Abstract
Ischemic preconditioning (IPC) provides protection against subsequent severe ischemic injury. A recent study found that cerebral IPC prolongs bleeding time. In this study, we examined whether IPC protects against intra-cerebral hemorrhage (ICH)-induced brain edema formation and whether IPC affects blood coagulation. There were three sets of experiments in this study. In the first set, male Sprague–Dawley rats were preconditioned with either 15 min of left middle cerebral artery occlusion, an IPC stimulus, or a sham operation. Three days later, rats received an infusion of autologous whole blood in the ipsilateral or contralateral caudate. Rats were killed 24 h later for brain water content measurement. In the second set, rats underwent 15 min of IPC or a sham operation. Three days later, rats were used for bleeding and thrombin clotting time tests. In the third set, the levels of p44/42 mitogen-activated protein kinases (MAPKs), heme oxygenase-1 (HO-1), transferrin (Tf), and transferrin receptor (TfR) in the brain 24 or 72 h after IPC were examined. We found that IPC reduced ICH-induced brain edema when blood was injected into the ipsilateral caudate but it did not when blood was injected into the contralateral caudate. IPC resulted in prolongation of bleeding time and thrombin clotting time. IPC also induced the activation of p44/42 MAPKs and upregulation of HO-1, Tf, and TfR levels in the ipsilateral caudate. These results suggest that IPC protects against ICH-induced brain edema formation and decreases blood coagulation. The protection of IPC against ICH is mainly due to local factors in the brain and may be related to activation of p44/42 MAPKs and upregulation of HO-1, Tf, and TfR.
Keywords: Brain edema, Ischemic preconditioning, p44/42 mitogen activated protein kinases, Heme oxygenase-1, Transferrin, Transferrin receptor, Intracerebral hemorrhage
Introduction
Ischemic preconditioning (IPC) refers to a phenomenon by which a short period of ischemia induces protection, or tolerance, against a subsequent prolonged ischemic event. Preconditioning occurs after a wide range of other distinct stimuli (e.g., hypoxia, hyperthermia, hyperbaric oxygen, low dose of thrombin, pharmacological agents) [1–4] and in a variety of organs including brain [5]. Many experimental studies have shown that cerebral IPC provides robust protection against later ischemic injury [6]. Our earlier study also found that cerebral IPC attenuates brain edema formation and cerebrovascular injury induced by permanent middle cerebral artery (MCA) occlusion [5]. However, it is not clear whether or not IPC can reduce brain edema formation after intracerebral hemorrhage (ICH).
Coagulation cascade activation plays a crucial role in early brain edema formation after ICH [7]. A recent mouse study found that cerebral IPC prolongs bleeding time which may be related to alterations in platelet aggregation [6]. It is well known that bleeding time is influenced by a number of factors including platelet count and function, blood coagulation, and blood vessel properties. To date, whether or not cerebral IPC affects the blood coagulation cascade is unknown.
A number of endogenous factors in the brain may also be involved in modulating brain edema formation after ICH. p44/42 mitogen-activated protein kinases (p44/42 MAPKs) are important mediators of the signal transduction that regulate cell response to external stimuli. Our previous studies showed that p44/42 MAPKs are activated after hyperbaric oxygen preconditioning and thrombin preconditioning, and the activation of p44/42 MAPKs is involved in both hyperbaric oxygen preconditioning- and thrombin-induced protection against ICH-induced brain edema [1, 8]. Heme-oxygenase-1 (HO-1) is a key enzyme in heme degradation. There is evidence that HO-1 can prevent cell death by augmenting iron efflux [9]. Transferrin (Tf) and transferrin receptor (TfR), iron transport proteins, play an important role in iron transport across the blood–brain barrier and neuronal membrane [10]. Our previous studies have demonstrated that iron is an important factor causing brain edema after ICH [11–13].
In the present study, therefore, we examined whether IPC protects against ICH-induced brain edema formation and whether IPC affects the blood coagulation cascade. In addition, we examined p44/42 MAPKs activation and changes in brain HO-1, Tf, and TfR after IPC.
Materials and Methods
Animal Preparation
The animal use protocol was approved by the University Committee on Use and Care of Animals, University of Michigan. Adult male Sprague–Dawley rats (275–325 g; Charles River Laboratories Portage, MI, USA) were used for this study. Rats were fasted overnight before surgery with free access of water. Anesthesia was induced with 5 % isoflurane. After the animals were sedated and intubated, anesthesia was maintained by 2.25 % isoflurane through mechanical ventilation with a rodent ventilator (model 683; Harvard Apparatus, S. Natick, MA). A poly-ethylene catheter (PE-50) was then inserted into the femoral artery to monitor arterial blood pressure and to obtain blood samples for analysis of pH, PaO2, PaCO2, and blood glucose concentration before the onset of ischemia. Body temperature was maintained at 37.5°C by using a feedback-controlled heating pad.
