Abstract
An auxiliary-assisted, copper catalyzed or promoted sulfenylation of benzoic acid derivative β-C–H bonds and benzylamine derivative γ-C–H bonds has been developed. The method employs disulfide reagents, copper (II) acetate, and DMSO solvent at 90–130 °C. Application of this methodology to the direct trifluoromethylsulfenylation of C–H bonds was demonstrated.
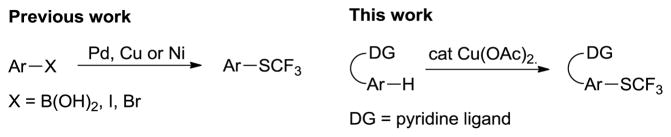
Aryl trifluoromethyl sulfides are important pharmaceuticals and agrochemicals.1 The introduction of trifluoromethylthio group into arenes leads to the increase of lipophilicity, thus enhancing bioavailability.1,2 Moreover, aryl trifluoromethyl sulfides are important intermediates for the synthesis of trifluoromethyl sulfones and sulfoxides.3 Conventional methods to introduce trifluoromethylthio moiety often require harsh reaction conditions and stoichiometric amounts of transition metals.1,4 Recently, efficient methods utilizing milder protocols have been developed. Buchwald demonstrated trifluoromethylthiolation of aryl bromides by employing a palladium catalyst and AgSCF3 reagent.5 Aryl bromides and iodides can also be converted to ArSCF3 by a nickel-catalyzed method developed by Vicic.6 More recently, Qing group reported the preparation of aryl trifluoromethyl sulfides from aryl boronic acids by a copper-catalyzed method.7 All these methods require prefunctionalized starting materials. Consequently, a method for direct trifluoromethylthiolation of aromatic C–H bonds would be highly advantageous due to potential shortening of synthetic pathways.8 We report here a method for an auxiliary-assisted sulfenylation of benzamide β-C–H bonds and benzylamine derivative γ-C–H bonds that is promoted by copper (II) acetate.
In recent years, abundant first-row transition metals such as copper have been explored as alternative catalysts for C–H bond functionalization.9–13 Yu showed that 2-phenylpyridine can be sulfenylated, aminated, halogenated, cyanated, and hydroxylated under copper catalysis.10 Deprotonative arylation and perfluoroalkylation of arenes and heterocycles using copper catalysis has also been developed.11 Several copper-catalyzed intramolecular heterocycle syntheses have been published.12 However, only a few methods for C–H bond sulfenylation have been published, and most of them involve functionalization of 2-phenylpyridine derivatives or deprotonative sulfenylation of acidic heterocycles.13 No examples of transition-metal catalyzed C–H bond trifluoromethylsulfenylation have been reported.
In 2005, we introduced picolinic acid and 8-aminoquinoline auxiliaries as removable directing groups for palladium-catalyzed C–H bond arylation.14a Subsequently, these directing groups have been employed in palladium-catalyzed arylation, alkylation, alkoxylation, and intramolecular amination of sp2 and sp3 C–H bonds.14 Reported methods for functionalization of 2-arylpyridines show that copper catalysis is possible even for non-acidic C–H bonds.9a,9f–i,10 However, the substrate scope is extremely limited due to presence of a non-removable pyridine moiety. We hypothesized that 8-aminoquinoline and picolinic acid auxiliaries would facilitate ortho-trifluoromethylsulfenylation of benzoic acid and benzylamine derivatives. Thus, 8-aminoquinoline was chosen as the auxiliary for the reaction optimization. Commercially available and easy-to-handle bis(trifluoromethyl)disulfide was chosen as the electrophile since most of trifluoromethylsulfide salts are either thermally unstable or not readily available.15 Moreover, bis(trifluoromethyl) disulfide can act as both thiolating reagent and oxidant. Dimethyl sulfoxide (DMSO) was selected as a solvent due to its ability to oxidize thiols to disulfides, allowing potential in situ regeneration of CF3SH to (CF3S)2.16
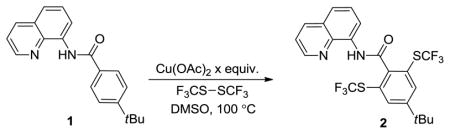
The reaction of 8-aminoquinoline 4-t-butylbenzoic acid amide with (CF3S)2 was investigated with respect to the amount of Cu(OAc)2 (Table 1). Stoichiometric copper acetate afforded good conversion to the desired product (Entry 1). Decreasing the amount of Cu(OAc)2 to 0.8 and 0.5 equivalents improved the yield of the product (Entries 2 and 3). However, if 0.2 equiv Cu(OAc)2 was employed, only 44% of 2 was formed (Entry 4). Consequently, 0.5 equivalents of Cu(OAc)2 was chosen for further studies.
