Abstract
Prolonged maternal deprivation during early infancy increases basal- and stress-induced corticosterone (CORT) levels, but the underlying mechanism is not clear. In general, stressors activate the hypothalamic-pituitary-adrenal (HPA) axis, with secretion and compensatory synthesis of hypothalamic corticotropin-releasing hormone (CRH). In the infant rat, we have demonstrated that maximally tolerated acute cold stress induced a robust elevation of plasma CORT throughout the first 2 postnatal weeks. However CRH messenger RNA (CRH-mRNA) abundance 4 h subsequent to cold stress was enhanced only in rats aged 9 days or older. This suggests a developmental regulation of the CRH component of the HPA-response to this stressor. The present study examined whether increased basal and cold stress-induced CORT levels after 24 h of maternal deprivation were due to enhanced CRH-mRNA abundance in the hypothalamic paraventricular nucleus (PVN). CRH-mRNA abundance, and basal- and cold-induced plasma CORT levels were measured in maternally deprived 6 and 9-day-old pups compared to non-deprived controls. Maternal deprivation increased basal and cold-induced CORT levels on both 6 and 9-day-old rats. CRH-mRNA abundance in the PVN of deprived rats did not differ from that in non-deprived rats. Our results indicate that the enhanced basal and stress-induced plasma CORT observed after 24 h maternal deprivation is not due to increased CRH-mRNA abundance in the PVN.
Keywords: Maternal deprivation, Stress, Infant rat, Development, Corticosterone, Corticotropin releasing hormone, Messenger RNA, Hypothalamic-pituitary-adrenal
Mechanisms of the activation of the hormonal stress response in the immature rat are not fully understood [7,10,14,18]. In the mature animal, a variety of stressors induce secretion of corticotropin-releasing hormone (CRH) from the hypothalamic paraventricular nucleus (PVN) causing ACTH release from the pituitary and increasing plasma glucocorticoids (GCs). This is followed by a ‘compensatory’ increase in CRH-mRNA abundance in the hypothalamic PVN [7,10,21]. We have established that CRH-mRNA abundance in the PVN does not increase subsequent to maximal tolerated acute-cold stress in rats aged 4 or 6 days, despite robust elevation in plasma GCs [19,21]. The onset of stress-induced increase of steady state CRH-mRNA abundance occurs on postnatal day (PND) 9 [21].
The infant hypothalamic-pituitary-adrenal (HPA) axis is under maternal regulation [6,9,13,16,17]. Prolonged, 24 h maternal deprivation has been found to increase basal plasma corticosterone (CORT) levels and to enhance HPA axis responsiveness to further stressors [6,9,16]. Shorter periods of maternal deprivation (1, 2, 4, and 8 h) have been shown to have only a minimal effect on basal CORT levels and the response to acute stress [9,16]. The mechanisms underlying increased basal and cold-induced plasma CORT subsequent to maternal deprivation have not been elucidated. The purpose of this study was to examine whether the enhanced basal HPA tone and the altered response of plasma CORT to acute stress observed after 24 h maternal deprivation were due to increased hypothalamic CRH-mRNA abundance.
Time pregnant Sprague–Dawley rats were purchased from Zivic-Miller (Zelienople, PA) and kept on a 12 h light/dark cycle with access to unlimited lab chow and water [4,21]. Day of birth was considered day 0, and litters were culled to 12 pups, and mixed and matched. Experiments were started at 0830–0930 h, to avoid diurnal variability in CRH-mRNA [20] and plasma CORT levels [5,19].
Maternal deprivation was carried out as follows. Pups on PND 5 or 8 were divided to 3 groups: (1) individual maternal deprivation (I-DEP) pups were placed individually in divided plastic cages; (2) maternal deprivation as a group (G-DEP) pups were kept with their littermates; (3) non-deprived (N-DEP) rats were left in home cages. During the 24 h deprivation period, cages were placed on a heating pad (30–33°C) under a 12 h light/dark cycle. Pups were weighed as a group before and after deprivation.
Groups of maternally deprived and of control rats were subjected to acute-cold-separation stress (on PND 6 or 9), as previously described [21]. Briefly, pups (except ‘time 0’ and controls) were separated from their mothers and placed individually in glass jars in a cold room (4°C) for 25–30 min on PND 6, and 40 min on PND 9. After re-warming on a heating pad, groups of pups (n = 6–12) were sacrificed 30, 60, 150 or 240 min subsequent to cold stress termination. Controls and ‘time 0’ rats were decapitated within 45 s of disturbance. Brains and trunk blood were collected.
