SYNOPSIS
Mother-infant interactions in rodents can be used to explore the biological basis of postnatal parental effects. There is emerging evidence from laboratory studies that variation in early life experiences can induce molecular changes in the developing brain which lead to activation or silencing of genes. These epigenetic effects may account for the stability of the effects of parenting on offspring development and the transmission of parenting from one generation to the next. In this article, we highlight evidence supporting a role for epigenetic mechanisms in the consequences, transmission, and variability in parenting. Although primarily drawn from laboratory studies in rodents, this evidence may also provide some insights into key questions within the study and practice of human parenting. We discuss these questions, highlighting both the challenges and benefits of using translational approaches.
INTRODUCTION
Experiences occurring early in life can set the stage for later development, leading to long-term changes in physiology, brain, and behavior. In mammals, the quality of the early life environment is affected by the interactions between parents and offspring. Although it is perhaps not surprising that extreme forms of early negative experiences, such as abuse or neglect, would induce significant changes in offspring development, studies in humans, primates, and laboratory rodents suggest that variations in parental care within the normal range can likewise shift developmental trajectories. Moreover, the quality of parental care received in infancy is a significant predictor of offspring and grand-offspring parental behavior, suggesting that these developmental effects persist across generations. These findings raise several questions which are critical to understanding the relation between parenting behavior and offspring development, including: (1) What are the neurobiological and molecular effects of parent-infant interactions? (2) What mechanisms allow for variations in parenting behavior to persist from one generation to the next? (3) What environmental factors lead to variations in parenting behavior?
Although studies in humans have provided some insight into these issues, experimental evidence for the neurobiological and molecular impact of early life experiences has relied primarily on laboratory studies in rodents. In this article, we discuss how studies of mother-infant interactions in rodents can be used to explore the biological basis of postnatal parental effects. Emerging evidence from these studies suggests that variation in early life experiences can induce molecular changes in the developing brain which lead to activation or silencing of genes involved in stress sensitivity, neurodevelopment, and social/reproductive behavior. These molecular changes to gene activity, referred to as “epigenetic” effects, may account for the stability of the effects of parenting on offspring development and the transmission of parenting behavior from one generation to the next. Here, we highlight evidence supporting a role for epigenetic mechanisms in the consequences, transmission, and variability in parenting. Although primarily drawn from laboratory studies in rodents, this evidence may also provide some insights into key issues within the study and practice of human parenting, such as (1) Are there optimal forms of parenting? (2) How can parenting practices, interventions, and social policy use this information? (3) How can future studies in this field be used to inform the study of parenting? We discuss these questions, highlighting both the challenges and benefits of using translational approaches.
VARIATION IN MATERNAL BEHAVIOR IN RODENTS
Our understanding of the neurobiological basis and consequence of maternal behavior is based primarily on studies of laboratory rodents (rats and mice). The use of rodents allows experimental manipulation of early life experiences, access to brain tissue for region-specific analysis, and the ability to study brain and behavior of mothers (dams) and offspring across multiple generations. The behavioral repertoire of postpartum rat dams is complex and involves nest building, retrieving pups to the nest, nursing, and licking/grooming (LG) pups, in addition to non-pup-directed behaviors including self-grooming, eating, and drinking. Extensive home cage observations of dams with their litters over the first postpartum week reveal a natural variation in maternal LG behavior (Champagne, Francis, Mar, & Meaney, 2003). Thus, based on observational data, females can be divided into categories based on the frequency with which they engage in pup LG, such that females are either Low or High LG dams, representing opposite ends within the natural spectrum of maternal behavior. These females and their offspring can then be compared.
