The photoacoustic or optoacoustic effect has seen two major renaissances since its discovery by Bell.[1] The first transformation was facilitated by lasers and piezoelectric transducers to create an ultra-sensitive spectroscopy technique to characterize the absorptive properties of gaseous, liquid, and solid materials in the form of bulk, powders and surface films.[2–3] It has also been very helpful in characterizing photo-induced chemical reactions,[4] and in chemical, thermal, and structural sensing,[5] or in the characterization of two-photon absorption cross sections.[6] Theoretical descriptions of various experimental situations were developed by a number of authors,[7–10] and summarized for instance in.[11] More recently, a second renewed interest has developed motivated by biomedical imaging and image-guided therapy,[12] although photoacoustic techniques have been used in the life sciences earlier.[13] This renaissance is based on the development of tomographic techniques,[14–19] and molecular sensing strategies using highly absorbing nanostructures for biomedical imaging.[20–24] The strength of the photoacoustic technique for scattering or non-radiating samples lies in the fact that it is not light that is detected but sound, which has a much longer wavelength. Although nanoparticle based photoacoustics is not fundamentally different from the many forms of photoacoustics developed so far, the small volume of nanoparticles introduces new phenomena that reflect the dominance of surface effects in these systems. The goal of this paper is to show that in many situations the photoacoustic response is not from the nano-sized absorber but the very low-absorbing surrounding material and therefore photoacoustic imaging can provide information about the nanoparticle surface, and the immediate environment surrounding the particle, which is of high importance to molecular imaging. The information that can be gained includes the interfacial resistance,[25] as well as the thermodynamic characteristic of the environment.
Nanoparticle-augmented photoacoustic imaging is a non-invasive diagnostic imaging modality capable of assessing tissue properties with good spatial resolution and a sufficient depth that is hard to achieve with common optical imaging techniques.[19, 17, 12] Photoacoustics uses a broadband ultrasound transducer to visualize biological tissue by measuring the photoacoustic transient – an ultrasound wave produced by localized thermal expansion of tissue absorbing the pulsed electromagnetic radiation. Using the duration of the radiation pulses shorter than the time needed for the generated heat and stress to propagate through the radiated area, the amplitude of the photoacoustic signal in optically homogeneous materials is determined by the optical absorption and thermal properties of the material, as well as the fluence of the impinging radiation.[26] Therefore, the contrast in the photoacoustic image of media, which are homogeneous on a length scale much larger than the thermal diffusion length, is mainly attributed to the optical absorption of the materials.
The introduction of plasmonic nanoparticles as photoacoustic contrast agents and their enhanced optical absorption due to surface plasmon polaritons brought molecular sensitivity to photoacoustic imaging.[27, 20–21] Small size, ease of surface functionalization and the ability to tune the peak absorption wavelength away from tissue absorption allows nanoparticles to molecularly target imaged tissue.[20, 22, 27–29] However, with the presence of nanoparticles, the medium containing the nano-sized strong optical absorbers in a weakly absorbing tissue is no longer optically homogeneous – the medium is heterogeneous on the scale of the thermal diffusion length of the tissue. Unlike in systems that are homogeneous or where the heterogeneities are much larger than the thermal diffusion length and therefore the photoacoustic signal is produced directly by the illuminated absorber,[30–31] the origin of the photoacoustic signal in the case of plasmonic nanoparticles is expected to depend on the heat transfer from the nanoparticle to the environment, and has not been explored in detail so far. In this study, we estimate the relative contribution from a nanoparticle and its liquid environment based on continuums mechanics, and show experimentally with gold nanospheres that the photoacoustic signal has the thermal signature of the colloidal solvent. Additionally, silica coating the gold nanospheres is shown to modulate the signal by influencing the heat transfer from gold particle to liquid.