Brains were rapidly frozen on powdered dry ice and stored at −80°C. They were cut into 20 μm coronal sections in a cryostat and mounted on gelatin-coated slides. Preparation and labeling of oligonucleotide probes and ISH have been described [1,3,4,21,22]. Briefly, sections were brought to room temperature, air-dried and fixed for 20 min in fresh 4% buffered PBS-paraformaldehyde. After a graded ethanol treatment, sections were exposed to acetic anhydride-triethanolamine and dehydrated through 100% ethanol. Sections were prehybridized for 1 h, then hybridized for 20 h at 40°C in a humidity chamber. Sections were washed (2× SSC for 15 min 4 times at 40°C, 1×, and 0.3× SSC for 30 min each at room temperature). Sections were dehydrated through ethanol, air dried and apposed to film (Hyperfilm B-Max, Amersham, IL ) for 24–48 h [3,4]. Quantitative analysis of CRH-mRNA was achieved using the MCID software image analysis system (Imaging Research, Ont., Canada) as described [4,21,22]. Each point was derived from 6–12 sections from a minimum of 4 individual rats. To eliminate background variability with age, both OD ratios of PVN/background and absolute brain-paste standardized values were obtained [4,21,22]. Statistical significance between groups was determined using two-way analysis of variance, followed by Duncan’s multiple range test.
Plasma CORT levels were determined by a commercial radioimmunoassay (ICN, Irvine, CA). Assay sensitivity was 0.5 μg/dl. Interassay variability was determined by two dilutions of adult rat plasma and averaged 15% [2].
Maternal deprivation for 24 h resulted in significantly higher basal (AM) plasma CORT levels (Figs. 1,2). On PND 6, basal plasma CORT level in the combined maternally deprived groups (G-DEP + I-DEP) was 2.9 ± 0.3 μg/dl compared with 1.6 ± 0.2 μg/dl of the non-deprived (N-DEP) group (P < 0.05). Plasma CORT was significantly higher in group-deprived rats than in individually deprived rats (3.5 ± 0.3 μg/dl versus 2.2 ±0.3, P < 0.05; Fig. 1). On PND 9, basal plasma CORT in the maternally deprived groups (G-DEP + I-DEP) was 6.8 ± 1.1 μg/dl compared with 2.0 ± 0.2 μg/dl in the non-deprived group (P <0.05; Fig. 2). Evaluated separately, both G-DEP plasma CORT levels (7.5 ± 1.0 μg/dl) and I-DEP plasma CORT levels (6.1 ± 1.1 μg/dl), were significantly higher than those of the N-DEP group (2.0 ±0.2 μg/dl, P < 0.05). Maximally tolerated acute cold stress induced a more robust and prolonged elevation of plasma CORT levels in deprived rats compared to non-deprived controls (Figs. 1,2). On PND 6, peak plasma CORT level was 11.2 ± 1.2 μg/dl in G-DEP rats, and 10.1 ± 1.4μg/dl in I-DEP rats, both significantly higher than N-DEP rats (7.6 ± 0.4 μg/dl, P<0.05; Fig. 1). On PND 9, peak plasma CORT was 19.4 ± 1.2 μg/dl in G-DEP rats, 13.9 ± 1.3 μg/dl in I-DEP rats, and 6.1 ± 0.6 μg/dl in N-DEP rats (P < 0.05, G-DEP or I-DEP versus N-DEP group, and G-DEP versus I-DEP; Fig. 2). The same pattern was observed 4h after cold stress (15.0 ± 1.7 μg/dl, 6.2 ± 1.1 μg/dl and 2.8 ±0.4 μg/dl for G-DEP, I-DEP and N-DEP, respectively).
Fig. 1.