POSTNATAL MATERNAL INFLUENCE ON DEVELOPMENT
Variation in postpartum maternal LG has implications for the developing infant brain. In laboratory studies using Long-Evans rats, offspring reared by a Low LG dam are found to exhibit heightened sensitivity to stressors in adulthood. Activation of the hypothalamic-pituitary-adrenal (HPA) axis stimulates physiological and behavioral responses to stressors, while glucocorticoid receptors (GR) in the hippocampus feed back to the HPA axis to reduce activity and stress response (Meaney et al., 1991). Offspring reared by Low LG dams are observed to have reduced levels of hippocampal GR in infancy which persist into adulthood and are associated with elevated adult HPA activity (Meaney, 2001). These neurobiological and physiological effects may account for the behavioral inhibition (reduced time exploring the more “anxiogenic” inner area of a novel environment) that is observed when offspring of Low LG dams are placed in a novel environment and may have implications for long-term health outcomes. These behavioral outcomes are likely to emerge developmentally (although there have yet to be developmental studies of stress/behavioral reactivity using this model) coincident with the maternal induced changes in neurobiological targets associated with the HPA response to stress.
The experience of Low LG compared to High LG has broad effects on the brain and behavior of offspring that extend beyond the changes in stress responsivity (Meaney, 2001). Low LG is associated with impaired learning/memory, reduced hippocampal plasticity, and changes in gene activity within the hippocampal region. Amongst female offspring, low levels of maternal LG are associated with decreased oxytocin receptor (OTR) levels, decreased sensitivity to estrogen, and decreased estrogen receptor alpha (ERα) protein and gene expression in the medial preoptic area of the hypothalamus (MPOA) (Champagne, 2008). The MPOA is a brain region that is critical for maternal care, and sensitivity to estrogens within this brain region is essential for the ability to “prime” the maternal brain. Consequent to these neurobiological effects, female offspring exhibit variations in maternal LG that are predicted by the frequency of LG experienced during infancy. Overall, it is evident that variation within the normal range of parent-offspring interactions can have profound effects on numerous neurobiological and neuroendocrine circuits leading to persistent changes in behavior. The challenge of this research is in understanding how such long-term effects can be achieved.
EPIGENETICS AND THE INFLUENCE OF PARENT-OFFSPRING INTERACTIONS
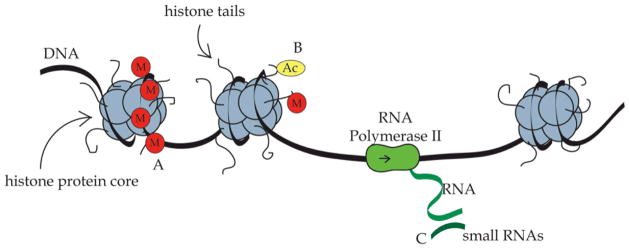
The neurodevelopmental and behavioral consequences of mother-infant interactions that have been observed amongst laboratory rodents has led to further exploration of the molecular mechanisms that may be associated with these effects. In many cases, these effects are observed in species where there is limited genetic variability and in experimental designs that use cross-fostering to illustrate the role of environmental rather than genetic factors in predicting the long-term outcomes. Thus, there would appear to be factors other than DNA sequence variation which are critical for these developmental effects. The activity or expression of genes can lead to a cascade of neurodevelopmental changes and is dependent on accessibility of the DNA to transcriptional machinery. Gene expression can be altered through several “epigenetic” mechanisms (molecular changes that alter DNA expression without altering DNA sequence), including DNA methylation, histone protein modification, and effects on transcription/translation via small RNAs (microRNAs; see Figure 1). DNA methylation is perhaps the most relevant of these mechanisms for understanding the stability and plasticity of parental effects and involves attachment of methyl chemical groups to cytosines within cytosine-guanine (CpG) nucleotide pairs in DNA. When DNA is methylated, access to the gene is decreased, leading to silencing of the gene (Razin, 1998). DNA methylation is a form of epigenetic gene regulation that is highly stable and heritable through cell division. Although epigenetic mechanisms are complex and our knowledge of the dynamics of these pathways is still in its infancy, emerging evidence suggests that mechanisms such as DNA methylation may provide a link between the experience of variation in early environmental experiences and persistent changes in gene activity, neurodevelopment, and behavior (Champagne, 2010).
Figure 1.
Epigenetic regulation of gene expression. DNA is wrapped around histone proteins and gene expression is dependent on the enzyme RNA Polymerase II accessing the DNA and creating RNA. (A) Attachment of methyl chemical groups (M) to the DNA sequence suppresses gene expression. (B) Histone proteins have “tails” that can be modified by attachment of acetyl (Ac) and methyl groups to specific sites. Histone tail acetylation increases gene transcription, while histone tail methylation typically decreases gene activity. (C) Small RNA molecules such as microRNAs can bind to and degrade newly transcribed RNA, repressing gene activity.
