Abstract
Uncomplicated urinary tract infections (UTIs) are common, with nearly half of all women experiencing at least one UTI in their lifetime. This high frequency of infection results in huge annual economic costs, decreased workforce productivity and high patient morbidity. At least 80% of these infections are caused by uropathogenic Escherichia coli (UPEC). UPEC can reside side by side with commensal strains in the gastrointestinal tract and gain access to the bladder via colonization of the urethra. Antibiotics represent the current standard treatment for UTI; however, even after treatment, patients frequently suffer from recurrent infection with the same or different strains. In addition, successful long-term treatment has been complicated by a rise in both the number of antibiotic-resistant strains and the prevalence of antibiotic-resistance mechanisms. As a result, preventative approaches to UTI, such as vaccination, have been sought. This review summarizes recent advances in UPEC vaccine development and outlines future directions for the field.
Keywords: ExPEC, extraintestinal pathogenic, immunization, UPEC, uropathogenic, UTI
UTI: a common & costly public health burden
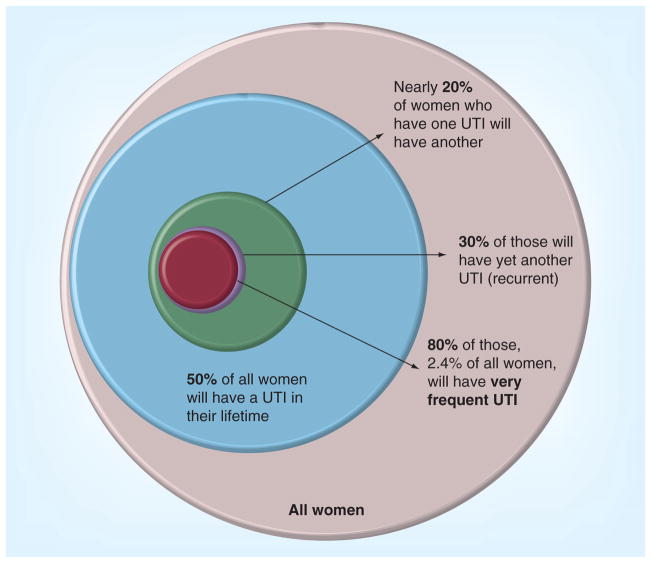
Despite the regular flow of urine, formidable physiological barriers and a robust array of host defenses, the human urinary tract remains one of the most common sites for bacterial infection [1]. Approximately half of all women and 12% of men will experience a urinary tract infection (UTI) in their life-time (Figure 1) [2]. Approximately a quarter of these women will have a recurrent infection within 6–12 months [3]. The high incidence of infection results in 11 million physician office visits and 1.7 million emergency room visits annually in the USA alone [4]. On a population scale, the consequence of frequent UTI amounts to a substantial fiscal public health burden. In 2000, Americans spent a staggering US$3.5 billion treating UTIs [5].
Figure 1. Urinary tract infection among women is extremely common; approximately 13% of women between the ages of 18 and 90 years will have an annual incidence of urinary tract infection.
Based on 2010 US census data, an estimated 15 million women will have a UTI annually in the USA [118]. Percentages are proportional to the area of the circles.
UTI: Urinary tract infection.
The majority of UTIs begin as a bladder infection, clinically termed cystitis, which results from pathogenic bacteria colonizing the perineum, traversing the urethra and successfully infecting the bladder. If cystitis is left untreated, colonizing bacteria can ascend the ureters to cause a secondary infection in the kidneys, acute pyelonephritis, which can result in renal scarring and permanent kidney damage [6]. In severe cases of pyelonephritis, invading bacteria can breach epithelial and endothelial barriers in the kidney to gain access to the bloodstream (bacteremia) leading to systemic infection and sepsis, a serious and sometimes fatal complication [7]. UTIs occurring in individuals with no physical abnormalities of the urinary tract or medical devices that would circumvent natural host defenses, such as a catheter, are classified as ‘uncomplicated’ and are the most common type of UTI [1]. Uncomplicated UTIs are unique among bacterial infections, as they occur most frequently among otherwise healthy women between the ages of 18 and 29 years [1].
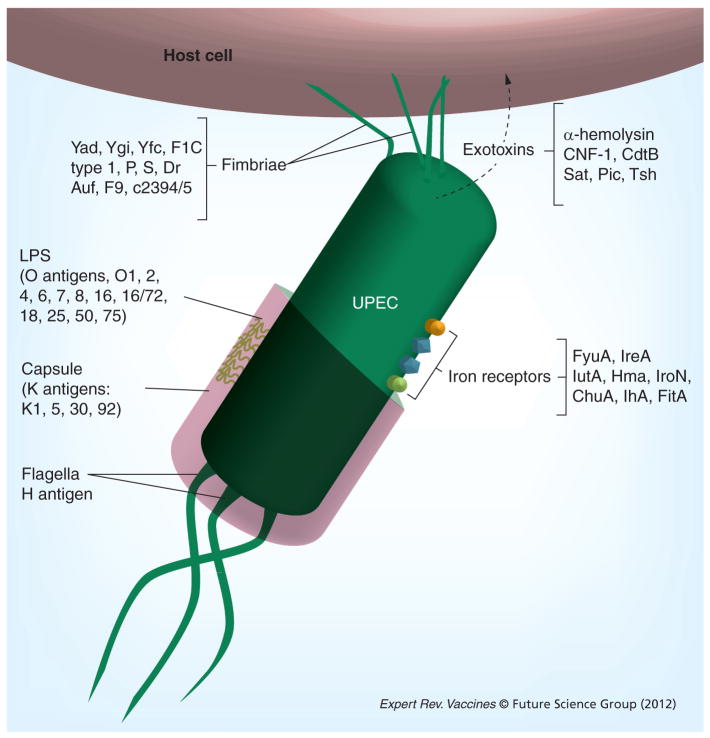
Although there are a number of bacterial genera that infect the human urinary tract, including Proteus, Enterococcus, Klebsiella, and Staphylococcus spp. more than 80% of uncomplicated UTIs are caused by a heterogeneous group of Escherichia coli strains, termed uropathogenic E. coli (UPEC) [8]. Genotypic and phylogenetic analyses provide evidence that UPEC is actually part of a larger group of pathogenic E. coli strains collectively termed extraintestinal pathogenic E. coli (ExPEC) that include meningitis-associated E. coli and sepsis-associated UTI [9]. UPEC are distinct from fecal commensal strains of E. coli found in the gastrointestinal tract, in that their genomes frequently encode a war chest of additional genes and virulence factors that facilitate infection of the host urinary tract [8,10,11]. These additional genes may encode toxins, adhesins, iron acquisition systems, additional metabolic enzymes and structural components that enable UPEC to take advantage of a unique and otherwise sterile host environmental niche (Figure 2) [12].
Figure 2. Classes of uropathogenic Escherichia coli vaccine targets include fimbrial adhesins, surface polysaccharides, outer membrane iron receptors and toxins.
LPS: Lipopolysaccharide; UPEC: Uropathogenic Escherichia coli.
Antibiotic therapy for UTI: rising resistance & inadequate outcomes
Antibiotics currently represent the most commonly prescribed treatment for UTI, and patients who suffer from recurrent infection, having three or more infections a year, may be prescribed antibiotics prophylactically [13]. In individuals plagued with persistently recurring UTI, which includes roughly 3% of women, rising rates of antibiotic resistance to first- and second-line therapies can make UTI treatment especially challenging, forcing physicians to reach for more expensive and sometimes less effective drugs (Figure 1) [14–16]. For example, in Turkey, the first-line antibiotic for UTI, trimethoprim–sulfamethoxazole (TMP–SMX), was used extensively in the early 1980s. By the early 1990s, uropathogen resistance rates to TMP–SMX had increased above 50%, leading to therapeutic failure and physicians to adopt quinolones as the preferred therapeutic for UTI [17]. In the decade following the shift toward quino-lones for UTI treatment, uropathogen resistance rates to quinolones in Turkey spiked at almost 30% [17]. In the USA and Canada, approximately 10–25% of uncomplicated UTI isolates are resistant to TMP–SMX, and in Spain and Portugal the resistance rate can be as high as 35% [15,18,19]. Even more troubling is the rate of multidrug resistance among UPEC isolates, which has risen as physicians adapt prescription choices to address shifting microbial susceptibilities [20]. A recent international ARESC documented over 10% of cystitis E. coli isolates to be resistant to at least three different classes of antimicrobial agents [21].
In addition to contributing to a rise in antibiotic resistance, repeat antibiotic treatment of UTI frequently results in added comorbidities that drive up medical costs and substantially diminish patient quality of life. Indeed, antibiotic therapy may deleteriously affect a patient’s commensal microbiota and lead to secondary infections post-treatment, such as vaginal yeast infection and gastrointestinal infection [22–24]. Individuals who undergo repeat antibiotic treatment are also at increased risk for carrying additional pathogens resistant to multiple antibiotics, further complicating medical care [25]. In an effort to provide a more effective and less costly alternative to antibiotic therapy for UTI management, progress has been made in the development of a preventive vaccine to target the most prominent uropathogen, UPEC.