Experimental Groups
There were three sets of experiments in this study. In the first set, rats underwent 15 min of transient MCA occlusion, an IPC stimulus, or a sham operation. Three days later, rats received an infusion of 100 μl autologous blood in the ipsilateral or contralateral caudate. Rats were sacrificed 24 h after ICH and the brains were used for brain water and ion content measurements. In the second set, rats underwent 15 min of IPC or a sham operation. Rats were used for thrombin clotting time and bleeding time measurement 72 h later. In the third set, rats received 15 min of IPC or a sham operation. Rats were killed 24 or 72 h later and brains were used for immunohistochemistry and Western blotting analysis.
MCA Occlusion
A lateral incision was made over the parietal bone. A 1.0-mm-diameter burr hole 6 mm lateral and 1 mm posterior to the bregma was drilled by a microsurgical drill without injury to the dura mater as previously described [14]. A laser doppler flowmetry (Laserflo BMP2; Vasamedics, Little Canada, MN) was used for measurement of the cortical blood flow before the onset of ischemia and during the ischemia. Transient (15 min) focal cerebral ischemia was induced by insertion of a 3–0 monofilament nylon suture via the left common carotid artery as previously described [15]. The suture was gently advanced into the internal carotid artery approximately 19 or 20 mm from the bifurcation to occlude the ostium of the MCA. After 15 min of MCA occlusion, reperfusion was achieved by withdrawal of the suture from the common carotid artery. The sham operation was identical except that the intraluminal suture was not inserted.
Intracerebral Infusion
Three days after IPC or a sham operation, animals were reanesthetized with isoflurane. The rats were positioned in a stereotactic frame (Kopf Instruments, Tujunga, CA). A cranial burr hole (1 mm) was drilled (coordinates: 0.2 mm anterior, 5.5 mm ventral, and 4.0 mm lateral to the bregma). Autologous blood was withdrawn from the femoral artery and infused (100 μl) immediately into the caudate nucleus through a 26-gauge needle at a rate of 10 μl/min with a microinfusion pump (Harvard Apparatus). After intracerebral infusion, the needle was removed and the skin incision closed.
Bleeding Time and Thrombin Clotting Time Test
Three days after 15 min of IPC or a sham operation, the rats were reanesthetized with pentobarbital (50 mg/kg, i.p.). The first 4 mm from the tip of the tail was incised. Tails were immersed in saline (37°C), and the time until bleeding stopped was measured. Blood samples were withdrawn from the abdominal aorta into plastic syringes containing 3.8 % sodium citrate solution (1/9 volume of the blood) and collected into plastic tubes. Plasma samples were obtained by centrifugation (for 10 min at 2000×g) and kept frozen until the day of measurement. Thrombin clotting time (TCT) was measured.
Brain Water and Ion Content Measurement
Rats were anesthetized with pentobarbital (50 mg/kg, i.p.) and decapitated 24 h after ICH for brain water and ion content measurement. The brains were immediately removed, and a 4-mm-thick coronal brain slice was cut 4 mm posterior to the frontal pole. The slice was divided into four samples: ipsilateral and contralateral basal ganglia, ipsilateral and contralateral cortex. The cerebellum was taken as a control. Tissue samples were weighed on an electronic analytical balance (Model AE 100; Mettler Instrument, Highstown, NJ) to obtain the wet weight. The tissue was then dried at 100°C for 24 h to determine the dry weight. Tissue water content (%) was calculated as [(WW − DW)/WW] × 100, where WW is the wet weight and DW is the dry weight. The dehydrated brain samples were digested in 1 ml of 1 N nitric acid for 1 week. Sodium and potassium contents were measured by flame photometry (model IL943; Instrumentation Laboratory, Lexington, MA). Ion contents were expressed in milliequivalents per kilogram brain dry weight (mEq/kg dry weight).
