraline dose was 50 mg (IQR = 50, 100).
Side effects
Untoward effects of each treatment were examined by patient ratings on a 36-item symptom checklist, which was obtained before and after treatment (e.g., headaches, dizziness, constipation, muscle pain and soreness, etc.). Symptoms were rated on a 5-point Likert scale ranging from 0 (never) to 4 (almost always). There were relatively few patients who reported a worsening of symptoms after treatment. Among the 36 side effects assessed, only two showed a significant group difference: 20% of patients receiving Sertraline reported worse post-treatment fatigue compared to 2.4% in Exercise and 8.8% in Placebo (p = 0.025) and 26% reported increased sexual problems compared to 2.4% in Exercise and 11.8% in Placebo (p = 0.005).
Changes in aerobic fitness
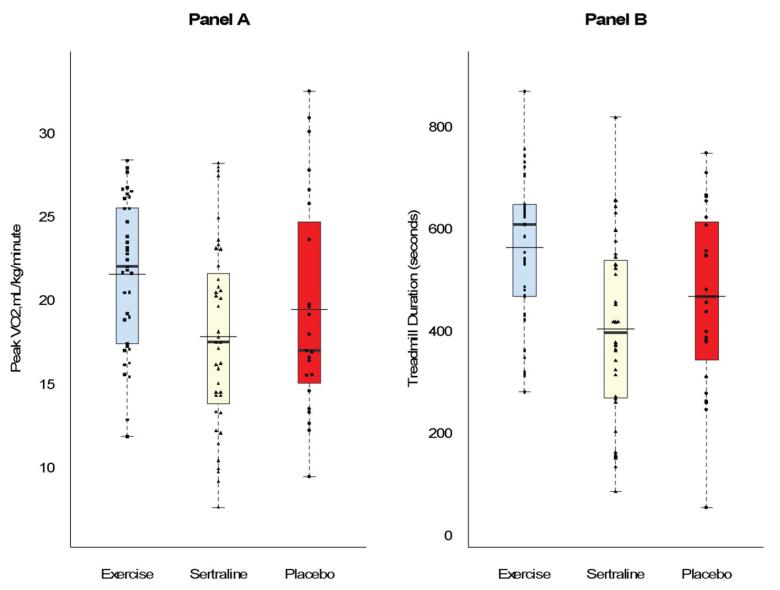
The Exercise group exhibited greater improvements in peak VO2 and exercise endurance compared to the Sertraline and Placebo groups. Adjusting for pretreatment levels, age, gender, and ethnicity, the mean post-treatment peak VO2 was greater in Exercise (21.3 ml/kg/min, 95% CI = 20.3, 22.3) compared to Sertraline (18.5 ml/kg/min, 95% CI = 17.5, 19.4, p < .001) and Placebo (18.5 ml/kg/min, 95% CI = 17.2, 19.8, p = .002) (Figure 2, Panel A). Participants in the Exercise group showed a 9.6% increase in peak VO2, compared to Sertraline (2.3%) and Placebo (2.1%). Similarly, at 16 weeks treadmill test duration was longer in Exercise (8.8 minutes, 95% CI = 8.3, 9.4) compared to Sertraline (7.1 minutes, 95% CI = 6.6, 7.7) (p < .001) and Placebo (7.2 minutes, 95% CI = 6.5, 8.0) (P < .001) (Figure 2, Panel B). The values represented a 19% increase from baseline for Exercise, and 0.5% and 1% decreases for Sertraline and Placebo, respectively. Body weight remained essentially constant throughout the study; there were no group differences in post-treatment BMI.
Figure 2. Aerobic capacity (Panel A) and treadmill time (Panel B) at 16 weeks, adjusted for age, gender, ethnicity, and baseline scores of the outcome.
Contrasts were planned and non-orthogonal: Exercise vs. Sertraline and Exercise vs. Placebo, with Bonferroni correction. Thicker horizontal line represents median; thinner, wider line represents mean. Box represents 25th and 75th percentiles. Following treatment, Exercise was associated with higher levels of aerobic capacity and longer treadmill time compared to either Sertraline or Placebo (P’s < .001).