UPEC vaccine development: improving UTI management through prevention
Although it appears that a prior UTI fails to elicit a protective host immune response and uropathogen heterogeneity complicates vaccine design, data from animal model studies offer encouragement for successful UPEC vaccine development [26,27]. Immunization with UPEC antigens can stimulate a mucosal immune response that may be effective at preventing experimental UTI and increases in urinary and serum antibody titers correlate with reductions in bladder bacterial load and infection duration [28–32]. With continued effort, these data provide encouragement that an effective UPEC vaccine can be developed.
A successful UPEC vaccine will likely require the following design considerations. The target of a UPEC vaccine will need to be highly immunogenic, expressed by the bacterium in vivo (i.e., during infection) and be surface-exposed on the bacterium in order to be accessible and recognized by the host immune system [33]. In addition, ideal vaccine candidates should be pathogen- specific, as to avoid targeting host commensal E. coli in the gastrointestinal tract. The heterogeneous nature of the UPEC population will also need to be considered, as a required core set of virulence factors common to all UPEC isolates has yet to be identified. Designing a UTI vaccine that can be effective against such a diverse pathogen population as UPEC may prove to be a considerable challenge. Furthermore, an effective vaccine for UTI will need to generate a robust mucosal adaptive immune response in the urinary tract. To better enable a strong mucosal immune response, research into novel antigen delivery systems, routes of immunization and adjuvants, such as modified heat-labile toxin, engineered outer membrane vesicles and mast cell activators, have been ongoing [34–38].
Much of the understanding of UPEC pathogenesis and the ability to evaluate UPEC vaccine designs have relied on the use of a mouse model of experimental UTI (for a recent review of mouse models of UTI see Hung et al. [39]). The use of mice to model UTI has proven to be an invaluable resource for advancing the knowledge of bacterial infection in the mammalian host. However, like all animal models of human disease, mouse models of UTI are not without limitations. Environmental, behavioral and physiological differences between mice and humans, including differences in the urinary tract, such as urine concentration and contents (mouse urine contains more protein and is more concentrated compared with human urine), require avoidance of direct comparison between human and mouse studies [40]. Indeed, studies comparing UPEC gene expression between bacteria obtained from experimental UTI in the mouse and active human UTI have identified differences, indicating unsurprisingly that artificial infection of the mouse does not directly equate with natural infection in humans [41]. Although mouse UTI models have been instrumental for UPEC vaccine design and data from both animal models and human studies will be included in this review, it is important to differentiate between data obtained through experimental infection in animals and those obtained through human studies. In the following sections, various UTI vaccination strategies, as well as the future directions of the UPEC vaccine field, will be discussed.
Strain diversity & molecular mimicry: vaccines targeting surface polysaccharides
The carbohydrate-rich cell surface of UPEC, such as all Gram-negative bacteria, contains an abundance of various polysaccharides. The external leaflet of the bacterium’s outer membrane contains lipopolysaccharide (LPS; O antigen), and virtually all UPEC strains are covered in a protective polysaccharide coat, or capsule (K antigen). O-antigenic LPS and, to a greater extent, K-antigenic capsular polysaccharide are virulence factors that allow UPEC to evade host immune system assaults, such as opsonophagocytosis, complement-mediated killing and damage by antimicrobial peptides [42,43]. Furthermore, recent work suggests that UPEC capsular polysaccharide and O antigen may cloak subcapsular epitopes on the bacterial cell surface, obscuring them from recognition by host antibodies, demonstrating yet another mechanism by which surface polysaccharides may interfere with the host immune response [44].
Early UTI vaccine studies focused on surface polysaccharides as targets for immunization, and some O and K antigen-based UPEC vaccines do elicit a protective immune response in animal models of ascending UTI (Table 1) [45–49]. However, substantial antigenic heterogeneity exists among E. coli surface polysaccharides, including 167 different O serogroups and greater than 80 polysaccharide K antigens [50]. Although certain K (K1, K5, 30 and 92) and O (O1, 2, 4, 6, 7, 8, 16, 16/72, 18, 25, 50 and 75) antigenic groups are more prevalent among uropathogenic strains, designing a surface polysaccharide-based UPEC vaccine that can be effective against all UPEC serotypes is extremely challenging [51]. In addition, many UPEC capsular polysaccharides are poorly immunogenic, camouflaged from the adaptive immune system by having a shared structural identity with the host. For example, the capsular antigen K1, which is present in an estimated 30% of pyelonephritis UPEC strains, is formed by repeating units of α-(2-8)-linked polysialic acid [51]. An identical host structure is found in the carbohydrate portion of human neonatal neural cell adhesion molecule, which plays an important role in the organization of neural tissue [52]. The adaptive immune system has protective mechanisms to prevent the generation of self-specific antibodies and, as a result, α-(2-8)-linked polysialic acid-specific antibodies are inefficiently produced, allowing K1-positive UPEC to avoid host adaptive immune detection [53]. Other UPEC surface polysaccharide antigens, such as capsular antigen K5, display similar molecular mimicry, and their poor immunogenicity coupled with high heterogeneity pose a substantial barrier to the development of a polysaccharide-based UTI vaccine [54].
Table 1.
Summary of uropathogenic Escherichia coli vaccine studies.
| Type/vaccine | Tested | Route | Adjuvant/delivery system | Challenge method/strain | Immune response† | Protection‡ | Ref. |
|---|---|---|---|---|---|---|---|
| Surface polysaccharides | |||||||
| O antigen (O6) | R | SC, SP | None | IU/E. coli O6 | IgG | B | [45] |
| K antigen (K13) | M (BALB/c) | SC | None | IU/E. coli O6:K13:H1 | IgG | NP | [47] |
| K antigen (K13) | M (BALB/c) | SC | DT | IU/E. coli O6:K13:H1 | IgG | K | [47] |
| Multistrain whole cell | |||||||
| Urovac® | H | IM | None | NC | – | Y | [57] |
| Urovac® | H | V | None | NC | sIgA, IgG | Y | [60,62,63,119] |
| Urovac® | P | V, IM | MO, none | VUR/JR1 | IgG | NP | [120] |
| Urovac® | R | IM | AP | VUR/ATCC 23500, 23513, 23504, 9018 | – | K | [29] |
| Urovac® | M (NMRI) | IP, IM | AP | IP/ATCC 23500, 23513, 23504, 9018 | sIgA, IgG | LC | [28,29] |
| Urovac® | M (BALB/c) (C57B1/6) | IP | None | VUR/1677 | – | B, K | [30] |
| Urovac® | M (BALB/c) (C57B1/6) | V | MO | VUR/1677 | ND | B | [30] |
| OM-89/Urovaxom® | H | O | None | NC | IgG | Y | [64,66,67] |
| UROstim | H | O | None | NC | IgA, IgG | – | [72] |
| Genetically engineered | |||||||
| UPEC CP9 live and killed | M (C57BL/6J) | IN | None | NC | IgG | – | [73] |
| UPEC CP923 live and killed | M (C57BL/6J) | IN | None | NC | IgG | – | [73] |
| UPEC NU14 | M (C57BL/6J) | TU | None | IU/NU14 | – | B | [74] |
| UPEC NU14 ΔwaaL | M (C57BL/6J) | TU | None | IU/NU14, CFT073 | – | B | [74] |
| Adherence | |||||||
| P fimbria | M (BALB/c) | IM | CFA, IFA | IV/J96 | IgG | K | [100] |
| PapDG | P | IP | AP | IU/DS17 | IgG | NP | [80] |
| Dr fimbria | M (C3H/HeJ) | ND | CFA, IFA | IU/IH11128 | IgG | NP | [84] |
| FimCH and FimHt | M (C3H/HeJ) | SC | CFA, IFA | IU/NU14 | IgG | B, K | [89] |
| FimCH | P | IM | MF59 | IU/NU14 | IgG | U | [90] |
| Toxins | |||||||
| Denatured α-hemolysin | M (BALB/c) | IM | CFA, IFA | IV/J96 | IgG | NP§ | [100] |
| Denatured α-hemolysin | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
| Iron acquisition | |||||||
| Denatured IroN | M (BALB/c) | SC | None | IV/CP9 | IgG | K | [106] |
| Denatured IroN | M (BALB/c) | SC | CFA | IP/S26 | IgG | LC | [107] |
| Denatured FyuA | M (BALB/c) | SC | CFA | IP/S26 | IgG | LC | [107] |
| IroN peptide | M (CBA/J) | IN | CT | IU/CFT073 | IgG | NP | [31] |
| IutA peptide | M (CBA/J) | IN | CT | IU/CFT073 | IgG | NP | [31] |
| Native IreA | M (CBA/J) | IN | CT | IU/CFT073 | sIgA, IgG | B | [31] |
| Native IutA | M (CBA/J) | IN | CT | IU/CFT073 | sIgA, IgG | B, K | [31] |
| Native Iha | M (CBA/J) | IN | CT | IU/CFT073 | sIgA, IgG | NP | [31] |
| Native Hma | M (CBA/J) | IN | CT | IU/CFT073 | sIgA, IgG | K | [31] |
| Native ChuA | M (CBA/J) | IN | CT | IU/CFT073 | sIgA, IgG | NP | [31] |
| Denatured ChuA | M (BALB/c) | SC | CFA | IP/S26 | ND | NP | [107] |
| ECOK1_3457 (FitA) | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
| Multiepitope subunit | |||||||
| Vol1 (FyuA, IutA, Iha, Usp) | M (BALB/c) | IN | CT | IP/CFT073 | sIgA, IgG | S, L | [111] |
| Vol2 (IroN, ChuA, IreA) | M (BALB/c) | IN | CT | IP/CFT073 | sIgA, IgG | L | [111] |
| pSTI (FyuA, IutA, Iha, Usp) | M (BALB/c) | O | T3SS | IP/CFT073 | ND | S, L | [112] |
| pST2 (IroN, ChuA, IreA) | M (BALB/c) | O | T3SS | IP/CFT073 | ND | NP | [112] |
| Hypothetical proteins | |||||||
| c1275 | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
| c5321 | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
| c0975 | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
| ECOK1_3385 | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
| ECOK1_3473 | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
| ECOK1_0290 | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
| ECOK1_3374 | M (CD1) | SC | CFA, IFA | IP/IHE3034, CFT073; IV/536 | ND | LC | [117] |
Significant increases detected.