Table 1.
Optimization of C–H Bond Trifluoromethylsulfenylationa
| ||
|---|---|---|
| Entry | x | % yield |
| 1 | 1 | 61 |
| 2 | 0.8 | 70 |
| 3 | 0.5 | 74 |
| 4 | 0.2 | 44 |
Yields were determined by NMR. See Supporting Information for details.
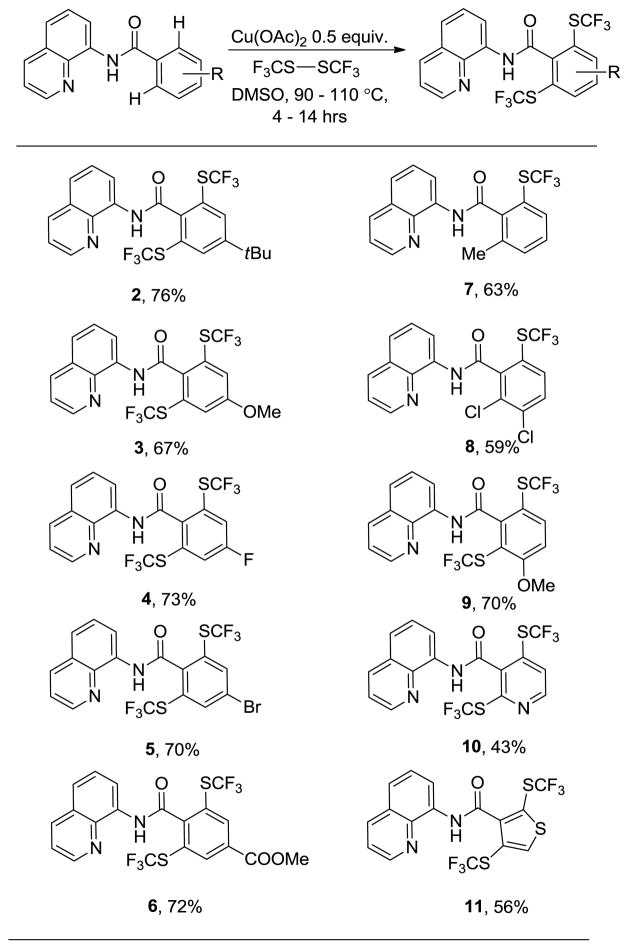
The scope of the reaction with respect to carboxylic acid derivatives is shown in Table 2. Various amides containing different electron donating and withdrawing groups are suitable substrates providing products in moderate to good yields. The reaction does not show any profound electronic preference, and both electron-rich (2, 3, 7) and electron-poor benzamides (4, 5, 6, 8) afford products in good yields. The reaction afforded difunctionalization products even with meta-substituted amides (9) in contrast with our previous palladium catalyzed arylation methodology where only monoarylated products were obtained in such cases.14a,b Moreover, the reaction demonstrated excellent functional group tolerance. Bromide (5), ester (6), and chloride functionalities (8) are tolerated. Both five- and six-membered heterocyclic substrates are also reactive. Nicotinamide (10) and 3-thiophenecarboxylic acid amide (11) were converted to disubstituted products in synthetically useful yields. Selective monotrifluoromethylsulfenylationation of N-(4-t-butylbenzoyl)-8-aminoquinoline could not be achieved under a variety of conditions. Either low conversion or about 1/1 mixture of mono and disubstituted products were obtained.17
Table 2.