Variation in postnatal maternal LG has been associated with epigenetic effects in offspring. Offspring reared by Low LG dams have reduced expression of hippocampal GR, and increased DNA methylation within the GR promoter region (DNA regions which control gene expression), which may account for this reduced gene activity (Weaver et al., 2004). Although it is unclear exactly how maternal LG induces this epigenetic effect, in the case of GR DNA methylation, high levels of LG are associated with an apparent demethylation of the GR promoter region whereas amongst offspring who receive low levels of LG, the high levels of DNA methylation that are observed at birth are maintained during the postnatal period and into adulthood. Maternal LG also affects γ-aminobutyric acid (GABA) circuits, and in a recent study, reduced hippocampal levels of glutamic acid decarboxylase (GAD1, an enzyme involved with GABA synthesis) were found in male offspring of Low LG dams and associated with increased DNA methylation within the GAD1 gene promoter (Zhang et al., 2010). The stability of DNA methylation may account for the persistence of these early rearing effects, and exploration of this epigenetic mechanism in rodent models of early-life maternal separation and abuse confirm the responsiveness of this epigenetic pathway to the quality of parent-offspring interactions (Champagne, 2010).
TRANSGENERATIONAL EFFECTS OF PARENTING
Parenting styles are often similar from one generation to the next. Amongst laboratory rats, mothers, daughters, and granddaughters all lick and groom their litters at similar frequencies (Champagne & Meaney, 2007), and cross-fostering studies support the hypothesis that this behavioral transmission of maternal LG is not dependent on the transmission of specific DNA variants (Champagne, et al., 2003). Epigenetic mechanisms may help to explain the persistence of maternal LG across generations. The level of LG a female experiences in infancy predicts the pattern of gene expression of OTR and ERα within the MPOA of the hypothalamus. The persistence of the maternal LG effects on ERα gene activity in offspring are associated with DNA methylation of the ERα gene promoter - daughters of Low LG dams have a highly methylated ERα gene promoter region, while daughters of High LG dams have a relatively unmethylated ERα promoter (Champagne, 2008). This epigenetic effect in daughters leads to altered LG behavior which then shapes the DNA methylation, gene expression, and neurobiological circuits involved in maternal care in grand-daughters.
Similar transgenerational effects on maternal LG have been observed in response to communal rearing in mice. In naturalistic settings, rodents frequently engage in communal care of offspring, where newborn pups from multiple litters are grouped together and nurtured by multiple caregivers. In the laboratory, communally nesting dams display increased frequencies of nursing and LG compared to standard non-communally housed dams. Female offspring who have experienced communal rearing in infancy exhibit increased maternal care in adulthood, as do the female offspring of these communally reared females (Curley, Davidson, Bateson, & Champagne, 2009). Thus, stability of maternal LG across generations may be a common occurrence across species of rodents. This stability in the transmission of variation in maternal care also has implications for the neurobiological and behavioral development of offspring and grand-offspring on measures of stress responsivity, response to novelty, and cognitive ability, as these aspects of development are shaped by the quality of maternal care experienced in infancy.
VARIATION IN THE MATERNAL BRAIN: INFLUENCE OF THE ENVIRONMENT
Evidence for the persistent and multigenerational effect of variation in parent-offspring interactions leads us to consider a critical question–What factors lead to variation in parental care? This question can be considered from both a neurobiological and environmental perspective. Studies of laboratory rodents suggest that several brain regions are critical for the expression of active maternal care behaviors, including the MPOA, lateral septum, bed nucleus of the stria terminalis (BNST), and central nucleus of the amygdala (see Figure 2). The neurobiological basis of variations in maternal LG can be explained, in part, by differences in gene expression and protein levels within these brain regions (Champagne, 2008; Meaney, 2001).
Figure 2.
