Abstract
Background
Obesity could attenuate diuretic effectiveness in treatment of acute decompensated heart failure (HF).
Methods
The DOSE trial randomized 308 subjects with acute HF to low vs. high intensification intravenous diuretic therapy. We tested for statistical interactions between obesity and dosing strategy across clinical end points.
Results
After 72 hours of treatment, obese subjects (BMI > 30 kg/m2; n=173) had greater volume loss than non-obese subjects (n=119), but similar improvements in dyspnea and freedom from congestion. Both groups had greater fluid loss with high intensification treatment. Obese subjects had a higher incidence of worsening renal function (WRF) at 72 hours with low intensification treatment compared to non-obese subjects. In contrast, non-obese and obese had similar incidence of WRF with high intensification treatment. There were no differences between obese and non-obese subjects in time to discharge, 60 day freedom from death, emergency department visit or rehospitalization.
Conclusions
The incidence of WRF was greater in obese than nonobese subjects with low intensification treatment. However, the frequency of WRF was equivalent in obese and nonobese subjects with high intensification treatment. Additional studies are needed to assess whether obese patients with acute HF benefit from an initial high intensification treatment strategy.
Keywords: obesity, congestive heart failure, diuretics, renal function, readmission
Introduction
The prevalence of obesity in most developed countries has approximately doubled over the past 2 decades1,2. Obesity is a risk factor for the development of heart failure (HF)3,4. Mechanisms accounting for the association between obesity and HF likely include premature and accelerated coronary atherosclerosis5, 6, hypertension7, 8, diabetes9, sleep disordered breathing and atrial fibrillation10. In a large registry of patients with acute decompensated HF, 38% of the patients were obese based on World Health Organization criteria11. Despite the very frequent coexistence of these conditions, it is unknown whether the treatment for HF should be altered based on the presence of obesity.
Intravenous loop diuretics are the most commonly used pharmacological agents in the treatment of acute HF because of their rapid action in alleviating volume overload and the consequent symptoms. Some retrospective studies have suggested that higher doses of diuretics are associated with harm12, 13, but the optimal dosing of intravenous loop diuretics has not been rigorously studied. Despite uncertain clinical significance, it is common practice to stop or reduce the dosage of diuretics in the setting of worsening renal function. There are theoretical reasons why obesity might attenuate the response to intravenous diuretics or exacerbate worsening renal function during diuretic treatment: 1) obese patients often have higher lean body mass as well as fat mass, and thus may have larger volume of distribution of drugs,14 2) obese patients often have increased intra-abdominal pressure and/or increased renal vein pressure and hence may have greater resistance to glomerular filtration15 and 3) obesity has been associated with a 3–4 fold increased lifetime risk of developing chronic renal failure, not all of which is attributable to diabetic nephropathy16–19. Few trials have directly examined whether obese subjects respond differently to treatment of acute HF compared to non-obese subjects.
The diuretic optimization strategies evaluation (DOSE) trial showed that among subjects with acute decompensated HF, there were no significant differences in subjects’ global assessment of symptoms or in the change in renal function when intravenous diuretic therapy was administered using high vs. low intensification strategy or with bolus vs. continuous infusion dosing20. We tested the hypothesis that obese subjects would require high intensification diuretic treatment to achieve adequate volume loss and symptomatic improvement, whereas non-obese subjects would improve in both the high and low intensification treatment arms. For the reasons described above, we also hypothesized that high intensification diuretic treatment would be associated with more worsening of renal function in obese compared to non-obese subjects.
Methods
The DOSE study design and primary outcomes have been reported previously21. The study was conducted by a National Heart, Lung and Blood Institute funded Heart Failure Clinical Research Network. All subjects provided written informed consent.
Study Participants
Patients with history of chronic HF were eligible for enrollment into the trial if they were within 24 hours of hospital admission with acute decompensated HF.
Randomization and Treatment Assignments
Subjects were randomized to either a low intensification strategy (total daily intravenous dose of 1.0 x their daily oral furosemide dose) or high intensification strategy (a total daily intravenous dose of 2.5 x their daily oral dose) and to dosing either by intravenous bolus every 12 hours or by continuous intravenous infusion. Since the main trial did not show differences between continuous and intermittent dosing, in this analysis we have focused only on high intensification vs. low intensification diuretic treatment. Blinded study treatment was continued for up to 72 hours and subjects were followed for clinical events to 60 days. All patients were recommended to receive standard heart failure medications and a low salt diet with fluid restriction.