For an optically heterogeneous medium the photoacoustic signal results in general from both the particles and the surrounding medium. Their relative magnitudes depend on the size of the absorber and the size of the heated region in the surrounding, as well as the temporal profile of the heating in the absorber and the surrounding. In order to show that the photoacoustic signal for a plasmonic nanoparticle in a liquid environment with good thermal conductivity is generated mostly from the surrounding, we consider a metallic sphere of radius a heated by light absorption to have the temperature Ts(t) (Figure 1). We use a continuums approach and make the following assumptions for our estimation: (1) the problem has spherical symmetry, so that transversal wave components can be neglected; (2) the sphere is small enough and the absorption time scales are long so that the sphere can be considered to be heated homogeneously, an assumption made frequently and justified by the very high thermal conductivity of the gold and other noble metals we are interested in;[29] (3) the temperature profile in the surrounding at time t and position r from the center of the sphere can be written as , with a characteristic decay length of r0. We then solve the equation of motion in each material in spherical coordinates (r, θ, φ):
| (1) |
for the radial displacement ur and the diagonal stress tensor with components σii = −KβT + K (εrr + 2ε θθ) + 2 μ[εii − (εrr + 2ε θθ)] /3; here β is the volumetric expansion coefficient, K and μ are the compressive and shear modulus, respectively, and ε is the strain tensor. With the longitudinal speed of sound and ρ the density, equation (1) then reduces to:
| (2) |
Figure 1.
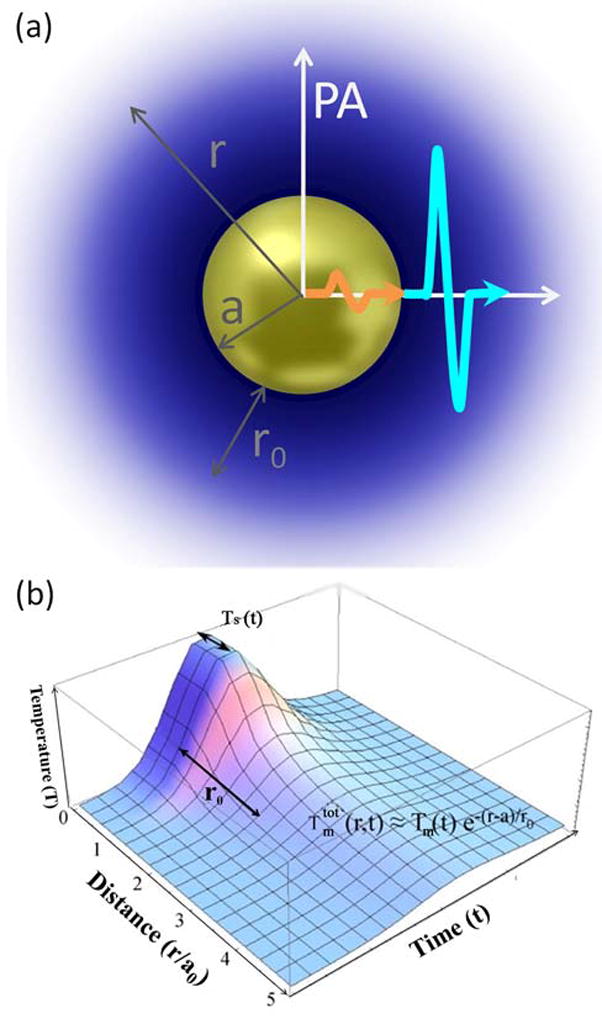
(a) Schematic representation of the photoacoustic response generated from a sphere (gold) and the surrounding liquid (blue). (b) Generalized temperature profile without interfacial resistance as a function of time and distance, summarizing the major assumptions made for the analytical pressure amplitude estimation.
Introducing the potential ϕ so that ∂ur /∂t = ∇ ϕ and , gives the photoacoustic wave equation that has to be solved in both media:
| (3) |
Boundary conditions at the interface between the sphere and surrounding fluid are that the radial stresses and displacements are continuous.[11] Additionally, the displacement at the sphere center needs to be finite (and zero), and no incoming wave (traveling from infinity) exists for the surrounding. With ψ = rϕ, the Laplace operator can be reduced to a simple second derivative in equation (3) and it can be solved in (temporal) Fourier space with F(r,ω) = F(ψ), Gs(ω) = F(Ts(t)), and Gm(ω) = F(Tm(t)), and F denotes the Fourier transform of the potential (ψ ), temperature in the particle (Ts), and temperature in the environment (Tm), respectively. The solution is given in the supplement. Since the pressure is measured in the medium far from the nanoparticle, the contributions from the solid sphere and the liquid environment can be separated by setting the liquid and the metal volume expansion coefficients, respectively, to zero. The ratio of the two pressure contributions is then approximately:
| (4) |
Here and are the pressure contribution at frequency ω from the sphere (s) and the medium (m) measured far away from the nanoparticle in the medium, and rn = r0/a. If no interfacial heat resistance exists between the nanosphere and the fluid, then spectral temperatures at the interface are equal and Gs(ω) = Gm(ω). If there is interfacial heat resistance at the interface, then Gs(ω) > Gm(ω), and the temperature undergoes a jump at the interface.