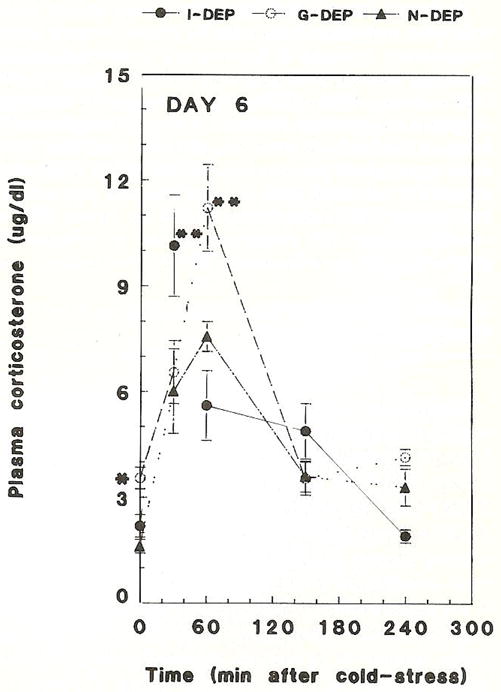
Time course of basal and cold stress-induced plasma corticosterone in 24 h maternally deprived 6-day-old rats. *Basal CORT was significantly elevated in individually deprived (I-DEP) and group-deprived rats (G-DEP) compared with non-deprived controls (N-DEP), P<0.05. **Basal CORT was significantly elevated in G-DEP compared with 1-DEP rats. Cold stress-induced peak plasma corticosterone was significantly higher in I-DEP or G-DEP versus N-DEP rats, (P < 0.05). Values represent the mean ± SEM of 6–8 rats per group.
Fig. 2.
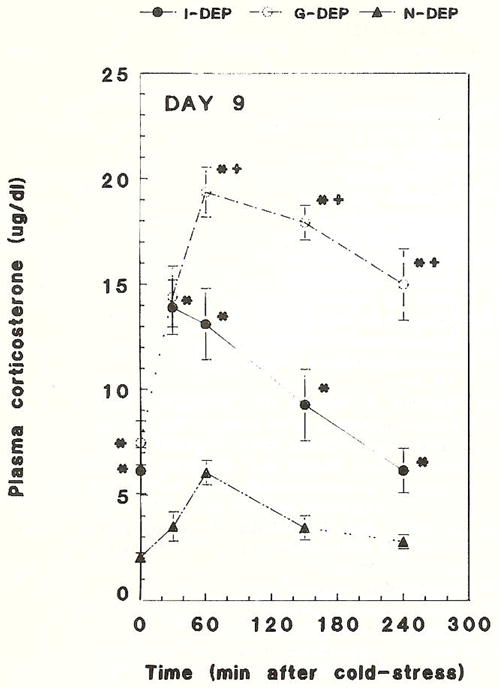
Time course of basal and cold stress-induced plasma corticosterone in 24 h maternally deprived 9-day-old rats. Basal CORT was significantly elevated in individually deprived rats (I-DEP) and group-deprived rats (G-DEP), compared with non-deprived controls (N-DEP). Evaluated separately, both G-DEP and I-DEP plasma corticosterone levels were significantly higher than those of the N-DEP group. Cold stress-induced peak plasma corticosterone was significantly higher and more sustained in G-DEP or I-DEP versus N-DEP rats and G-DEP versus I-DEP rats. Values are the mean ± SEM of 6–8 rats per group. *Significantly different from N-DEP (P < 0.05); +Significantly different from I-DEP (P < 0.05).
CRH-mRNA abundance in PVN, measured in arbitrary units, in G-DEP, I-DEP and N-DEP, is shown in Fig. 3. As is evident from the figure, CRH-mRNA levels were similar in maternally deprived and non-deprived rats on PND 9 (P > 0.05).
Fig. 3.
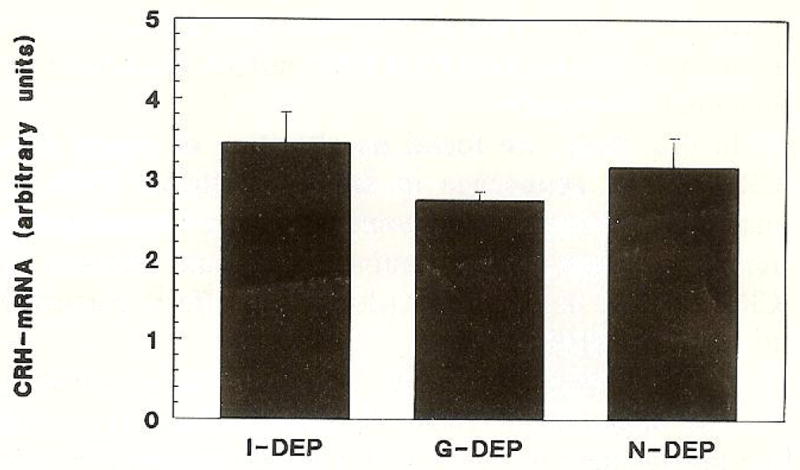
Steady-state CRH messenger RNA abundance in the paraventricular nucleus of 9-day-old rats. Groups were: individually deprived (I-DEP); group-deprived (G-DEP); and controls (N-DEP). Differences among groups were not statistically significant (P > 0.05). Values are expressed in arbitrary units and presented as mean ± SEM of 6–12 sections from a minimum 4 rats.