Endpoints
The endpoints of this substudy were similar to those of the main DOSE trial. The primary efficacy endpoint was subject reported global assessment of symptoms assessed using a visual analog scale and quantified as the area under the curve from baseline to 72 hours. The primary safety endpoint was the change in serum creatinine from baseline to 72 hours. Secondary endpoints included: 1) subject reported dyspnea; 2) changes in net fluid loss and body weight; 3) the proportion of subjects free from congestion; 4) worsening renal function defined as an increase in serum creatinine greater than 0.3 mg/dL at any time from randomization to 72 hours. 5) worsening or persistent heart failure defined as the need for additional therapy between randomization and 72 hours; 6) changes in cystatin C and NTproBNP, 7) composite of death, rehospitalization, or emergency room visit within 60 days.
Statistical Analysis
All comparisons involving the different diuretic treatment strategies were based on “intention to treat.” Continuous variables are presented as mean and standard deviation or median and interquartile range, and categorical variables are presented as frequencies and percentages. The baseline characteristics of subject groups defined by presence or absence of obesity were compared using the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables. The effects of high intensity diuretics and obesity with respect to the primary and secondary endpoints were estimated and assessed using a general linear model (continuous endpoints), logistic regression (binary endpoints) or Cox model and Kaplan-Meier curves (event-time endpoints), as appropriate. A test for the presence of an interaction between the treatment strategy and presence of obesity was also performed within the statistical modeling framework appropriate for each individual endpoint, including whether the interaction was independent of other relevant clinical factors. Differences between groups were considered statistically significant if two-sided p<0.05.
Results
Subject Characteristics
The DOSE study enrolled 308 subjects between March 2008 and November 2009. Two hundred ninety two subjects had data available to calculate BMI. Median BMI in subjects enrolled in the DOSE study was 31.9 kg/m2 (interquartile range 25.6–34.7), and 59% of the subjects met World Health Organization criteria for obesity (BMI > 30 kg/m2) at enrollment. Baseline characteristics for obese (median BMI 35.5 interquartile range 32.8–40.6 kg/m2 and median weight 236 interquartile range 210–268 lbs) and non-obese (median BMI 25.7 interquartile range 24.1–27.9 kg/m2 and median weight 171 interquartile range 151–190 lbs) subjects are provided in Table 1. Obese subjects were younger than non-obese subjects (median age 66 vs. 72 years, p<0.01). Obese and non-obese subjects had similar gender distribution, etiology of heart failure, qualifying furosemide dosage, number of HF hospitalizations in the past 12 months, history of atrial fibrillation and baseline serum creatinine. Obese subjects had a higher median left ventricular EF (31.5 interquartile range 20–55 vs. 25.0 interquartile range 20–45%, p=0.03). However, the proportions of subjects with an ejection fraction ≥ 50% were not significantly different between the 2 groups (31% vs. 23%, p=0.13). There was a significantly higher proportion of subjects with diabetes in the obese compared to the non-obese cohort (61 vs. 38%, p<0.01). There was no difference in the use of essential heart failure medications in obese vs. non-obese subjects at enrollment. Although within the normal range in both groups, the median systolic blood pressure was higher in obese subjects (median [25th, 75th] 119 [106,135] vs.112 [101,128] mmHg, p<0.01). Admission median NTproBNP was significantly lower in obese than non-obese subjects (median [25th, 75th] 3404 [2005,6929] vs. 7492 [4190,14374] ng/ml, p< 0.01).
Table 1.
Baseline demographics and clinical characteristics.
