Abstract
Activation of neurokinin-1 (NK-1) receptors in the rostral ventromedial medulla (RVM) can facilitate pain transmission in conditions such as inflammation, and thereby contribute to hyperalgesia. Since blockade of NK-1 receptors in the RVM can attenuate hyperalgesia produced by prolonged inflammation, we examined the role of NK-1 receptors in changes of response properties of RVM neurons following 4 days of hind paw inflammation with complete Freund’s adjuvant (CFA). Recording were made from functionally identified ON, OFF and NEUTRAL cells in the RVM. Spontaneous activity and responses evoked by a series of mechanical (10, 15, 26, 60, 100, and 180 g) and heat (34–50°C) stimuli applied to the inflamed and non-inflamed hind paws were determined before and at 15 and 60 min after injection of the NK1-antagonist L-733,060 or vehicle into the RVM. Prolonged inflammation did not alter the proportions of functionally-identified ON, OFF and NEUTRAL cells. ON cells exhibited enhanced responses to mechanical (60–100 g) and heat (48°–50°C) stimuli applied to the inflamed paw, which were attenuated by L-733,060 but not by vehicle. Inhibitory responses of OFF cells evoked by mechanical stimuli applied to the inflamed paw were also inhibited by L-733,060, but responses evoked by stimulation of the contralateral paw were increased. Heat-evoked responses of OFF cells were not altered by L-733,060. Also, neither L-733,060 nor vehicle altered spontaneous ongoing discharge rate of RVM neurons. These data indicate that NK-1 receptors modulate excitability of ON cells which contribute to both mechanical and heat hyperalgesia, whereas NK-1 modulation of OFF cells contributes to mechanical hyperalgesia during prolonged inflammation.
1. INTRODUCTION
Processing of nociceptive transmission in the spinal cord is modulated by descending projections from the brain stem, including the rostral ventromedial medulla (RVM) (Tracey, 1995; Duggan and Morton, 1988; Jones and Gebhart, 1987; Aimone and Gebhart, 1986; McCreery et al., 1979; Pubols et al., 1991). Descending pathways were initially shown to decrease pain transmission and produce antinociception (Aimone and Gebhart, 1986; Criswell, 1976; Dostrovsky et al., 1983; Basbaum and Fields, 1984; Basbaum and Fields, 1978; Behbehani, 1995). However, it is now accepted that descending systems can also facilitate nociceptive transmission and contribute to hyperalgesia (see reviews by Porreca et al., 2002; Vanegas, 2004; Suzuki et al., 2004).
The rostral ventromedial medulla (RVM), which includes the Nucleus Raphe Magnus, the Nucleus Reticularis Gigantocellularis, the Nucleus Gigantocellularis pars alpha, and the Nucleus Paragigantocellularis lateralis, plays an important role in spinal nociceptive processing as a relay structure of descending modulation (Basbaum and Fields, 1984; Fields et al., 1991; Watkins et al., 1998; Urban et al., 1999; Urban et al., 1999; Urban and Gebhart, 1999; Urban and Gebhart, 1999; Millan, 2002), including facilitation of nociceptive transmission and the development of hyperalgesia. Studies have shown that the RVM contributes to hyperalgesia produced by inflammation (Montagne-Clavel and Oliveras, 1994; Hamity et al., 2010; Hurley et al., 2003; Pacharinsak et al., 2008; Wiertelak et al., 1994; Watkins et al., 1994; Hurley and Hammond, 2000; Hurley and Hammond, 2000), nerve injury (Burgess et al., 2002; Pertovaara et al., 1996), chronic opioid administration (Kaplan and Fields, 1991; Meng and Harasawa, 2007), cancer (Donovan-Rodriguez et al., 2006), and visceral pain (Coutinho et al., 1998; Zhuo and Gebhart, 2002; Zhuo et al., 2002).
Neurons in the RVM are classified physiologically as ON, OFF, NEUTRAL, and serotonergic cells. ON cells are thought to facilitate nociceptive transmission because they demonstrate bursting discharge in response to noxious stimuli just prior to nociceptive reflex and are inhibited by morphine. In contrast, OFF cells are thought to inhibit nociceptive transmission since their spontaneous discharge is transiently inhibited by noxious stimuli just prior to a nociceptive reflex and are excited by morphine (Fields et al., 1983; Fields and Heinricher, 1985; Fields, 2004; Heinricher et al., 1994; Heinricher et al., 2009; Heinricher et al., 1989). NEUTRAL cells are unresponsive to noxious cutaneous stimuli and their role in pain modulation is unclear. It was suggested that during hyperalgesia NEUTRAL cells might convert to ON- or OFF-like cells and thereby contribute to descending modulation of nociceptive transmission (Miki et al., 2002). Also, involvement of NEUTRAL cells in the modulation of nociceptive visceral inputs has been suggested (Brink et al., 2006; Brink and Mason, 2003). Serotonergic neurons appear to be a separate group of RVM cells that exhibit steady slow discharge rates, do not have immediate response to noxious stimuli, and exhibit distinct neurochemistry (Gao et al., 1997; Mason, 1997; Potrebic et al., 1994). These neurons may be involved in tonic, rather than phasic, modulation of different spinal processes (for detailed review see Mason, 1999; Mason, 2012).
Importantly, pronociceptive and antinociceptive activity of ON and OFF cells are altered in various models of hyperalgesia (Kaplan and Fields, 1991; Meng and Harasawa, 2007; Bederson et al., 1990; Carlson et al., 2007; Goncalves et al., 2007; Budai et al., 2007; Sanoja et al., 2010). Several lines of evidence indicate that substance P (SP) via neurokinin-1 (NK-1) receptors in the RVM engages ON cell activity and drives descending facilitation of nociceptive transmission. NK-1 receptors are found on neurons in the RVM (Budai et al., 2007; Ljungdahl et al., 1978; Marson and Loewy, 1985; Nakaya et al., 1994; Saffroy et al., 2003; Saffroy et al., 1988). ON cells were excited by iontophoretic application of SP or intraplantar injection of capsaicin through activation of NK-1 receptors (Budai et al., 2007). In behavioral studies, NK-1 receptor antagonists attenuated hyperalgesia produced by capsaicin (Pacharinsak et al., 2008) and inflammation (Hamity et al., 2010; Lagraize et al., 2010), and administration of SP into the RVM produced hyperalgesia (Hamity et al., 2010; Lagraize et al., 2010). Moreover, SP facilitates glutamate excitatory transmission in the RVM during prolonged inflammation (Zhang and Hammond, 2009).
We recently showed that excitatory responses of ON and inhibitory responses of OFF cells became transiently sensitized to mechanical and heat stimuli after an intraplantar injection of capsaicin, and this was, at least in part, dependent on NK-1 receptors (Brink et al., 2012). Since blockade of NK-1 receptors in the RVM attenuated hyperalgesia produced during prolonged inflammation (Hamity et al., 2010), we investigated changes in response properties of identified RVM neurons following four days of inflammation and the contribution of NK-1 receptors.
2. EXPERIMENTAL PROCEDURES
2.1. Animals
Adult, male Sprague-Dawley rats (250–375g) (Harlan Industries, Indianapolis, IN) were housed and maintained in a climate-controlled room on a 12-h dark/light cycle with food and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and were performed in accordance with the guidelines recommended by the International Association for the Study of Pain.
2.2. Inflammatory hyperalgesia
Inflammation was produced by a single intraplantar injection of undiluted Complete Freund’s Adjuvant (CFA, 0.1 ml) into the left hind paw four days prior to electrophysiology experiments. To determine the development of mechanical hyperalgesia, a von Frey monofilament with a calibrated bending force of 26 g was applied to the plantar surface of each hind paw 10 times (2–3 sec each). This monofilament produces withdrawal response frequencies of ~10–30% in naïve rats. Hyperalgesia was defined as a withdrawal response frequency ≥ 60%. Only rats that exhibited hyperalgesia on the inflamed paw were used. From among 129 rats pretreated with CFA, 7 animals (5.4%) did not meet this criterion and were excluded from the study
2.3. Surgical preparation
Rats were initially anesthetized by an intraperitoneal injection of Xylazine (10 mg/kg,) and Ketamine HCL (75 mg/kg,). The trachea was cannulated to maintain a clear airway and the jugular vein was catheterized for supplemental anesthesia with Brevital (1.0–1.5 ml/hr). A catheter was inserted into a carotid artery for continuous monitoring of blood pressure. Rats were placed in a stereotaxic apparatus and a craniotomy ~2 mm in diameter was made 11.5 mm caudal to bregma for access to the RVM. Body temperature was maintained at ~37°C using a feedback-controlled heating pad (Harvard Apparatus, Holliston, MA). Needle electrodes were inserted into the biceps femoris to record electromyographic (EMG) activity, which was used to identify withdrawal responses. Following surgery, rats were stabilized and monitored for one hour before recordings. The level of anesthesia was adjusted so that EMG responses were present during noxious stimulation of the hind paws.
