Abstract
The steroid hormone, progesterone, modulates neuroendocrine functions in the central nervous system resulting in integration of reproduction and reproductive behaviors in female mammals. Although it is widely recognized that progesterone’s effects on female sex behavior are mediated by the classical neural progestin receptors (PRs) functioning as “ligand-dependent” transcription factors to regulate genes and genomic networks, additional mechanisms of PR activation also contribute to the behavioral response. Cellular and molecular evidence indicates that PRs can be activated in a ligand-independent manner by neurotransmitters, growth factors, cyclic nucleotides, progestin metabolites and mating stimuli. The rapid responses of progesterone may be mediated by a variety of PR types, including membrane-associated PRs or extra-nuclear PRs. Furthermore, these rapid, non-classical progesterone actions involving cytoplasmic kinase signaling and/or extra-nuclear PRs also converge with classical PR-mediated, transcription dependent pathway to regulate reproductive behaviors. In this review, we summarize some of the history of the study of the role of PRs in reproductive behaviors, and update the status of PR-mediated mechanisms involved in the facilitation of female sex behavior. We present an integrative model of PR activation via crosstalk and convergence of multiple signaling pathways.
Keywords: Progesterone, Progestin Receptors, Dopamine, Non-classical, Signaling, Cross-talk
Introduction
The dominant, model system for studying the cellular mechanisms of progesterone action in the brain remains the estradiol (E2)/progesterone (P) induction of female sexual behavior in guinea pigs, rats and mice. In the 1930s, a series of experiments performed by W.C. Young and his collaborators demonstrated that female sexual behavior in both rats and guinea pigs required the sequential exposure to E2 followed a day or two later with P [1–4]. These findings were subsequently corroborated in mice [5]. To understand the importance of this work, which provided the model used by many research groups, it is necessary to bear in mind that these studies preceded the availability of chemical tools for assaying blood levels of steroid hormones. The findings that P was essential were not widely accepted [6], because sexual behavior is expressed during the estrous cycle prior to ovulation. However, P was believed to be secreted only by the corpus luteum, a structure that is formed at the time of ovulation. This work predicted the discovery of P secretion from the ovaries prior to ovulation, which was confirmed three decades later by gas chromatography [7, 8] followed by radioimmunoassay [9, 10].
Early hypotheses of P’s cellular mechanisms suggested that P acted by a non-receptor-mediated mechanism. It was originally believed that for Pto act as quickly as it had been shown to facilitate female sexual behavior after intravenous injection in rats (<10 minutes: [11]; < 30 minutes:[12]; 30 minutes [13, 14], the hormone probably acted via a mechanism distinct from that of E2[15], probably by a general membrane stabilization mechanism.
Progesterone action in brain
Although earlier signs from studies using in vivo uptake, in vitro binding and autoradiographic technique susing radioactively labeled progestins indicated that neural progestin receptors (PRs), which are similar to the previously characterized neural estrogen receptors (ERs), exist in some brain areas [16–22], studies of the physiological and behavioral relevance of the receptors began in earnest with the work of MacLusky and McEwen in 1978 [23]. Using a novelin vitro binding assay for brain PRs, these investigators demonstrated that PRs, physicochemically similar to those in peripheral reproductive tissues, were induced by E2 in some brain areas of rats (pooled hypothalamus-preoptic area-septum), and independent of E2 in other areas, including the cerebral cortex and amygdala.
Shortly after the characterization of these receptors in rats, a close relationship was reported between E2-induced PRs in the pooled hypothalamus-preoptic area-septum, midbrain and hypothalamus and P-facilitated behavioral responsiveness in rats and guinea pigs [24–29]. To summarize, when PRs in guinea pig or rat brain are elevated by E2, animals respond to P; when they are depressed by prior P treatment, they are less responsive (sequential inhibition/ refractoriness). This suggested that PRs are essential for mediating both the facilitatory and inhibitory effects of P on female sexual behavior in guinea pigs and rats. Behavioral refractoriness to P is thought to involve degradation of PRs by 26S proteosome activity within the hypothalamus and preoptic areas (POA)[30].