Significant reduction detected.
Did not prevent kidney colonization, but immunized mice had less renal damage.
–: Not determined; AP: Aluminum phosphate; B: Bladder colonization; CFA: Complete Freund’s adjuvant; CT: Cholera toxin; DT: Purified diphtheria toxoid; H: Human; IFA: Incomplete Freund’s adjuvant; IgA: Serum IgA; IgG: Serum IgG; im.: Intramuscular; in.: Intranasal; ip.: Intraperitoneal; iu.: Intraurethral; iv.: Intravascular; K: Kidney colonization; L: Liver colonization; LC: Lethal challenge; M: Mouse; MO: Mineral oil; NC: No challenge; ND: None detected; NP: No protection; O: Oral; P: Nonhuman primates; R: Rat; S: Spleen colonization; sc.: Subcutaneous; sIgA: Urinary IgA; SP: Surgical procedure; T3SS: Salmonella Type 3 secretion system; tu.: Transuretheral; U: Urinary tract infection as determined by analysis of urine; UPEC: Uropathogenic Escherichia coli; V: Vaginal; VUR: Intravesically per urethra reflux; Y: Urinary tract infection incidence.
Adapted with permission from [121].
Uropathogen cocktails: multistrain whole-cell/cell lysate UTI vaccines
Vaccinating with whole or lysed fractions of inactivated pathogens can be an effective method to generate protective immunity, and a number of successful vaccines against human pathogens, including Bordetella pertussis (whooping cough), Vibrio cholerae (cholera) and Salmonella Typhi (typhus) contain killed whole bacteria [55]. There are four standardized whole-cell/cell lysate-based vaccines that have been tried for UTI with limited success (Table 1).
Urovac® (Solco Basel AG, Birsfelden, Switzerland and Protein Express, Cincinnati, OH, USA) was designed to provide broad protection by containing ten heat-killed uropathogens: six UPEC strains, and one strain each of Proteus mirabilis, Morganella morganii, Enterococcus faecalis and Klebsiella pneumoniae. The UPEC strains added to the Urovac® formulation possess several virulence factors, including hemolysin, type 1, P, and S fimbrial adhesins, CNF-1, several siderophores and the E. coli CFT073 pathogenicity island marker, and display the serotypes O 1, 4, 6, 17, 75, 77, K 1, 3, 5, 13, 95 and H: 1, 5, 7, 33 [56]. Rodents immunized with Urovac intramuscularly were protected for up to 20 weeks from experimental challenge by Urovac homologous strains and some heterologous strains (strains that do not share any of the same O, K or H antigens as the vaccine strains) [28,29]. Women volunteers with a history of recurrent UTI experienced a statistically significant reduction in recurrent infection (28 infections in 23 patients out of 202 immunized) in the 12 months following intramuscular injections of Urovac (once weekly for 3 consecutive weeks), than women who did not receive the vaccine (84 infections in 47 patients out of 198) [57]. Adverse side effects reported after Urovac injection were comparable with other bacterial vaccines, such as the DTaP vaccine for diphtheria, tetanus and pertussis, including redness at the injection site (25% of patients), pressure (9%), pain (5%) and fever to 38°C (3.5%) [57,58]. Subsequent trials delivered Urovac vaginally, either with a vaginal suppository or an oil emulsion, to reduce the risk of endotoxin toxicity and adverse side effects, and to stimulate a more robust local mucosal immune response in the urinary tract [59]. Phase II and extended Phase II clinical trials evaluating the efficacy of vaginally administered Urovac® found that women who received six total doses of vaccine, on weeks 0, 1, 2, 6, 10 and 14, gained short-term protection from infection, having significant delays to reinfection during the first 8 weeks of the study in comparison with women who received placebos [60–63]. However, over the full course of the 6-month study, Urovac immunization did not provide significant long-term protection from UTI or increase mean levels of UPEC-specific serum, urinary or vaginal antibodies [60–63]. Interestingly, although the average number of UTIs caused by any bacterial strain during the 6-month trial period was not significantly different between the vaccinated and placebo groups with 1.1 and 1.5 infections per patient, respectively, the number of E. coli-caused UTIs was significantly decreased in the Urovac vaccinated group [60–63]. Of the women receiving Urovac, 72% remained free from UTIs caused by E. coli, compared with women given placebos, of which only 30% remained free from E. coli-caused UTIs, suggesting that Urovac may be successful in reducing the incidence of E. coli-caused UTIs in susceptible women. However, even with a vaginal route of delivery, some of the women reported adverse side effects, including single occurrences of low-grade fever (8%; four out of 50), a burning sensation shortly after treatment (12%; six out of 50), nausea (8%; four out of 50), vaginal bleeding (8%; four out of 50) or vaginal rash (8%; four out of 50) [63].
OM-89/Uro-Vaxom® (OM Pharma, Myerlin, Switzerland): is the second such standardized formula, a lyophilized mix of membrane proteins from 18 UPEC strains that has been approved for use as a human therapeutic in Switzerland since 1988 and marketed and sold in almost 40 countries worldwide, excluding the USA and Canada. Uro-Vaxom is prescribed as a daily oral capsule, and several double-blind, placebo-controlled clinical studies have evaluated the vaccine’s safety and efficacy [64–68]. A statistical meta-analysis of five clinical studies, involving 601 female participants given 90 days of treatment, showed Uro-Vaxom to be significantly more effective than placebo in preventing recurrent UTI [69]. In a multicenter clinical trial funded by OM Pharma, women with a history of recurrent UTI were administered a daily oral capsule of Uro-Vaxom for 90 days, given 3 months without treatment and then boosted with a daily dose for 10 days in the beginning of months 7, 8 and 9 [67]. After 12 months, women given Uro-Vaxom experienced a statistically significant reduction in UTI recurrences (185 recurrences in 93 patients out of 220 in total) in comparison with those given placebos (276 recurrences in 122 patients out of 215 in total) [67]. In addition, patients given Uro-Vaxom experienced a reduction in the frequency in the signs and symptoms of UTI, including dysuria (painful urination), bacteriuria (presence of bacteria in the urine) and leukocyturia (presence of leukocytes in the urine) in comparison with women receiving placebos; although this reduction was only statistically significant during one of the six follow-up visits [67]. Patient tolerance of Uro-Vaxom is reported to be good with the most frequent adverse events being headache followed by gastrointestinal events, including pain and nausea and skin reactions [67,68]. Although Uro-Vaxom does appear to reduce the incidence of recurrent UTI, with limited toxicity issues, the required daily administration may create problems with patient compliance.
Urvakol® (Institute of Microbiology, Prague, Czech Republic) and Urostim (National Center of Infectious and Parasitic Diseases, Sofia, Bulgaria), the third and fourth standardized whole-cell vaccines, are administered as daily oral tablets containing mixtures of inactivated uropathogens. Both formulations contain strains of E. coli, P. mirabilis and E. faecalis, although Urvakol also includes a strain of Pseudomonas aeruginosa, whereas UROstim contains K. pneumoniae. Data from animal and patient studies demonstrate that Urvakol and UROstim have immunostimulating activity as measured by cytokine production and the presence of vaccine-specific antibodies in the serum, urine and saliva of patients after immunization [70–72]. However, the ability of either vaccine to prevent recurrent UTI has not been established as well-structured clinical trials have yet to be completed.