2.4. Electrophysiological recording from RVM neurons
Recordings from single RVM neurons were made extracellularly using a stainless steel microelectrodes (10 MΩ, Frederick Haer and Co., Brunswick, ME) alone or attached to a single glass micropipette (outer tip diameter 50 – 80 μm) for combined recording and drug infusion (Heinricher and Neubert, 2004). The tip of the microelectrode extended ~300 μm beyond the tip of pipette. The infusion micropipette was connected to a 1-μl Hamilton syringe (Reno, NV) by PE-50 tubing for either drug or vehicle injection. The microelectrode (alone or with the pipette) was lowered into the RVM in 5-μm steps using an electronic microdrive (David Kopf Instruments, Tujunga, CA). The ranges of stereotaxic coordinates for all cells studied were: AP = −9.9 to −11.8 mm from bregma; DV = +9.4 to +10.6 mm from the cranial surface; L = −1.1 to +1.1 mm from midline (Paxinos and Watson, 1998). Neurons were initially isolated by ongoing discharge, and action potentials were amplified, displayed on a storage oscilloscope, audio-monitored, and discriminated according to amplitude and waveform. Only neurons whose action potentials were easily discriminated according to amplitude and shape were studied. Neurons in the RVM were classified functionally as ON, OFF, or NEUTRAL cells according to their responses evoked by noxious pinch or heat (50°C for 5 sec) applied to the non-inflamed paw. Cells were classified as ON cells if they exhibited an abrupt increase in discharge rate, as OFF cells if there was an abrupt decrease in discharge rate, and as NEUTRAL cells if discharge rates were unaltered during noxious stimulation. It is likely that our sample of NEUTRAL cells included serotonergeic cells since this cell types were not classified beyond the absence of an evoked response to noxious stimulation. Evoked responses of RVM neurons were typically accompanied by a withdrawal response (EMG activity). Action potentials, EMG activity, stimulus temperature, and time of mechanical stimulation were collected using a customized data acquisition program (Spike2, Cambridge Electronic Design, Cambridge, UK) and stored on a computer for off-line analyses.
2.5. Drug solutions
A stock solution (10 mM) of the non-peptide NK-1 receptor antagonist, L-733,060 (Tocris, Ellisville, MO) was prepared in distilled water and dissolved in PBS to its final concentration (1.5 pmol) on the day of the experiment. This concentration of L-733,060 was chosen because it reduced hyperalgesia (Pacharinsak et al., 2008) and sensitization of RVM neurons produced by capsaicin (Brink et al., 2012). Injections of L-733,060 or vehicle were given into the RVM in a volume of 0.5 μl (Pacharinsak et al., 2008; Lagraize et al., 2010; Brink et al., 2012) administered over a period of 3–5 minutes.
2.6. Experimental design
In the first set of experiments we determined whether hind paw inflammation altered the proportion of ON, OFF and NEUTRAL cells and their ongoing spontaneous activity. Following functional classification, spontaneous activity was sampled continuously for 5 min. Since multiple cells were often characterized in a single electrode track, stereotaxic coordinates were recorded for each cell for histological identification of recording sites.
In separate experiments, we determined the effects of injecting the NK-1 receptor antagonist, L-733,060, or vehicle into the RVM on evoked responses of RVM neurons. Once a neuron was classified, controlled mechanical and heat stimuli were applied to the plantar surface of each hind paw beginning with the non-inflamed paw. von Frey monofilaments with bending forces of 10, 15, 26, 60, 100, and 180 g were applied in ascending order, each for a duration of 5 sec and a 20 sec interstimulus interval. Next, heat stimuli of 34–50°C, each of 5 sec duration, were delivered in ascending order of 2°C increments from a base temperature of 30°C using a computer-controlled Peltier thermode with a 1 cm2 contact area (Yale Instrumentation Facility). Each heat stimulus was separated by 60 sec. All stimuli were first applied to the non-inflamed hind paw followed by the paw injected with CFA. Spontaneous activity (collected during 120 s period) and responses evoked by mechanical and heat stimuli were determined before and at 15 and 60 min after injection of the NK-1 receptor antagonist, L-733,060, or vehicle injection into the RVM. Only one neuron was studied in each animal. Since it was not always possible to complete all tests from each paw we occasionally had incomplete data sets for individual neurons. Incomplete data were used in our analyses with a few restrictions: 1) comparisons of side differences in responses to each modality of stimuli (mechanical or heat) were conducted only with neurons tested by the full series of stimuli of the same modality on both paws before any injection into the RVM; 2) for the analysis of effects of RVM injections on responses to stimuli of different modalities, only neurons that were fully tested by mechanical or heat stimuli from each paw before and at 15 and 60 minutes following injection were used.
2.7. Histological verification of recording sites
At the end of each experiment, a microlesion was made at the recording site by passing positive current (20 μA for 20 s) through the recording electrode. Animals were euthanized with an overdose of Brevital and perfused intracardially with physiological saline followed by 10% formalin containing 1% potassium ferrocyanide to cause a Prussion blue reaction. Recording sites were identified histologically in 50 μm sections. Locations of Prussion blue marks, microlesions, and stereotaxic coordinates were reconstructed using a stereotaxic atlas (Paxinos and Watson, 1998).
2.8. Data analysis
Changes in the mean ongoing and stimulus evoked activities of ON, OFF, and NEUTRAL cells following injection of vehicle or L-733,060 were determined using two-way ANOVA with repeated measures. Responses evoked by mechanical stimuli were determined by subtracting the number of spontaneous impulses that occurred for 5 s prior to each stimulus from the number of impulses evoked during the stimulus (5 sec). Similarly, responses to heat stimuli were determined by subtracting the number of impulses that occurred during 20 sec preceding each stimulus temperature from the number of impulses evoked for 20 sec from the beginning of the stimulus. Since OFF cells exhibit ongoing activity that is decreased by noxious stimuli, their evoked responses are described as a negative number of impulses. The effects of L-733,060 and vehicle on responses evoked by mechanical and heat stimuli applied to each hind paw were determined using one- or two-way ANOVAs with repeated measures followed by Student Neuman-Keuls comparisons, Chi-square tests and unpaired t-tests where appropriate since data were normally distributed. A p value of < 0.05 was considered significant in all cases. Mean (±SEM) values are reported throughout the results unless otherwise stated.
3. RESULTS
A total of 149 rats were used for these experiments. For comparisons between control and inflamed rats in proportions of ON OFF and NEUTRAL cells and spontaneous activity, 20 naïve and 22 animals with hind paw inflammation were used. For studding the effects of inflammation on evoked responses of RVM neurons and modulation of RVM neurons by L-733,060 or vehicle into the RVM, 107 CFA-treated rats were used.
3.1. Proportions and spontaneous activity of functionally-identified neurons in the RVM
We examined whether long lasting inflammation altered the proportions and spontaneous activity of functionally-classified neurons in the RVM. In 80 neurons identified in naïve animals, 28 (35%) were classed as ON, 15 (19%) as OFF, and 37 (46%) as NEUTRAL cells. Of the 71 neurons studied in CFA-treated rats, 25 (35%) were classified as ON, 13 (18%) as OFF, and 33 (47%) as NEUTRAL cells. These data indicate that long lasting inflammation did not alter the distribution of functional subtypes of neurons in the RVM (Chi-square test, p = 0.998). The observation that the proportion of functionally-identified NEUTRAL cells was not changed by prolonged inflammation suggests that these cells did not develop excitatory (ON-like) or inhibitory (OFF-like) responses to mechanical or heat stimuli.
Prolonged inflammation increased spontaneous activity of ON cells. The mean discharge rate of ON cells was 7.8 ± 1.6 imp/sec, and this was greater than the discharge rate of ON cells in naïve rats (3.9 ± 0.7 imp/sec; unpaired t-test, p< 0.05). Discharge rates of OFF cells were not altered by inflammation and were 8.7 ±1.7 imps/sec in naïve rats and 10.8 ±1.7 imps/sec in inflamed rats (unpaired t-test, p= 0.39). Similarly, the mean spontaneous activity of NEUTRAL cells in naïve rats was 17.4 ± 2.6 imp/sec and did not differ from the mean rate of spontaneous activity in inflamed rats (20.2 ± 2.6 imp/sec; unpaired t-test, p= 0.45).
3.2. Changes in evoked responses of RVM neurons during prolonged inflammation: comparisons between inflamed and control hind paws and effects of L-733,060
In separate experiments, we determined the effects of hind paw inflammation on responses of RVM neurons evoked by noxious stimuli applied to the inflamed and non-inflamed hind paw, and the effects of the NK-1 antagonist, L-733,060, on evoked responses of RVM neurons.