Changes in Depression
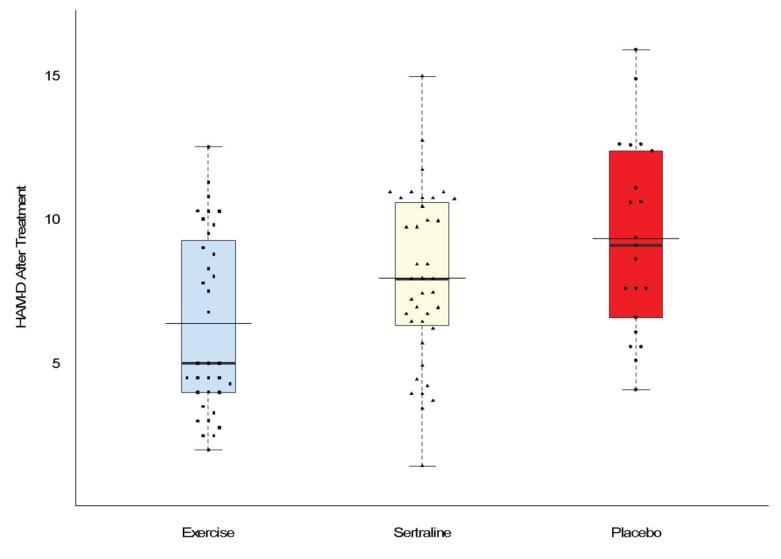
After 16 weeks, all groups showed improvement on HAM-D scores. The raw change in score from baseline to 16 weeks among participants with complete data was: Exercise, −7.5 (95% CI = −9.8, −5.0); Sertraline, −6.1 (95% = −8.4, −3.9), and Placebo, −4.5 (95% CI = −7.6, −1.5). The combined active treatments showed lower HAM-D scores at 16 weeks compared to Placebo (p = .034) and there was no difference between Exercise and Sertraline (p = .607) (Figure 3). Based on the ITT analysis, the HAM-D scores after 16 weeks in Exercise were 3.3 points lower compared to Placebo, while the average score in Sertraline was 1.7 points lower compared to Placebo.
Figure 3. HAM-D scores adjusted for age, gender, ethnicity, and baseline scores of the outcome.
Thicker horizontal line represents median; thinner, wider line represents mean. Box represents 25th and 75th percentiles. HAM-D scores were lower for the active treatments (Exercise and Sertraline) compared to Placebo, P = .034, but were not different from each other P = .607.
Among those patients who were diagnosed with MDD initially and who were available for follow-up (N=44), we examined rates of remission at 16 weeks, defining remission as the absence of DSM-IV criteria for MDD and a HAM-D score < 8 (34). Among the Exercise group, 40% (6/15) were remitted, compared to 10% (2/20) in the Sertraline group, and no participants (0/9) in the Placebo group (chi-square [2 df] = 7.70, p = .021). Among participants who did not meet criteria for MDD at baseline, one participant in Exercise and one participant in Placebo met MDD criteria at 16 weeks. We also examined HAM-D scores among the same subgroup of patients initially diagnosed with MDD. Again, the contrast for active treatments vs. Placebo was significant (p = .042), while the contrast between Exercise and Sertraline was not (p = .579). The mean adjusted HAM-D score at 16 weeks was 8.8 (95% CI = 6.1, 11.6) for Exercise, 9.9 (95% CI = 7.4, 12.3) for Sertraline, and 13.6 (95% CI = 10.0, 17.2) in Placebo. Among this subgroup, the change in HAM-D scores from baseline to 16 weeks was −9.4 (95% CI = −12.4, −6.4) in Exercise, −8.6 (95% CI = −11.1, −6.1) in Sertraline, and −4.5 (95% CI = −8.4, −0.7) in Placebo.