Immunohistochemistry
Immunohistochemistry was performed as previously described [8]. Briefly, rats were reanesthetized with pentobarbital (60 mg/kg, i.p.) and underwent intracardiac perfusion with 4 % paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). The brains were removed and kept in 4 % paraformaldehyde overnight, then were immersed in 25 % sucrose for 3 to 4 days at 4°C. Specimens were then placed in embedding compound and sectioned (18 μm slices) on a cryostat. Immunohistochemistry staining was performed by avidin–biotin complex technique. The primary antibodies were polyclonal rabbit anti-phospho-p44/42 MAPK (1:200 dilution, Cell Signaling Technology, Beverly, MA) and rabbit anti-rat HO-1 (1:400 dilution, StressGen, Victoria, Canada). Normal rabbit immunoglobulin G was used as a negative control.
Western Blot Analysis
Rats were anesthetized with pentobarbital (60 mg/kg, i.p.) and underwent intracardiac perfusion with 0.1 M phosphate-buffered saline (pH 7.4). The brains were then removed and sampled as described for water content measurement. Brain samples were sonicated with Western blot lysis buffer. Protein concentration was determined using a Bio-Rad protein assay kit. Fifty-μg protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a hybond-C-pure nitrocellulose membrane. Membranes were blocked in Carnation nonfat milk and probed with the primary and secondary antibodies. The primary antibodies were polyclonal rabbit anti-phospho-p44/42 MAPK (1:2,000 dilution, Cell Signaling Technology), rabbit anti-p44/42 (1:2000 dilution, Cell Signaling Technology), rabbit anti-rat HO-1 (1:1000 dilution, StressGen), monoclonal mouse anti-human TfR (1:2000 dilution, ZYMED Laboratories, CA), polyclonal rabbit anti-human Tf (1:2000 dilution, DAKO, Danmark). The antigen–antibody complexes were observed with the ECL chemiluminescence system (Amersham Pharmacia, Piscatataway, NJ) and exposed to Kodak (Rochester, NY.) X-OMAT film. The relative densities of bands were analyzed using NIH Image (Version 1.61).
Statistical Analysis
All data in this study are presented as mean±S.D. Data were analyzed with Student’s t-test. Statistical significance was set at 0.05.
Results
Systemic physiological variables including blood pH, PaO2, PaCO2, and glucose were measured before the onset of MCA occlusion and were controlled within normal ranges. The combined mean physiological variables are shown in Table 1. This was a blinded study. Surgeries were preformed by one research fellow (MK). Water content, blood test, immunohistochemistry, and Western blotting were assessed by another research fellow (YH).