At the time of the characterization of neural PRs, it was widely held that unoccupied steroid hormone receptors were localized in the cytoplasm of cells, and the binding of ligand caused translocation to the cell nucleus where these occupied receptors could be measured by binding assays. It was subsequently reported that many steroid receptors, including unoccupied receptors, reside in the cell nucleus [31, 32]. The development of an assay for what were then called “nuclear PRs,” but should more appropriately be referred to as “occupied PRs”, led to the observation that the animal’s behavioral response to P was dependent on the level of hypothalamic, occupied PRs [13, 33–36].
Autoradiographic techniques provided better anatomical resolution than in vitro ligand binding techniques, albeit with low sensitivity. [3H]Progestin binding was detected in parts of the POA, including its suprachiasmatic and periventricular aspect, as well as the ventromedial nucleus of the hypothalamus (VMN) and arcuate nucleus in rats [37, 38] and guinea pigs[21, 22].
The punch microdissection technique provided reasonable anatomical resolution as well as good sensitivity [39, 40]. With this technique, the regions with the highest abundance of E2-induced PRs were the arcuate nucleus, the VMN, periventricular POA, the periventricular hypothalamus(PVN), the suprachiasmatic preoptic area (SC-POA), and the medial preoptic area (MPOA). Each of these areas also has a high concentration of ERs [41], and the list includes areas important in the regulation of female sexual behavior.
The advent of immunocytochemical techniques for PRs [42, 43] provided greater precision in the localization of the progestin-responsive cells. Consistent with the earlier findings that a subset of PRs was E2-induced, in virtually all cells in which PR-immunoreactivity (-ir) is dependent onE2, also have ERs [44, 45].E2-induced PRs were seen in a variety of brain areas in guinea pigs related to reproduction and reproductive behavior, including the bed nucleus of stria terminalis, periventricular preoptic regions, medial preoptic nucleus, MPOA, ventrolateral nucleus of the hypothalamus (homologous with the VMN in rats), lateral hypothalamus, premammillary nucleus, arcuate nucleus [43, 46, 47], and the midbrain central gray and tegmentum[46]. Other PR-containing areas include the peripeduncular region, parabrachial nucleus of guinea pigs[46]and the hippocampus of rats [48, 49].It is important to note that many of the PR-ir cells in the ventrolateral area of the hypothalamus in guinea pigs [43, 50] and in the VMH in other species that we have examined (Blaustein, unpublished observations) lie outside of the Nissl-defined nuclei. In guinea pigs, they extend upwards in a crescent in proximity to the fornix.
A recent comprehensive analysis of the localization of PR mRNA by in situ hybridization (ISH) in E2-treated, ovariectomized rats found the highest levels of PRs in some of the neural areas that are believed to be involved in reproduction or reproductive behaviors, including medial nucleus of the amygdala, anteroventral periventricular nucleus, arcuate nucleus, MPOA, median preoptic nucleus, anterior hypothalamic area, VMN, periaqueductal gray [51]. Numerous other areas not directly involved in reproduction have high levels, including CA3 pyramidal layer of the hippocampus, zonaincerta, interpeduncular nucleus, and nucleus of the oculomotor cranial nerve, and many other areas have moderate levels of PR mRNA (the reader is referred to Table 1 of reference: [51] for a comprehensive analysis).