Biomarker Endpoints
All biomarker endpoint data are reported in Table 2. For SDNN, our measure of HRV, the contrast between the active treatments versus Placebo approached significance (p = .052). At the conclusion of treatment, the Exercise group displayed higher 24-hr SDNN compared to the Placebo group. For BRS, the active treatments combined were not different from Placebo (p = .515), but exercise was lower compared to Sertraline (p = .046). For inflammatory biomarkers, patients randomized to exercise had lower levels of IL-6 post-treatment compared to those treated with sertraline (p = .040), but the active treatments combined were not different from Placebo (p = .828). There were no treatment group differences for FMD, CRP, βTG or PF4.
Table 2.
Biomarkers before and after treatment.
| Contrast p-value | |||||
|---|---|---|---|---|---|
|
| |||||
| Biomarker variable | Exercise Only | Sertraline | Placebo | All Active Treatment vs. Placebo |
Exercise vs. Sertraline |
| SDNN (msec)a | |||||
| Before | 116 | 120 | 123 | ||
| After | 122 | 118 | 112 | .052 | .093 |
| Change | +6 | −2 | −11 | ||
| BRS (msec/mmHg) b | |||||
| Before | 4.8 | 4.7 | 4.9 | ||
| After | 4.0 | 5.1 | 4.1 | .515 | .046 |
| Change | −0.8 | +0.4 | −0.7 | ||
| FMD (%) | |||||
| Before | 2.8 | 2.2 | 3.3 | ||
| After | 3.0 | 4.1 | 3.3 | .727 | .131 |
| Change | +0.2 | +0.9 | 0 | ||
| CRP (μg/ml) | |||||
| Before | 2.5 | 2.5 | 2.4 | ||
| After | 2.1 | 2.5 | 2.5 | .567 | .343 |
| Change | −0.4 | 0 | +0.1 | ||
| IL6 (pg/ml) | |||||
| Before | 1.98 | 1.7 | 1.95 | ||
| After | 1.80 | 2.1 | 1.85 | .828 | .040 |
| Change | −0.10 | +0.4 | −0.10 | ||
| PF4 (IU/ml) | |||||
| Before | 44.1 | 34.1 | 32.0 | ||
| After | 35.5 | 34.0 | 34.4 | .995 | .712 |
| Change | −8.6 | −0.1 | +2.4 | ||
| Beta-TG | |||||
| Before | 137.0 | 102.5 | 103.1 | ||
| After | 134.3 | 129.0 | 125.0 | .830 | .968 |
| Change | −2.7 | +16.5 | +22.1 | ||
Values in rows labeled before are pre-treatment means. Values in rows labeled after are the adjusted post-treatment means. With the exception of FMD, analyses were performed on imputed rank-transformed values, adjusted for baseline level of corresponding outcome, age, gender, and race. Pre- and post treatment means in the table were estimated from the ranks, using the nearest value in the original metric that corresponded to the rank. Pre-treatment values are based on the raw ranks, while post-treatment means were estimated from the adjusted post-treatment ranks. Analysis of treatment effect on FMD was performed on imputed values in original metric, adjusted for baseline level of FMD, baseline arterial diameter, age, gender, and race.
Analysis based on 93 participants with valid baseline measurements.
Analysis based on 92 participants with valid baseline measurements.
DISCUSSION
The results of the UPBEAT trial confirm and extend evidence for the value of aerobic exercise to treat depression. Participants with CHD and elevated depressive symptoms who were prescribed aerobic exercise 3 times per week for 30-45 minutes per session achieved significantly greater reductions in depressive symptoms compared to placebo controls; the reduction in depressive symptoms associated with exercise was comparable to that observed in patients receiving antidepressant medication. Furthermore, exercise was even more effective in reducing clinical depression in the subset of patients who were diagnosed with MDD at study entry. Although the sample was small, 40% of patients with MDD at the time of randomization were remitted at the end of 16 weeks of exercise, compared to 10% of those who received sertraline and none of the patients who received placebo. These data add to the growing literature suggesting that exercise may be a viable alternative to traditional psychopharmacologic treatments of depression.