Table 1.
Physiological parameters
| Variable | Sham (n=15) | IPC (n=15) |
|---|---|---|
| pH | 7.42±0.03 | 7.42±0.03 |
| PaO2 (mm Hg) | 91.2±9.4 | 92.3±7.0 |
| PaCo2 (mm Hg) | 42.4±3.4 | 40.5±3.3 |
| Blood glucose (mg/dl) | 114.3±23.9 | 113.1±28.7 |
IPC Attenuated Brain Edema Formation After ICH
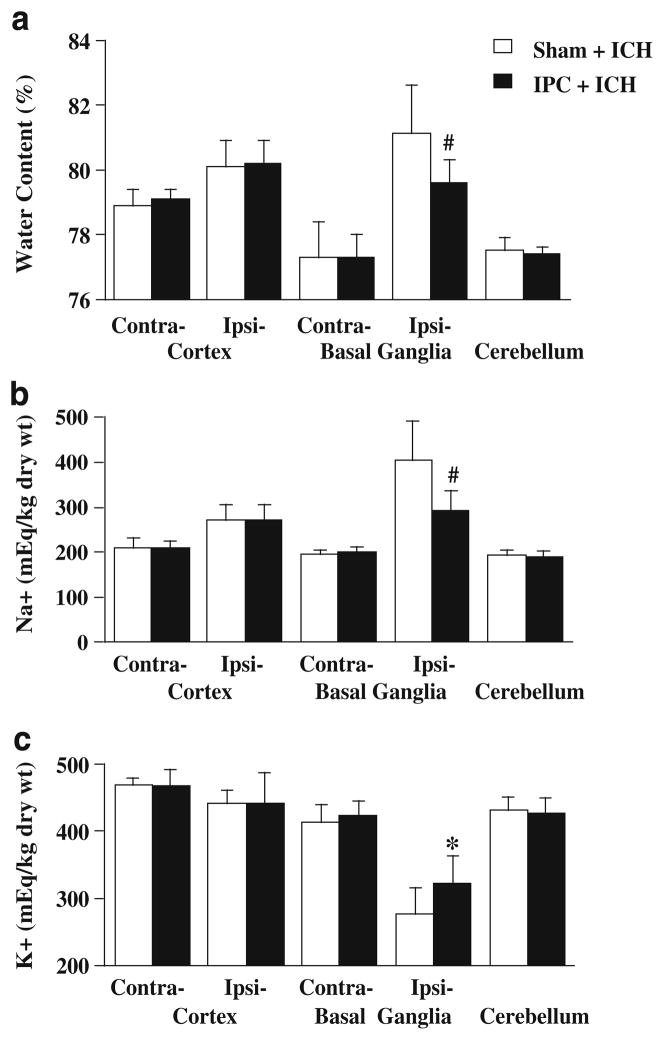
To investigate the effects of IPC on brain edema formation after ICH, rats received an infusion of 100 μl autologous blood into the ipsilateral caudate 72 h after 15 min of IPC or a sham operation and were killed 24 h after ICH. IPC significantly reduced brain edema in the ipsilateral basal ganglia (79.6 ±0.7 % vs. 81.1±1.5 % in sham, p<0.05; Fig. 1a) after ICH. The reduction of water content was accompanied by less sodium ion accumulation (293±44 vs. 404±87 mEq/kg dry weight in sham, p<0.01, Fig. 1b) and less potassium ion loss (322±41 vs. 277±40 mEq/kg dry weight in sham, p<0.05; Fig. 1c). Water and ion contents in the ipsilateral and contralateral cortex, contralateral basal ganglia and cerebellum were not significantly different between the IPC and sham groups (Fig. 1).
Fig. 1.
Brain water (a), sodium (b), and potassium (c) contents 24 h after intracerebral infusion of 100 μl autologous whole blood into the ipsilateral caudate. Rats received 15 min of IPC (n=10) or a sham operation (n=11) 72 h before ICH. Contra- Contralateral, Ipsi- ipsilateral. Values are expressed as mean ± SD. #p<0.01, *p<0.05 vs. sham
IPC Prolonged TCT and Bleeding Time
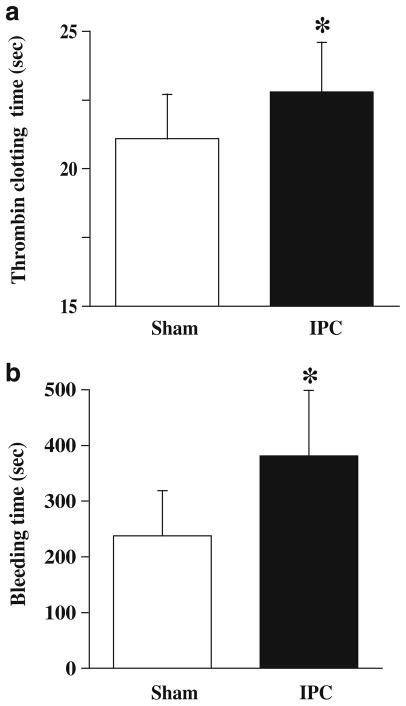
To examine the effects of IPC on blood coagulation, rats received IPC or a sham operation. Three days later, rats were used for bleeding time and TCT tests. The IPC group animals had a significantly longer TCT (22.8±1.8 vs. 21.1±1.6 s in shams, p<0.05; Fig. 2a) and bleeding time (381±118 vs. 238±81 s in shams, p<0.05; Fig. 2b).
Fig. 2.
Thrombin clotting times (a) and bleeding times (b) 72 h after 15 min of IPC (n=9) or a sham operation (n=8). Values are expressed as mean ± SD. *p<0.05 vs. sham
Protection of IPC Against ICH Was Mainly Due to Local Rather Than Systemic Factors
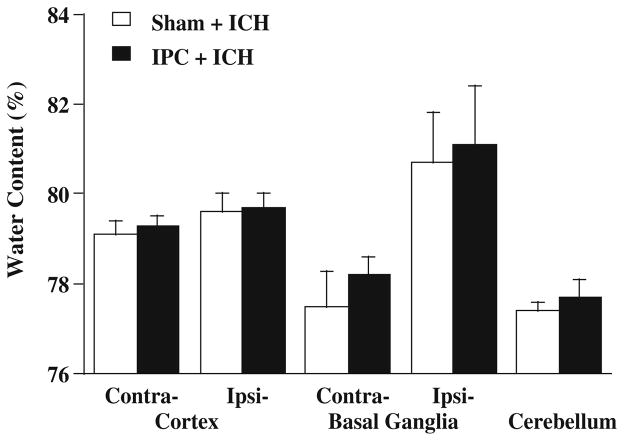
To determine whether protection of IPC against ICH-induced brain edema formation is due to the alteration in blood coagulation, rats received an infusion of 100 μl autologous blood into the contralateral caudate 72 h after 15 min of IPC or a sham operation. Rats were killed 24 h after ICH and the brains were used for water content analysis. There were no significant differences in water content between the IPC and sham groups (Fig. 3).