Genetically engineered vaccines: improving immunization through genetic modification
In addition to the whole-cell/cell lysate, inactivated UTI vaccines being designed and tested in Europe, vaccines based on genetically modified strains of UPEC are also in development. To determine if UPEC’s poorly immunogenic surface polysaccharides impede the generation of an optimal host humoral response, Russo and colleagues immunized mice with a genetically modified isogenic strain of pathogenic E. coli, termed CP923, that is unable to produce capsule or the O antigen of LPS due to a transposon insertion into a gene that encodes for an enzyme involved in rhamnose biosynthesis [73]. Mice immunized intranasally with formalin-killed CP923 generated a significantly greater overall humoral immune response, as measured by serum antibody levels, than mice immunized with the wild-type parental strain CP9 [73]. In addition, immunization with CP923 generated significantly greater levels of antibodies directed against noncapsular and non-O-antigenic epitopes, a desirable outcome for a vaccine directed against the highly heterologous UPEC population [73]. However, when the formalin-killed CP923 vaccine was tested in an intravenous sepsis model of infection, mice intranasally immunized with CP923 were not significantly protected from experimental infection by CP9, in comparison with phosphate-buffered saline-immunized controls [44]. Data from in vitro binding and bactericidal assays suggest that the failure of the CP923 vaccine to protect against CP9 infection, despite generating high serum antibody titers, may be due to interference by CP9 surface polysaccharides [44]. Since UPEC strains are generally positive for capsule and O antigen, the potential of surface polysaccharides to mask nonpolysaccharide epitopes and reduce the efficiency of antibody binding may provide additional motivation to identify capsular antigens or vaccine targets that are external to the capsule.
Employing a vaccine strategy with a similar genetically modified E. coli strain with impaired surface polysaccharide expression, Billips and coworkers assessed the use of a live-attenuated UPEC vaccine to prevent UTI [74]. The authors produced an attenuated mutant UPEC strain unable to persist in the host urinary tract due to a targeted deletion of a gene that encodes an O antigen ligase (waaL), an enzyme required for joining the variable O antigen to the LPS lipid A-core during biosynthesis [74]. Mice inoculated with the live-attenuated UPEC strain (NU14 ΔwaaL), via a urinary catheter to the bladder, were protected from experimental UTI challenge with the wild-type parental strain NU14, as well as a range of clinical UPEC isolates, including CFT073 [74]. Promisingly for UPEC vaccine development, although the live-attenuated vaccine strain NU14 ΔwaaL failed to persist for longer than 2 weeks in the mouse bladder after inoculation, the protective immunity generated postimmunization lasted for at least 8 weeks after vaccination [74].
Sticky structures: vaccines targeting adherence
UPEC encode a wide variety of adhesins, a general term used to describe extracellular proteins that facilitate bacterial attachment to, or invasion of, host cells, such as fimbriae or pili. Indeed, fimbrial adhesins were among the first recognized UPEC virulence factors and the genomes of UPEC isolates generally contain many more fimbrial operons (8.3 ± 1.3) than those of commensal E. coli strains (2.0 ± 2.3) [75]. For example, the genome of the prototypical UPEC strain CFT073 encodes 12 putative fimbrial operons [11]. Many of these fimbriae enable colonization of the host urinary tract, and it is theorized that blocking bacterial adherence to host epithelial cells by the binding of antibodies could prevent colonization [76]. Naturally, the critical role of adherence during infection has made fimbrial adhesins a prime target for UPEC vaccines. Thus far, however, only a small subset of UPEC fimbriae has been evaluated for use in a UTI vaccine, including P, Dr and type 1 fimbriae.
The UPEC adhesin, PapG, which is the tip adhesin on P fimbriae, binds to Gal(α1-4)Gal-specific glycosphingolipids on kidney epithelium (P blood group antigen) and likely plays an important role in UPEC human kidney colonization [77]. Vaccination studies using P fimbrial subunits in murine and primate models of ascending UTI found it to be effective in preventing kidney infection [78–80]. However, P fimbriae have a limited role during UPEC bladder colonization and, as a result, vaccines targeting the P fimbrial adhesin, PapG, will need to be combined with additional components that can provide adequate protection from infection in the bladder.
Dr fimbriae bind to type 4 collagen and DAF/CD55 on the tubular basement membrane and Bowman’s capsule of the human kidney, and contribute to UPEC pathogenesis in animal models of UTI [81,82]. Mice experimentally infected with a strain of UPEC, positive for Dr fimbriae, maintained higher and more prolonged kidney bacterial loads and developed significantly more tubulointerstitial nephritis than mice infected with a Dr fimbriae knockout mutant [83]. Vaccinating mice with purified Dr fimbriae produced high titers of serum anti-Dr antibodies and significantly reduced experimental UTI-associated mortality, but did not affect the rate of bladder or renal colonization [84]. Preincubating Dr-positive UPEC with Dr-immunized mouse sera resulted in an observable reduction in bacterial adherence to mouse bladders and kidneys, although the same UPEC antibinding activity was not observed when Dr-positive UPEC were pre-incubated with the urine from Dr-immunized mice [84]. Further studies are needed to fully test the potential of Dr fimbria as a UPEC vaccine target as it is possible that modifying the method or route of Dr antigen delivery may improve vaccine efficacy.
The type 1 fimbrial adhesion FimH mediates UPEC adherence to bladder epithelial cells by binding to mannose residues on uroplakin, a major structural component of the bladder epithelium [85]. In mice, type 1 fimbria is a particularly important UPEC virulence factor, as its expression greatly enhances UPEC binding and colonization of the murine bladder during experimental UTI, a process that can be outcompeted by administering a competitive inhibitor of FimH, such as methyl α-D-mannopyranoside, with the challenging UPEC strain at the time of inoculation [86–88]. A knockout mutant UPEC strain deficient in FimH production, due to a targeted deletion of the fimH gene, failed to bind human and mouse bladder epithelial tissues, a phenotype that could be restored by complementing fimH expression off a plasmid [89]. The discovery that cystitis could potentially be prevented or treated through disrupting type 1 fimbria-mediated UPEC adherence to bladder epithelium, either pharmacologically or with host-generated antibodies, galvanized efforts to develop FimH-based vaccine designs. Indeed, mice immunized with FimHt, a man-nose-binding truncated form of FimH, or a complex containing the periplasmic chaperone FimC bound to the full-length FimH protein, termed FimCH, were significantly protected from experimental UPEC infection in the bladder, exhibiting a 100- to 1000- fold reduction in the number of colonizing bacteria in comparison with adjuvant-only immunized controls [89]. Immunization with the FimHt vaccine was able to generate a substantial and long-lasting humoral immune response as immunized mice had significantly higher levels of FimH-specific urinary IgG, an outcome that correlated with the levels of protection from infection in the bladder [89]. Cynomolgus monkeys immunized with the FimCH vaccine elicited a strong systemic humoral response and three out of four were protected from experimental UTI 48 h postinoculation, in comparison with zero out of four monkeys in the adjuvant-only control group [90,91].
In 1999, MedImmune, Inc. (MA, USA) brought the UPEC type 1 fimbria subunit vaccine, FimCH, to Phase II clinical trials with women volunteers. Unfortunately, MedImmune formally announced in early 2003 the discontinuation of further research and development of the FimCH vaccine, citing that the previous clinical trials failed to demonstrate a sufficient level of efficacy in prevention of UTIs to warrant additional larger Phase III studies [92,201] [MedImmune Medical Affairs Department, Pers. Comm.]. Since the data from the FimCH Phase II clinical trials have not been published, the level of efficacy of the FimCH vaccine in humans remains unclear. In the decade since the start of FimCH clinical trials, additional research on UPEC type 1 fimbriae has further complicated the understanding of its role during human infection. Microarray and transcriptional analysis data from women with active UTI suggest that genes encoding type 1 fimbrial subunits may not be highly expressed during UPEC infection of the human urinary tract [41,93]. In addition, the expression of type 1 fimbriae is phase variable, as its promoter region resides on an invertible element that switches fim expression between on and off [94,95]. Variation in type 1 fimbriae expression may allow invading UPEC to avoid detection from an adaptive immune response targeting this organelle. In addition, recent evidence suggests that antibodies generated against the adhesive tip of type 1 fimbriae, FimH, do not target the mannose-binding pocket or block FimH adhesion, may actually enhance the binding affinity of FimH for mannose, and are shed as mannose-bound FimH undergoes a conformational shift during binding [96]. While these results may be at odds with the previous understanding of FimH-mediated vaccine protection, they may suggest an alternative explanation for FimH-based vaccine efficacy in mice. The recent evidence suggesting UPEC gene expression may be different between mouse and human infection, and the experience with the FimCH vaccine highlights the need to better understand UPEC antigen expression in the human host. Although the mouse model of ascending UTI is a valuable resource for testing vaccine efficacy and design, the ability to choose promising UPEC vaccine candidates would benefit enormously from having a more complete understanding of UPEC virulence gene expression during UTIs in humans.
Toxin-based UPEC vaccines
Vaccines containing inactivated bacterial toxins, termed toxoids, have been effective against a select number of bacterial pathogens and many current routine childhood vaccinations are toxoid-based, including the diphtheria, tetanus and acellular pertussis vaccine (DTaP), which contains both diphtheria and tetanus toxoids [55]. UPEC genomes frequently contain genes that encode toxins, including α-hemolysin, CNF-1, cytolethal distending toxin, and secreted autotransporter toxins Sat, Pic and Tsh [97]. Many of these toxins have been associated with symptom severity during UTI, such as increased inflammation, bladder epithelial cell shedding and renal damage, but none have been shown to be required for infection and thus may represent less-than-ideal vaccine candidates [98,99]. When the pore-forming cytolytic toxin, α-hemolysin, was administered intramuscularly to mice, which were then challenged with an intravesicular injection of UPEC, no significant protection was acquired [100]. It was noted, however, that the α-hemolysin-immunized mice did suffer significantly less renal damage than their phosphate-buffered saline immunized counterparts, and although toxoid-based vaccines may not be applicable for reducing the incidence of UTI, they may be effective at reducing infection severity in certain high-risk patient populations.