3.2.1. Changes in evoked responses of RVM neurons during prolonged inflammation
Responses to mechanical stimuli
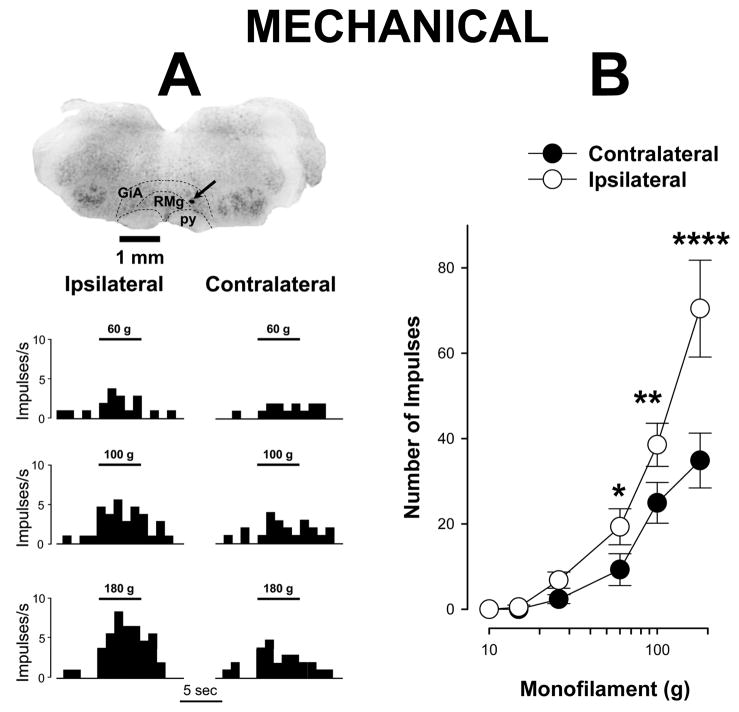
Following 4 days of unilateral hind paw inflammation, ON cells exhibited increased responses to suprathreshold mechanical stimuli following stimulation of the inflamed hind paw. Examples of responses evoked by mechanical stimulation of the inflamed and contralateral paws are shown for a single ON cell in Figure 1A. As shown in Figure 1B, mean responses evoked by the strongest mechanical stimuli, 60, 100, and 180 g, were significantly greater for the inflamed hind paw as compared to the contralateral paw. For example, the mean numbers of impulses evoked by the 60, 100 and 180 g stimuli applied to the inflamed paw were 19.3 ± 4.2, 38.6 ± 5.1, and 70.5 ± 11.4 impulses, respectively whereas application of these stimuli to the contralateral paw evoked smaller responses (9.3 ± 3.7, 24.9 ± 4.8, and 34.9 ± 6.4 impulses, respectively) (two-way repeated measures ANOVA, p < 0.001, n=32).
Figure 1.
Prolonged inflammation increases responses of ON cells evoked by mechanical stimulation of the inflamed paw. A) Examples of responses of a single ON cell and location of its recording site in the RVM. Responses to mechanical stimuli applied to the inflamed paw were greater than those produced by stimulation of the contralateral paw. Structures of the brain stem are indicated by dashed lines according to the atlas of the rat brain (Paxinos and Watson, 1998), GiA – Gigantocellular Reticular nucleus, pars Alpha, RMg – Raphe Magnus nucleus, py – pyramidal tract. The site of recording, marked by prussian blue, is indicated by arrow. Responses are presented as frequency histograms. Mechanical stimuli of 5 sec and bending force of applied von Frey monofilaments are indicated above histograms. B) Mean (± sem) number of impulses evoked by different intensities of mechanical stimulation with von Frey monofilaments (each of 5 s duration) applied to the plantar surface of the inflamed and contralateral paws for all ON cells (n=32). * indicates a significant difference between the inflamed and contralateral (* p < 0.05, ** p < 0.01, **** p < 0.001).
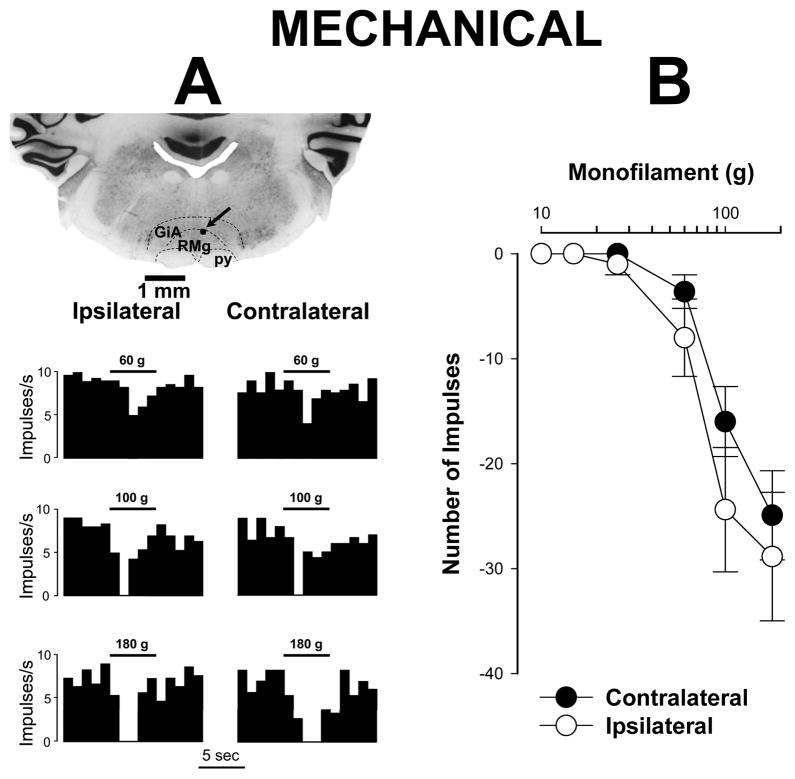
Unlike ON cells, differences in responses of OFF cells evoked by stimulation of the inflamed and contralateral paws did not differ (Figure 2; two-way repeated measures ANOVA, p = 0.056, n=17). The mean number of impulses evoked by stimuli of 60, 100 and 180 g applied to the inflamed hind paw were −8.0 ± 3.7, −24.4 ± 6.0, and −28.9 ± 6.1 impulses, respectively, for OFF cells. These responses did not differ from those evoked by mechanical stimuli applied to the contralateral paw (−3.6 ± 1.6, −16.0 ± 3.3, and −24.9 ± 4.3 impulses, respectively).
Figure 2.
Responses of OFF cells to mechanical stimuli during prolonged inflammation. A) Examples of mechanically-evoked responses (frequency histograms) and the recording site for a single OFF cell. Responses to mechanical stimuli did not differ between inflamed and contralateral paws. Structures of the brain stem are indicated as in Figure 1A. The recording site is indicated by the arrow. Periods of mechanical stimuli and bending force of applied by the von Frey monofilaments are indicated above the histograms. B) Mean (± sem) number of impulses evoked by different intensities of mechanical stimulation with von Frey monofilaments (each of 5 s duration) applied to the plantar surface of the inflamed and contralateral paws (n=17). There were no differences in responses evoked by stimulation of the inflamed and contralateral paws.
Responses to heat stimuli
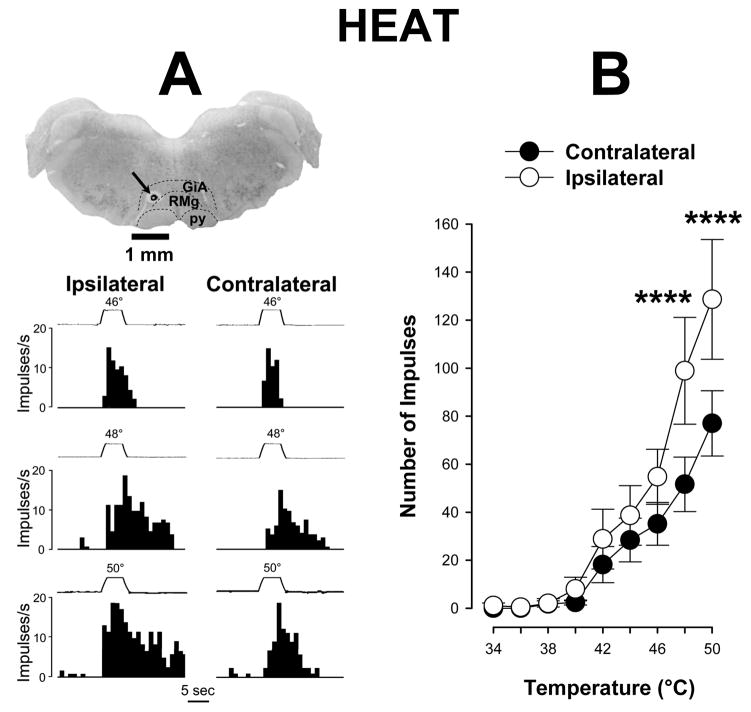
Differences were observed in responses of ON cells to heat applied to the inflamed and contralateral paws (Figure 3A). Responses of ON cells evoked by heat stimuli applied to the inflamed hind paw were significantly greater than those evoked by heat stimuli applied to the contralateral, non-inflamed paw (two-way repeated measures ANOVA, p < 0.001, n=27). Although there were no differences in responses of ON cells evoked by low stimulus temperatures applied to each paw, stimuli of 48 and 50°C applied on the inflamed hind paw evoked greater mean responses (98.9 ± 22.3 and 128.7 ± 25.0 impulses, respectively) as compared to the non-inflamed paw (51.6 ± 11.4 and 77.0 ± 13.6 impulses, respectively) (Figure 3B).