In an initial study from our group, exercise was shown to be as effective as antidepressant medication in non-cardiac patients with MDD (21). Because there was no control condition, however, the benefits could not be attributed to exercise. In a subsequent study (22), both group exercise and home-based exercise proved comparable to antidepressant medication, and were superior to placebo controls in non-CHD patients diagnosed with MDD. The present findings extend these prior reports by demonstrating that exercise reduces depressive symptoms in patients with stable CHD. These results may be particularly important in light of the growing evidence that depression is associated with increased risk of fatal and non-fatal events in a wide range of CHD populations (3,4,7,9).
To date, there is limited evidence that traditional treatments are effective for depressed CHD patients. The SADHART trial reported that sertraline was no better than placebo in reducing depression in the entire sample of patients with acute coronary syndrome, but was more effective compared to placebo in reducing depressive symptoms in the subgroup of patients with more severe MDD (14). The subsequent SADHART-HF trial showed that sertraline performed no better than placebo in patients with heart failure after 12 weeks of treatment, and did not improve clinical outcomes (15). The CREATE trial showed that citalopram was associated with greater reductions in depressive symptoms compared to placebo in CHD patients, but participants also received concomitant counseling from nurse clinicians, and such counseling was found to be more effective than interpersonal psychotherapy (16). In the ENRICHD trial (18), post-MI patients who received cognitive behavior therapy exhibited greater reductions in HAM-D scores compared to health education controls, although there were no treatment group differences in clinical outcomes. Interestingly, secondary analyses of the ENRICHD trial found that exercise (36) and antidepressant medications (37) were associated with reduced mortality and non-fatal MI. However, because the patients were not randomly assigned to exercise or medication, it was unclear whether either treatment was responsible for the observed effects. Milani and colleagues (38) reported that exercise reduced depression in CHD patients, but because participants received concurrent treatments and were not randomized, reduced depressive symptoms could not be attributed to exercise. To our knowledge, UPBEAT is the first RCT to show that exercise improves depression in CHD patients with MDD or subclinical depressive symptoms.
In addition to reduced depression, the two active treatments, especially exercise, resulted in increased HRV in study participants compared to placebo controls. These findings are consistent with studies of exercise training in healthy adults (39) and in patients with CHD (40). Prior studies evaluating antidepressant medications have found either no effect or possible unfavorable effects on HRV (14,41).
No improvements were observed in BRS in the treatment groups compared to placebo controls, although sertraline resulted in improved BRS compared to exercise. We also found no evidence for improvements in measures of chronic inflammation in the active treatments compared to placebo, although exercise resulted in greater improvements in IL-6 compared to sertraline. Previously we found that vascular endothelial function was improved by exercise (42), but no such benefit was observed in the present study. In SADHART, depressed CHD patients treated with sertraline had lower levels of βTG compared to those treated with placebo, although there was no difference in PF4. We found no effect of drug therapy on either of these markers of platelet activation.
Although there were few side effects associated with treatment, sertraline was associated with more fatigue and greater sexual dysfunction relative to placebo and exercise. Sexual dysfunction is a common symptom of depression (43) and may be a common side effect of SSRI’s (44), while aerobic exercise has been associated with improved sexual function among men with erectile dysfunction (45) and with better overall sexual function compared to sertraline or placebo pill (46).
Limitations
Our relatively small sample size likely limited our ability to detect small treatment effects for the biomarkers. Several issues may affect the generalizability of our findings. The extent to which UPBEAT patient volunteers are representative of the general population of depressed CHD patients is uncertain. Participants had to be willing to accept the condition to which they were randomly assigned, and patients who were not interested in exercise or taking an antidepressant were unlikely to have volunteered. We also note that our sample was highly educated, with three quarters of the sample attending at least some college, which may help to explain the high adherence rates observed in our trial.