Fig. 3.
Brain water content 24 h after intracerebral infusion of 100 μl autologous whole blood into the caudate contralateral to IPC. Rats received IPC (n=5) or a sham operation (n=6) 72 h before ICH. Contra Contralateral; Ipsi ipsilateral. Values are expressed as mean ± SD
IPC Activated p44/42 MAPKs Pathway
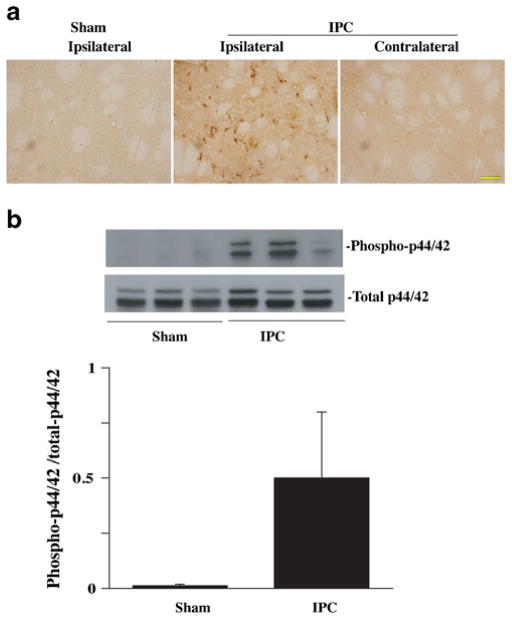
The p44/42 MAPKs signaling pathway is important in regulating cell response to external stimuli. To examine p44/42 MAPKs pathway activation, brain levels of phosphorylated p44/42 MAPKs were measured 24 h after IPC by immunohistochemistry and Western blotting. Immunohistochemistry showed phosphorylated p44/42 MAPK-positive cells in the ipsilateral hemisphere 24 h after IPC (Fig. 4a). There were no phosphorylated p44/42-positive cells in the contralateral hemisphere or in the ipsilateral hemisphere of sham-operated rats. Western blot analysis showed that the ratio of phosphorylated p44/42 MAPKs/total p44/42 MAPKs was significantly increased in the ipsilateral basal ganglia 24 h after IPC (0.5±0.3 vs. 0.01±0.007 in shams; p <0.05, Fig. 4b).
Fig. 4.
a Phosphorylated p44/42 immunoreactivity in the ipsilateral and contralateral basal ganglia at 24 h after 15 min of IPC or a sham operation. Scale bar = 50 μm. b Western blot analysis of phosphorylated and total p44/42 protein levels in the ipsilateral basal ganglia at 24 h after 15 min of IPC or a sham operation. Value are expressed as mean ± SD. *p<0.05 vs. sham
IPC-Induced Upregulation of HO-1, Tf, and TfR Levels
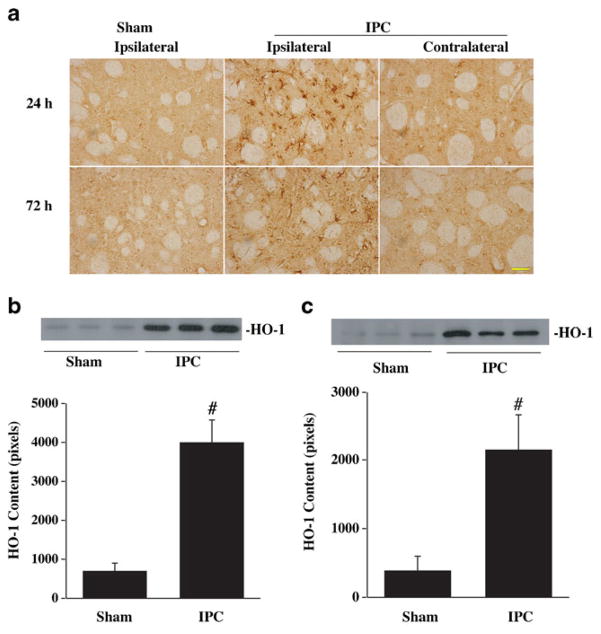
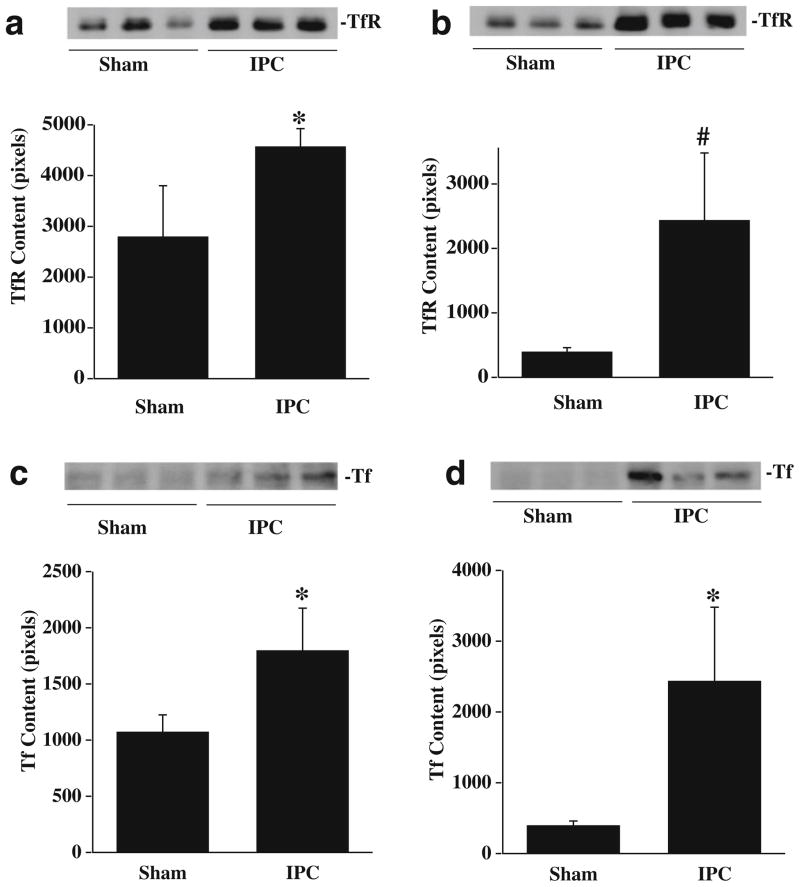