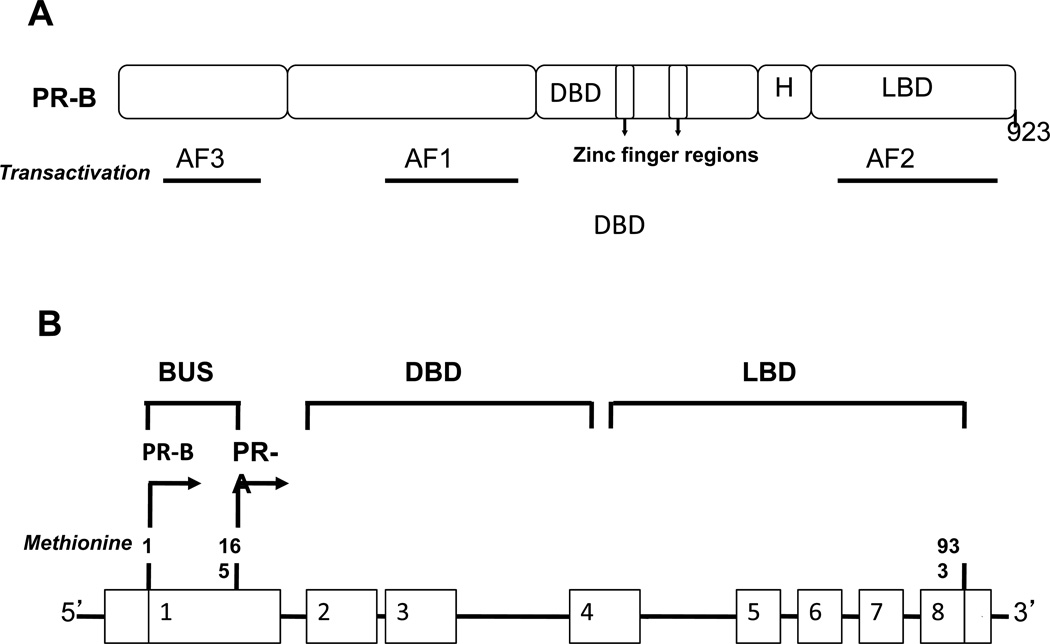
The PR is expressed in two isoforms, PR-A and PR-B, that are synthesized from alternate estrogen-inducible promoters on the same gene, as well as alternate transcription start codons (Fig.1; [52, 53]). Tools used in the experiments discussed thus far could not discriminate between these two isoforms, because they bind progestins with equal affinity and are identical with the exception of 164 amino acids on the amino-terminus of PR-B that is absent from PR-A [54]. Relatively little is known about the role of each isoform in brain. However, the two isoforms are expressed in the rat brain [55], and that the ratio of the two isoforms varies under different hormonal [56–59] and mating [60] conditions. Mani et al. [61] used PR isoform-specific knockout strains of mice to determine the relative contribution of each isoform to P-facilitated female sexual behavior. P-facilitated lordosis was completely eliminated in the PR-A null mutant mouse. PR-B null mutant mice showed a trend of suppression of P-facilitated sexual behavior. Collectively the data suggest that PR-A is essential for P-facilitated lordosis, and both isoforms are required for optimal facilitation by P.
Fig. 1.
Structure and functional organization of progestin receptor (A) and isoforms (B). (A). Progestin receptor (PR) has a conserved DNA-binding domain (DBD) and a ligand binding domain (LBD) connected by a hinge region (H). The N-terminal region contains a transactivation function 1 (AF1) and the LBD contains the AF2 domain. AF3, present in the N-terminal domain is unique to PR-B isoform. (B) Schematic representation of PR isoforms and splice variants. Classical PR gene is composed of 8 exons with 3100bp coding region and 5’- and 3’- untranslated regions. PR-B and PR-A isoforms are transcribed from two alternate transcription initiation sites.
Although PRs bind directly to hormone-response elements on the promoters of target genes, PRs, like other steroid hormone receptors, do not act alone. Rather, a complex of coregulators (coactivators that enhance gene expression and corepressor that repress it) interacts to fine-tune gene expression [62]. Since the characterization of the first steroid hormone receptor co-activator, steroid receptor co-activator-1 (SRC-1) in 1995 [63], over 300 coregulators have been discovered [62]. Very few of these coregulators have been studied within the context of ER or PR action in the brain; however, a decrease in SRC-1 expression by infusion of antisense oligonucleotides into the brain decreases the expression of E2-induced PR-ir in the VMH and the expression of female sexual behavior [64]. SRC-1 and another co-activator, CREB binding protein (CBP) influence the activity of both, ERα and PR [65], in the hormonal regulation of female sexual behaviors. Furthermore, SRC-1 from either hypothalamus or hippocampus, interacts in pull-down assays with ligand-occupied, ERα, ERβ, PR-A and PR-B in a receptor-specific and brain region-specific manner [66], perhaps helping to explain some of the diverse actions of steroid hormones on the brain. A related co-activator, SRC-2, from hypothalamus and hippocampus interacted with ligand-occupied ERα, PR-B in pull-down assays, but had very little interaction with ERβ and did not interact with PR-A [67].