In order to emphasize the importance of the heat transfer from the solid sphere to the fluid environment a Gaussian temperature profile of width δ for both the sphere and the medium, such as T(t) = (2π)−1/2 δ−1 exp(−t2/2δ2) can be assumed. The ratio of the contributions to the pressure measured some distance away from the source is then
| (5) |
From equation (5), it is clear that the spatial decay of the temperature profile, the temporal broadening due to a finite heat transfer through the interface, and the characteristics of the interface influence the balance of the two contributions. It should be pointed out that equation (5) assumes a temporal temperature profile in the sphere; for the situation of constant fluence, however, the temperature in the sphere is higher with large interfacial resistance than with lower resistance. This effect is included in the Gaussian profile to the order of approximation used here. For a gold sphere in water and rn ≈ 1, little broadening of a nanosecond laser pulse δm ≈ δs, and the range of frequencies used in photoacoustic imaging with ultrasound transducers (f = 2πω = 1 – 50 MHz), so that and βs /βm ≈ 0.2 at 20° C in aqueous solvent, and equation (5) predicts the pressure ratio between the sphere and the water medium of about one percent. Therefore, the photoacoustic signal is generated mostly in the fluid surrounding the spherical particle.
Water is ideally suited to study the origin of the photoacoustic signal from a nanoparticle colloid because the thermal expansion of water at atmospheric pressure vanishes at 3.98 °C, and therefore any photoacoustic signal generated by the heating of water at this temperature vanishes, while sound generated by the sphere will still be transported.[32] Experiments were performed with polyethylene glycol stabilized gold spheres, and silica coated gold spheres with silica thickness of 6, 18 and 38 nm (Figure 2a, and Figure 2b) using a flow capillary suspended in a thermostated bath, 5 ns pulses from a Nd:YAG pumped OPO laser, and imaging with a single element focused 7.5 MHz ultrasound transducer (Figure 2c and Figure 2d). The PEGylated gold nanospheres were produced from citrate stabilized gold nanospheres by exchanging citrate with the biocompatible mPEG-thiol, and silica coated gold nanospheres were prepared by coating silica directly on PEGylated gold nanospheres via a modified Stöber method.[33–34, 25] The peak optical density of each sample was adjusted to 1.5 to achieve equal absorption, and the laser was tuned to the peak wavelength of each sample. We verified with Mie theory that at the extinction peak the scattering contribution to the extinction cross-section due to the addition of a silica shell was well below 10% for silica shells between 0 nm and 40 nm thickness (see supplement for the experimental extinction spectra of gold nanosphere solutions). A laser fluence of 5 mJ/cm2 was chosen to prevent bubble formation in organic solvents, and to avoid damage to the nanoparticles. We have not found that the fluence used here causes any damage to the bare or coated nanospheres, and the PA signal remained stable over at least 128 laser pulses.
Figure 2.
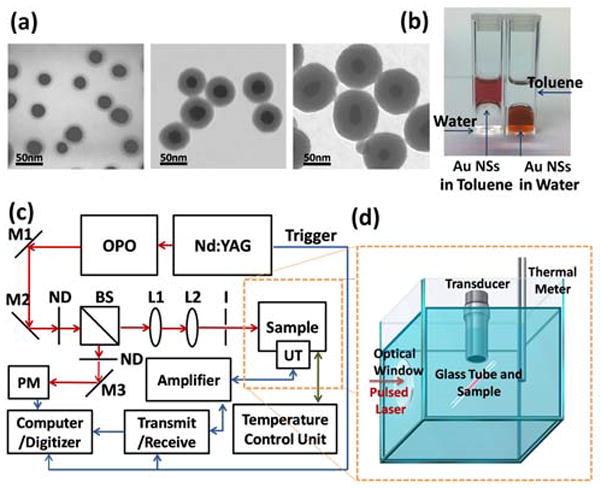
(a) Transmission electron microscopy images of silica coated gold nanospheres (core radius: 13 ± 3 nm) with 6 ± 0.8 nm, 20 ± 2.8 nm, 38 ± 3.9 nm of silica shell. (b) Photographic image of organic solvent soluble gold nanospheres (left) and of aqueous soluble gold nanospheres (right). (c) Schematic illustration of combined photoacoustic/ultrasound system used in this study, where M1–3 represent mirror 1 to 3; L1–2 are lens 1–2; ND represent neutral density filters; BS represent beam splitter; PM represent power meter, and UT represent ultrasound transducer. (d) ) Close-up schematic illustration of the sample irradiated by a pulsed laser beam while photoacoustic transients were measured using the ultrasound transducer.