HO-1, Tf, and TfR are involved in iron metabolism and our previous studies have shown that iron is an important factor causing brain edema and oxidative injury after ICH. We, therefore, measured brain HO-1, Tf, and TfR protein levels 24 and 72 h after IPC by immunohistochemistry and Western blotting. By immunohistochemistry, HO-1-positive cells were detected in the ipsilalateral basal ganglia 24 and 72 h after IPC. There were no HO-1-positive cells in the contralateral hemisphere or in the ipsilateral hemisphere of sham-operated rats (Fig. 5a). Western blot analysis showed that HO-1 protein levels in the ipsilateral basal ganglia were significantly increased at 24 (Fig. 5b) and 72 h (Fig. 5c) after IPC. Transferrin and transferrin receptor levels in the ipsilateral basal ganglia were also upregulated after IPC (Fig. 6).
Fig. 5.
HO-1 immunoreactivity (a) in the ipsilateral and contralateral basal ganglia 24 and 72 h after 15 min of IPC or a sham operation. Scale bar = 50 μm. Western blot analysis of HO-1 levels in the ipsilateral basal ganglia 24 (b) and 72 h (c) after 15 min of IPC or a sham operation. Value are expressed as mean ± SD. #p<0.01 vs. sham
Fig. 6.
Western blot analysis of transferrin receptor (a, b) and transferrin (c, d) levels in the ipsilateral basal ganglia 24 (a, c) and 72 h (b, d) after 15 min of IPC or a sham operation. Value are expressed as mean ± SD. #p<0.01, *p<0.05 vs. sham
Discussion
The major findings of this study are: (1) IPC attenuated ICH-induced brain edema formation; (2) IPC prolonged bleeding time and TCT; (3) The protection of IPC against hemorrhagic injury was mainly due to local rather than systemic factors; (4) IPC activated the p44/42 pathway and upregulated HO-1, Tf, and TfR levels in the ipsilateral hemisphere.