Figure 3.
Responses of ON cells evoked by heat during prolonged inflammation. A) Examples of responses (frequency histograms) of a single ON cell to heat stimuli and the location of its recording site in the RVM. Responses were greater for stimuli applied to the inflamed paw. Structures of the brain stem are indicated as in Figure 1A. The recording site is indicated by arrow. Traces of stimulus temperatures (5-s duration) are indicated above the histograms. B) Mean (± sem) number of impulses evoked by heat stimuli applied to the inflamed and contralateral paws, Responses evoked by 48° and 50°C were greater for stimuli applied to the inflamed paw (n=27). **** indicates a significant difference between the inflamed and contralateral paws (p < 0.001).
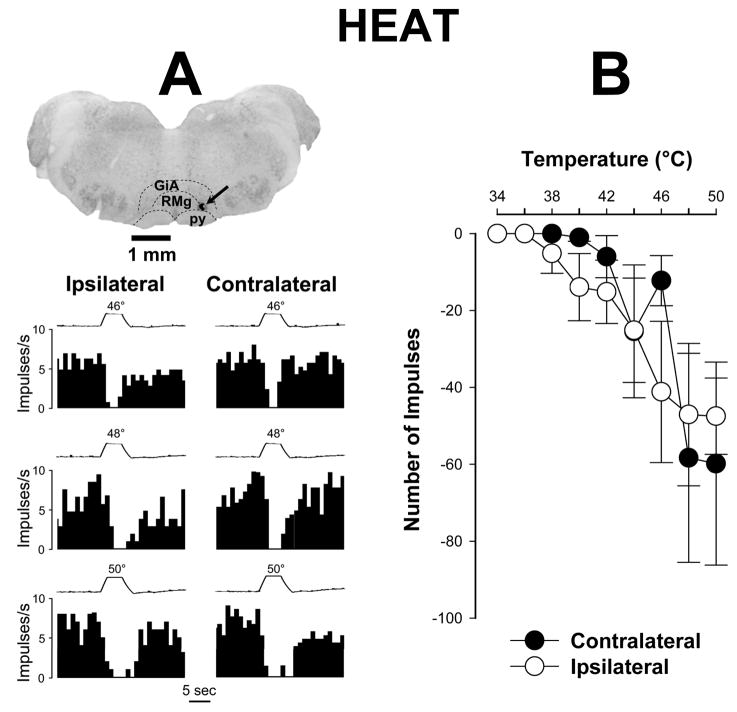
As for responses evoked by mechanical stimuli, OFF cells did not exhibit any differences in their inhibitory responses to heat stimuli between the inflamed and non-inflamed hind paws (two way repeated measures ANOVA, p = 0.21, n=16) (Figure 4A). For example, the mean number of impulses evoked by application of 50°C to the inflamed and contralateral hind paws was −47.5 ± 10.0 and −59.8 ± 26.4 impulses, respectively (Figure 4B). Collectively, these data suggest that ON cells, but not OFF cells, play a major role in the facilitation of mechanical and heat hyperalgesia produced by prolonged inflammation.
Figure 4.
Responses of OFF cells evoked by heat stimuli during prolonged inflammation. A) Examples of responses (frequency histograms) to heat and the recording site for a single OFF cell. Responses to heat stimuli did not differ between paws. Brain stem structures are indicated as in Figure 1A. The recording site is indicated by arrow. Traces of stimulus temperature (5-s duration) are provided above each histogram. B) Mean (± sem) number of impulses evoked by different intensities of heat stimulation (each of 5 s duration) applied to the plantar surface of the inflamed and contralateral paws (n=16). No differences in responses evoked by heat applied to the inflamed and contralateral paws.
3.2.2. Effects L-733,060 on spontaneous activity of RVM neurons
The discharge rate of spontaneous activity of ON cells was not altered following injection of L-733,060 or vehicle into the RVM. The mean discharge rate of spontaneous activity for ON cells (n=20) before injection of L-733,060 was 6.0 ± 1.0 imps/sec, and the rate was similar at 15 (6.2 ± 0.9 imps/sec) and 60 min (6.7 ± 1.0 imps/sec) after injection. In 6 ON cells tested following injection of vehicle into the RVM, mean discharge rates before and at 15 and 60 min after injection were 5.1 ± 1.7 imps/sec, 5.4 ± 1.8 imps/sec and 5.3 ± 1.7 imps/sec, respectively. There were no differences between groups (one-way ANOVA, p = 0.942).
Similarly, the rate of spontaneous activity for OFF cells (n=10) was not altered following injection of L-733,060 or vehicle into the RVM. The mean discharge rate of spontaneous activity before injection of L-733,060 was 10.7 ± 3.5 imps/sec and was not altered at 15 (9.5 ± 3.6 imps/sec) or 60 (11.0 ± 2.8 imps/sec) min after injection. These discharge rates of spontaneous activity were similar to those before and at 15 and 60 min following injection of vehicle (n=6) (11.7 ± 5.7 imps/sec, 9.1 ± 5.3 imps/sec, and 10.3 ± 4.6 imps/sec, respectively; one-way ANOVA between groups, p = 0.998).
Injection of L-733,060 or vehicle into the RVM also did not alter the ongoing spontaneous activity of NEUTRAL cells. The mean rate of spontaneous activity for NEUTRAL cells (n=10) before and at 15 and 60 min after injection of L-733,060 into the RVM was 21.9 ± 4.9 imps/sec, 21.6 ± 2.7 imps/sec, and 23.9 ± 4.3 imps/sec, respectively. These rates of spontaneous activity of NEUTRAL cells (n=6) obtained before and at 15 and 60 min after injection of vehicle into the RVM were 18.5 ± 4.5 imps/sec, 18 ± 3.8 imps/sec and 21.1 ± 3.9, respectively (one-way ANOVA, p = 0.835). Since spontaneous activity of NEUTRAL cells were not altered following injection of L-733,060 or vehicle into the RVM, and they did not exhibit responses to mechanical or heat stimuli at any time, these cells were not further analyzed.
3.2.3. Effects of L-733,060 on responses of RVM neurons evoked by mechanical stimuli
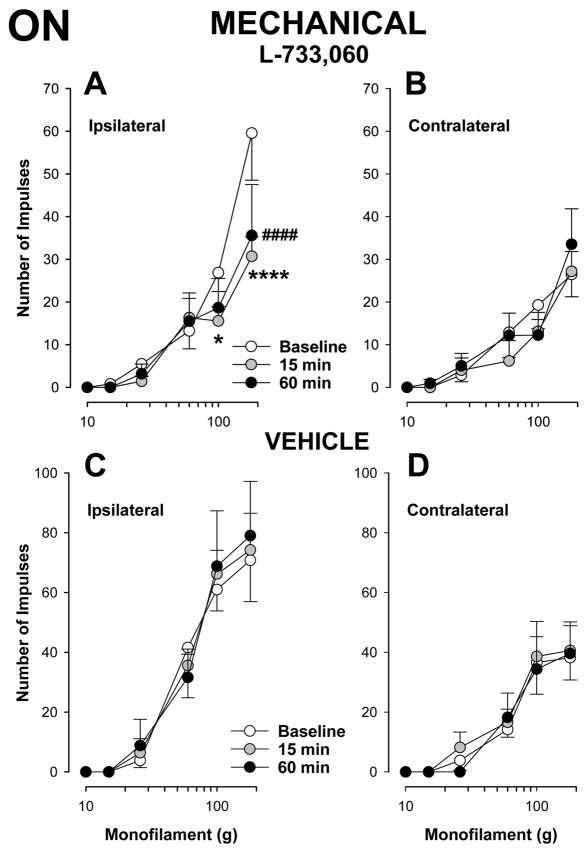
As shown in Figure 5A, injection of the NK-1 receptor antagonist, L-733,060 into the RVM decreased responses of ON cells (n=20) evoked by suprathreshold mechanical stimuli at 15 and 60 min after the injection, compared to baseline (two-way repeated measures ANOVA, p < 0.05 at 15 and 60 min). Moreover, the decrease in mechanically-evoked responses of ON cells was observed only when stimuli were applied to the ipsilateral, inflamed hind paw. For example, the mean number of impulses evoked by application of the strongest stimulus, 180 g, to the inflamed hind paw before injection was 59.5 ± 11.0 impulses and this decreased to 30.7 ± 4.5 and 35.6 ± 12.0 impulses at 15 and 60 min after injection, respectively. In contrast, the mean number of impulses evoked by the same stimulus applied to the contralateral paw was 26.4 ± 5.2 impulses before injection and 27.2 ± 4.7 and 33.5 ± 8.3 impulses at 15 and 60 min after injection, respectively (Figure 5B). Importantly, following injection of L-733,060 responses evoked by mechanical stimuli applied to the inflamed hind paw did not differ from responses evoked on the contralateral paw at anytime after injection (two-way repeated measures ANOVA, p = 0.366 at 15 min and p = 0.154 at 60 min). Injection of the vehicle into the RVM did not alter responses of ON cells (n=6) evoked by mechanical stimuli applied to either the inflamed or contralateral hind paw (Figure 5C and 5D). These results demonstrate that the enhanced responses of ON cells to mechanical stimuli following prolonged inflammation were dependent on NK-1 receptor activation and abolished by injection of L-733,060 into the RVM.