Figure 3a shows that the photoacoustic signal of PEGylated and silica-coated gold nanospheres in aqueous solution indeed falls below noise level around 4 °1C. The photoacoustic signal increases nearly linearly with temperature with a slope that depends on the silica thickness. The slope is lowest for the PEGylated gold nanospheres and is maximal for 18 nm silica. An approximately linear dependence of the photoacoustic signal with temperature owing to the raise of the Grüneisen parameter of water has been seen for gold nanospheres in water above 25 °C,[35] and for homogeneous aqueous absorbers without nanoparticles, where the photoacoustic signal also disappeared at 4 °C.[32] The photoacoustic signal therefore originates mostly from the surrounding water, and the signal from the absorber is below the noise level even if the size of the nanoparticle is increased 2-fold by the silica coating. The relative contributions of the nanoparticle, including shell, and the water can be estimated by fitting the measured photoacoustic signal to the equation Ptotout = PNP+bΓH2O(T), assuming that the nanoparticle contribution PNP, including the PEG-thiol capping and binding layer, is constant, and the Grüneisen parameter’s temperature dependence Γ(T) is dominated by that of the expansion coefficient, as demonstrated in Figure 3d. The proportionality factor b is a function of shell thickness, and the nanoparticle contribution to the signal PNP, which is below noise level for the fluence used here, also varies linearly with thickness (see Figure S1 in the supplement). The good fit to the thermal expansion of water, the magnitude of the nanoparticle contribution, and its linear dependence with thickness all confirm the trends of the theoretical estimate for a simple sphere with and without a shell (see supplement).
Figure 3.
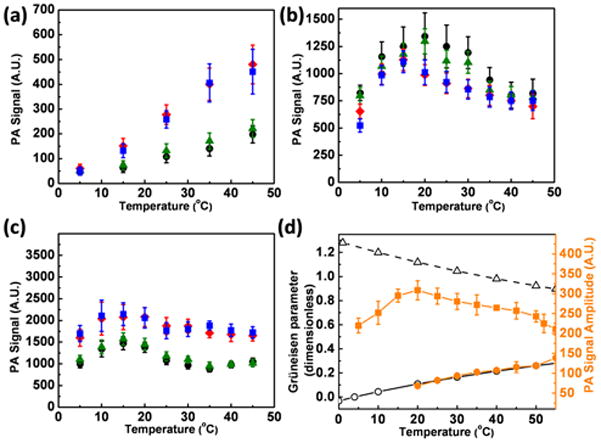
Photoacoustic signal amplitude of gold nanospheres with varying silica shell thickness as a function of temperature in different environments: (a) water (b) silicon oil (c) toluene. The silica thickness is: 0 nm (circle), 6 nm (triangle), 18 nm (square), and 38 nm (rhombus). (d) Temperature dependence of the Gruneisen parameter (left axis) for water (open black circle) and toluene (open black triangle), and of the measured photoacoustic signal (right axis) of water (solid orange circle) and silicon oil (solid orange square). The error bar represents the standard error of 16 signals of eight pulses each.
The increase in slope reflects the increase in amplitude of the photoacoustic signal at 20 °C, which we have recently reported for the silica-coated nanorods.[36] The amplitude of the photoacoustic signal is determined by the Grüneisen parameter of the solvent, as well as the details of the profile of heat transferred from the nanoparticles to the liquid, which depends on the optical absorption of the nanoparticles, the laser fluence, and the interfacial heat conductivities between the two materials. We had concluded there that the signal increase due to silica coating was mainly due to changes in the interfacial heat resistance between the gold and water, and an enhanced efficiency of the heat transfer between the solid and water due to the coating, since all other parameters were approximately constant.