| BMI > 30 (N=173) | BMI <= 30 (N=119) | P-Value | |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age | <0.01 | ||
| Median (25th, 75th) | 66.0 (54.0, 75.0) | 72.0 (62.0, 80.0) | |
| Mean (SD) | 64.3 ± 13.5 | 69.7 ± 13.1 | |
|
| |||
| White race | 126 (72.8%) | 94 (79.0%) | 0.23 |
|
| |||
| Male | 123 (71.1%) | 89 (74.8%) | 0.49 |
|
| |||
| Qualifying Furosemide Dose | 0.64 | ||
| Median (25th, 75th) | 120.0 (80.0, 160.0) | 120.0 (80.0, 160.0) | |
| Mean (SD) | 132.3 ± 52.4 | 128.9 ± 51.3 | |
|
| |||
| HF Hospitalization within 12 months prior to randomization | 130 (76.5%) | 81 (68.1%) | 0.11 |
|
| |||
| LVEF* | 0.03 | ||
| Median (25th, 75th) | 31.5 (20.0, 55.0) | 25.0 (20.0, 45.0) | |
| Mean (SD) | 36.6 ± 17.9 | 32.5 ± 16.7 | |
| ≥50% | 52 (31.0%) | 27 (22.9%) | 0.13 |
|
| |||
| Medical history | |||
|
| |||
| Ischemic Etiology | 95 (54.9%) | 77 (64.7%) | 0.09 |
|
| |||
| Diabetes Mellitus | 106 (61.3%) | 45 (37.8%) | <0.01 |
|
| |||
| Atrial Fibrillation/Flutter | 90 (52.0%) | 69 (58.0%) | 0.31 |
|
| |||
| Baseline medications | |||
|
| |||
| ACE or ARB | 111 (64.2%) | 72 (60.5%) | 0.53 |
|
| |||
| Aldosterone Antagonists | 46 (26.6%) | 38 (31.9%) | 0.32 |
|
| |||
| Beta blockers | 143 (82.7%) | 99 (83.2%) | 0.91 |
|
| |||
| Physical and laboratory findings | |||
|
| |||
| Systolic blood pressure | <0.01 | ||
| Median (25th, 75th) | 119.0 (106.0, 135.0) | 112.0 (101.0, 128.0) | |
| Mean (SD) | 121.3 ± 20.7 | 114.2 ± 16.6 | |
|
| |||
| Heart rate* | 0.65 | ||
| Median (25th, 75th) | 76.0 (67.0, 86.0) | 76.0 (70.0, 84.0) | |
| Mean (SD) | 78.3 ± 17.2 | 78.3 ± 13.5 | |
|
| |||
| Rales* | 100 (58.1%) | 69 (58.5%) | 0.95 |
|
| |||
| JVP >= 8cm* | 147 (91.3%) | 108 (93.1%) | 0.58 |
|
| |||
| SPO2 (%)* | 0.51 | ||
| Median (25th, 75th) | 96.0 (94.0, 98.0) | 96.0 (94.0, 99.0) | |
| Mean (SD) | 95.9 ± 3.0 | 96.1 ± 3.0 | |
|
| |||
| Serum creatinine (mg/dL) | 0.95 | ||
| Median (25th, 75th) | 1.40 (1.11, 1.82) | 1.44 (1.08, 1.85) | |
| Mean (SD) | 1.49 ± 0.50 | 1.50 ± 0.55 | |
|
| |||
| Serum cystatin C (mg/L) | 0.53 | ||
| Median (25th, 75th) | 1.51 (1.16, 1.92) | 1.45 (1.08, 1.89) | |
| Mean (SD) | 1.58 (0.56) | 1.54 ± 0.56 | |
|
| |||
| Sodium (mEq/L) | <0.01 | ||
| Median (25th, 75th) | 139.0 (137.0, 141.0) | 138.0 (135.0, 140.0) | |
| Mean (SD) | 138.7 ± 3.9 | 137.6 ± 3.4 | |
|
| |||
| NTproBNP (pg/mL)* | <0.01 | ||
| Median (25th, 75th) | 3404.0 (2005.0, 6929.0) | 7491.5 (4190.0, 14374.0) | |
| Mean (SD) | 5630.3 ± 6479.4 | 10135.0 ± 7898.0 | |
P-values for continuous variables are based on Wilcoxon rank-sum test. P-values for categorical variables are based upon Chi-square test.
Small number of responses missing; percentages are based on total known responses
Study Drug Administration & Randomization
The median time from presentation to randomization (14.3 hours vs. 15.5 hours, p=0.80), duration of study drug administration (67.7 vs. 60.5 hours, p=0.10) and change in drug strategy at 48 hours (57% vs. 63%, p=0.31) were similar in obese and non-obese subjects. The proportions of subjects randomized to high intensification strategy among obese and non-obese subjects were similar (49% vs. 52%, p=0.58).
Primary endpoints
Obese subjects were not statistically different from non-obese in terms of overall symptoms at 72 hours expressed as visual analog scale area under the curve (Table 2). There was a difference, however, between the two groups in the primary safety endpoint of change in serum creatinine from baseline to 72 hours. The mean serum creatinine increased in obese subjects (+0.09 mg/dl) but did not change (0.00 mg/dl) in the non-obese subjects (p<0.01). At 60 days, there was no longer a significant difference in the change of serum creatinine between the groups (+0.11 mg/dL vs. +0.05 mg/dl, respectively, p=0.37; Table 2).
Table 2.