Required for growth in the host: iron acquisition system-based UPEC vaccines
Nearly all forms of life require iron. Iron is an essential cofactor for enzymes involved in primary and secondary cellular metabolism and a critical component of normal cell physiology. UPEC’s ability to colonize the host urinary tract is dependent on iron acquisition [101]. Although the human body contains substantial iron, the majority is inaccessible to invading micro-organisms as it is bound to the oxygen-carrying heme group within hemoglobin or sequestered by iron storage molecules, such as ferritin and hemosiderin. The scarcity of free iron in the host is a major obstacle for microbial growth, and the ability to circumvent this barrier is a hallmark of many successful bacterial pathogens [102].
UPEC survives in the iron-limited host urinary tract by upregulating the expression of molecular iron acquisition systems that synthesize and secrete small organic iron-chelating molecules called siderophores [41,103]. Siderophores have extremely high affinity for ferric iron and can successfully compete with host proteins for iron resources. Once iron is complexed, the ferrisiderophore is imported back into the bacterial cell through their respective outer membrane receptors, thereby allowing iron to be extracted from the host and used by the bacterium for its survival [104]. In addition to expressing outer membrane iron receptors specific for its own siderophores, UPEC opportunistically expresses outer membrane receptors for iron-containing host molecules, such as heme, and the siderophores of other microorganisms, such as the fungal siderophore, ferrichrome.
In contrast to most commensal E. coli strains that encode few iron acquisition systems, UPEC expresses a large arsenal of iron acquisition systems [105]. For example, the genome of prototypical pyelonephritis UPEC strain CFT073 encodes 14 characterized outer membrane iron compound receptors, as well as the biosynthesis machinery for three different siderophores: catecholates enterobactin and salmochelin, and the hydroxamate aerobactin [11]. Many outer membrane iron compound receptors share the characteristics of an ideal UPEC vaccine target: they are surface-exposed on the bacterium; expressed during infection; and prevalent among pathogenic E. coli strains. Thus far, seven UPEC outer membrane iron compound receptors (IroN, IreA, IutA, FyuA, Iha, Hma and ChuA) have been evaluated as vaccine candidates to prevent UTI, five of which (IroN, IreA, IutA, FyuA and Hma) significantly protect in a mouse model of infection, demonstrating that outer membrane iron receptors are a promising new class of UPEC vaccine targets [31,106,107].
Of the four possible siderophores produced by E. coli clinical isolates, three are more often produced by pathogens: aerobactin, yersiniabactin and salmochelin [105,108]. As a result, the outer membrane receptors for these siderophores have been investigated as prospective targets for vaccination. Subcutaneous immunization with a denatured form of the UPEC salmochelin outer membrane receptor, IroN, conferred significant protection from experimental UPEC infection in mouse kidneys and produced a significant IroN-specific serum IgG response [106]. However, IroN immunization did not induce a significant systemic or mucosal IgA response or protect from experimental infection in the bladder, an outcome that could possibly be improved on through the use of an adjuvant or alternative route of vaccine administration. Intranasal immunization with the aerobactin receptor, IutA, conjugated to cholera toxin as adjuvant, significantly protected mice from experimental UPEC infection in both the bladder and kidneys, and induced a significant increase in IutA-specific urinary IgA [31]. Although aerobactin production is pathogen-associated, gene expression data using mRNA isolated directly from bacteria in urine of women with active UTIs suggest that aerobactin production may not be widely abundant among UPEC strains, limiting the usefulness of an IutA-based UPEC vaccine [41]. Subcutaneous immunization with the UPEC yersiniabactin receptor, FyuA, combined with Freund’s adjuvant, significantly protected mice from death in a lethal sepsis model of pathogenic E. coli infection [107]. Passive immunization with purified sera from FyuA-immunized rabbits demonstrated the protection to be immunoglobulin-mediated.
In addition to the outer membrane receptors that facilitate ferrisiderophore import, UPEC vaccines have also targeted bacterial outer membrane receptors for host heme, the iron-containing prosthetic group of hemoglobin, and other hemoproteins. Intranasal immunization with the heme receptor Hma conjugated to cholera toxin as adjuvant, significantly protected mice from experimental infection with UPEC in the kidney, but not the bladder [31]. Surprisingly, immunization with an alternative UPEC heme receptor, ChuA, did not provide significant protection from experimental infection [31,107]. Although iron acquisition is required for UPEC colonization of the host urinary tract, no single essential iron acquisition system has yet to be identified. Indeed, many UPEC iron acquisition systems may be functionally redundant and unequally expressed among UPEC strains, requiring a successful iron receptor-based UTI vaccine to target more than one iron acquisition system to be broadly effective [41,109,110]. Multiepitope vaccines that contain domains from many outer membrane iron receptors address this dilemma by generating protective immunity against several UPEC iron acquisition systems. Recently, Wieser and colleagues [111] constructed two such multiepitope subunit vaccines (designated Vol1 and Vol2) containing domains from six E. coli outer membrane iron receptors (FyuA, IroN, ChuA, IreA, IutA and Iha) and the uropathogenic-specific protein (Usp) connected by spacer domains on single recombinant proteins. Intranasal immunization of mice with either Vol1 or Vol2, conjugated to cholera toxin as adjuvant, was able to significantly reduce UPEC bacterial loads in the liver 48 h after experimental intraperitoneal UPEC challenge [111]. However, only immunization with the Vol1 construct, containing domains from FyuA, IutA, Iha and Usp, was able to significantly decrease experimental UPEC bacterial loads in the spleen [111]. Similar results were obtained when the multiepitope vaccines were administered via a live bacterial antigen delivery system based on the Salmonella type 3 secretion system [112]. In agreement with earlier results, immunization with the Vol1 (pST1) construct significantly reduced bacterial loads in the murine liver and spleen, in contrast to the Vol2 (pST2), which did not induce significant protection [112]. These data from the multiepitope and iron receptor-based immunization studies are encouraging for UTI vaccine development and continued research into UPEC iron acquisition during infection may help inform future vaccine designs that target UPEC, as well as other pathogens that depend on iron acquisition to survive within the host.
Expert commentary & five-year view: UPEC heterogeneity & pathogenesis, mucosal immunity & the discovery of novel UTI vaccine candidates
Although select individual antigen-based UPEC vaccines may successfully prevent experimental infection in animal models, a broadly effective UTI vaccine may need to target more than one virulence factor to be clinically useful against the highly heterogeneous UPEC population. Substantial diversity exists within classes of UPEC virulence factors, such that not every UPEC strain expresses the exact set of virulence-associated genes during infection [41]. Although the genomes of pathogenic E. coli frequently encode many more virulence factors than commensal E. coli strains, the absence of a required core set of virulence factors complicates UTI vaccine design [11,75]. Targeting a single virulence factor may only be effective against a select group of UPEC strains. Vaccine strategies that target multiple virulence factors, such as the multiepitope vaccines constructed by Wieser and coworkers [111], provide a solution to the problem of UPEC strain diversity. Through targeting multiple members of a class of virulence factors, such as multiple fimbrial adhesins or multiple iron receptors, rather than an individual, it may be possible to overcome UPEC diversity and design a clinically effective vaccine for UTI.
In addition to tackling the ongoing challenge presented by UPEC strain diversity, UTI vaccine development could be better guided through further understanding UPEC pathogenesis and the host mucosal immune response to infection. It is still unclear as to why we are unable to generate an effective adaptive immune response after an initial UPEC infection, leaving us susceptible to repeat infection with the same UPEC strain. Future UTI vaccine development strategies may need to include considerations for mechanisms by which UPEC may be impeding the host’s generation or implementation of a protective adaptive immune response. Mechanisms by which UPEC may be subverting the host immune response include interference with Toll-like receptor signaling, the formation of intracellular bacterial communities, the suppression of cytokine secretion, impedance of antibody binding and reduction of the secretion of secretory IgA [26,44,113–115]. Understanding UPEC immune-modulating mechanisms could help guide strategies to combat repeat infection. In addition, better understanding the mucosal immune response to infection could provide guidance to more effective vaccine delivery systems and adjuvants. Defining what specific factors influence the robustness and longevity of a mucosal immune response in the urinary tract would allow vaccine delivery systems and adjuvants to be tailored more effectively. The development of novel adjuvants to improve the mucosal immune response to vaccines, as well as increasing the knowledge of how UPEC interacts with the host immune system, could help inform rational UTI vaccine design.