Many experimental studies have shown that a short period of ischemia provides robust protection against a subsequent prolonged ischemic event. This phenomenon, known as IPC, occurs in multiple species and in a variety of organs including brain [16–18]. Cerebral IPC in animal models of stroke induces striking protection against later severe ischemic injury [6]. There is also some evidence of IPC in humans with transient ischemic attacks attentuating later stroke severity [19]. We used IPC in this study because it has previously been shown to prolong bleeding time [6] and coagulation plays an important role in intracerebral hemorrhage (ICH)-induced brain edema formation. We chose 15 min of MCA occlusion as an IPC stimulus and induction of the hemorrhage 72 h after IPC based on our previous study [5]. Most IPC studies have been focused on ischemic injury. It is not clear whether IPC can protect hemorrhagic brain injury. In the present study, we showed that cerebral IPC induces protection against ICH-induced brain edema formation. Our earlier study have also demonstrated that that cerebral IPC attenuates brain edema formation and cerebrovascular injury induced by permanent MCA occlusion [5]. In combination with the current results, these findings indicate that IPC can reduce both ischemia and ICH-induced brain edema formation. Changes in % brain water content can be somewhat misleading in assessing the magnitude of the edema formation. In the non-IPC rats, the perihematomal % water content increased from about 77.3 % to 81.1 %, which is equivalent to a change from 3.41 g water/g dry weight to 4.29 g/g dry weight, a 26 % increase. For the IPC rats, the perihematomal % water content was only 79.6 % or 3.90 g/g dry weight, a 14.6 % increase compared to contralateral, approximately half the increase found without IPC.
In this study, we found that TCT was prolonged after IPC. TCT is defined as the time required for formation of a plasma fibrin clot upon addition of thrombin [20]. It is well established that conversion of fibrinogen to fibrin constitutes the third and final stage of blood coagulation. The prolonged TCT after IPC indicates that IPC affects blood coagulation cascade. In addition, we found that cerebral IPC prolonged bleeding time. This is consistent with a previous study in mice [6]. Safety is a major concern when translating preconditioning study to the clinic [21]. The prolonged TCT and bleeding time after IPC may raise a safety concern when utilizing IPC in clinical practice, particularly for ICH. In the current study, we used a blood injection ICH model, which lacks a ruptured blood vessel. Future studies should test whether or not IPC causes hematomal enlargement in a collagenase injection ICH model. However, it should be pointed out that TCT was slightly prolonged after IPC and no hemorrhagic complications, such as subarachnoid hemorrhage and scalp hematomas, were associated with this effect. The mechanisms of prolonged TCT and bleeding time after IPC are not clear. Stenzel-Poore et al. [6] reported that the prolonged bleeding time is associated with alternations in platelet aggregation. Our results suggest that alterations in the coagulation cascade after IPC may also contribute to the prolonged bleeding time, which is influenced by not only platelet count and function but also blood coagulation. Further studies are required to investigate the mechanisms of hypocoagulation after IPC.
Although many factors are involved in ICH-induced edema formation, coagulation cascade activation, especially thrombin production, plays a crucial role in early brain edema formation [22]. Cerebral IPC protects ICH-induced brain edema formation and decreases blood coagulation. To determine whether IPC-induced protection against hemorrhagic edema formation is due to blood hypocoagulation, we examined the effects of IPC on contralateral rather than ipsilateral caudate ICH. That there was no effect on contralateral ICH suggests that IPC-induced protection against hemorrhagic edema formation is mainly due to local factors in the brain, rather than alterations in systemic factors.
The precise mechanisms of neuroprotection of IPC are not well understood. Our recent study found that cerebral IPC upregulates neuronal hemoglobin which may help neurons to survive cerebral ischemia [23]. The present study demonstrated that cerebral IPC activates the p44/42 MAPKs pathway and upregulates HO-1, Tf, and TfR levels in the ipsilateral caudate. The p44/42 MAP kinases play an important role in regulating the cell response to external stimuli. Our recent studies showed that p44/42 MAP kinases are activated after hyperbaric oxygen preconditioning and thrombin preconditioning and that this activation is involved in the induced protection against injury after ICH [1, 8]. HO-1 is a stress protein that degrades heme to biliverdin, iron, and carbon monoxide. Biliverdin/bilirubin and carbon monoxide have antioxidant and anti-inflammatory properties [24]. Furthermore, there is evidence that HO-1 plays an important role in iron efflux from cells and provides cyto-protection by augmenting iron efflux [9]. Transferrin and the transferrin receptor, iron transport proteins, are involved in maintaining brain iron homeostasis and are upregulated in brain after ICH [25]. It is well known that brain iron overload occurs after ICH and causes brain edema and oxidative injury. Modification of HO-1, Tf, and TfR after IPC may change brain iron homeostasis during ICH. Further studies are required to determine whether protection of IPC against ICH-induced brain edema is mediated by p44/42 MAPKs, HO-1, Tf, and TfR.