The effect of the heat transfer between nanoparticle and solvent on the photoacoustic signal was further investigated by replacing the aqueous solvent with two non-polar solvents with different thermal properties: polydimethylsiloxane (silicon oil) and toluene. The surface functionalization of the bare and silica-coated nanoparticles was changed to be able to suspend them in non-aqueous solvent. As shown in Figure 2b, the citrate of the citrate-stabilized nanospheres was replaced by octadecane-1-thiol, and the silica surface was functionalized with trimethoxy(octadecyl)silane by co-polymerization (see supplement for details).[37]
Silicon oil (Figure 3b) shows three different features of the photoacoustic signal compared to water: a non-zero signal at 4 °C, a bi-phasic and mostly opposite slope of the temperature dependence, and a decrease of the signal amplitude with silicon coating thickness. The signal follows the bi-phasic profile of the Grüneisen parameter of pure silicon oil, measured using the intrinsic absorption of the oil without nanoparticles (Figure 3d), signaling that the oil is the major signal-generating medium despite the differences to water. However, the temperature dependence is more complex, as the signal is not proportional to the measured Grüneisen coefficient of oil, and there is a leveling off at high temperatures. One possible reason may be residues of water in the silica shell left from the solvent exchange. The fact that silica coating the nanospheres reduces the photoacoustic signal in the oil over most of the temperature range is in agreement with our recent findings at 20 °C.[36]
Toluene is known to have a very high interfacial resistance with citrate coated gold nanospheres and could therefore be a good test of the role that thermal interfacial transfer has on the balance of the contributions from gold and fluid to the signal. Figure 3c shows the photoacoustic signal as a function of temperature in toluene. Between 15 and 45 °C the signal follows the trend of the temperature dependent Grüneisen parameter of toluene (Figure 3d) with a slight negative slope. A small signal amplification due to the addition of a silica shell can be seen, which differs from our finding for nanorods at 25°C.[25] This difference may come in part from changes in the absorption cross section of gold spheres when a silica shell is added, which are not present for the longitudinal resonance of nanorods.
In conclusion, the photoacoustic signal of a solution of gold nanospheres in different solvents shows that the dominant photoacoustic signal is produced by its surrounding medium and hence carries the information of the thermal properties of the surrounding medium. This is despite the fact that the heat is generated by the absorption of light in the nanoparticle and therefore carries also the signature of the absorptivity of the gold nanoparticle and its interface to the solvent by determining the amount and time-dependence of the heat provided to the solvent. Photoacoustics can therefore be a new tool for investigating the thermal properties and transport through nanoparticle interfaces.
Experimental Section
Chemicals
gold(III) chloride hydrate (HAuCl4(aq), Aldrich), Trisodium citrate (Aldrich), O-(2-mercaptoethyl)-O′-methyl-hexa(ethylene glycol) (mPEG-thiol, MW. 5,000, Laysan Bio), ammonia (Fisher Scientific), 2-propanol (Fisher Scientific), and tetraethyl orthosilicate (TEOS, Aldrich), octadecyltrimethoxysilane (OTMS, Aldrich), silicon oil (Fisher Scientific), Toluene (Aldrich), Tetrahydrofuran (THF, Sigma-Aldrich). All chemcials were used as recieved.