Figure 5.
L-733,060 decreased responses of ON cells to mechanical stimuli during prolonged inflammation The mean (± sem) number of impulses evoked by mechanical stimulation of the inflamed (A) but not the contralateral (B) paw were reduced following injection of L-733,060 in the RVM (n=20). Responses evoked by stimulation of the inflamed (C) or contralateral (D) paw were not altered following injection of vehicle into the RVM (n=6). * indicates a significant difference from baseline value at 15 min and # indicates a significant difference from baseline at 60 min (*p < 0.05, ****p < 0.001, ####p < 0.001).
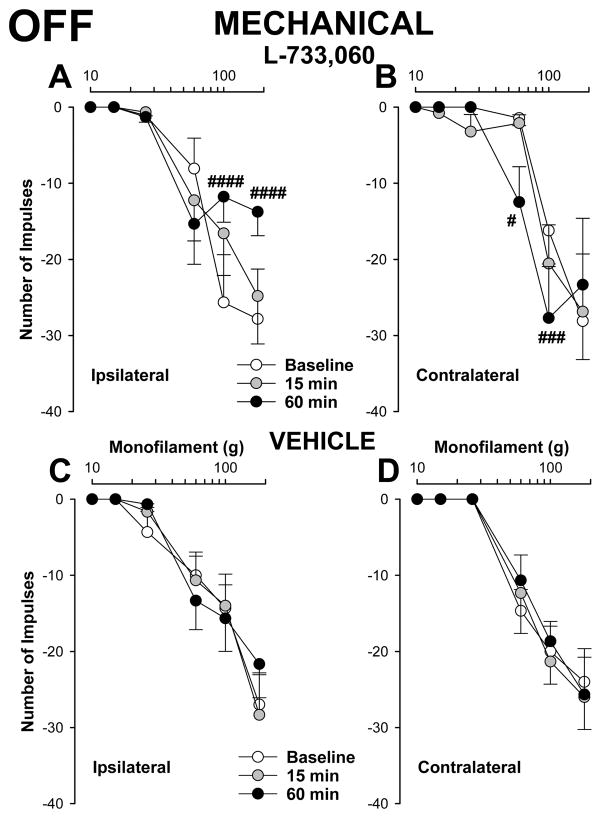
Injection of L-733,060 into the RVM also decreased responses of OFF cells (n=10) evoked by suprathreshold mechanical stimuli applied to the inflamed hind paw, but only at 60 min following injection (two-way repeated measures ANOVA, p= 0.271 at 15 min, and p <0.05 at 60 min). Mean responses of OFF cells evoked by stimuli 100 and 180 g before and at 60 min after injection were −25.7 ± 6.3 and −27.8 ± 6.6 before injection and −11.8 ± 3.4 and −13.8 ± 3.1 at 60 min after injection, respectively (Figure 6A).
Figure 6.
Effects of L-733,060 injection into the RVM on responses of OFF cells to mechanical stimuli. The inhibitory response evoked by mechanical stimulation decreased when stimuli were applied to the inflamed paw (A) but increased when applied to the contralateral paw (B) following injection of L-733,060 into the RVM (n=10). Responses evoked by stimulation of the inflamed (C) or contralateral (D) paw were not altered following injection of vehicle into the RVM (n=6). Data are expressed as the mean (± sem) number of impulses. # indicate significant differences from baseline values (# p < 0.05, ### p < 0.005, #### p < 0.001).
Interestingly, although inhibitory responses of OFF cells evoked by mechanical stimuli applied to the contralateral paw were unchanged at 15 min after injection of L-733,060 (two-way repeated measures ANOVA, p = 0.969), inhibitory responses evoked by 60 and 100g were enhanced at 60 min after injection (p < 0.001) (Figure 6B).
Injection of vehicle into the RVM did not alter responses of 6 OFF cells evoked by mechanical stimuli applied to either the inflamed or non-inflamed paw (Figures 6C and 6D). These data indicate that with prolonged inflammation, there may be NK-1 receptor dependent modulation of OFF cells that could contribute to hyperalgesia on the inflamed hind paw as well as to potential changes in sensitivity of the contralateral hind paw.
3.2.4. Effects of L-733,060 on responses of RVM neurons evoked by heat stimuli
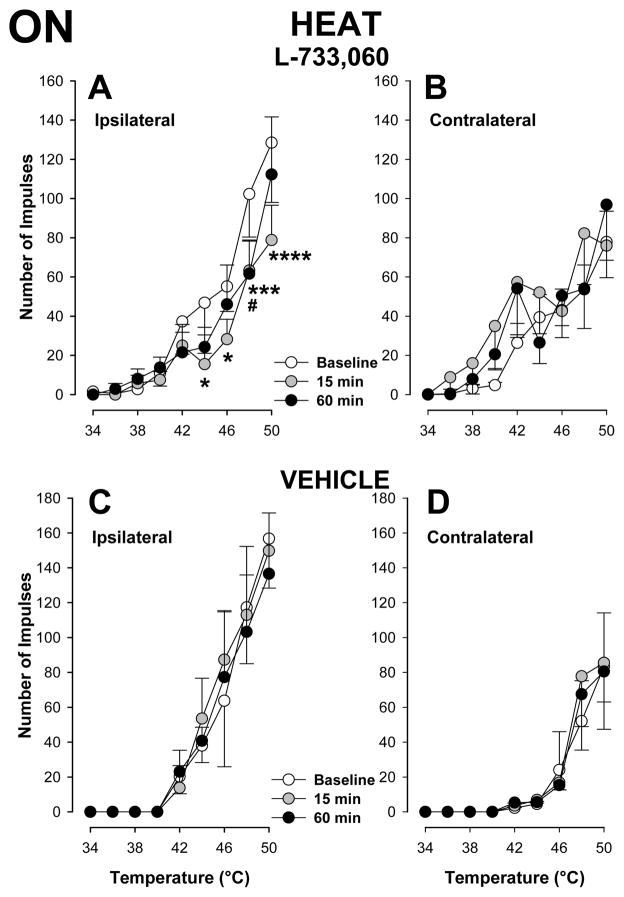
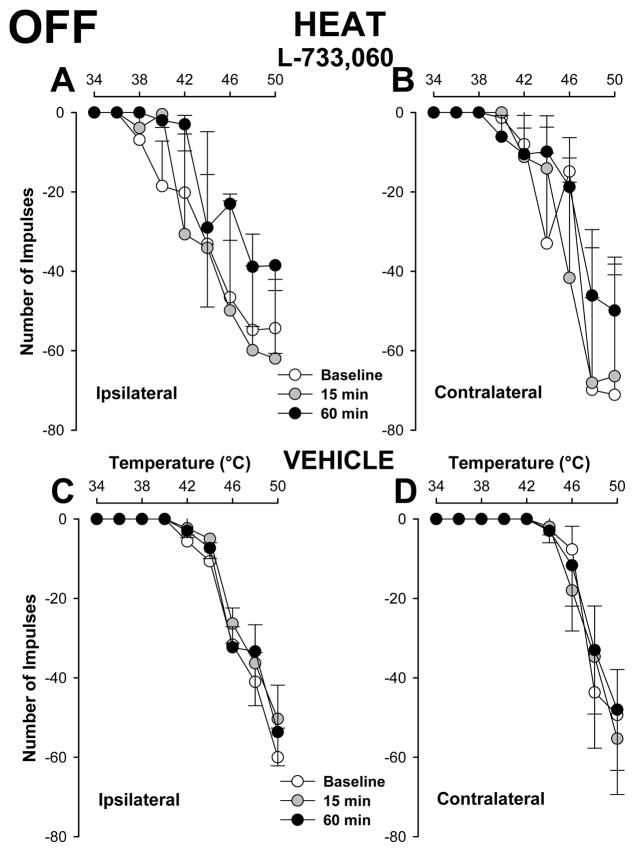
As shown in Figure 7A, responses of ON cells (n=20) evoked by suprathreshold heat stimuli applied to the inflamed hind paw were significantly decreased at 15 and 60 min after injection of L-733,060 into the RVM (two way repeated measures ANOVA, p < 0.01 at 15 min and p < 0.05 at 60 min after injection). The number of impulses evoked by the strongest stimuli, 48 and 50°C, were reduced after L-733,060. For example, the mean number of impulses evoked by 48°C applied to the inflamed hind paw was 102.3 ± 21.9 impulses before injection and 63.3 ± 15.0 and 61.6 ± 17.2 impulses at 15 and 60 min following injection, respectively. After injection of L-733,060, responses to heat applied to the inflamed hind paw were comparable to those evoked from the contralateral hind paw before or at anytime after injection (two-way repeated measures ANOVA, p = 0.251 at 15 min and p = 0.214 at 60 min). Responses to heat stimuli applied to the contralateral hind paw were not altered at any time following injection of L-733,060 into the RVM (Figure 7B). Also, injection of vehicle into the RVM did not alter responses of ON cells (n=6) evoked by heat stimuli applied to the inflamed (Figure 7C) or contralateral (Figure 7D) hind paw. Figures 8A and 8B show that injection of L-733,060 into the RVM did not alter the inhibitory responses of OFF cells (n=10) evoked by heat stimuli applied to either hind paw. In addition, responses of 6 OFF cells to heat stimuli applied to either hind paw were not altered following injection of vehicle into the RVM (Figures 8C and 8D).