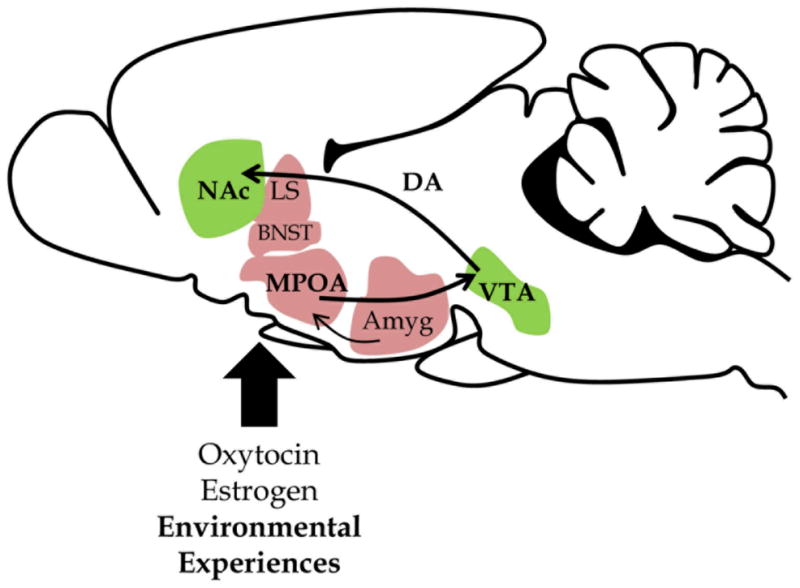
Neurobiology of the rodent maternal brain. The neurobiological circuits involved in variation in maternal LG include the medial preoptic area (MPOA), bed nucleus of the stria terminalis (BNST), lateral septum (LS), central amygdala (Amyg), ventral tegmental area (VTA), and nucleus accumbens (NAc). Levels of oxytocin and estrogen receptors in the MPOA influence the frequency of maternal LG care displayed. These hypothalamic effects have downstream consequences for activation within the mesolimbic dopamine (DA) system, which includes projections from the VTA to NAc. Environmental experiences shape the development of these circuits leading to altered LG behavior.
High LG dams have increased levels of OTR in the MPOA, lateral septum, BNST, and central amygdala, as well as elevated levels of ERα in the MPOA, compared to Low LG dams (Champagne, Diorio, Sharma, & Meaney, 2001). Variations in maternal behavior are also accompanied by differences in neural pathways targeted by the MPOA. Oxytocin neurons in the MPOA project to the ventral tegmental area (VTA), a region of the ventral midbrain that is rich in dopamine neurons and one of the main centers of reward processing. High LG dams have greater numbers of oxytocin neurons projecting from the MPOA onto dopamine neurons in the VTA compared to Low LG dams (Shahrokh, Zhang, Diorio, Gratton, & Meaney, 2010). During pup LG, more dopamine is released from the VTA into the nucleus accumbens (NAc) of High LG dams compared to Low LG dams (Champagne et al., 2004). These studies suggest that Low and High LG mothers may differ in their motivation to interact with their pups. Thus variation in hormone sensitive pathways (MPOA) which then alter reward/motivation pathways (dopamine neurons) may lead to variation in LG during the postpartum period.
Although it is clear that variation in maternal LG is shaped by the neurobiological differences between Low LG and High LG dams, the question that remains is How are these neurobiological differences in the maternal brain achieved? The transmission of maternal behavior from one generation to the next, described in the previous section, suggests that variation in maternal LG experienced in infancy is a significant predictor of variation in the maternal brain. However, other environmental experiences occurring during the lifespan of an individual can also induce shifts in maternal behavior. Although offspring reared by High LG dams would be expected to exhibit high levels of LG in adulthood, the experience of social isolation stress throughout the juvenile period of development reduces LG amongst the offspring of High LG dams and is associated with decreases in OTR in the MPOA and amygdala (Champagne & Meaney, 2007). Conversely, juvenile social and environmental enrichment (group-housed living in an environment with toys etc.) increases, or “rescues,” the LG behavior of offspring reared by Low LG dams, and is associated with increased in OTR in the MPOA and amygdala. This plasticity in the maternal brain continues into adulthood. Chronic stress during pregnancy decreases the LG frequency of High LG dams, an effect that is persistent across subsequent mating accompanied by decreases in OTR in the MPOA, BNST, and amygdala (Champagne & Meaney, 2006). This plasticity may indicate a lack of critical period in environmental induced changes in the maternal brain, although further studies are necessary to determine critical periods for the effects of particular stimuli (maternal care, isolation, enrichment) and whether the epigenetic mechanisms targeted by early life experiences (e.g., DNA methylation) are likewise recruited to achieve the effects of later life experiences.