In order for these coactivators to influence steroid receptor action directly and thereby influence reproductive behaviors, the co-activators must be co-expressed in cells containing steroid hormone receptors in brain areas involved in reproductive behaviors. SRC-1-ir is present in most cells containing estradiol-induced PRs in the VMN, MPOA, and arcuate nucleus, and many in the midbrain central gray [68]. Most cells containing E2-induced PRs in these areas also express CBP-ir. SRC-2 is also highly expressed within these regions [67] and is expressed in many ERα-ir cells (many of which are likely to co-express PR-ir). Thus, co-activators confer another, complex level of fine-tuning of steroid hormone response systems. Response to P is dependent not only on the presence of sufficient levels of PRs in relevant cells, but also dependent upon, and fine-tuned by the co-expression of a variety of steroid receptor co-activators.
Like ER-ir[69], PR-ir is observed in both cell nuclear, and extranuclear, sites [43] within hypothalamic areas, suggesting extra-nuclear sites of action as well as the classical nuclear site. The notion that ERs and PRs were shunted to distal subcellular sites was supported by work showing that the microtubule inhibitor, colchicine, induced the appearance of ER-ir and PR-ir in some brain areas in which they were not typically seen [70]. These extranuclear receptors may represent receptors en route to membrane sites and may explain some of the reported membrane effects of P on sexual behavior [71].
Ligand-independent action of progestin receptors
Although P-dependent activation of neural PRs remains the prevalent model in the regulation of female sexual behavior, alternate mechanisms by which PRs can be indirectly activated cannot be ignored. As early as in the 1970s, non-steroidal agents, gonadotropin releasing hormone (GnRH; [72]) and prostaglandins (PGE2; [73]) were shown to influence female sexual behavior in rodents, independent of P. Subsequent studies demonstrated that a large number of peptide hormones (e.g., oxytocin, melanocyte stimulating hormone, prolactin, adenocorticotropic hormone), neurotransmitters (e.g., noradrenaline, dopamine, acetylcholine, gamma-aminobutyric acid)and cyclic nucleotides also facilitate female sexual behavior (reviewed in [74–76]).In this context, it is interesting to note that levels of hypothalamic GnRH, PGE2, oxytocin and dopamine peak in estrous cycling rats concurrently with the rise in P levels, that is, at about the time that the animal exhibits sexual behavior [77–81].However, unlike P, these agents do not bind to PRs. Instead, they acton G-protein coupled membrane receptors to elevate hypothalamic levels of second messengers(cyclic AMP, cyclic GMP, calcium), suggesting that alternate pathways mediated by second messengers indirectly influence female sex behavior[74].We now know that such second messengers can substitute for P in the facilitation of female sex behavior[82]. These observations do not diminish the importance of classical mechanism through which P activated neural PRs mediate transcription-dependent genomic actions to influence sexual behavior. Indeed, studies using PR antagonists, protein and RNA synthesis inhibitors [83–86], antisense oligonucleotides to PR[87–89], and mutant mice with targeted deletion of PR gene[90] provide substantial proof that PR- mediated classical mechanism plays an important role in the facilitation of female sexual behavior.
Studies demonstrating that factors, other than its cognate ligands, can activate PRs have led to a better understanding of the mechanisms involved in the regulation of female sex behavior. This mechanism termed “Ligand-independent activation” was first demonstrated in dopamine (DA) facilitation of sexual behavior. Although it was known that DA could substitute for P in the facilitation of female sexual behavior [91], the demonstration that this behavior involved ligand-independent activation of PRs by DA provided the molecular underpinnings for the observed effects and highlighted a great degree of cross-talk between P- and DA-initiated pathways [90, 92].
Using biochemical and molecular tools, Mani et al.[93] demonstrated that both P and DA initiate second messenger signaling cascades involving increases in 3’-5’-cyclic adenosine mono phosphate(cAMP) levels, activation of protein kinase A (PKA) and phosphorylation of neuronal phosphoprotein, dopamine and cAMP regulated phosphoprotein-32 (DARPP-32), leading to the alterations in phosphorylation dynamics and activation of PRs and/or its coregulators in the hypothalamus. Homozygous mice carrying a null mutation for DARPP-32 gene exhibited significantly reduced P- and DA-facilitated female sexual receptive behavior compared to their wild type littermates, reinforcing the obligatory role of DARPP-32 in PR-mediated ligand-independent activation mechanism[93]. While the observations indicate that DARPP-32 activation is an obligatory step in PR regulation of sexual receptivity, the subsequent sequence of events leading to the activation of PR have yet to be defined. It is likely that the mechanisms include, not only a direct phosphorylation and activation of PR, but also enhanced phosphorylation of a distinct, yet diverse, set of PR-associated coactivators leading to rapid efficient transcriptional activation [54, 94–97]. Furthermore, ligand-independent activation by DA requires the expression of both PR-A and PR-B isoforms, since both PR-A and PR-B mutant mice displayed reduced female sexual behavior [61].