Another limitation is that we did not examine the optimal dose of exercise necessary to improve depression. All patients received an exercise prescription of approximately 90 min of exercise per week (i.e., 30 min, 3 times/week). Patients were also on relatively low doses of sertraline, with only 30% of patients receiving >100 mg of sertraline by the end of the trial. Higher doses may have produced more favorable effects on the biomarkers, but were difficult to justify in participants who did not meet criteria for MDD. Because exercise training was supervised group exercise, it was confounded with social support, which may have augmented the beneficial effects of exercise. However, our previous work showed that unsupervised, home-based exercise produced similar reductions in depressive symptoms compared to supervised exercise (22), and the DOSE study established that exercise was effective in improving depression independent of social support (47).
In summary, the present findings from UPBEAT indicate that depression can be improved in cardiac patients. Because of the paucity of evidence that depression can be successfully treated, some have questioned the merits of assessing depression in CHD patients (10). We believe that the evidence documenting the association of depression with poor prognosis and the present findings that depressive symptoms can be reduced, support the recommendation that routine monitoring of depressive symptoms in CHD patients should be performed. While improvements in biomarkers do not necessarily translate into improved clinical outcomes (48), our finding that HRV may be improved in depressed cardiac patients is encouraging. Although further research with larger samples is warranted, these findings provide preliminary evidence that cardiac patients with elevated depressive symptoms are likely to benefit from the antidepressant effects of exercise in addition to the well-documented cardiopulmonary benefits.
ACKNOWLEDGEMENTS
This study was supported by Grant HL080664-01A1 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, and grant M01-RR-30 from the General Clinical Research Center program, National Center for Research Resources, National Institutes of Health. Medication and matched placebo pills were provided by a grant from Pfizer Pharmaceuticals, Inc. Thanks also are extended to Dr. William Kraus and the staff at the Duke Center for Living, Durham, NC; Dr. Paula Miller and the staff at the UNC Wellness Center at Meadowmont, Chapel Hill, NC; Dr. William Sessions and the staff at the Maria Parham Medical Center, Henderson, NC; Dr. Dwayne Callwood at HeartTrack-Alamance Regional Medical Center, Burlington, NC; and Drs. Lawrence Liao and Stephen Robinson at Duke Raleigh Hospital Cardiac Rehabilitation for their participation in the UPBEAT trial.
Financial Support: This study was supported by Grant HL080664 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, and grant M01-RR-30 from the General Clinical Research Center program, National Center for Research Resources, National Institutes of Health.
ABBREVIATIONS
- βTG
beta thromboglobulin
- BRS
baroreflex sensitivity
- BDI-II
Beck Depression Inventory-II
- CRP
C-reactive protein
- FMD
flow mediated dilation
- HAM-D
Hamilton Depression Rating Scale
- HRV
heart rate variability
- Interleuken 6
(IL-6)
- MDD
Major Depressive Disorder
- PF 4
Platelet factor 4
- SDNN
Standard deviation of the normal-to-normal R-R intervals
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 2.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53:897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 3.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:304–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 4.Lichtman JH, Bigger JT, Jr., Blumenthal JA, et al. AHA science advisory. Depression and coronary heart disease. Recommendations for screening, referral, and treatment. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 5.Thombs BD, Bass EB, Ford DE, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21:30–8. doi: 10.1111/j.1525-1497.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesperance F, Frasure-Smith N, Talajic M, et al. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105(9):1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 7.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–41. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 8.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 9.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 10.Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol. 2009;54:886–90. doi: 10.1016/j.jacc.2009.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumenthal JA, O’Connor C. No laughing matter. J Am Coll Cardiol. 2010;55:836–7. doi: 10.1016/j.jacc.2009.09.048. [DOI] [PubMed] [Google Scholar]