On both PND 6 and PND 9, group-deprived rats lost 4.5–5.8% of their body weight, while individually deprived pups lost 5.3–6.0% (P > 0.05).
These results indicate that 24 h maternal and sibling deprivation during early infancy was a major stressor resulting in significant alteration of the HPA axis in the infant rat. Maternal deprivation affected ‘basal’ HPA tone resulting in a significant increase in AM plasma CORT levels on both PND 6 and 9. This is in accord with reports by Stanton et al. [16], Levine et al. [9] and Kuhn et al. [6]. Maternal deprivation also enhanced the responsiveness of the HPA axis to further stressors. Stanton [16] and Levine [9] reported an enhanced plasma CORT elevation subsequent to saline injection or novelty in maternally deprived rats. We demonstrated enhancement of both peak and duration of plasma CORT, using acute cold-separation stress [21]. Peak plasma CORT level subsequent to acute maximally tolerated cold stress in maternally deprived rats represented an increase of 150% on PND 6, and 200–300% on PND 9. Cold is not a strong stressor in the adult [7,10]. However, immature thermoregulation [15] and the absence of fur makes cold exposure a powerful stressor in the infant rat [21].
We distinguished between individually deprived rats (I-DEP), deprived from both mother and siblings, and group-deprived rats (G-DEP) deprived from their mother but kept with their littermates. Using this paradigm, maternal deprivation alone resulted in an increased basal and acute stress-induced plasma CORT level compared to maternal and sibling deprivation. This was not associated with excess weight loss in either group (see above), excluding thermal-loss as the cause of the enhanced stress response.
What mechanisms [8,9,12–14,19] mediated the increased basal and cold-induced plasma CORT subsequent to maternal deprivation? An increase of basal plasma CORT and PVN-CRH-mRNA abundance in adult rats subjected to maternal separation in infancy has been reported [16]. The same authors also found an enhanced CORT elevation and greater release of CRH in response to acute restraint stress in these adults, maternally deprived as infants. This is consistent with a long-term alteration of steady state PVN-CRH-mRNA abundance by maternal deprivation.
In this study, we found no alteration of steady state CRH-mRNA abundance in the hypothalamic PVN by maternal deprivation. However, we used a somewhat different paradigm, and examined for acute changes in CRH-mRNA, as opposed to long-term effects persisting to adulthood [16].
CORT upregulation after 24 h maternal deprivation without alteration of PVN-CRH-mRNA levels, suggests the involvement of other ACTH secretagogues. The role of hypothalamic arginine vasopressin (AVP) in the neonatal stress response has been demonstrated by Muret et al. [23]. These authors showed that both on PND 8 and 20, passive immunization against AVP abolished ACTH secretion after insulin-induced hypoglycemia, while no change was observed after passive immunization against CRH [23]. Widmaier [24] suggested that hypoglycemia-induced plasma ACTH elevation in 8 and 11-day-old rats was independent of CRH, since the stressor did not alter hypothalamic CRH in vitro. Interaction of AVP and CRH in regulating the neonatal stress response was documented by Paulmyer-Lacroix et al. [25]. They demonstrated increased CRH synthesis with hypoglycemia only in an AVP positive subset of parvocellular neurons. Our analysis, also using ISH, did not distinguish between AVP positive and negative neurons.
An alternative mechanism may derive from alterations in CRH receptors: Recently, maternal deprivation has been shown to increase CRH-receptor abundance in certain limbic structures [11]. Enhancement of receptor number or synthesis could induce elevated basal and acute stress-induced plasma CORT in the absence of altered PVN-CRH-mRNA abundance.