Copper-catalyzed Trifluoromethylsulfenylation of Carboxylic Acid Derivativesa
|
Yields are isolated yields. Amide (1 equiv), CF3SSCF3 (2–2.5 equiv), Cu(OAc)2 (0.5 equiv), DMSO, 90–110 °C.
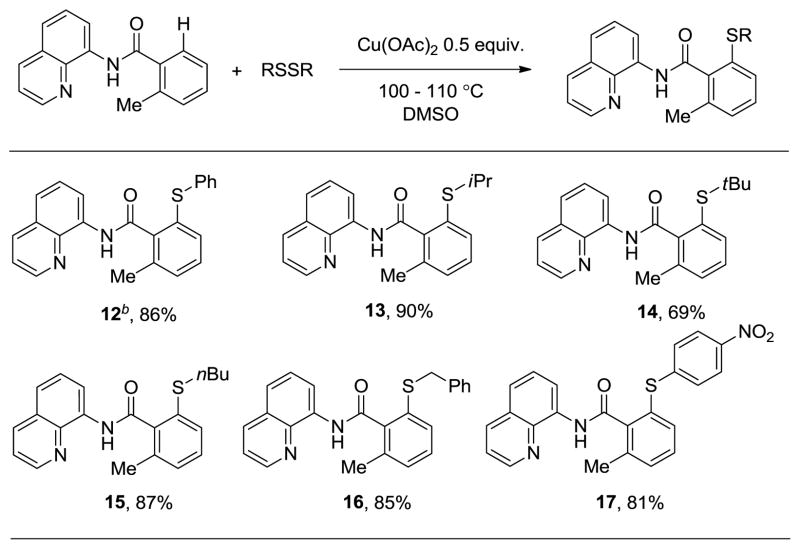
We were pleased to discover that other aryl and alkyl disulfides are also active sulfenylating reagents. Reactions of o-toluic acid amide with various disulfides are presented in Table 3. A short optimization revealed that the reaction using diphenyl disulfide reagent requires K2CO3 base in order to achieve high conversion (12). Various dialkyl (13–16) and diaryl disulfides (12, 17) are reactive and products were obtained in excellent yields. Slightly lower yield was obtained by using di-t-butyl disulfide (14), presumably due to increased steric bulk. Nitro group is tolerated under the reaction conditions (17), further demonstrating the excellent functional group tolerance of the method.
Table 3.
Disulfide Scope in C–H Bond Sulfenylationa
|
Yields are isolated yields. Amide (1 equiv), RSSR (2–2.5 equiv), Cu(OAc)2 (0.5 equiv), DMSO, 100–110 °C.
K2CO3 additive (1 equiv). Please see Supporting Information for details.