Figure 7.
Attenuation of responses of ON cells to heat stimuli following injection of L-733,060 into the RVM. The mean (± sem) number of impulses evoked by heat stimuli of 44°–50°C applied to the inflamed paw (A) but t not the contralateral paw (B) was decreased at 15 min following injection of L-733,060 (n=20). Responses to heat stimuli following injection of vehicle (n=6) into the RVM were not altered (C and D). * and # indicate a significant difference from baseline at 15 and 60 min after injection, respectively (*p < 0.05, #p < 0.05, ***p < 0.005, ****p < 0.001).
Figure 8.
Effects of L-733,060 injection into the RVM on responses of OFF cells to heat stimuli. Responses of OFF cells evoked by heat stimuli applied to either paw were not altered at any time following injection of either 733,060 (A and B, n=10) or vehicle (C and D, n=6) into the RVM.
4. DISCUSSION
We examined the contribution of NK-1 receptors in the RVM to changes in response properties of RVM neurons during persistent inflammation of the hind paw. Following 4 days of inflammation, it was found that 1) responses of ON cells evoked by mechanical and heat stimuli applied to the inflamed hind paw were increased as compared to the contralateral, non-inflamed hind paw, 2) inhibitory responses of OFF cells evoked by mechanical and heat stimuli applied to each hind paw were similar; 3) injection of the NK-1 receptor antagonist, L-733,060, into the RVM attenuated the enhanced responses of ON cells to mechanical and heat stimuli; and 4) inhibitory responses of OFF cells evoked by mechanical stimuli applied to the inflamed hind paw were attenuated whereas mechanically-evoked responses applied to the non-inflamed hind paw were increased following injection of L-733,060 into the RVM. We did not conduct studies using an enantiomer of L-733,060 and we did not examine the effects of L-733,060 injected outside the RVM because these had no effects in behavioral studies (Pacharinsak et al., 2008). Our results suggest that changes in NK-1 signaling in the RVM alter response properties of RVM neurons during prolonged inflammation that may contribute to persistent hyperalgesia.
One concern is the potential effect of anesthesia on response characteristics of RVM neurons. We (Brink et al., 2012) and others (Jinks et al., 2004) have shown that increasing the depth of anesthesia decreases the responsiveness of ON and OFF cells without changing their functional properties. Considering that barbiturates, including Brevital that was used in our experiments, produce stronger inhibition of spinal motor neurons than interneurons and ascending projection neurons in the dorsal horn (Paik et al., 1989; Soja et al., 2002; Namjoshi et al., 2009), and the usual presence of a stimulus-evoked withdrawal response (EMG activity), suggests that anesthesia did not greatly influence responses of RVM neurons.
4.1. Functional alterations of RVM neurons that contribute to descending facilitation of pain during prolonged inflammation
Prolonged inflammation of the hind paw is accompanied by robust mechanical and heat hyperalgesia of the inflamed paw (Ren and Dubner, 1999; Simone et al., 2008; Potenzieri et al., 2008), activation of descending facilitatory pathways (Ren and Dubner, 1996), and sensitization of nociceptive spinal neurons (Ren et al., 1992). During inflammation, the RVM undergoes pronounced neurochemical (Miki et al., 2002; Guan et al., 2003; Terayama et al., 2000; Terayama et al., 2002; Guo et al., 2006; Imbe et al., 2008) and physiological plasticity (Terayama et al., 2002). Cellular changes in the RVM may lead to changes in function and the relative proportions of ON, OFF and NEUTRAL cells that may contribute to descending facilitation. Indeed, greater proportions of ON and OFF cells were found following 24 h of hind paw inflammation. (Miki et al., 2002), suggesting that NEUTRAL cells may develop ON- or OFF-like properties. In contrast, we did not observe changes in the proportions of functionally identified RVM neurons following 4 days of inflammation, suggesting that changes in the relative proportions of functionally-identified RVM neurons may be transient and contribute to descending facilitation soon after inflammation. Although it is likely that intrinsic changes within the RVM cause sensitization of RVM neurons (Budai et al., 2007), modification of ascending nociceptive input during inflammation, due to sensitization of nociceptors (Andrew and Greenspan, 1999) and enhanced responses of dorsal horn neurons (Ren et al., 1992; Hylden et al., 1989), may also contribute to the changes in RVM output (Khasabov et al., 2005; Heinricher and Drasner, 1991).
Our data demonstrate that prolonged inflammation increased spontaneous activity of ON cells without altering the ongoing discharge rates of OFF and NEUTRAL cells. In a previous study (Brink et al., 2012) we examined responses of RVM neurons before and after intradermal injection of capsaicin. The spontaneous discharge rate of ON cells under normal conditions in those studies (before capsaicin) was also lower than that of ON cells studied in CFA-treated rats in the present study. Since ongoing activity of OFF and NEUTRAL cells was not altered, it appears that ongoing pain associated with prolonged inflammation is in part mediated by a descending facilitation of nociceptive transmission without alterations in descending inhibition. However, it should be noted that intraplantar injection of capsaicin, a model of acute pain and hyperalgesia, increased ongoing activity of ON cells and decreased ongoing activity of OFF cells, but the decrease in OFF cell activity was transient and peaked soon after injection (Brink et al., 2012). This suggests that an increase in descending facilitation together with a decrease in descending inhibition may contribute to enhanced pain.
The role of ongoing spontaneous activity of RVM neurons in pain is unclear. Earlier studies have shown that removing descending controls increased activity of nociceptive dorsal horn neurons (Pubols et al., 1991; Khasabov et al., 2005; Li et al., 1998; Sandkuhler et al., 1987), suggesting that the net output from the RVM under normal conditions causes tonic inhibition of nociceptive transmission. However, morphine did not inhibit spontaneous activity of ON cells in awake rats but still strongly reduced responses of these cells evoke by to noxious stimuli (Martin et al., 1992). This finding raised the possibility that ongoing spontaneous discharge of ON cells in the RVM neurons does not play a major role in modulating spinal nociceptive transmission in awake animals (Mason, 2012). The role of spontaneous activity in descending modulation of nociceptive transmission, and particularly how spontaneous activity in the RVM relates to ongoing pain, is unclear and needs further study.
Prolonged inflammation increased responses primarily of ON cells evoked by mechanical and heat stimuli applied to the inflamed paw and are in agreement with previous studies (Miki et al., 2002). The effects of prolonged inflammation on evoked responses of RVM neurons are different from those produced by intraplantar injection of capsaicin, which clearly increased the excitatory responses of ON cells as well as the inhibitory responses of OFF cells evoked by mechanical and heat stimuli applied to the injected paw (Brink et al., 2012). It is difficult to determine the extent to which inhibitory responses of OFF cells were enhanced during inflammation since responses to mechanical and heat stimuli applied to either hind paw were not different. However, it is clear that inflammatory hyperalgesia was associated with increased activity of ON cells.