Acknowledgments
Supported by NINDS NS28912 (to T.Z.B) and a BRSG award (to S.A.E).
References
- 1.Baram TZ, Lerner SP. Corticotropin releasing hormone – ontogeny of gene expression in rat hypothalamus. Int J Dev Neurosci. 1991;9:473–478. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 2.Baram TZ, Schultz L. Fetal and maternal levels of corticosterone and ACTH after pharmacological adrenalectomy. Life Sci. 1990;47:485–489. doi: 10.1016/0024-3205(90)90607-s. [DOI] [PubMed] [Google Scholar]
- 3.Baram TZ, Schultz L. Ontogeny of somatostatin gene expression in rat diencephalon: comparison with CRH. Dev Neurosci. 1991;13:176–180. doi: 10.1159/000112155. [DOI] [PubMed] [Google Scholar]
- 4.Baram TZ, Schultz L. CRH gene expression in the fetal rat is not increased after pharmacological adrenalectomy. Neurosci Lett. 1992;142:215–218. doi: 10.1016/0304-3940(92)90376-i. [DOI] [PubMed] [Google Scholar]
- 5.Dallman MF, Akana SF, Casico CS, Darlington DN, Jacobson L, Levine N. Regulation of ACTH: variation on a theme of B. Rec Prog Horm Res. 1987;43:113–131. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn C, Pauk J, Schanberg S. Endocrine response to mother-infant separation in developing rats. Dev Psychobiol. 1990;23:395–410. doi: 10.1002/dev.420230503. [DOI] [PubMed] [Google Scholar]
- 7.Harbuz MS, Lightman SL. Response of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- 8.Levine S. Critical period for the effects of infantile experience on the maturation of the stress response. Science. 1957;129:42–43. doi: 10.1126/science.129.3340.42. [DOI] [PubMed] [Google Scholar]
- 9.Levine S, Hutchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol. 1992;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- 10.Lightman SL, Harbuz MS. Corticotropin Releasing Factor. Wiley; Chicester: 1993. Expression of corticotropin-releasing factor mRNA in response to stress; pp. 173–198. [DOI] [PubMed] [Google Scholar]
- 11.Nemeroff CB, Owens MJ, Plott SJ, Levine S. Increased density of regional brain CRF binding sites after maternal deprivation. Soc Neurosci Abstr. 1993;19:6.2. [Google Scholar]
- 12.Plotsky PM, Meany MJ. Early neonatal experience alters hypothalamic CRF-mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld P, Wetmore JB, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol Behav. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt I, Kaul R, Heldmaier G. Thermoregulation and diurnal rhythms in 1-week-old rat pups. Can J Physiol Pharmacol. 1987;65:105–109. doi: 10.1139/y87-214. [DOI] [PubMed] [Google Scholar]
- 16.Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- 17.Sucheki D, Mozaffarian D, Graziella G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57:204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- 18.Walker CD, Perrin P, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- 19.Walker CD, Scribner KA, Casico CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1990;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 20.Watts AG, Swanson LW. Diurnal variation in the content of prepro-corlicotropin releasing hormone mRNA in the hypothalamic paraventricular nucleus of rats of both sexes as measured by in situ hybridization. Endocrinology. 1989;125:17734–1738. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- 21.Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi SJ, Masters JN, Baram TZ. Effects of specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- 23.Muret L, Priou A, Oliver C, Grino M. Stimulation of adreno-corticotropin secretion by insulin-induced hypoglycemia in the developing rat involves arginine vasopressin but not corticotropin-releasing factor. Endocrinology. 1992;130:2725–2732. doi: 10.1210/endo.130.5.1315256. [DOI] [PubMed] [Google Scholar]
- 24.Widmaier EP. Glucose homeostasis and hypothalamic-pituitary-adrenocortical axis during development in rats. Am J Physiol. 1990:601–613. doi: 10.1152/ajpendo.1990.259.5.E601. [DOI] [PubMed] [Google Scholar]
- 25.Paulmyer-Lacroix O, Anglade G, Grino M. Stress regulates differently the arginine vasopressin (AVP)-containing and AVP-deficient corticotropin-releasing-factor synthesizing cell bodies in the hypothalamic paraventricular nucleus of the developing rat. Endocrine. 1994;2:1037–1043. [Google Scholar]