IMPLICATIONS AND TRANSLATION OF LABORATORY STUDIES
Studies examining the consequences, transmission, and variation in parenting behavior using laboratory rodents have provided some novel insights into the biological pathways linking experiences, occurring either in infancy or in later life, and the frequency of nurturing behaviors. A general question that is raised by these findings concerns the relevance to human parenting. Although humans and primates do not lick their offspring as a primary form of parental care, there are certainly parallels between the effects of LG and the frequency of parent-infant contact (holding, carrying, skin-to-skin contact) that are more typical of human parenting. As such, it would appear that the tactile experiences of the developing infant are important for neurodevelopment and growth (Weiss, Wilson, & Morrison, 2004). In rodents, there is also significant role for the neurobiological pathways involved in motivation (e.g., dopaminergic inputs from the VTA to the NAc) in regulating LG, and these same pathways have been implicated in the response of human parents to infant stimuli (Swain, Lorberbaum, Kose, & Strathearn, 2007). Given these parallels, specific questions can be raised regarding the value and translation of studies of natural variations in maternal LG in laboratory rats to our understanding of human parenting. Here we explore three questions that are relevant to the science and practice of parenting.
Are There Optimal Forms of Parenting?
When considering the consequences of natural variations in LG in rodents, it is tempting to apply the labels of “good” or “bad” to High LG and Low LG, respectively. The outcomes of Low LG, such as heightened stress responsivity and reduced cognitive ability would certainly be consequences for offspring that are considered “not-optimal”. However, it is important to note that natural variations in maternal LG do not lead to increased mortality. Offspring of Low LG and High LG dams are equivalent on gross measures of health and welfare. Interestingly, despite performing more poorly on cognitive measures under “standard” testing conditions, when offspring of Low LG dams are in a heighted physiological state of stress, they exhibit enhanced learning and hippocampal plasticity (Champagne et al., 2008). Thus, determining whether a rearing environment is “optimal” may depend on the quality of the environment experienced later in life. Low LG experience may be beneficial to the functioning of offspring living in stressful environments (e.g., enhancing stress-induced cognition or reacting to threats more rapidly due to enhanced HPA activity), whereas High LG leads to adaptive functioning in low-stress environments (e.g., increasing exploration leading to increased access to potential resources). Related to the idea of context-dependent benefits of high or low parenting is evidence for trade-offs in reproduction that are observed in the female offspring of High LG and Low LG dams. Although female offspring of Low LG dams exhibit reduced maternal care, there is emerging evidence that these females have heightened sexual receptivity and produce more litters than female offspring of High LG dams (Cameron et al., 2008). This enhancement in sexual behavior amongst offspring of Low LG dams is accompanied by up-regulation of the neuroendocrine systems which are associated with this aspect of reproduction. These findings suggest that reproductive strategies are shaped by mother-infant interactions and that, although decreases in one aspect of reproduction may occur, there are compensatory increases in other aspects of reproduction that allow offspring to successfully reproduce. Offspring of Low LG dams appear to mature more rapidly, enter puberty at an earlier age, and thus can compensate for the conditions of a high stress/high mortality environment through increased reproductive output. Thus, the question of “optimal” in the context of natural variations in parenting may need to be reformulated into questions regarding the adaptiveness and “match” of offspring characteristics to their environment and reproductive opportunities.
How Can Parenting Practices, Interventions, and Social Policy Use this Information?