Synthesis
The PEGylated gold nanospheres were produced from citrate stabilized gold nanospheres by exchanging citrate with the biocompatible mPEG-thiol, and silica coated gold nanospheres were prepared by coating silica directly on PEGylated gold nanospheres via a modified Stöber method, as described earlier.[38–39, 34] Briefly, 95 mL of HAuCl4(aq) solution (0.27 mM) solution was heated to boiling temperature, and 5 mL of trisodium citrate solution (34 mM) was added. The transparency of the solution changed to blue in 30 seconds and burgundy red within 90 seconds. The solution then aged for another 1 hours at refluxing before being centrifuged at 30,000 g for 45 min, twice. The collected citrate gold nanospheres were re-dispersed in ultrafiltrated (18 MΩ cm, Thermo Scientific Barnstead Diamond water purification systems), deionized water. The stabilization agent, citrate, on the surface of the gold nanospheres was replaced by mPEG-thiol through ligand exchange. Briefly, the citrate-stabilized gold nanosphere dispersion was added to an equal volume of mPEG-thiol (0.2 mM) aqueous solution under vigorous stirring. The mixture was sonicated for 5 minutes and left to react for 8 hours. Excess mPEG-thiol molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min and the PEGylated gold nanospheres were re-suspended in water. For silica coating, the PEGylated gold nanosphere suspension (1.2 mL) was added under vigorous stirring to 1.8 mL of isopropanol, then an ammonia solution (3.8%) in isopropanol was added slowly under vigorous stirring until the solution reached pH = 11. Finally, 0.04 mL–0.40 mL of a solution of TEOS in isopropanol (100 mM) was added under gentle stirring, depending on the desired shell thickness. The reaction mixture was allowed to react for 2 hours. The above procedure produces silica shells with an adjustable thickness from 6 nm to 18 nm. The 38 nm coating was achieved by extending the reaction time to 4 hours with the highest concentration of TEOS (0.4mL of TEOS).
Hydrophobic nanospheres for non-aqueous solvents were prepared by replacing the citrate of citrate-stabilized nanospheres by octadecane-1-thiol, and the organic soluble silica coated nanospheres was prepared by functionalizing the silica surface with trimethoxy(octadecyl)silane. The detailed procedure of the nanoparticle synthesis has been described earlier.[40–41] Briefly, the citrate-stabilized gold nanosphere dispersion was added to an equal volume of mPEG-thiol (0.1 mM) aqueous solution under vigorous stirring. The mixture was sonicated for 30 seconds and left to react for 1 hour. Excess mPEG-thiol molecules were removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min and the PEGylated gold nanospheres were re-suspended in THF. The PEGylated gold nanospheres in THF were mixed with an equal volume of ethanol solution of dodecanethiol (5 mg/mL). The mixture was sonicated for 30 min at room temperature and then 60 min at 50 °C and then allowed to react overnight at room temperature. The functionalized gold nanospheres were centrifuged at 8,000 g for 20 min, twice, then re-suspended into toluene or into silicon oil. For the hydrophobation of silica coated nanospheres, 300 μL of OTMS in chloroform solution (2.4%) was added dropwise into 3 mL of silica coated gold nanospheres solution (0.78 mM (in ethanol) containing 30 μL of NH4OH (32%) under vigorous stirring. The mixture was allowed to react for 24 hours. Ethanol and excess OTMS was removed by centrifugation filtration (Amicon ultra-15, Millipore) at 3,000 g for 10 min and the hydrophobic silica coated gold nanospheres were re-suspended in organic solutions, such as toluene and silicon oil.
Photoacoustic Measurements and Analysis
A custom-made ultrasound and photoacoustic imaging system was used to test the photoacoustic effect of the nanoparticle solutions. As illustrated in Figure 2d, a 1 mm diameter glass tube was fixed in an acrylate water tank with an optical window inlay. The glass tube contains an inlet and an outlet for injecting the samples into the glass tube without moving the tube during the experiment. At the fluences used here, the photoacoustic signal generated in the glass wall is negligible. The water tank also has an outlet and an inlet which connects to the temperature controlled water circulator. During the experiment, 50 μL of gold nanoparticle solution, each with a peak optical density (O.D.) of 1.5 were injected into the tube through the outlet. The water temperature was set at either 4 °C or 15 °C and then increased stepwise until 45 °C. When the desired temperature was reached, it was stabilized for at least 5 minutes at each temperature to ensure equilibration of the temperature of the colloidal solution. A single element focused ultrasound transducer (Panametrics Inc., V320) with 7.5 MHz center frequency, 50.4 mm focal distance, and 13 mm aperture was mounted on a one dimensional positioning stage. The nanoparticle solution was aligned with the focus of the transducer, and the distance between transducer and glass tube was kept constant during the entire experiment. Seven nanosecond laser pulses produced by a Nd:YAG pumped OPO (Spectra Physics) laser were introduced through the optical window into the water tank and uniformly irradiated the sample. A laser fluence of 5 mJ/cm2 was chosen to prevent bubble formation. The wavelength was matched to the peak absorption of each nanoparticle solution. For each temperature, the photoacoustic signal of each pulse was captured and stored for off-line processing. The amplitude of the recorded photoacoustic signal from each pulse was first compensated by the fluence fluctuation factor (calculated from recorded power meter readings per pulse), then normalized to the maximum photoacoustic signal recorded. 128 pulses were recorded and separated evenly to sixteen sets with eight pulses per set. Each set of signals was averaged and the peak amplitude of the averaged signal of each set was recorded. The mean value and the standard error of sixteen averaged peak amplitudes were calculated. Photoacoustic signals from pure solvents were recorded in the same way as those with nanoparticles, but using a wavelength of 920 nm for silicon oil and 1180 nm for water, and fluences of 30 mJ/cm2, respectively. Measurements for pure toluene failed because no absorption bands were available within the wavelength range of the laser system.