Primary and secondary endpoints
| BMI > 30 (N=173) | BMI <= 30 (N=119) | P-Value | |
|---|---|---|---|
|
| |||
| Global VAS AUC (72 Hours) | 0.67 | ||
| N | 171 | 116 | |
| Median (25th, 75th) | 4497 (3371, 5429) | 4340 (3561, 5157) | |
| Mean (SD) | 4329 ± 1417 | 4180 ± 1452 | |
|
| |||
| Dyspnea VAS AUC (72 Hours) | 0.67 | ||
| N | 171 | 116 | |
| Median (25th, 75th) | 4803 (3483, 5759) | 4719 (3900, 5461) | |
| Mean (SD) | 4588 ± 1558 | 4529 ± 1517 | |
|
| |||
| Free from congestion (%, 72 hours) | 24/169 (14.2%) | 16/114 (14.0%) | 0.97 |
|
| |||
| Fluid loss (ml, 72 hours) | 0.02 | ||
| N | 141 | 95 | |
| Median (25th, 75th) | 4114 (2501, 6262) | 3193 (1404, 5306) | |
| Mean (SD) | 4631 ± 3184 | 3654 ± 3036 | |
|
| |||
| Change in NTproBNP (72 hours) | 0.83 | ||
| N | 143 | 93 | |
| Median (25th, 75th) | −580 (−1856, 30) | −1669 (−4166, −103) | |
| Mean (SD) | −1153 ± 3731 | −2165 ± 4665 | |
|
| |||
| Creatinine change (72 hours) | 0.01 | ||
| N | 170 | 115 | |
| Median (25th, 75th) | 0.05 (−0.10, 0.22) | −0.02 (−0.14, 0.15) | |
| Mean (SD) | 0.09 ± 0.31 | −0.00 ± 0.28 | |
|
| |||
| Creatinine change (60 days) | 0.37 | ||
| N | 131 | 89 | |
| Median (25th, 75th) | 0.05 (−0.15, 0.26) | 0.03 (−0.14, 0.25) | |
| Mean (SD) | 0.11 ± 0.45 | 0.05 ± 0.41 | |
|
| |||
| Cystatin C change (72 hours) | 0.04 | ||
| N | 143 | 94 | |
| Median (25th, 75th) | 0.14 (0.01, 0.29) | 0.06 (−0.07, 0.16) | |
| Mean (SD) | 0.17 ± 0.36 | 0.08 ± 0.28 | |
|
| |||
| Cystatin C change (60 days) | 0.43 | ||
| N | 106 | 74 | |
| Median (25th, 75th) | 0.11 (−0.08, 0.22) | 0.05 (−0.10, 0.38) | |
| Mean (SD) | 0.21 ± 0.54 | 0.15 ± 0.36 | |
|
| |||
| Worsening renal function (%, 72 hours) | 37/170 (21.8%) | 15/115 (13.0%) | 0.06 |
|
| |||
| Worsening renal function (%, 60 days) | 28/128 (21.9%) | 19/85 (22.4%) | 0.93 |
|
| |||
| Time to discharge (days) | 0.74 | ||
| N | 173 | 119 | |
| Median (25th, 75th) | 6.0 (4.0, 9.0) | 5.0 (3.0, 9.0) | |
| Mean (SD) | 7.9 ± 7.3 | 7.4 ± 7.8 | |
Secondary endpoints
Compared to non-obese subjects, the obese subjects had greater average volume loss at 72 hours (4631 vs. 3654 ml, p=0.02). There was a trend toward a greater incidence of worsening renal function, defined as an increase in creatinine of >0.3 mg/dl, within 72 hours of randomization in the obese vs. the non-obese subjects (21.8% vs. 13.0%, respectively, p=0.06; Table 2). Serum cystatin C was measured at randomization, 72 hours and 60 days. Similar to the changes in serum creatinine, obese patients had a greater rise in cystatin C at 72 hours compared to nonobese patients (Table 2). There were no significant differences between obese and non-obese subjects in freedom from congestion at 72 hours, change of serum NTproBNP between baseline and 72 hours, or length of hospital stay.