- 12.Depression Guideline Panel . Depression in Primary Care. No. 5. Volume 2. Treatment of Depression, Clinical Practice Guideline. U.S.Department of Health and Human Services,Public Health Service,Agency for Health Care Policy and Research,HHCPR; Rockville, MD: 1993. [Google Scholar]
- 13.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–9. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–9. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesperance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367–79. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 17.van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–6. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 18.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 19.Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev. 2009:CD004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- 20.Freemont J, Craighead LW. Aerobic exercise and cognitive therapy in the treatment of dysphoric moods. Cog Ther Res. 1987;11:241. [Google Scholar]
- 21.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–56. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–96. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–4. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- 24.Watkins LL, Grossman P. Association of depressive symptoms with reduced baroreflex cardiac control in coronary artery disease. Am Heart J. 1999;137:453–457. doi: 10.1016/s0002-8703(99)70491-6. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood A, Blumenthal JA, Hinderliter A. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. JACC. 2005;46:456–459. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 26.Kop WJ, Gottdiener JS, Tangen CM, et al. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–424. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 27.Whooley MA, de Jong P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. The Psychological Corporation; San Antonio,TX: 1996. [Google Scholar]
- 29.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 30.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–7. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 31.Blumenthal JA, Rejeski WJ, Walsh-Riddle M, et al. Comparison of high- and low-intensity exercise training early after acute myocardial infarction. Am J Cardiol. 1988;61:26–30. doi: 10.1016/0002-9149(88)91298-2. [DOI] [PubMed] [Google Scholar]
- 32.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 33.Task Force on DSM-IV Diagnostic and statistical manual of mental disorders (DSM-IV-TR) American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- 34.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 35.Conover WJI. R.L. Rank transformation as a bridge between parametric and nonparametric statistics. Am Statistician. 1981;35:124. [Google Scholar]
- 36.Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD trial. Med Sci Sports Exerc. 2004;36:746–755. doi: 10.1249/01.mss.0000125997.63493.13. [DOI] [PubMed] [Google Scholar]
- 37.Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792–8. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 38.Milani RV, Lavie CJ, Cassidy MM. Effects of cardiac rehabilitation and exercise training programs on depression in patients after major coronary events. Am Heart J. 1996;132:726–32. doi: 10.1016/s0002-8703(96)90304-x. [DOI] [PubMed] [Google Scholar]
- 39.Levy WC, Cerqueira MD, Harp GD, et al. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol. 1998;82:1236–41. doi: 10.1016/s0002-9149(98)00611-0. [DOI] [PubMed] [Google Scholar]
- 40.Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: A randomized, controlled study. Circulation. 2000;102:2588–92. doi: 10.1161/01.cir.102.21.2588. [DOI] [PubMed] [Google Scholar]
- 41.van Zyl LT, Hasegawa T, Nagata K. Effects of antidepressant treatment on heart rate variability in major depression: a quantitative review. Biopsychosoc Med. 2008;2:12. doi: 10.1186/1751-0759-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: A randomized controlled trial. JAMA. 2005;293:1626–1634. doi: 10.1001/jama.293.13.1626. [DOI] [PubMed] [Google Scholar]
- 43.Clayton A, Keller A, McGarvey EL. Burden of phase-specific sexual dysfunction with SSRIs. J Affective Dis. 2006;91:27–32. doi: 10.1016/j.jad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Bonierbale M, Lancon C, Tignol J. The ELIXIR study: evaluation of sexual dysfunction in 4557 depressed patients in France. Current Medical Research andOpinion. 2003;19:114–124. doi: 10.1185/030079902125001461. [DOI] [PubMed] [Google Scholar]
- 45.Esposito K, Giugliano F, Di PC, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman MB, Babyak MA, Sherwood A, et al. Effects of aerobic exercise on sexual functioning in depressed adults. Ment Hlth Phys Act. 2009;2:23–25. [Google Scholar]
- 47.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 48.DeMets DL, Califf RM. Lessons learned from recent cardiovascular clinical trials: Part I. Circulation. 2002;106:746–751. doi: 10.1161/01.cir.0000023219.51483.66. [DOI] [PubMed] [Google Scholar]