Supplementary Material
Acknowledgments
Partial support from National Institutes of Health under grants CA 149740, EB 008101 and HL 096981 is acknowledged. Authors thank the National Science Foundation (Grant No. 0821312) for funding the Hitachi S-5500 SEM/STEM used in this work.
Footnotes
Supporting Information is available on the WWW under http://www.small-journal.com
Contributor Information
Yun-Sheng Chen, Department of Biomedical Engineering, University of Texas at Austin, 1 University Station C0800, Austin, TX 78712 (USA). Department of Electrical and Computer Engineering, University of Texas at Austin.
Dr. Wolfgang Frey, Department of Biomedical Engineering, University of Texas at Austin, 1 University Station C0800, Austin, TX 78712 (USA)
Dr. Salavat Aglyamov, Department of Biomedical Engineering, University of Texas at Austin, 1 University Station C0800, Austin, TX 78712 (USA)
Prof. Stanislav Emelianov, Email: emelian@mail.utexas.edu, Department of Biomedical Engineering, University of Texas at Austin, 1 University Station C0800, Austin, TX 78712 (USA). Department of Electrical and Computer Engineering, University of Texas at Austin
References
- 1.Bell AG. Am J Sci. 1880;20:305. [Google Scholar]
- 2.Patel CKN, Tam AC. Rev Mod Phys. 1981;53:517. [Google Scholar]
- 3.Rosencwaig A. Annu Rev Biophys Bioeng. 1980;9:31. doi: 10.1146/annurev.bb.09.060180.000335. [DOI] [PubMed] [Google Scholar]
- 4.Braslavsky SE, Heibel GE. Chem Rev. 1992;92:1381. [Google Scholar]
- 5.Tam AC. Rev Mod Phys. 1986;58:381. [Google Scholar]
- 6.Tam AC, Patel CKN. Nature. 1979;280:304. [Google Scholar]
- 7.Rosencwaig A, Gersho A. Science. 1975;190:556. [Google Scholar]
- 8.Rosencwaig A, Gersho A. J Appl Phys. 1976;47:64. [Google Scholar]
- 9.Adams M, Kirkbright G. The Analyst. 1977;102:281. [Google Scholar]
- 10.McDonald FA, Wetsel GC. J Appl Phys. 1978;49:2313. [Google Scholar]
- 11.Gusev VE, Karabutov AA. Laser Optoacoustics. AIP; New York: 1993. [Google Scholar]
- 12.Emelianov SY, Li PC, O’Donnell M. Phys Today. 2009;62:34. doi: 10.1063/1.3141939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cahen D, Bults G, Garty H, Malkin S. J Biochem Bioph Methods. 1980;3:293. doi: 10.1016/0165-022x(80)90010-x. [DOI] [PubMed] [Google Scholar]
- 14.Bowen T. presented at Ultrasonics Symposium; 1981. [Google Scholar]
- 15.Kruger RA, Liu PY. Medical Physics. 1994;21:1179. doi: 10.1118/1.597399. [DOI] [PubMed] [Google Scholar]
- 16.Oraevsky AA, Jacques SL, Esenaliev RO, Tittel FK. OSA Proceedings on Advances in Optical Imaging and Photon Migration. Vol. 21. OSA; Washington: 1994. p. 161. [Google Scholar]
- 17.Wang XD, Pang YJ, Ku G, Xie XY, Stoica G, Wang LHV. Nat Biotechnol. 2003;21:803. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 18.Sethuraman S, Amirian JH, Litovsky SH, Smalling RW, Emelianov SY. Opt Express. 2007;15:16657. doi: 10.1364/oe.15.016657. [DOI] [PubMed] [Google Scholar]