Low vs. high intensification diuretic strategy
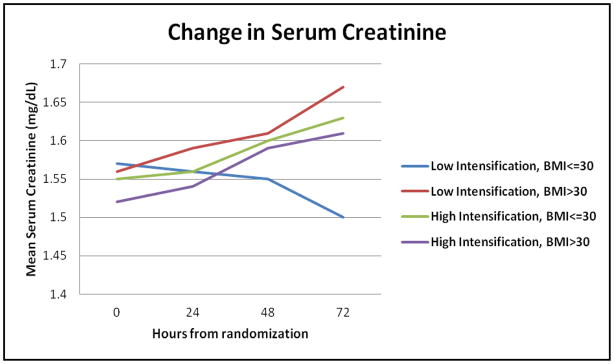
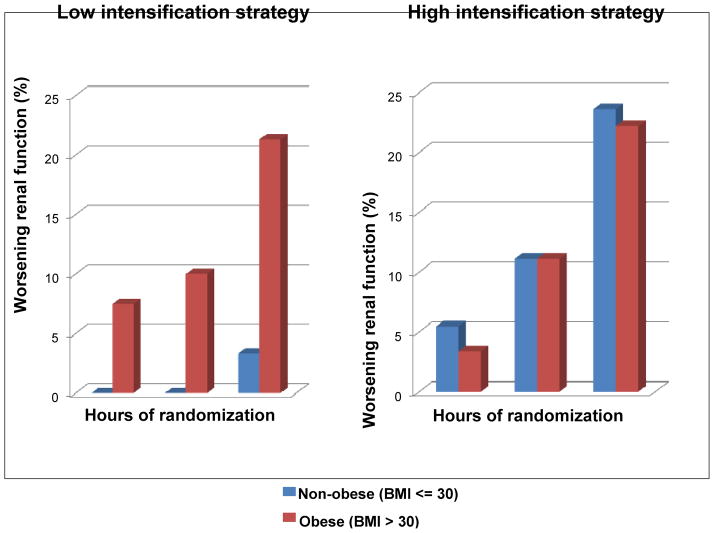
The current analysis extends the findings of the original report by showing that the additional fluid losses achieved with high intensification treatment were similar between the obese and non-obese subjects (Table 3). There was an increase in serum creatinine at 72 hours in obese subjects randomized to low intensification strategy (mean increase +0.09 mg/dL) compared to a decrease in non-obese subjects randomized to low intensification strategy (mean −0.04 mg/dL), p<0.01. Both obese and non-obese subjects randomized to high intensification strategy experienced increases in serum creatinine at 72 hours after randomization (+0.10 mg/dL and +0.04 mg/dL, respectively, p=0.34); Figure 1. A statistically significant interaction between obesity and diuretic intensification strategy was seen for the end point of worsening renal function. In obese subjects, both high and low intensification strategy resulted in a similar incidence of worsening renal function (22.2% vs. 21.3%, respectively, p=NS). In the non-obese subjects, high intensification strategy resulted in a higher incidence of worsening renal function compared to the low intensification strategy (23.6% vs. 3.3%, respectively, p-value for BMI x intensification strategy interaction = 0.01; Figure 2). To ascertain that this interaction was not a result of confounding, we constructed a multivariable logistic regression model that included age, gender, history of diabetes mellitus and systolic blood pressure as additional clinically meaningful covariates. The interaction between BMI and intensification strategy remained significant even after this adjustment (p=0.03). Diabetes mellitus also represented an independent risk factor for worsening renal function in this model - OR (95% CI) = 2.3 (1.14, 4.5), p=0.01.
Table 3.
Fluid loss with low vs. high intensification diuretic strategy.
| Low Intensification (N=144) | High Intensification (N=148) | P-Value | |
|---|---|---|---|
|
| |||
| Fluid loss (ml, 72 hours) | <0.01 | ||
| N | 118 | 118 | |
| Median (25th, 75th) | 3177 (1760, 4744) | 4298 (2201, 7229) | |
| Mean (SD) | 3540 ± 2585 | 4935 ± 3512 | |
|
| |||
| BMI <= 30 | (N=61) | (N=58) | |
|
| |||
| Fluid loss (ml, 72 hours) | 0.02 | ||
| N | 49 | 46 | |
| Median (25th, 75th) | 2880 (1495, 4574) | 3834 (1334, 6390) | |
| Mean (SD) | 2945 ± 2268 | 4409 ± 3555 | |
|
| |||
| BMI > 30 | (N=83) | (N=90) | |
|
| |||
| Fluid loss (ml, 72 hours) | 0.01 | ||
| N | 69 | 72 | |
| Median (25th, 75th) | 3220 (2475, 5320) | 4904 (2663, 7434) | |
| Mean (SD) | 3962 ± 2726 | 5272 ± 3467 | |
Figure 1. Change in serum creatinine from baseline to 72 hours.
Serum creatinine was measured at baseline, 24, 48 and 72 hours after admission. Data are shown for obese and non-obese subjects randomized to low and high intensification diuretic treatment. Baseline creatinine was not different between the 4 groups. At 72 hours, obese subjects had a greater increase in creatinine than non-obese subjects (P<0.01). Obese subjects receiving low intensification treatment had an increase in creatinine compared to a decrease in the non-obese (p<0.01). Both obese and non-obese assigned to high intensification treatment had a similar rise in creatinine (p=0.34).
Figure 2. Frequency of worsening renal function at 24, 48 and 72 hours after admission.
Worsening renal function was defined as increase of serum creatinine by > 0.3 mg/dl above baseline measurement. In the low intensification treatment group, the obese subjects experienced a higher incidence of worsening renal function compared to non-obese. In the high intensification treatment arm, both obese and non-obese subjects had similar incidence of worsening renal function. P-value for BMI x intensification strategy interaction = 0.01.