As well as further exploring the potential of already identified promising vaccine candidates, novel vaccine candidate discovery screens are helping to identify previously unrecognized UTI vaccine targets. Vaxign, a web-based vaccine design program developed by He and colleagues, was used to predict new UPEC vaccine targets based on a reverse vaccinology strategy [116]. In addition to Vaxign, other discovery screens have been used to identify novel vaccine targets. In a ‘subtractive reverse vaccinology screen’ by Moriel and coworkers, vaccine antigens were predicted using a bioinformatic analysis of three ExPEC strains (CFT073, 536 and IHE3034) [117]. Antigens from CFT073, 536 and IHE3034, predicted to be surface associated or secreted, with three or fewer transmembrane domains, were selected and compared against nonpathogenic E. coli strains (MG1655, DH10B and W3110) for exclusion. By this approach, 230 potential antigens were identified and tested in a mouse model of sepsis, nine of which were newly identified and found to protect against experimental ExPEC infection [117]. Despite ongoing challenges, the progress toward discovering and testing novel vaccine candidates, adjuvants and delivery methods is promising, and we are hopeful for the future development of a vaccine to prevent UTI.
Key issues.
Urinary tract infections (UTIs) are caused when pathogenic bacteria, present in fecal matter, traverse the urethra and colonize the normally sterile host urinary tract, which can lead to permanent kidney damage and sepsis in the most serious cases.
Out of all uncomplicated UTIs, 80% are caused by a highly heterogeneous group of Escherichia coli strains termed uropathogenic E. coli (UPEC).
Frequent UTIs amount to a substantial fiscal and public healthcare burden, as half of all women and 12% of men will have at least one UTI in their lifetime.
Recurrent UTI, defined by having three or more infections within 1 year, can be caused by the same or different UPEC strains.
Antibiotic therapy is the current standard treatment for UTI, but a rise in the number of antibiotic-resistant strains and the prevalence of antibiotic-resistant mechanisms has complicated treatment.
The development of a vaccine to prevent UTI would be highly desirable, but none are currently licensed for use in the USA.
The high degree of diversity among the UPEC population complicates vaccine design, as no individual or core set of virulence factors is known to be required for UTI.
Although multiple classes of UPEC virulence factors have been targets of UTI vaccinea, including bacterial surface polysaccharides, fimbrial adhesins, toxins and outer membrane iron receptors, a successful vaccine for UTI may need to target more than one UPEC virulence factor to be effective against such a diverse pathogen population.
Acknowledgments
The authors would like to thank C Alteri for valuable discussion and critical comments on the review.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work was supported by Public Health Service Grants AI43365 and AI059722. HLT Mobley serves as a consultant to Novartis AG. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1•.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7(12):653–660. doi: 10.1038/nrurol.2010.190. A recent review on the epidemiology of urinary tract infections (UTIs) [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17(2):227–241. doi: 10.1016/s0891-5520(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151(12):1194–1205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 4.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5:1–20. [PubMed] [Google Scholar]
- 5.Litwin MS, Saigal CS, Yano EM, et al. Urologic Diseases in America Project. Urologic diseases in America Project: analytical methods and principal findings. J Urol. 2005;173(3):933–937. doi: 10.1097/01.ju.0000152365.43125.3b. [DOI] [PubMed] [Google Scholar]
- 6.Mühldorfer I. Emerging bacterial pathogens. Preface. Contrib Microbiol. 2001;8:XI–XIV. [PubMed] [Google Scholar]
- 7.Mehnert-Kay SA. Diagnosis and management of uncomplicated urinary tract infections. Am Fam Physician. 2005;72(3):451–456. [PubMed] [Google Scholar]
- 8.Zhang L, Foxman B. Molecular epidemiology of Escherichia coli mediated urinary tract infections. Front Biosci. 2003;8:e235–e244. doi: 10.2741/1007. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JR, Russo TA. Uropathogenic Escherichia coli as agents of diverse non-urinary tract extraintestinal infections. J Infect Dis. 2002;186(6):859–864. doi: 10.1086/342490. [DOI] [PubMed] [Google Scholar]
- 10.Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85(1):11–19. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch RA, Burland V, Plunkett G, 3rd, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic. Escherichia coli Proc Natl Acad Sci USA. 2002;99(26):17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright KJ, Hultgren SJ. Sticky fibers and uropathogenesis: bacterial adhesins in the urinary tract. Future Microbiol. 2006;1(1):75–87. doi: 10.2217/17460913.1.1.75. [DOI] [PubMed] [Google Scholar]
- 13.Kodner CM, Thomas Gupton EK. Recurrent urinary tract infections in women: diagnosis and management. Am Fam Physician. 2010;82(6):638–643. [PubMed] [Google Scholar]
- 14.Gupta K, Hooton TM, Naber KG, et al. Infectious Diseases Society of America European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 15.Moura A, Nicolau A, Hooton T, Azeredo J. Antibiotherapy and pathogenesis of uncomplicated UTI: difficult relationships. J Appl Microbiol. 2009;106(6):1779–1791. doi: 10.1111/j.1365-2672.2008.04115.x. [DOI] [PubMed] [Google Scholar]
- 16.Foxman B, Ki M, Brown P. Antibiotic resistance and pyelonephritis. Clin Infect Dis. 2007;45(3):281–283. doi: 10.1086/519267. [DOI] [PubMed] [Google Scholar]
- 17.Karaca Y, Coplu N, Gozalan A, Oncul O, Citil BE, Esen B. Co-trimoxazole and quinolone resistance in Escherichia coli isolated from urinary tract infections over the last 10 years. Int J Antimicrob Agents. 2005;26(1):75–77. doi: 10.1016/j.ijantimicag.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135(1):41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 19.Zhanel GG, Hisanaga TL, Laing NM, et al. NAUTICA Group. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA) Int J Antimicrob Agents. 2005;26(5):380–388. doi: 10.1016/j.ijantimicag.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Karlowsky JA, Hoban DJ, Decorby MR, Laing NM, Zhanel GG. Fluoroquinolone-resistant urinary isolates of Escherichia coli from outpatients are frequently multidrug resistant: results from the North American Urinary Tract Infection Collaborative Alliance-Quinolone Resistance study. Antimicrob Agents Chemother. 2006;50(6):2251–2254. doi: 10.1128/AAC.00123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schito GC, Naber KG, Botto H, et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34(5):407–413. doi: 10.1016/j.ijantimicag.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald TM, Beardon PH, McGilchrist MM, Duncan ID, McKendrick AD, McDevitt DG. The risks of symptomatic vaginal candidiasis after oral antibiotic therapy. Q J Med. 1993;86(7):419–424. [PubMed] [Google Scholar]
- 23.Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758–764. doi: 10.7326/0003-4819-145-10-200611210-00008. [DOI] [PubMed] [Google Scholar]
- 24.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Eldere J, Mera RM, Miller LA, Poupard JA, Amrine-Madsen H. Risk factors for development of multiple-class resistance to Streptococcus pneumoniae strains in Belgium over a 10-year period: antimicrobial consumption, population density, and geographic location. Antimicrob Agents Chemother. 2007;51(10):3491–3497. doi: 10.1128/AAC.01581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billips BK, Schaeffer AJ, Klumpp DJ. Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect Immun. 2008;76(9):3891–3900. doi: 10.1128/IAI.00069-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivick KE, Schaller MA, Smith SN, Mobley HL. The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J Immunol. 2010;184(4):2065–2075. doi: 10.4049/jimmunol.0902386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruze D, Holzbecher K, Andrial M, Bossart W. Urinary antibody response after immunisation with a vaccine against urinary tract infection. Urol Res. 1989;17(6):361–366. doi: 10.1007/BF00510527. [DOI] [PubMed] [Google Scholar]
- 29.Kruze D, Biro K, Holzbecher K, Andrial M, Bossart W. Protection by a polyvalent vaccine against challenge infection and pyelonephritis. Urol Res. 1992;20(2):177–181. doi: 10.1007/BF00296534. [DOI] [PubMed] [Google Scholar]
- 30.Uehling DT, James LJ, Hopkins WJ, Balish E. Immunization against urinary tract infection with a multi-valent vaginal vaccine. J Urol. 1991;146(1):223–226. doi: 10.1016/s0022-5347(17)37756-x. [DOI] [PubMed] [Google Scholar]
- 31•.Alteri CJ, Hagan EC, Sivick KE, Smith SN, Mobley HL. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009;5(9):e1000586. doi: 10.1371/journal.ppat.1000586. Evaluates six uropathogenic Escherichia coli outer membrane iron receptors in a mouse model of UTI and establishes a correlate of protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thumbikat P, Waltenbaugh C, Schaeffer AJ, Klumpp DJ. Antigen-specific responses accelerate bacterial clearance in the bladder. J Immunol. 2006;176(5):3080–3086. doi: 10.4049/jimmunol.176.5.3080. [DOI] [PubMed] [Google Scholar]