- 19.Wang LV. Nat Photonics. 2009;3:503. doi: 10.1038/nphoton.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallidi S, Larson T, Aaron J, Sokolov K, Emelianov S. Opt Express. 2007;15:6583. doi: 10.1364/oe.15.006583. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Yantsen E, Larson T, Karpiouk AB, Sethuraman S, Su JL, Sokolov K, Emelianov SY. Nano Lett. 2009;9:2212. doi: 10.1021/nl801852e. [DOI] [PubMed] [Google Scholar]
- 22.Homan K, Kim S, Chen YS, Wang B, Mallidi S, Emelianov S. Opt Lett. 2010;35:2663. doi: 10.1364/OL.35.002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C, Favazza C, Wang LHV. Chem Rev. 2010;110:2756. doi: 10.1021/cr900266s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallidi S, Luke GP, Emelianov S. Trends Biotechnol. 2011;29:213. doi: 10.1016/j.tibtech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y-S, Frey W, Kim S, Kruizinga P, Homan K, Emelianov S. Nano Lett. 2011;11:348. doi: 10.1021/nl1042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu MH, Wang LHV. Rev Sci Instrum. 2006;77:041101. [Google Scholar]
- 27.Oraevsky AA, Karabutov AA, Savateeva EV. Proc SPIE. Vol. 4434. SPIE; Bellingham: 2001. p. 60. [Google Scholar]
- 28.Mallidi S, Larson T, Tam J, Joshi PP, Karpiouk A, Sokolov K, Emelianov S. Nano Lett. 2009;9:2825. doi: 10.1021/nl802929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egerev S, Ermilov S, Ovchinnikov O, Fokin A, Guzatov D, Kilmov V, Kanavin A, Oraevsky A. Appl Opt. 2009;48:C38. doi: 10.1364/ao.48.000c38. [DOI] [PubMed] [Google Scholar]
- 30.Diebold GJ, Sun T, Khan MI. Phys Rev Lett. 1991;67:3384. doi: 10.1103/PhysRevLett.67.3384. [DOI] [PubMed] [Google Scholar]
- 31.Khan MI, Diebold GJ. Ultrasonics. 1995;33:265. [Google Scholar]
- 32.Hunter SD, Jones WV, Malbrough DJ, Vanburen AL, Liboff A, Bowen T, Jones JJ, Learned JG, Bradner H, Pfeffer L, March R, Camerini U. J Acoust Soc Am. 1981;69:1557. [Google Scholar]
- 33.Stober W, Fink A, Bohn E. J Colloid Interface Sci. 1968;26:62. [Google Scholar]
- 34.Frens G. Nature-Physical Science. 1973;241:20. [Google Scholar]
- 35.Shah J, Park S, Aglyamov S, Larson T, Ma L, Sokolov K, Johnston K, Milner T, Emelianov SY. J Biomed Opt. 2008;13:034024. doi: 10.1117/1.294036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y-S, Frey W, Kim S, Kruizinga P, Homan K, Emelianov S. Nano Lett. 2010;11:348. doi: 10.1021/nl1042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y-S, Kruizinga PP, Joshia P, Kim S, Homan K, Sokolov K, Frey W, Emelianov S. Proc SPIE. Vol. 7564. SPIE; San Fransisco: 2010. p. 75641Q. [Google Scholar]
- 38.Jana NR, Gearheart L, Murphy CJ. Adv Mater. 2001;13:1389. [Google Scholar]
- 39.Nikoobakht B, El-Sayed MA. Chem Mater. 2003;15:1957. [Google Scholar]
- 40.Pastoriza-Santos I, Perez-Juste J, Liz-Marzan LM. Chem Mater. 2006;18:2465. [Google Scholar]
- 41.Thierry B, Ng J, Krieg T, Griesser HJ. Chem Commun. 2009;13:1724. doi: 10.1039/b820137d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