Ligand-independent activation of PRs is also observed in other physiological processes associated with female sexual behavior. Mating related stimuli induced by copulatory attempts by a male rodent, or manually by the experimenter, activate PRs in the absence of P [98–101]. Since mating stimulation induces DA release[102–105]and immediate early gene response (Fos) in PR-containing neurons [106], it is possible that DA activates PRs via ligand-independent activation mechanism. Ligand-independent activation of PRs also appears to play a role in VCS-, GnRH-, PGE2-, δ opioid-, nitric oxide-, and α1 adrenergic receptor-facilitation of sexual behavior in rats, since PR antagonists inhibit the behavior [107–111]. Thus, ligand-independent activation of PRs by non-ligands, involving cross-talk with second messenger cascades, appears to be a common mechanism mediating female sex behavior in rats and mice and is discussed in the following sections.
Non-classical mechanisms of P action
P also exhibits short-latency effects via modulation of putative cell surface PR receptors, ion channels and mechanisms coupled to cytoplasmic second messenger signaling cascades, independent of gene transcription [75, 112, 113]. Since these non-classical effects occur rapidly (in seconds or minutes) and are triggered at the membrane surface, the classical model of nuclear PR-mediation is inadequate to explain these effects. The identification of two types of novel membrane proteins unrelated to classical PRs, membrane PRs (mPRs) and progesterone receptor membrane component 1 (PGRMC1), in the brain suggests the possibility of these proteins in mediating effects [51, 114–117]. mPRs, originally discovered in teleost ovaries, are G-protein coupled receptors, which belong to the seven trans-membrane adiponectin Q receptor (PAQR) family and comprise of at least three subtypes α, β and γ [118, 119]. PGRMC1 (25Dx), a single trans-membrane protein, was originally isolated from porcine liver membranes [120] and has been shown to be regulated by E2 and P in the VMH of female rat [121].
Real time reverse transcriptase-polymerase chain reaction (RTPCR) and ISH studies indicate that both these membrane proteins are present in the rat brain. Sleiteret al have reported the presence of mPRα and mPRβ message in the medial basal hypothalamus [115]. mRNA levels of PRB, mPRα and mPRβ, but not of mPRγ and PGRMC1 levels, are elevated in the mediobasal hypothalamus on the afternoon of proestrus, around the time of pre-ovulatory peak of P in cycling rats, suggesting that P action could involve both rapid and classical genomic mechanisms around that period [117]). In contrast, neuroanatomical distribution studies using ISH demonstrated low and homogenous expression of mPRs in the hypothalamus and robust expression in the thalamic nuclei and cortex of estradiol-treated, ovariectomized female rat. In addition, PGRMC1, PGRMC2 and classical PR mRNAs were highly expressed and displayed extensive overlap in the preoptic and hypothalamic nuclei and their projection sites [51]. Furthermore, using realtime RTPCR, Intlekofer and Peterson [116] also demonstrated that P treatment resulted in a significant increase in PGRMC1 mRNA levels in the ventrolateral region of VMN and sexually dimorphic nucleus of the POA, the areas known to be involved in female sexual behavior. While the functional role of these putative membrane receptors remains to be determined, the findings suggest possible interactions of membrane PRs and classical PRs within the same neurons in mediating the effects of P.
In addition to the factors discussed above, ring-A reduced metabolites of P, 5α-dihydroprogesterone and allopregnanolone, facilitate lordosis response in ovariectomized, E2-primed female rats [122–125]. Since ring-A reduced progestins have decreased affinity for the PRs, direct PR binding is probably not involved in this response. Furthermore, inhibition of this behavioral response by RU 38486, suggests that pathways antagonized by this compound could be involved in the facilitation of sexual behavior [111, 123, 126].Accumulating evidence suggests that several of these agents influence female sexual behavior, by activating extra-nuclear protein kinases A, C and (PKA, PKC, PKC), calcium and calmodulin kinase II (CaMKII) and mitogen activated protein kinase (MAPK) in the VMH and POA in the female rat [30, 74, 111, 127–137].