Although epidemiological studies in humans have certainly provided support for the hypothesis that adverse environments can lead to reduced parenting behavior with consequences for the development of offspring, the confirmation of these effects under controlled experimental conditions can be very convincing when developing policy. Studies of laboratory rodents suggest that the maternal brain and consequent mother-infant interactions are sensitive to the changing quality of the environment (isolation, stress, enrichment/communal rearing). Thus, a policy aimed at increasing the quality of parent-infant interactions to enhance child development would need to consider the context of parenting and the variety of environmental experiences that alter the quality of nurturing behaviors. In humans, these experiences are likely to include socioecomonic status, stability in the home, and degree of social support from family and/or the community. The neurobiological plasticity within both parents and offspring demonstrated in laboratory studies also suggest that, when adversity is experienced early in development, the quality of the later life environment can compensate or shift development in such a way that deficits can be ameliorated. This finding suggests that intervention within the lifespan of an individual is possible and may disrupt the continuity of the effects of adversity across generations. However, it would also appear that to maintain the benefits of a shift in parenting or offspring development, the change in the environment that induced the shift would also have to be maintained. Finally, it should be noted that natural variations in parenting exist even under controlled laboratory conditions. Variation will be likely to exist within any context of parenting and when designing or evaluating an intervention/policy, this variability will need to be considered–particularly when predicting the magnitude of the effect of an intervention.
How Can Future Studies in this Field be Used to Inform the Study of Parenting?
Translation of findings from animal studies is challenging and requires careful consideration of both the overall themes of the research and the details of the mechanistic pathways thought to be relevant. The themes of parental influences on child development, continuity of these effects across generations, and environmental regulation of parenting have support within the studies of human parenting. However, there are critical questions relevant to these themes which need to be addressed, such as (1) determining the periods of sensitivity to caregiver behavior and (2) determining the intensity and duration of environmental experiences that are capable of shifting the quality of parent-offspring interactions within and across generations. These are questions that can be addressed in future studies using laboratory rodents, although it should be noted that the timeline of development in rodents compared to humans will need to be carefully considered in the interpretation of these experiments. Mechanistic questions, particularly those examining the role of epigenetic changes in maintaining the effects of parenting on offspring brain development and behavior, will need to be more fully explored in rodent models. Our knowledge of the dynamics and environmental regulation of epigenetic marks is in its infancy. However, future directions in this research that would be particularly informative in the study of parenting would be determining the plasticity of epigenetic changes in response to “interventions” in later life and establishing the value of peripheral biological markers of developmental changes in offspring in response to variation in parental care. Overall, these studies would provide tools which can be applied to the better understanding of human parenting.
DISCUSSION
The use of laboratory rodent models to explore the consequences, transmission, and origins of variation in parental behavior has expanded our understanding of parenting and provided a tool for better understanding the biological pathways involved in these processes. The interplay between genes and the environment that is evident in the epigenetic effects of parenting is a critical new insight into the mechanisms linking our experiences to changes in the brain. These epigenetic factors may confer both the stability and plasticity needed to account for the regulation of complex neurobiological circuits mediating parenting behaviors, the fluctuations in parenting observed in changing environments, and the behavioral consequences of individual differences in parent-offspring interactions Future translational studies combining use of basic laboratory experiments and clinical interventions may prove to be an optimal approach to better understand the emerging scientific and practical questions within the study of parenting.
Acknowledgments
The authors acknowledge research funding support from Grant Number DP2OD001674 from the Office of the Director, National Institutes of Health
Contributor Information
Catherine L. Jensen, Columbia University
Frances A. Champagne, Email: fac2105@columbia.edu, Columbia University, Department of Psychology, 1190 Amsterdam Avenue, Room 406 Schermerhorn Hall, New York NY 10027.
References
- Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS ONE. 2008;3(5):e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28(23):6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29(3):386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52(4):299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79(3):359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59(12):1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational Effects of Social Environment on Variations in Maternal Care and Behavioral Response to Novelty. Behav Neurosci. 2007;121(6):1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Viau V, Bhatnagar S, Betito K, Iny LJ, O'Donnell D, et al. Cellular mechanisms underlying the development and expression of individual differences in the hypothalamic-pituitary-adrenal stress response. J Steroid Biochem Mol Biol. 1991;39(2):265–274. doi: 10.1016/0960-0760(91)90072-d. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. Embo J. 1998;17(17):4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48(3–4):262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weiss SJ, Wilson P, Morrison D. Maternal tactile stimulation and the neurodevelopment of low birth weight infants. Infancy. 2004;5(1):85–107. [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 2010;30(39):13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]