Clinical outcomes at 60 days
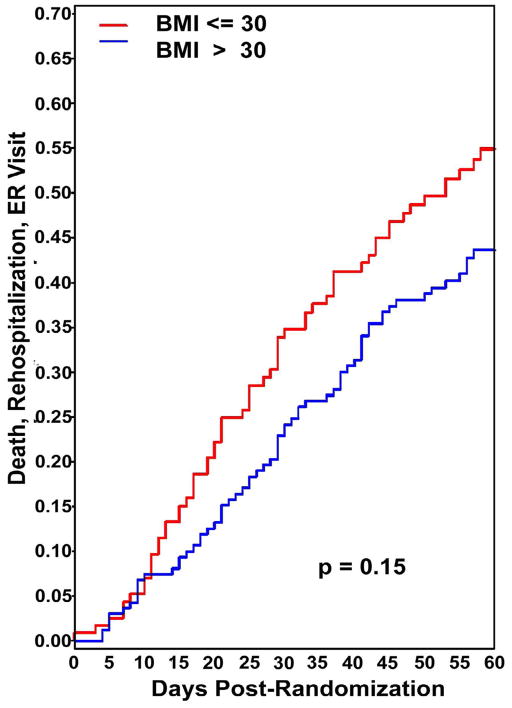
Despite the differences in serum creatinine at 72 hours after randomization, at 60 days there were no significant differences in serum creatinine or in the number of subjects with worsening renal function in obese vs. non-obese subjects, regardless of diuretic intensification strategy (Table 2). The number of days free from HF hospitalization or death was not different in the obese and non-obese groups (45.1±14.1 vs. 43.7±15.9 day, respectively, p=0.45). Similarly, the time to composite endpoint of emergency room visit, rehospitalization or death was not statistically different between the groups (Figure 3).
Figure 3.
Kaplan Meier plot of time to composite endpoint (emergency room visit, rehospitalization or death). There was no significant difference in the 60 day event rate between the obese and non-obese groups.
Discussion
The prevalence of obesity in our study is higher than that in previous work such as the ADHERE registry11. Despite wide recognition of the continuing obesity epidemic, there is very little literature that specifically addresses the optimal treatment of obese subjects with HF. In this study, we examined whether obese subjects with HF differed from non-obese in terms of demographics, clinical characteristics, and response to the treatment strategies tested in the DOSE trial. The main findings of our analyses are that: 1) high intensification compared to low intensification diuretic treatment resulted in greater fluid loss in both obese and non-obese subjects compared to low intensity treatment; 2) non-obese subjects had a low incidence of worsening renal function with the low intensification treatment strategy, but high intensification diuretic treatment in the non-obese subjects was associated with a greater incidence of worsening renal function; 3) in contrast, obese subjects had a higher incidence of worsening renal function with low intensification treatment compared to non-obese subjects; further, high intensification diuretic treatment in obese subjects did not result in any additional incidence of worsening renal function compared to the low intensification strategy; 4) although obese subjects more often had worsening renal function than nonobese subjects when considering both treatment strategies, this did not translate into any worsening of clinical outcomes including the degree of symptomatic improvement, length of hospital stay or 60-day rehospitalization or death. These findings provide some insights into important questions regarding obesity and HF.
Are obese patients with acute decompensated HF different from non-obese patients? In this study, obese subjects were younger, and had a higher prevalence of diabetes. These differences between the obese and nonobese groups are unlikely to account for the differences in fluid loss or changes in kidney function since obesity was associated with worsening renal function even after multivariable adjustment for diabetes, age and gender. The issue of age in HF patients is clinically relevant since GFR tends to decrease with age, and the rise in prevalence of obesity is most pronounced in young adults and children22. Therefore, it is likely that we will see increasing numbers of younger and more obese patients with heart failure. On average, the obese group had higher LV ejection fraction. While obesity has been associated with the syndrome of HF with normal ejection fraction, most subjects in this study had LV systolic dysfunction. Identifying the optimal treatment of patients with HF with normal ejection fraction has proven to be a difficult target. The current results may not be applicable to that patient population. Taken together, our data imply that obesity in and of itself may be a factor that can modify the response to HF treatments.