In conclusion, our present results demonstrate that IPC attenuates ICH-induced brain edema formation and induces blood hypocoagulation. The protective effects of IPC on hemorrhagic injury are mainly due to local factors in the brain and may be related to the upregulation of brain HO-1, Tf, and TfR.
Acknowledgments
This study was supported by grants NS-039866, NS-057539, and NS-073595 from the National Institutes of Health (NIH) and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
References
- 1.Qin Z, Song S, Xi G, Silbergleit R, Keep RF, Hoff JT, et al. Preconditioning with hyperbaric oxygen attenuates brain edema after experimental intracerebral hemorrhage. Neurosurg Focus. 2007;22(5):E13. doi: 10.3171/foc.2007.22.5.14. [DOI] [PubMed] [Google Scholar]
- 2.Hua Y, Keep RF, Hoff JT, Xi G. Thrombin preconditioning attenuates brain edema induced by erythrocytes and iron. J Cerebr Blood Flow Metabol. 2003;23(12):1448–54. doi: 10.1097/01.WCB.0000090621.86921.D5. [DOI] [PubMed] [Google Scholar]
- 3.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–48. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 4.Gidday JM. Pharmacologic preconditioning: translating the promise. Transl Stroke Res. 2010;1(1):19–30. doi: 10.1007/s12975-010-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masada T, Hua Y, Xi G, Ennis SR, Keep RF. Attenuation of ischemic brain edema and cerebrovascular injury after ischemic preconditioning in the rat. J Cerebr Blood Flow Metabol. 2001;21 (1):22–33. doi: 10.1097/00004647-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia–tolerant states. Lancet. 2003;362(9389):1028–37. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 7.Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, et al. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29(12):2580–6. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 8.Xi G, Hua Y, Keep RF, Duong HK, Hoff JT. Activation of p44/42 mitogen activated protein kinases in thrombin-induced brain tolerance. Brain Res. 2001;895(1–2):153–9. doi: 10.1016/s0006-8993(01)02064-9. [DOI] [PubMed] [Google Scholar]
- 9.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nature Cell Biol. 1999;1(3):152–7. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 10.Connor JR, Menzies SL, Burdo JR, Boyer PJ. Iron and iron management proteins in neurobiology. Pediatr Neurol. 2001;25 (2):118–29. doi: 10.1016/s0887-8994(01)00303-4. [DOI] [PubMed] [Google Scholar]
- 11.Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, et al. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104(2):305–12. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100(4):672–8. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- 13.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41 (2):375–82. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg. 2000;93(1 Suppl):71–6. doi: 10.3171/spi.2000.93.1.0071. [DOI] [PubMed] [Google Scholar]
- 15.Karabiyikoglu M, Hua Y, Keep RF, Ennis SR, Xi G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cerebr Blood Flow Metabol. 2004;24(2):159–66. doi: 10.1097/01.WCB.0000100062.36077.84. [DOI] [PubMed] [Google Scholar]
- 16.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circ. 1986;74 (5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 17.Chien CT, Chen CF, Hsu SM, Lee PH, Lai MK. Protective mechanism of preconditioning hypoxia attenuates apoptosis formation during renal ischemia/reperfusion phase. Transplant Proc. 1999;31(5):2012–3. doi: 10.1016/s0041-1345(99)00250-x. [DOI] [PubMed] [Google Scholar]
- 18.Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatol. 1999;30(5):1223–31. doi: 10.1002/hep.510300513. [DOI] [PubMed] [Google Scholar]
- 19.Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, et al. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke. 1999;30(9):1851–4. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- 20.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Heamatol. 1957;17(4):237–46. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 21.Keep RF, Wang MM, Xiang J, Hua Y, Xi G. Is there a place for cerebral preconditioning in the clinic? Transl Stroke Res. 2010;1 (1):4–18. doi: 10.1007/s12975-009-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 23.He Y, Hua Y, Liu W, Hu H, Keep RF, Xi G. Effects of cerebral ischemia on neuronal hemoglobin. J Cerebr Blood Flow Metabol. 2009;29(3):596–605. doi: 10.1038/jcbfm.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia–reperfusion. Cell Death Differ. 2008;15(4):686–90. doi: 10.1038/cdd.2008.13. [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34(12):2964–9. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]