4.2. Modulation of RVM neurons by NK-1 receptors
In the present study we found that administration of the NK-1 receptor antagonist, L-733,060, into the RVM attenuated the enhanced responses of ON cells evoked by mechanical and heat stimuli applied to the inflamed paw. Because responses evoked by only the most intense mechanical and heat stimuli were decreased following L-733,060, we conclude that during prolonged inflammation NK-1 receptors modulate activity produced by intense stimuli only, as was shown for NK-1 receptors in the spinal cord (Cao et al., 1998). Interestingly, blockade of NK-1 receptors in the RVM also reduced the inhibitory responses of OFF cells to mechanical stimuli applied to the inflamed paw but enhanced the inhibitory responses produced by mechanical stimuli applied to the contralateral paw without affecting responses to heat. Although blockade of NK-1 receptors in the RVM reduced hyperalgesia on the inflamed paw (Hamity et al., 2010; Pacharinsak et al., 2008; Lagraize et al., 2010), there is evidence that under certain conditions activation of NK-1 receptors in the RVM may produce antinociception (Hamity et al., 2010). The increased inhibitory response of OFF cells following blockade of NK-1 receptors following prolonged inflammation may indicate changes in OFF cell activity that are related to antinociception. However, further studies are needed to define the functional correlates of these responses. Under normal conditions, inhibition of NK-1 receptors had no effect on behavioral measures of nociception (Hamity et al., 2010; Pacharinsak et al., 2008; Lagraize et al., 2010) and on evoked responses of RVM neurons (Hamity et al., 2010; Pacharinsak et al., 2008; Budai et al., 2007; Brink et al., 2012), indicating that there is normally no tonic release of SP in RVM and that NK-1 receptors may not play a role in the modulation of acute pain. In the present study, we also found that blockade of NK-1 receptors in the RVM did not alter spontaneous activity of ON, OFF, or NEUTRAL cells, suggesting that tonic release of SP in the RVM is unlikely to occur with prolonged inflammation. Rather, NK-1 receptors play a significant role in the sensitization of evoked responses of RVM neurons, and thereby contribute to descending facilitation and hyperalgesia. A similar function for NK-1 receptors occurs in nociceptive neurons in the spinal cord (Dougherty et al., 1994; Khasabov et al., 2002). It is likely that up-regulation of NK-1 receptors (Lagraize et al., 2010) and an increase in the number of RVM neurons that express NK-1 receptors (Hamity and Hammond, 2011) play a significant role in descending facilitation during prolong inflammation particularly since NK-1 receptor activation is associated with activation and sensitization of ON cells (Budai et al., 2007)
5. Conclusions
Results of the present study confirm and extend earlier behavioral and electrophysiological studies showing that NK-1 receptors in the RVM modulate activity of RVM neurons and play a significant role in descending facilitation of pain. Dickenson and colleagues originally proposed ascending-descending circuitry through which excitation of NK-1 expressing neurons in the spinal dorsal horn leads to excitation of neurons in the RVM and descending facilitation of nociceptive transmission (Suzuki et al., 2002). It was further shown that 5-HT3 receptors (Suzuki et al., 2005; Suzuki et al., 2004) as well as NMDA receptors (Lagraize et al., 2010) in the spinal cord contribute to the facilitation of nociceptive transmission originating from the RVM. It should be noted that SP is not the only neurotransmitter system in the RVM involved in descending facilitation of pain. For example, neurotensin or cholecystokinin administered into the RVM produced hyperalgesia and excited ON cells (Heinricher and Neubert, 2004; Kovelowski et al., 2000; Neubert et al., 2004). In addition, there is evidence that brain-derived neurotrophic factor (Guo et al., 2006) and glial activation in the RVM (Guo et al., 2007; Wei et al., 2008; Roberts et al., 2009) also contributes to descending facilitation. Unraveling the mechanisms in the RVM and spinal cord that drive descending facilitation may identify new targets for treating chronic pains.
Highlights.
We examined the modulation of RVM neurons by NK-1 receptors during inflammation.
Hind paw inflammation increased evoked responses of ON and OFF cells in the RVM.
The NK-1 antagonist, L-733,060, or vehicle was injected into the RVM.
Responses of primarily ON cells to mechanical and heat stimuli were decreased by L-733,060
RVM contributes to inflammatory hyperalgesia primarily through sensitization of ON cells via NK-1 receptors
Acknowledgments
The authors thank Drs. Donna Hammond and Darryl Hamamoto for reading an earlier version of the manuscript. This work was supported by NIH grants DA011471 and CA091007 (D.A. Simone), and DA023576 (D.L. Hammond). T.S. Brink was supported by the National Institute of Dental and Craniofacial Research (T32-DE007288).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone LD, Gebhart GF. Stimulation-produced spinal inhibition from the midbrain in the rat is mediated by an excitatory amino acid neurotransmitter in the medial medulla. J Neurosci. 1986;6:1803–1813. doi: 10.1523/JNEUROSCI.06-06-01803.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Brink TS, Hellman KM, Lambert AM, Mason P. Raphe magnus neurons help protect reactions to visceral pain from interruption by cutaneous pain. J Neurophysiol. 2006;96:3423–3432. doi: 10.1152/jn.00793.2006. [DOI] [PubMed] [Google Scholar]
- Brink TS, Mason P. Raphe magnus neurons respond to noxious colorectal distension. J Neurophysiol. 2003;89:2506–2515. doi: 10.1152/jn.00825.2002. [DOI] [PubMed] [Google Scholar]
- Brink TS, Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. Differential modulation of neurons in the rostral ventromedial medulla by neurokinin-1 receptors. J Neurophysiol. 2012;107:1210–1221. doi: 10.1152/jn.00678.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budai D, Khasabov SG, Mantyh PW, Simone DA. NK-1 receptors modulate the excitability of ON cells in the rostral ventromedial medulla. J Neurophysiol. 2007;97:1388–1395. doi: 10.1152/jn.00450.2006. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–294. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–13231. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- Criswell HE. Analgesia and hyperractivity following morphine microinjection into mouse brain. Pharmacol Biochem Behav. 1976;4:23–26. doi: 10.1016/0091-3057(76)90170-2. [DOI] [PubMed] [Google Scholar]
- Donovan-Rodriguez T, Urch CE, Dickenson AH. Evidence of a role for descending serotonergic facilitation in a rat model of cancer-induced bone pain. Neurosci Lett. 2006;393:237–242. doi: 10.1016/j.neulet.2005.09.073. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Shah Y, Gray BG. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. II. Effects on medullary dorsal horn nociceptive and nonnociceptive neurons. J Neurophysiol. 1983;49:948–960. doi: 10.1152/jn.1983.49.4.948. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Willis WD. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol. 1994;72:1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Morton CR. Tonic descending inhibition and spinal nociceptive transmission. Prog Brain Res. 1988;77:193–211. doi: 10.1016/s0079-6123(08)62786-7. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Gao K, Kim YH, Mason P. Serotonergic pontomedullary neurons are not activated by antinociceptive stimulation in the periaqueductal gray. J Neurosci. 1997;17:3285–3292. doi: 10.1523/JNEUROSCI.17-09-03285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves L, Almeida A, Pertovaara A. Pronociceptive changes in response properties of rostroventromedial medullary neurons in a rat model of peripheral neuropathy. Eur J Neurosci. 2007;26:2188–2195. doi: 10.1111/j.1460-9568.2007.05832.x. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Zou SP, Dubner R, Ren K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain. 2003;104:401–413. doi: 10.1016/s0304-3959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamity MV, Hammond DL. Neuroscience Meeting Planner Program No. 805.05. 2011. Persistent peripheral inflammation increases the number of neurokinin-1 receptor (NK1-R) expressing neurons in the rostral ventromedial medulla (RVM) [Google Scholar]