Should obese patients with acute decompensated HF be treated differently from non-obese patients? Our initial hypothesis was that obese subjects would require higher doses of diuretics to achieve the same amount of fluid loss. This hypothesis was based on the following: i) obese patients generally have higher lean and fat body mass, and thus may have larger volumes of distribution14; and ii) obese subjects often have increased intra-abdominal pressure and/or increased renal vein pressure and hence may have greater resistance to glomerular filtration15. Counter to our hypothesis, we observed that obese subjects had greater fluid loss than non-obese with low intensification treatment and both obese and non-obese subjects had significantly better diuresis with high intensification treatment. Glomerular hyperfiltration occurs in obesity and has been proposed as a possible cause of kidney dysfunction23. It is plausible that this phenomenon could account for the enhanced diuresis observed in the obese subjects. The data from the present study do not allow us to evaluate whether glomerular hyperfiltration influenced the response to intravenous diuretic treatment. The additional diuresis achieved through high intensification treatment was associated with a higher rate of early worsening renal function in the non-obese group. In obese subjects, however, there was no additional worsening of renal function in the high intensification diuretic strategy group. Thus, higher doses of diuretics in obese subjects produced more volume loss with no additional worsening of renal function. Even though the rise in serum creatinine was greater in obese subjects, creatinine rose more in the obese only with the low intensification therapy. Furthermore, there was no significant difference between obese and non-obese subjects with respect to length of hospital stay or the composite of death, emergency department visit or rehospitalization at 60 days. Whether early worsening of renal function after initiation of diuresis results in long-term adverse effects is uncertain. In clinical practice, however, worsening of renal function often triggers a reduction in the diuretic dose being administered. The findings of our study suggest that an initial high intensification diuretic treatment strategy in obese HF patients appears to be justified because of the extra fluid loss that can be expected without the incremental “cost” of increasing the incidence of worsening renal function.
Does worsening renal function affect outcomes during treatment of acute HF? The significance of changes in renal function during treatment of acute HF has recently been reviewed24. There is some evidence that worsening renal function during treatment for acute HF may result in higher long-term mortality. There is less convincing evidence that worsening renal function predicts rehospitalization. No prior studies have examined the issue of whether worsening renal function carries different prognostic significance in obese vs. non-obese patients. Although the DOSE trial was not powered to detect mortality differences between treatment groups, we found that 60-day clinical outcomes in obese subjects were not different compared to nonobese subjects (Figure 3).
What is the role of NTproBNP measurements in obese patients with heart failure? Serum NTproBNP levels were more than 50% lower in obese vs. non-obese subjects. Previous work has shown similar findings in other populations25–27. These differences in NTproBNP may have significant implications for establishing the diagnosis of HF when this biomarker is used as a criterion. Both obese and non-obese subjects had decreases in NTproBNP levels during treatment. The absolute decrease in NTproBNP was less in the obese, but as a percent of baseline levels, the change in BNP at 72 hours was comparable between the two groups. These differences should be taken into consideration if NTproBNP is used as an outcome measure during treatment of acute HF.
Limitations
This was a subgroup analysis of a randomized trial and as such should be considered exploratory and hypothesis generating. We used weight at the time of enrollment to define obesity. Because all subjects had evidence of fluid retention at baseline, misclassification of obesity status would have occurred if calculated BMI dropped below 30 kg/m2 after diuresis. Average weight loss at 72 hours was 6.1 lbs in the low and 8.7 lbs in the high intensification arm. Thus, a maximum of 11% of the subjects in this study were misclassified as obese when using initial vs. final weight (i.e. weight at discharge or 7 days, whichever came first). All but one of these subjects was still in the overweight range (BMI 25–29.9 kg/m2) at study end. It is unlikely that this small change in classification affected our main conclusions. In addition, BMI measured on presentation to the hospital is the most practical measurement upon which treatment decisions could be made. Another potential concern is the use of BMI rather than waist circumference to define obesity. Although there are data showing that waist circumference is modestly superior to BMI for predicting cardiac events in population studies28, in other large investigations, BMI performs almost as well as waist circumference as a predictor of events 29, 30. Some authors have proposed that blood urea nitrogen levels in the serum are the best predictors of worsening renal function31. However, changes in serum creatinine are still the most commonly used means for defining worsening renal function.
Summary
Despite the growing recognition of obesity as a major global health care issue, few studies have specifically addressed whether treatments should be tailored based on adiposity. We found that high intensification intravenous diuretic treatment for acute HF produced greater fluid loss over the first 72 hours of hospitalization in both obese and nonobese subjects. However, the frequency of worsening renal function over the same time frame was related to both the presence of obesity and the treatment strategy. These data suggest that clinicians may need to consider the effects of obesity when choosing a treatment strategy for patients with acute HF. Specifically, our findings support the hypothesis that an initial treatment strategy of high intensification therapy in obese subjects with acute HF may be preferred.