- 33.Sivick KE, Mobley HL. An “omics” approach to uropathogenic Escherichia coli vaccinology. Trends Microbiol. 2009;17(10):431–432. doi: 10.1016/j.tim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lycke N. Targeted vaccine adjuvants based on modified cholera toxin. Curr Mol Med. 2005;5(6):591–597. doi: 10.2174/1566524054863898. [DOI] [PubMed] [Google Scholar]
- 35.Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003;21(Suppl 2):S89–S95. doi: 10.1016/s0264-410x(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 36.Scavone P, Rial A, Umpierrez A, Chabalgoity A, Zunino P. Effects of the administration of cholera toxin as a mucosal adjuvant on the immune and protective response induced by Proteus mirabilis MrpA fimbrial protein in the urinary tract. Microbiol Immunol. 2009;53(4):233–240. doi: 10.1111/j.1348-0421.2009.00111.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen DJ, Osterrieder N, Metzger SM, et al. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc Natl Acad Sci USA. 2010;107(7):3099–3104. doi: 10.1073/pnas.0805532107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLachlan JB, Shelburne CP, Hart JP, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14(5):536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 39.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4(8):1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox JG. The Mouse in Biomedical Research. Elsevier; Amsterdam, The Netherlands: 2007. [Google Scholar]
- 41.Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HL. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 2010;6(11):e1001187. doi: 10.1371/journal.ppat.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 43.Buckles EL, Wang X, Lane MC, et al. Role of the K2 capsule in Escherichia coli urinary tract infection and serum resistance. J Infect Dis. 2009;199(11):1689–1697. doi: 10.1086/598524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Russo TA, Beanan JM, Olson R, MacDonald U, Cope JJ. Capsular polysaccharide and the O-specific antigen impede antibody binding: a potential obstacle for the successful development of an extraintestinal pathogenic Escherichia coli vaccine. Vaccine. 2009;27(3):388–395. doi: 10.1016/j.vaccine.2008.10.082. Demonstrates a necessary consideration for vaccine design. [DOI] [PubMed] [Google Scholar]
- 45.Uehling DT, Wolf L. Enhancement of the bladder defense mechanism by immunization. Invest Urol. 1969;6(5):520–526. [PubMed] [Google Scholar]
- 46.Kaijser B, Ahlstedt S. Protective capacity of antibodies against Escherichia coli and K antigens. Infect Immun. 1977;17(2):286–289. doi: 10.1128/iai.17.2.286-289.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar V, Ganguly N, Joshi K, et al. Protective efficacy and immunogenicity of Escherichia coli K13 diphtheria toxoid conjugate against experimental ascending pyelonephritis. Med Microbiol Immunol. 2005;194(4):211–217. doi: 10.1007/s00430-005-0241-x. [DOI] [PubMed] [Google Scholar]
- 48.Kaijser B, Larsson P, Olling S, Schneerson R. Protection against acute, ascending pyelonephritis caused by Escherichia coli in rats, using isolated capsular antigen conjugated to bovine serum albumin. Infect Immun. 1983;39(1):142–146. doi: 10.1128/iai.39.1.142-146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaijser B, Larsson P, Nimmich W, Söderström T. Antibodies to Escherichia coli K and O antigens in protection against acute pyelonephritis. Prog Allergy. 1983;33:275–288. doi: 10.1159/000318336. [DOI] [PubMed] [Google Scholar]
- 50.Whitfield C, Roberts IS. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol Microbiol. 1999;31(5):1307–1319. doi: 10.1046/j.1365-2958.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 51.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffman S, Sorkin BC, White PC, et al. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982;257(13):7720–7729. [PubMed] [Google Scholar]
- 53.Jann K, Jann B. Polysaccharide antigens of Escherichia coli. Rev Infect Dis. 1987;9(Suppl 5):S517–S526. doi: 10.1093/clinids/9.supplement_5.s517. [DOI] [PubMed] [Google Scholar]
- 54.Vann WF, Schmidt MA, Jann B, Jann K. The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur J Biochem. 1981;116(2):359–364. doi: 10.1111/j.1432-1033.1981.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 55•.Rappuoli R, Bagnoli F. Vaccine Design: Innovative Approaches and Novel Strategies. Caister Academic; Norfolk, UK: 2011. Well-organized review of vaccine design strategies, including reverse vaccinology. [Google Scholar]
- 56.Johnson JR, Kuskowski MA, Gajewski A, et al. Extended virulence genotypes and phylogenetic background of Escherichia coli isolates from patients with cystitis, pyelonephritis, or prostatitis. J Infect Dis. 2005;191(1):46–50. doi: 10.1086/426450. [DOI] [PubMed] [Google Scholar]
- 57.Grischke EM, Rüttgers H. Treatment of bacterial infections of the female urinary tract by immunization of the patients. Urol Int. 1987;42(5):338–341. doi: 10.1159/000281988. [DOI] [PubMed] [Google Scholar]
- 58.Knuf M, Habermehl P, Faber J, et al. Assessment of nine candidate DTP-vaccines with reduced amount of antigen and/or without adjuvant as a fourth (booster-) dose in the second year of life. Vaccine. 2006;24(27–28):5627–5636. doi: 10.1016/j.vaccine.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 59.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 60.Uehling DT, Hopkins WJ, Balish E, Xing Y, Heisey DM. Vaginal mucosal immunization for recurrent urinary tract infection: Phase II clinical trial. J Urol. 1997;157(6):2049–2052. [PubMed] [Google Scholar]
- 61.Uehling DT, Hopkins WJ, Beierle LM, Kryger JV, Heisey DM. Vaginal mucosal immunization for recurrent urinary tract infection: extended Phase II clinical trial. J Infect Dis. 2001;183(Suppl 1):S81–S83. [Google Scholar]
- 62.Uehling DT, Hopkins WJ, Elkahwaji JE, Schmidt DM, Leverson GE. Phase 2 clinical trial of a vaginal mucosal vaccine for urinary tract infections. J Urol. 2003;170(3):867–869. doi: 10.1097/01.ju.0000075094.54767.6e. [DOI] [PubMed] [Google Scholar]
- 63.Hopkins WJ, Elkahwaji J, Beierle LM, Leverson GE, Uehling DT. Vaginal mucosal vaccine for recurrent urinary tract infections in women: results of a Phase 2 clinical trial. J Urol. 2007;177(4):1349–1353. doi: 10.1016/j.juro.2006.11.093. quiz 1591. [DOI] [PubMed] [Google Scholar]
- 64.Frey C, Obolensky W, Wyss H. Treatment of recurrent urinary tract infections: efficacy of an orally administered biological response modifier. Urol Int. 1986;41(6):444–446. doi: 10.1159/000281253. [DOI] [PubMed] [Google Scholar]
- 65.Hachen HJ. Oral immunotherapy in paraplegic patients with chronic urinary tract infections: a double-blind, placebo-controlled trial. J Urol. 1990;143(4):759–762. doi: 10.1016/s0022-5347(17)40084-x. discussion 762. [DOI] [PubMed] [Google Scholar]
- 66.Schulman CC, Corbusier A, Michiels H, Taenzer HJ. Oral immunotherapy of recurrent urinary tract infections: a double-blind placebo-controlled multicenter study. J Urol. 1993;150(3):917–921. doi: 10.1016/s0022-5347(17)35648-3. [DOI] [PubMed] [Google Scholar]
- 67.Bauer HW, Alloussi S, Egger G, Blümlein HM, Cozma G, Schulman CC Multicenter UTI Study Group. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol. 2005;47(4):542–548. doi: 10.1016/j.eururo.2004.12.009. discussion 548. [DOI] [PubMed] [Google Scholar]
- 68.Kim KS, Kim JY, Jeong IG, et al. A prospective multi-center trial of Escherichia coli extract for the prophylactic treatment of patients with chronically recurrent cystitis. J Korean Med Sci. 2010;25(3):435–439. doi: 10.3346/jkms.2010.25.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bauer HW, Rahlfs VW, Lauener PA, Blessmann GS. Prevention of recurrent urinary tract infections with immunoactive E. coli fractions: a meta-analysis of five placebo-controlled double-blind studies. Int J Antimicrob Agents. 2002;19(6):451–456. doi: 10.1016/s0924-8579(02)00106-1. [DOI] [PubMed] [Google Scholar]
- 70.Koukalova D, Krocova Z, Vitek P, Macela A, Hajek V. Immunostimulatory activity of the vaccine used in the treatment of recurrent urinary infections. II. Bratisl Lek Listy. 1999;100(4):215–217. [PubMed] [Google Scholar]
- 71.Koukalová D, Reif R, Hájek V, et al. Immunomodulation of recurrent urinary tract infections with Urvakol vaccine. Bratisl Lek Listy. 1999;100(5):246–251. [PubMed] [Google Scholar]
- 72.Marinova S, Nenkov P, Markova R, et al. Cellular and humoral systemic and mucosal immune responses stimulated by an oral polybacterial immunomodulator in patients with chronic urinary tract infections. Int J Immunopathol Pharmacol. 2005;18(3):457–473. doi: 10.1177/039463200501800306. [DOI] [PubMed] [Google Scholar]
- 73.Russo TA, Beanan JM, Olson R, et al. A killed, genetically engineered derivative of a wild-type extraintestinal pathogenic E. coli strain is a vaccine candidate. Vaccine. 2007;25(19):3859–3870. doi: 10.1016/j.vaccine.2007.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Billips BK, Yaggie RE, Cashy JP, Schaeffer AJ, Klumpp DJ. A live-attenuated vaccine for the treatment of urinary tract infection by uropathogenic Escherichia coli. J Infect Dis. 2009;200(2):263–272. doi: 10.1086/599839. [DOI] [PubMed] [Google Scholar]
- 75.Spurbeck RR, Stapleton AE, Johnson JR, Walk ST, Hooton TM, Mobley HL. Fimbrial profiles predict virulence of uropathogenic Escherichia coli strains: contribution of ygi and yad fimbriae. Infect Immun. 2011;79(12):4753–4763. doi: 10.1128/IAI.05621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thankavel K, Madison B, Ikeda T, et al. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J Clin Invest. 1997;100(5):1123–1136. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lane MC, Mobley HL. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 2007;72(1):19–25. doi: 10.1038/sj.ki.5002230. [DOI] [PubMed] [Google Scholar]