Integration of mechanisms
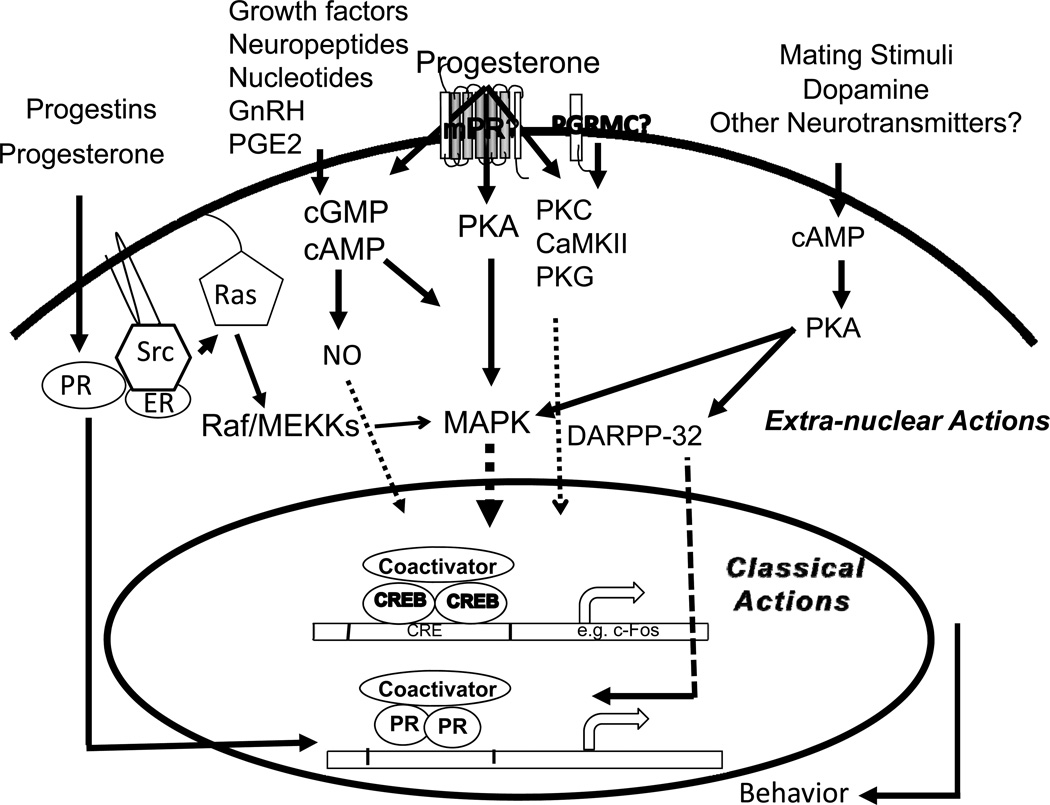
Studies till date have identified a high degree of cross-talk between various kinase-initiated pathways (by neurotransmitters, nucleotides and neuropeptides) and nuclear PRs in the brain, suggesting that integration of both rapid membrane and slower genomic actions is an essential component of female sexual behavior. A model depicting the interactions is shown in Figure 2. Classical actions of P, mediated by intracellular PRs functioning as transcription factors, remain the primary mechanism of P action in female sex behavior [65, 90].
Fig. 2.
A schematic representation of the crosstalk between classical and non-classical mechanisms in female reproductive behavior. Classical mechanism of action by progesterone- and ring-A class of progestins, mediated by classical PRs, promotes interactions with coactivators and plays a predominant role. Progesterone effects mediated by second messengers (cAMP, cGMP) and extra-nuclear signaling kinases (PKA, PKC, PKG, CaMKII) activates MAPK signal transduction cascade, phosphorylation of nuclear transcription factors, PRs/ PR coactivators and CREB. Progesterone and progestins, can act via the Src kinase, interact with extra-nuclear PRs to activate MAPK cascade. Progesterone acting via the extra-nuclear PKA/MAPK/DARPP-32 pathway can cause a decrease in phosphatase activity and an increase in phosphorylation of PR and/or its coactivators. Mating stimuli (VCS), dopamine or other neurotransmitters can stimulate PKA activation, phosphorylates DARPP-32, leading to the activation of CREB/PR/coactivators. VCS-stimulated PKA activation can also interact with MAPK cascade. Neuropeptides, nucleotides, growth factors, GnRH and PGE2 can act through various receptor- and/or second messengers (cAMP, cGMP, NO) and transmit signals to the nuclear PRs or other transcription factors. Interactions between multiple pathways may serve as an amplification mechanism to converge on nuclear transcription factors and/or coactivators to regulate gene transcription and translation, to facilitate female sex behavior.