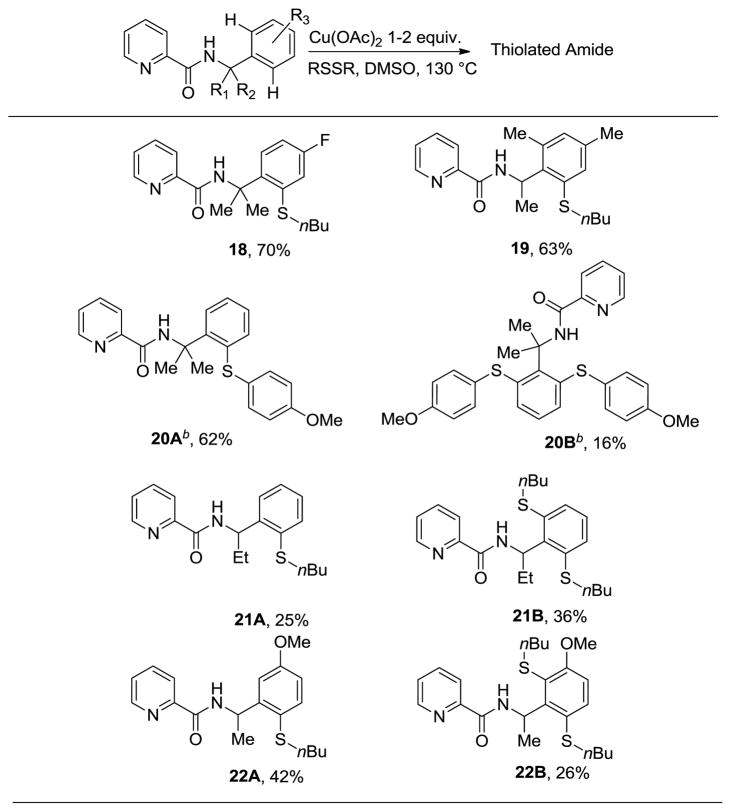
Benzylamine derivatives can also be sulfenylated by employing Cu(OAc)2, disulfide reagents, and a picolinic acid directing group. In contrast with 8-aminoquinoline benzamides, benzylamine picolinamides require stoichiometric amount of copper(II) acetate promoter and higher reaction temperatures. Using the optimized conditions, various benzylamine derivatives were sulfenylated at γ-sp2 C–H bonds in moderate to good yields (Table 4). p-Fluoro-α,α-dimethylbenzylamine and 2,4-dimethyl-α-methylbenzylamine amides were mono-n-butylsulfenylated in good yields (18 and 19). p-Methoxyphenylsulfenylation of α,α-dimethylbenzylamine picolinamide affords monosubstitution product in 62% yield accompanied by disubstitution product in 16% yield (20A and 20B).
Table 4.
Copper-mediated Sulfenylation of Amine Derivativesa
|
Yields are isolated yields. Amide (1 equiv.), RSSR (2–2.5 equiv), Cu(OAc)2 (1–2 equiv), DMSO, 130 °C.
K2CO3 additive (1 equiv).
α-Ethylbenzylamine and α–methyl-3-methoxybenzylamine amides gave a mixture of approximately equal amounts of mono- and disubstitution products (21A, 21B, 22A, and 22B). Trifluoromethylsulfenylation of N-(1-methyl-1(4-fluorophenyl)ethyl)picolinamide gave 5% yield of monosubstitution product.
While the mechanism of the reaction is unclear at this moment, two relevant observations may be discussed. First, it is known that sulfide ligands stabilize high-valent Cu(III).18 In some cases, disulfides can oxidize Cu(I) to Cu(III).18b Second, Stahl has shown that nucleophiles can react with arylcopper (III) species affording C-heteroatom coupling products.19
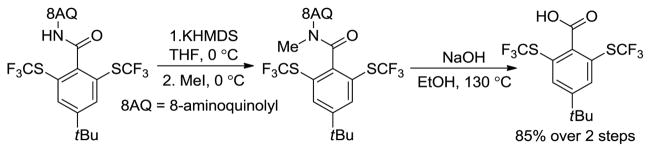
The 8-aminoquinoline group can be efficiently removed in a two-step procedure by amide N-methylation followed by base hydrolysis. The trifluoromethylthiolated acid was obtained in high yield (Scheme 2).
Scheme 2.
Removal of 8-Aminoquinoline Auxiliary
In order to confirm the role of the copper as an active catalyst, control experiments were performed. With reagent grade and ultra-pure Cu(OAc)2 nearly identical results were obtained, showing that catalysis by contaminants is unlikely. If copper salt was omitted, no product was obtained (Scheme 3).
Scheme 3.
Control Experiments
In conclusion, we have developed a method for direct, auxiliary-assisted sulfenylation of β-sp2 C–H bonds of benzoic acid derivatives and γ-sp2 C–H bonds of benzylamine derivatives. The reaction employs catalytic or stoichiometric Cu(OAc)2, disulfide reagent, and DMSO solvent at elevated temperatures. The utilization of inexpensive copper acetate and removable directing group are significant advantages that allow for an increased usefulness of the reaction. The reaction shows high generality, excellent selectivity towards ortho C–H bonds, as well as good functional group tolerance. This method provides a novel and straightforward way for the preparation of aryl trifluoromethyl thioethers. Mechanistic studies of the transformation as well as attempts to isolate reaction intermediates are in progress.