- 78.O’Hanley P, Lark D, Falkow S, Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roberts JA, Hardaway K, Kaack B, Fussell EN, Baskin G. Prevention of pyelonephritis by immunization with P-fimbriae. J Urol. 1984;131(3):602–607. doi: 10.1016/s0022-5347(17)50513-3. [DOI] [PubMed] [Google Scholar]
- 80.Roberts JA, Kaack MB, Baskin G, et al. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J Urol. 2004;171(4):1682–1685. doi: 10.1097/01.ju.0000116123.05160.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carnoy C, Moseley SL. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol Microbiol. 1997;23(2):365–379. doi: 10.1046/j.1365-2958.1997.2231590.x. [DOI] [PubMed] [Google Scholar]
- 82.Westerlund B, Kuusela P, Risteli J, et al. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989;3(3):329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 83.Goluszko P, Moseley SL, Truong LD, et al. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J Clin Invest. 1997;99(7):1662–1672. doi: 10.1172/JCI119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goluszko P, Goluszko E, Nowicki B, Nowicki S, Popov V, Wang HQ. Vaccination with purified Dr fimbriae reduces mortality associated with chronic urinary tract infection due to Escherichia coli bearing Dr adhesin. Infect Immun. 2005;73(1):627–631. doi: 10.1128/IAI.73.1.627-631.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou G, Mo WJ, Sebbel P, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001;114(Pt 22):4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 86.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93(18):9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iwahi T, Abe Y, Nakao M, Imada A, Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by Escherichia coli in mice. Infect Immun. 1983;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aronson M, Medalia O, Schori L, Mirelman D, Sharon N, Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D- mannopyranoside. J Infect Dis. 1979;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- 89.Langermann S, Palaszynski S, Barnhart M, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276(5312):607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 90.Langermann S, Möllby R, Burlein JE, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000;181(2):774–778. doi: 10.1086/315258. [DOI] [PubMed] [Google Scholar]
- 91.Langermann S, Ballou WR., Jr Vaccination utilizing the FimCH complex as a strategy to prevent Escherichia coli urinary tract infections. J Infect Dis. 2001;183(Suppl 1):S84–S86. [Google Scholar]
- 92.Meiland R, Geerlings SE, Langermann S, Brouwer EC, Coenjaerts FE, Hoepelman AI. Fimch antiserum inhibits the adherence of Escherichia coli to cells collected by voided urine specimens of diabetic women. J Urol. 2004;171(4):1589–1593. doi: 10.1097/01.ju.0000118402.01034.fb. [DOI] [PubMed] [Google Scholar]
- 93.Lim JK, Gunther NW, 4th, Zhao H, Johnson DE, Keay SK, Mobley HL. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect Immun. 1998;66(7):3303–3310. doi: 10.1128/iai.66.7.3303-3310.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eisenstein BI. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- 95.Nowicki B, Rhen M, Väisänen-Rhen V, Pere A, Korhonen TK. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J Bacteriol. 1984;160(2):691–695. doi: 10.1128/jb.160.2.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tchesnokova V, Aprikian P, Kisiela D, et al. type 1 fimbrial adhesin FimH elicits an immune response that enhances cell adhesion of Escherichia coli. Infect Immun. 2011;79(10):3895–3904. doi: 10.1128/IAI.05169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nielubowicz GR, Mobley HL. Host–pathogen interactions in urinary tract infection. Nat Rev Urol. 2010;7(8):430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 98.Rippere-Lampe KE, Lang M, Ceri H, Olson M, Lockman HA, O’Brien AD. Cytotoxic necrotizing factor type 1-positive Escherichia coli causes increased inflammation and tissue damage to the prostate in a rat prostatitis model. Infect Immun. 2001;69(10):6515–6519. doi: 10.1128/IAI.69.10.6515-6519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith YC, Rasmussen SB, Grande KK, Conran RM, O’Brien AD. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect Immun. 2008;76(7):2978–2990. doi: 10.1128/IAI.00075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Hanley P, Lalonde G, Ji G. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: efficacy of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect Immun. 1991;59(3):1153–1161. doi: 10.1128/iai.59.3.1153-1161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Torres AG, Redford P, Welch RA, Payne SM. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun. 2001;69(10):6179–6185. doi: 10.1128/IAI.69.10.6179-6185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102•.Chu BC, Garcia-Herrero A, Johanson TH, et al. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals. 2010;23(4):601–611. doi: 10.1007/s10534-010-9361-x. Good review of bacterial iron uptake during infection. [DOI] [PubMed] [Google Scholar]
- 103.Snyder JA, Haugen BJ, Buckles EL, et al. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun. 2004;72(11):6373–6381. doi: 10.1128/IAI.72.11.6373-6381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Faraldo-Gómez JD, Sansom MS. Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol. 2003;4(2):105–116. doi: 10.1038/nrm1015. [DOI] [PubMed] [Google Scholar]
- 105.Henderson JP, Crowley JR, Pinkner JS, et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009;5(2):e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Russo TA, McFadden CD, Carlino-MacDonald UB, Beanan JM, Olson R, Wilding GE. The Siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect Immun. 2003;71(12):7164–7169. doi: 10.1128/IAI.71.12.7164-7169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Durant L, Metais A, Soulama-Mouze C, Genevard JM, Nassif X, Escaich S. Identification of candidates for a subunit vaccine against extraintestinal pathogenic Escherichia coli. Infect Immun. 2007;75(4):1916–1925. doi: 10.1128/IAI.01269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carbonetti NH, Boonchai S, Parry SH, Väisänen-Rhen V, Korhonen TK, Williams PH. Aerobactin-mediated iron uptake by Escherichia coli isolates from human extraintestinal infections. Infect Immun. 1986;51(3):966–968. doi: 10.1128/iai.51.3.966-968.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia EC, Brumbaugh AR, Mobley HL. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun. 2011;79(3):1225–1235. doi: 10.1128/IAI.01222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watts RE, Totsika M, Challinor VL, et al. Contribution of siderophore systems to growth and urinary tract colonization of asymptomatic bacteriuria Escherichia coli. Infect Immun. 2012;80(1):333–344. doi: 10.1128/IAI.05594-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111•.Wieser A, Romann E, Magistro G, et al. A multiepitope subunit vaccine conveys protection against extraintestinal pathogenic Escherichia coli in mice. Infect Immun. 2010;78(8):3432–3442. doi: 10.1128/IAI.00174-10. Presents a multiepitope vaccine strategy for UTI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wieser A, Magistro G, Nörenberg D, Hoffmann C, Schubert S. First multiepitope subunit vaccine against extraintestinal pathogenic Escherichia coli delivered by a bacterial type-3 secretion system (T3SS) Int J Med Microbiol. 2012;302(1):10–18. doi: 10.1016/j.ijmm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 113.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 114.Rice JC, Peng T, Spence JS, et al. Pyelonephritic Escherichia coli expressing P fimbriae decrease immune response of the mouse kidney. J Am Soc Nephrol. 2005;16(12):3583–3591. doi: 10.1681/ASN.2005030243. [DOI] [PubMed] [Google Scholar]
- 115.Cirl C, Wieser A, Yadav M, et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008;14(4):399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 116.He Y, Xiang Z, Mobley HL. Vaxign: the first web-based vaccine design program for reverse vaccinology and applications for vaccine development. J Biomed Biotechnol. 2010;2010:297. doi: 10.1155/2010/297505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moriel DG, Bertoldi I, Spagnuolo A, et al. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic Escherichia coli. Proc Natl Acad Sci USA. 2010;107(20):9072–9077. doi: 10.1073/pnas.0915077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80(3):331–333. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uehling DT, Hopkins WJ, Dahmer LA, Balish E. Phase I clinical trial of vaginal mucosal immunization for recurrent urinary tract infection. J Urol. 1994;152(6 Pt 2):2308–2311. doi: 10.1016/s0022-5347(17)31664-6. [DOI] [PubMed] [Google Scholar]
- 120.Uehling DT, Hopkins WJ, James LJ, Balish E. Vaginal immunization of monkeys against urinary tract infection with a multi-strain vaccine. J Urol. 1994;151(1):214–216. doi: 10.1016/s0022-5347(17)34919-4. [DOI] [PubMed] [Google Scholar]
- 121.Sivick KE, Mobley HL. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun. 2010;78(2):568–585. doi: 10.1128/IAI.01000-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.Medimmune, Inc. [Accessed on 6 June 2012];Annual Report. 2002 www.astrazeneca.com/Investors/Annual-reports.