Acknowledgments
(Funded by the National Heart, Lung, and Blood Institute; ClinicalTrials.gov number, NCT00577135)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Howel D. Trends in the prevalence of obesity and overweight in english adults by age and birth cohort, 1991–2006. Public Health Nutr. 2011;14:27–33. doi: 10.1017/S136898001000056X. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S, Gaziano JM, Vasan RS. Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Med Clin North Am. 2004;88:1273–1294. doi: 10.1016/j.mcna.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Baena-Diez JM, Byram AO, Grau M, Gomez-Fernandez C, Vidal-Solsona M, Ledesma-Ulloa G, Gonzalez-Casafont I, Vasquez-Lazo J, Subirana I, Schroder H. Obesity is an independent risk factor for heart failure: Zona franca cohort study. Clin Cardiol. 2010;33:760–764. doi: 10.1002/clc.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenson GS, Srinivasan SR. Emergence of obesity and cardiovascular risk for coronary artery disease: The bogalusa heart study. Prev Cardiol. 2001;4:116–121. doi: 10.1111/j.1520-037x.2001.00537.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao SV, Donahue M, Pi-Sunyer FX, Fuster V. Results of expert meetings: Obesity and cardiovascular disease. Obesity as a risk factor in coronary artery disease. Am Heart J. 2001;142:1102–1107. doi: 10.1067/mhj.2001.119419. [DOI] [PubMed] [Google Scholar]
- 7.Reisin E, Jack AV. Obesity and hypertension: Mechanisms, cardio-renal consequences, and therapeutic approaches. Med Clin North Am. 2009;93:733–751. doi: 10.1016/j.mcna.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Lakoski SG, Cushman M, Siscovick DS, Blumenthal RS, Palmas W, Burke G, Herrington DM. The relationship between inflammation, obesity and risk for hypertension in the multi-ethnic study of atherosclerosis (mesa) J Hum Hypertens. 2011;25:73–79. doi: 10.1038/jhh.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89:309–319. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: Analysis of body mass index and inhospital mortality for 108,927 patients in the acute decompensated heart failure national registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF, Jr, Califf RM, Gheorghiade M. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: Results from the outcomes of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure (optime-chf) study. Circulation. 2005;111:2454–2460. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
- 13.Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation. 1999;100:1311–1315. doi: 10.1161/01.cir.100.12.1311. [DOI] [PubMed] [Google Scholar]
- 14.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH. Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 17.Cirillo M, Senigalliesi L, Laurenzi M, Alfieri R, Stamler J, Stamler R, Panarelli W, De Santo NG. Microalbuminuria in nondiabetic adults: Relation of blood pressure, body mass index, plasma cholesterol levels, and smoking: The gubbio population study. Archives of internal medicine. 1998;158:1933–1939. doi: 10.1001/archinte.158.17.1933. [DOI] [PubMed] [Google Scholar]
- 18.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA : the journal of the American Medical Association. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Annals of internal medicine. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 20.Felker GM, O’Connor CM, Braunwald E. Loop diuretics in acute decompensated heart failure: Necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in us children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 23.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol. 2008;294:F685–696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Chirovsky D, Phatak H, McNeill A, Cody R. Renal function, health outcomes, and resource utilization in acute heart failure: A systematic review. Circ Heart Fail. 2010;3:726–745. doi: 10.1161/CIRCHEARTFAILURE.109.920298. [DOI] [PubMed] [Google Scholar]
- 25.Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed b-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 26.Rogers RK, Stoddard GJ, Greene T, Michaels AD, Fernandez G, Freeman A, Nord J, Stehlik J. Usefulness of adjusting for clinical covariates to improve the ability of b-type natriuretic peptide to distinguish cardiac from noncardiac dyspnea. Am J Cardiol. 2009;104:689–694. doi: 10.1016/j.amjcard.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 27.Kevin Rogers R, Stehlik J, Stoddard GJ, Greene T, Collins SP, Peacock WF, Maisel AD, Clopton P, Michaels AD. Adjusting for clinical covariates improves the ability of b-type natriuretic peptide to distinguish cardiac from non-cardiac dyspnoea: A sub-study of heard-it. Eur J Heart Fail. 2009;11:1043–1049. doi: 10.1093/eurjhf/hfp127. [DOI] [PubMed] [Google Scholar]
- 28.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litwin SE. Which measures of obesity best predict cardiovascular risk? J Am Coll Cardiol. 2008;52:616–619. doi: 10.1016/j.jacc.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Gelber RP, Gaziano JM, Orav EJ, Manson JE, Buring JE, Kurth T. Measures of obesity and cardiovascular risk among men and women. J Am Coll Cardiol. 2008;52:605–615. doi: 10.1016/j.jacc.2008.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58:375–382. doi: 10.1016/j.jacc.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]