Supplementary Material
Scheme 1.
Trifluoromethylsulfenylation
Acknowledgments
We thank the Welch Foundation (Grant No. E-1571), National Institute of General Medical Science (Grant No. R01GM077635), Camille and Henry Dreyfus Foundation, and Norman Hackerman Advanced Research Program for supporting this research.
Footnotes
Detailed experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.(a) Leroux F, Jeschke P, Schlosser M. Chem Rev. 2005;105:827–856. doi: 10.1021/cr040075b. [DOI] [PubMed] [Google Scholar]; (b) Boiko VN, Beilstein J. Org Chem. 2010;6:880–921. doi: 10.3762/bjoc.6.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Jeschke P. Pest Manag Sci. 2010;66:10–27. doi: 10.1002/ps.1829. [DOI] [PubMed] [Google Scholar]; (b) Yagupol’skii LM, Il’chenko AYa, Kondratenko NV. Russ Chem Rev. 1974;43:64–94. [Google Scholar]; (c) Hansch C, Leo A, Unger SH, Kim KH, Nikaitani D, Lien EJ. J Med Chem. 1973;16:1207–1216. doi: 10.1021/jm00269a003. [DOI] [PubMed] [Google Scholar]
- 3.(a) Xu L, Cheng J, Trudell ML. J Org Chem. 2003;68:5388. doi: 10.1021/jo030031n. [DOI] [PubMed] [Google Scholar]; (b) Tang RY, Zhong P, Lin QL. J Fluorine Chem. 2007;128:636–640. [Google Scholar]; (c) Marom H, Antonov S, Popowski Y, Gozin M. J Org Chem. 2011;76:5240–5246. doi: 10.1021/jo2001808. [DOI] [PubMed] [Google Scholar]; (d) Kirsch P, Lenges M, Kühne D, Wanczek KP. Eur J Org Chem. 2005:797–802. [Google Scholar]
- 4.Manteau B, Pazenok S, Vors JP, Leroux FR. J Fluorine Chem. 2010;131:140–158.Electrophilic trifluoromethylsulfenylation: Baert F, Colomb J, Billard T. Angew Chem Int Ed. 2012;51:10382. doi: 10.1002/anie.201205156.
- 5.Teverovskiy G, Surry DS, Buchwald SL. Angew Chem Int Ed. 2011;50:7312–7314. doi: 10.1002/anie.201102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Zhang CP, Vicic DA. J Am Chem Soc. 2012;134:183–185. doi: 10.1021/ja210364r. [DOI] [PubMed] [Google Scholar]; (b) Zhang CP, Vicic DA. Chem Asian J. 2012;7:1756–1758. doi: 10.1002/asia.201200347. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Xie Y, Chu L, Wang R-W, Zhang X, Qing F-L. Angew Chem Int Ed. 2012;51:2492–2495. doi: 10.1002/anie.201108663. [DOI] [PubMed] [Google Scholar]
- 8.Reviews: Colby DA, Bergman RG, Ellman JA. Chem Rev. 2010;110:624–655. doi: 10.1021/cr900005n.Ackermann L, Vicente R, Kapdi AR. Angew Chem, Int Ed. 2009;48:9792–9826. doi: 10.1002/anie.200902996.Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem, Int Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273.Seregin IV, Gevorgyan V. Chem Soc Rev. 2007;36:1173–1193. doi: 10.1039/b606984n.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e.Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074–1086. doi: 10.1021/ar9000058.Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174–238. doi: 10.1021/cr0509760.Messaoudi S, Brion JD, Alami M. Eur J Org Chem. 2010:6495–6516.Yeung CS, Dong VM. Chem Rev. 2011;111:1215–1292. doi: 10.1021/cr100280d.Sun CL, Li BJ, Shi ZJ. Chem Commun. 2010:677–685. doi: 10.1039/b908581e.Li CJ. Acc Chem Res. 2009;42:335–344. doi: 10.1021/ar800164n.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem-Eur J. 2010;16:2654–2672. doi: 10.1002/chem.200902374.Catellani M, Motti M, Della Ca′ N, Ferraccioli R. Eur J Org Chem. 2007:4153–4165.