- Hamity MV, White SR, Hammond DL. Effects of neurokinin-1 receptor agonism and antagonism in the rostral ventromedial medulla of rats with acute or persistent inflammatory nociception. Neuroscience. 2010;165:902–913. doi: 10.1016/j.neuroscience.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Drasner K. Lumbar intrathecal morphine alters activity of putative nociceptive modulatory neurons in rostral ventromedial medulla. Brain Res. 1991;549:338–341. doi: 10.1016/0006-8993(91)90478-e. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–1989. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Banfor P, Hammond DL. Spinal pharmacology of antinociception produced by microinjection of mu or delta opioid receptor agonists in the ventromedial medulla of the rat. Neuroscience. 2003;118:789–796. doi: 10.1016/s0306-4522(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 1989;37:229–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Imbe H, Kimura A, Okamoto K, Donishi T, Aikawa F, Senba E, Tamai Y. Activation of ERK in the rostral ventromedial medulla is involved in hyperalgesia during peripheral inflammation. Brain Res. 2008;1187:103–110. doi: 10.1016/j.brainres.2007.10.075. [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E, Antognini JF. Isoflurane differentially modulates medullary on and off neurons while suppressing hind-limb motor withdrawals. Anesthesiology. 2004;100:1224–1234. doi: 10.1097/00000542-200405000-00026. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Spinal pathways mediating tonic, coeruleospinal, and raphe-spinal descending inhibition in the rat. J Neurophysiol. 1987;58:138–159. doi: 10.1152/jn.1987.58.1.138. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci. 1991;11:1433–1439. doi: 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabov SG, Ghilardi JR, Mantyh PW, Simone DA. Spinal neurons that express NK-1 receptors modulate descending controls that project through the dorsolateral funiculus. J Neurophysiol. 2005;93:998–1006. doi: 10.1152/jn.01160.2003. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–9098. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87:265–273. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- Lagraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neuroscience. 2010;171:1341–1356. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Monhemius R, Simpson BA, Roberts MH. Supraspinal inhibition of nociceptive dorsal horn neurones in the anaesthetized rat: tonic or dynamic? J Physiol (Lond) 1998;506 (Pt 2):459–69. doi: 10.1111/j.1469-7793.1998.459bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl A, Hokfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--I. Cell bodies and nerve terminals. Neuroscience. 1978;3:861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Marson L, Loewy AD. Topographic organization of substance P and monoamine cells in the ventral medulla of the cat. J Auton Nerv Syst. 1985;14:271–285. doi: 10.1016/0165-1838(85)90116-x. [DOI] [PubMed] [Google Scholar]
- Martin G, Montagne-Clavel J, Oliveras JL. Involvement of ventromedial medulla “multimodal, multireceptive” neurons in opiate spinal descending control system: a single-unit study of the effect of morphine in the awake, freely moving rat. J Neurosci. 1992;12:1511–1522. doi: 10.1523/JNEUROSCI.12-04-01511.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Medullary circuits for nociceptive modulation. Curr Opin Neurobiol. 2012 doi: 10.1016/j.conb.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Central mechanisms of pain modulation. Curr Opin Neurobiol. 1999;9:436–41. doi: 10.1016/S0959-4388(99)80065-8. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- McCreery DB, Bloedel JR, Hames EG. Effects of stimulating in raphe nuclei and in reticular formation on response of spinothalamic neurons to mechanical stimuli. J Neurophysiol. 1979;42:166–182. doi: 10.1152/jn.1979.42.1.166. [DOI] [PubMed] [Google Scholar]
- Meng ID, Harasawa I. Chronic morphine exposure increases the proportion of on-cells in the rostral ventromedial medulla in rats. Life Sci. 2007;80:1915–1920. doi: 10.1016/j.lfs.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL. Are ventromedial medulla neuronal properties modified by chronic peripheral inflammation? A single-unit study in the awake, freely moving polyarthritic rat. Brain Res. 1994;657:92–104. doi: 10.1016/0006-8993(94)90957-1. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Namjoshi DR, McErlane SA, Taepavarapruk N, Soja PJ. Network actions of pentobarbital in the rat mesopontine tegmentum on sensory inflow through the spinothalamic tract. J Neurophysiol. 2009;102:700–713. doi: 10.1152/jn.90933.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. NK-1 receptors in the rostral ventromedial medulla contribute to hyperalgesia produced by intraplantar injection of capsaicin. Pain. 2008;139:34–46. doi: 10.1016/j.pain.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Paik KS, Nam SC, Chung JM. Different classes of cat spinal neurons display differential sensitivity to sodium pentobarbital. J Neurosci Res. 1989;23:107–115. doi: 10.1002/jnr.490230114. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York, NY: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Wei H, Hamalainen MM. Lidocaine in the rostroventromedial medulla and the periaqueductal gray attenuates allodynia in neuropathic rats. Neurosci Lett. 1996;218:127–130. doi: 10.1016/s0304-3940(96)13136-0. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in Neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Potenzieri C, Brink TS, Pacharinsak C, Simone DA. Cannabinoid modulation of cutaneous Adelta nociceptors during inflammation. J Neurophysiol. 2008;100:2794–2806. doi: 10.1152/jn.90809.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci. 1994;14:1655–1665. doi: 10.1523/JNEUROSCI.14-03-01655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pubols LM, Simone DA, Bernau NA, Atkinson JD. Anesthetic blockade of the dorsolateral funiculus enhances evoked activity of spinal cord dorsal horn neurons. J Neurophysiol. 1991;66:140–152. doi: 10.1152/jn.1991.66.1.140. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999;40:111–118. doi: 10.1093/ilar.40.3.111. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J Neurophysiol. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JL, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci. 2009;30:229–241. doi: 10.1111/j.1460-9568.2009.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffroy M, Beaujouan JC, Torrens Y, Besseyre J, Bergstrom L, Glowinski J. Localization of tachykinin binding sites (NK1, NK2, NK3 ligands) in the rat brain. Peptides. 1988;9:227–241. doi: 10.1016/0196-9781(88)90255-0. [DOI] [PubMed] [Google Scholar]
- Saffroy M, Torrens Y, Glowinski J, Beaujouan JC. Autoradiographic distribution of tachykinin NK2 binding sites in the rat brain: comparison with NK1 and NK3 binding sites. Neuroscience. 2003;116:761–773. doi: 10.1016/s0306-4522(02)00748-0. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Fu QG, Zimmermann M. Spinal pathways mediating tonic or stimulation-produced descending inhibition from the periaqueductal gray or nucleus raphe magnus are separate in the cat. J Neurophysiol. 1987;58:327–341. doi: 10.1152/jn.1987.58.2.327. [DOI] [PubMed] [Google Scholar]
- Sanoja R, Tortorici V, Fernandez C, Price TJ, Cervero F. Role of RVM neurons in capsaicin-evoked visceral nociception and referred hyperalgesia. Eur J Pain. 2010;14:120.e1–120.e9. doi: 10.1016/j.ejpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Khasabov SG, Hamamoto DT. Changes in response properties of nociceptive dorsal horn neurons in a murine model of cancer pain. Sheng Li Xue Bao. 2008;60:635–644. [PubMed] [Google Scholar]
- Soja PJ, Taepavarapruk N, Pang W, Cairns BE, McErlane SA, Fragoso MC. Transmission through the dorsal spinocerebellar and spinoreticular tracts: wakefulness versus thiopental anesthesia. Anesthesiology. 2002;97:1178–1188. doi: 10.1097/00000542-200211000-00023. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nature Neuroscience. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res. 2004;1019:68–76. doi: 10.1016/j.brainres.2004.05.108. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Rygh LJ, Webber M, Hunt SP, Dickenson AH. Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin. Pain. 2005;117:292–303. doi: 10.1016/j.pain.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Terayama R, Dubner R, Ren K. The roles of NMDA receptor activation and nucleus reticularis gigantocellularis in the time-dependent changes in descending inhibition after inflammation. Pain. 2002;97:171–181. doi: 10.1016/s0304-3959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport. 2000;11:1915–1919. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- Tracey DJ. Ascending and descending pathways in the spinal cord. In: Paxinos G, editor. The rat nervous system. San diego, New York, Boston, London Sydney, Tokyo, Toronto: Academic Press; 1995. [Google Scholar]
- Urban MO, Coutinho SV, Gebhart GF. Involvement of excitatory amino acid receptors and nitric oxide in the rostral ventromedial medulla in modulating secondary hyperalgesia produced by mustard oil. Pain. 1999;81:45–55. doi: 10.1016/s0304-3959(98)00265-6. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Zahn PK, Gebhart GF. Descending facilitatory influences from the rostral medial medulla mediate secondary, but not primary hyperalgesia in the rat. Neuroscience. 1999;90:349–352. doi: 10.1016/s0306-4522(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Vanegas H. To the descending pain-control system in rats, inflammation-induced primary and secondary hyperalgesia are two different things. Neurosci Lett. 2004;361:225–228. doi: 10.1016/j.neulet.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, McGorry M, Martinez J, Schwartz B, Sisk D, Maier SF. Neurocircuitry of conditioned inhibition of analgesia: effects of amygdala, dorsal raphe, ventral medullary, and spinal cord lesions on antianalgesia in the rat. Behavioral Neuroscience. 1998;112:360–378. doi: 10.1037//0735-7044.112.2.360. [DOI] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertelak EP, Furness LE, Horan R, Martinez J, Maier SF, Watkins LR. Subcutaneous formalin produces centrifugal hyperalgesia at a non-injected site via the NMDA-nitric oxide cascade. Brain Res. 1994;649:19–26. doi: 10.1016/0006-8993(94)91044-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hammond DL. Substance P enhances excitatory synaptic transmission on spinally projecting neurons in the rostral ventromedial medulla after inflammatory injury. J Neurophysiol. 2009;102:1139–1151. doi: 10.1152/jn.91337.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002;122:1007–1019. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol. 2002;87:2225–2236. doi: 10.1152/jn.2002.87.5.2225. [DOI] [PubMed] [Google Scholar]