- 9.(a) Wendlandt AE, Suess AM, Stahl SS. Angew Chem Int Ed. 2011;50:11062–11087. doi: 10.1002/anie.201103945. [DOI] [PubMed] [Google Scholar]; (b) Kitahara M, Umeda N, Hirano K, Satoh T, Miura M. J Am Chem Soc. 2011;133:2160–2162. doi: 10.1021/ja111401h. [DOI] [PubMed] [Google Scholar]; (c) Nishino M, Hirano K, Satoh T, Miura M. Angew Chem Int Ed. 2012;51:6993–6997. doi: 10.1002/anie.201201491. [DOI] [PubMed] [Google Scholar]; (d) Li Z, Bohle DS, Li CJ. Proc Natl Acad Sci USA. 2006;103:8928–8933. doi: 10.1073/pnas.0601687103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ban I, Sudo T, Taniguchi T, Itami K. Org Lett. 2008;10:3607–3609. doi: 10.1021/ol8013717. [DOI] [PubMed] [Google Scholar]; (f) Wang W, Luo F, Zhang S, Cheng J. J Org Chem. 2010;75:2415–2418. doi: 10.1021/jo1000719. [DOI] [PubMed] [Google Scholar]; (g) Wang W, Pan C, Chen F, Cheng J. Chem Commun. 2011;47:3978–3980. doi: 10.1039/c0cc05557c. [DOI] [PubMed] [Google Scholar]; (h) Uemura T, Imoto S, Chatani N. Chem Lett. 2006;35:842–843. [Google Scholar]; (i) John A, Nicholas KM. J Org Chem. 2011;76:4158–4162. doi: 10.1021/jo200409h. [DOI] [PubMed] [Google Scholar]; (j) Wang Q, Schreiber SL. Org Lett. 2009;11:5178–5180. doi: 10.1021/ol902079g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) King AE, Huffman LM, Casitas A, Costas M, Ribas X, Stahl SS. J Am Chem Soc. 2010;132:12068–12073. doi: 10.1021/ja1045378. [DOI] [PubMed] [Google Scholar]; (m) Usui S, Hashimoto Y, Morey JV, Wheatley AEH, Uchiyama M. J Am Chem Soc. 2007;129:15102–15103. doi: 10.1021/ja074669i. [DOI] [PubMed] [Google Scholar]; (p) Holland PL, Rodgers KR, Tolman WB. Angew Chem Int Ed. 1999;38:1139–1142. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1139::AID-ANIE1139>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.(a) Chen X, Hao XS, Goodhue CE, Yu JQ. J Am Chem Soc. 2006;128:6790–6791. doi: 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]; (b) Chen X, Dobereiner G, Hao X-S, Giri R, Maugel N, Yu JQ. Tetrahedron. 2009;65:3085–3089. [Google Scholar]
- 11.(a) Do HQ, Daugulis O. J Am Chem Soc. 2007;129:12404–12405. doi: 10.1021/ja075802+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Do HQ, Khan RMK, Daugulis O. J Am Chem Soc. 2008;130:15185–15192. doi: 10.1021/ja805688p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Do HQ, Daugulis O. J Am Chem Soc. 2009;131:17052–17053. doi: 10.1021/ja907479j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Do HQ, Daugulis O. J Am Chem Soc. 2011;133:13577–13586. doi: 10.1021/ja2047717. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Popov I, Lindeman S, Daugulis O. J Am Chem Soc. 2011;133:9286–9289. doi: 10.1021/ja2041942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Brasche G, Buchwald SL. Angew Chem Int Ed. 2008;47:1932–1934. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]; (b) Bernini R, Fabrizi G, Sferrazza A, Cacchi S. Angew Chem Int Ed. 2009;48:8087–8081. doi: 10.1002/anie.200902440. [DOI] [PubMed] [Google Scholar]; (c) Ueda S, Nagasawa H. Angew Chem Int Ed. 2008;47:6411–6413. doi: 10.1002/anie.200801240. [DOI] [PubMed] [Google Scholar]; (d) Ueda S, Nagasawa H. J Org Chem. 2009;74:4272–4277. doi: 10.1021/jo900513z. [DOI] [PubMed] [Google Scholar]; (e) Guru MM, Ali MA, Punniyamurthy T. Org Lett. 2011;13:1194–1197. doi: 10.1021/ol2000809. [DOI] [PubMed] [Google Scholar]; Wang H, Wang Y, Peng C, Zhang J, Zhu Q. J Am Chem Soc. 2010;132:13217–13219. doi: 10.1021/ja1067993. [DOI] [PubMed] [Google Scholar]
- 13.(a) Zhang S, Qian P, Zhang M, Hu M, Cheng J. J Org Chem. 2010;75:6732–6735. doi: 10.1021/jo1014849. [DOI] [PubMed] [Google Scholar]; (b) Chu L, Yue X, Qing FL. Org Lett. 2010;12:1644–1647. doi: 10.1021/ol100449c. [DOI] [PubMed] [Google Scholar]; (c) Ranjit S, Lee R, Heryadi D, Shen C, Wu J, Zhang P, Huang K-W, Liu X. J Org Chem. 2011;76:8999–9007. doi: 10.1021/jo2017444. [DOI] [PubMed] [Google Scholar]; (d) Dai C, Xu Z, Huang F, Yu Z, Gao YF. J Org Chem. 2012;77:4414–4419. doi: 10.1021/jo202624s. [DOI] [PubMed] [Google Scholar]
- 14.(a) Zaitsev VG, Shabashov D, Daugulis O. J Am Chem Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; (b) Shabashov D, Daugulis O. J Am Chem Soc. 2010;132:3965–3972. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nadres ET, Daugulis O. J Am Chem Soc. 2012;134:7–10. doi: 10.1021/ja210959p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) He G, Zhao Y, Zhang S, Lu C, Chen G. J Am Chem Soc. 2012;134:3–6. doi: 10.1021/ja210660g. [DOI] [PubMed] [Google Scholar]
- 15.(a) Tyrra W, Naumann D, Hoge B, Yagupolskii YLJ. Fluorine Chem. 2003;119:101–107. [Google Scholar]; (b) Tavener S, Adams DJ, Clark JHJ. Fluorine Chem. 1999;95:171–176. [Google Scholar]; (c) Adams DJ, Clark JH, Heath PA, Hansen LB, Sanders VC, Tavener SJJ. Fluorine Chem. 2000;101:187–191. [Google Scholar]; (d) Clark JH, Smith HJ. Fluorine Chem. 1993;61:223–231. [Google Scholar]; (e) Dmowski W, Hass A. J Chem Soc Perkin Trans I. 1987:2119–2124. [Google Scholar]; (f) Emeléus HJ, MacDuffie DE. J Chem Soc. 1961:2597–2599. [Google Scholar]
- 16.Wallace TJ. J Am Chem Soc. 1964;86:2018–2021. [Google Scholar]
- 17.Please see Supporting Information for details.
- 18.(a) Beurskens PT, Cras JA, Steggerda JJ. Inorg Chem. 1968;7:810–813. [Google Scholar]; (b) Willert-Porada MA, Burton DJ, Baenziger NC. J Chem Soc, Chem Commun. 1989:1633–1634. [Google Scholar]
- 19.Huffman LM, Stahl SS. J Am Chem Soc. 2008;130:9196–9197. doi: 10.1021/ja802123p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.