Non-classical activation of cytoplasmic signaling pathways, mediated by kinases, whether initiated by non-steroidal agents or by progestins, can affect both transcription-dependent and –independent actions [94, 138–141]. Interactions between membrane-initiated signaling pathways and intracellular classical PRs resulting in transcription-dependent actions have been demonstrated in the ligand-independent activation of PRs by various factors [82, 111, 123]. Transcription-independent response of PR has been reported to involve the interaction of membrane-initiated signaling cascade Src/Ras/Raf/MAPK with the Src homology3 (SH3) domain of Src tyrosine kinases through the PXXXP motif at the N-terminal domain of PRs [140]. Recently, Gonzalez-Flores [30] reported that a specific inhibitor of Src kinase family blocked P-facilitated sex behavior in E2-primed rat. While this study suggests that Src kinase could be involved, its activation and its interactions with PRs in mediating transcription-independent actions remain unknown. The downstream mechanisms could involve gene expression via multiple transcription factors or transcription coactivators [142, 143].P has been shown to induce transcription of immediate early genes containing CRE-sequences such as c-fos and c-jun[144] to regulate downstream gene expression, by acting on target AP-1 DNA recognition sequences near promoter elements. In addition, integration of the signaling pathways could occur at the level of steroid receptor coregulators through phosphorylation of coactivators [141].
Summary and conclusions
The original two-step classical model of PR activation has undergone substantial modifications and evolved into a highly complex integrative model involving multiple signaling pathways. Recent studies have provided insights into ligand-dependent and ligand-independent mechanisms of receptor activation and provided a blueprint for the integrative model for PR activation in the regulation of female sexual behavior. It is also becoming abundantly clear that multiple intra- and inter-cellular mechanisms share signaling components that potentially amplify and integrate signals from a variety of stimuli to achieve neuroendocrine integration required for complex behaviors like reproductive behaviors. It will be critical to understand how neuronal kinases and phosphatases, activated by neurotransmitters, regulate the equilibrium between transcriptionally active and inactive states of PRs and their coregulators in relevant areas of the brain that contribute to the regulation of female sexual behavior. Furthermore, the molecular mechanisms by which this equilibrium could be fine-tuned by second and third messengers functioning as signal amplifiers remain to be established. Future studies will likely reveal further insights into the mechanisms by which the multiple signals converge and reinforce neuronal responses to environmental and behavioral events to alter steroid hormone effects on female reproductive behavior.
Acknowledgements
Work from the authors’ laboratories was supported by the following United States Public Health Service grants from the National Institutes of Health: MH57442 and MH63954 (SKM) and NS19327 (JDB).
References
- 1.Boling J, Young WC, Dempsey EW. Miscellaneous experiments on the estrogen progesterone induction of heat in the spayed guinea pig. Endocrinology. 1938;23:182–187. [Google Scholar]
- 2.Collins VJ, Boling JI, Dempsey EW, Young WC. Quantitative studies of experimentally induced sexual receptivity in the spayed guinea pig. Endocrinology. 1938;23:188–196. [Google Scholar]
- 3.Dempsey EW, Hertz R, Young WC. The experimental induction of oestrus (sexual receptivity) in the normal and ovariectomized guinea pig. Am J Physiol. 1936;116:201–209. [Google Scholar]
- 4.Boling JL, Blandau RJ. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinology. 1939;25:359–364. [Google Scholar]
- 5.Ring JR. The estrogen-progesterone induction of sexual receptivity in the spayed female mouse. Endocrinology. 1944;34:269–275. [Google Scholar]
- 6.Beach FA. Historical origins of modern research on hormones and behavior. Horm Behav. 1981;15:325–376. doi: 10.1016/0018-506x(81)90001-5. [DOI] [PubMed] [Google Scholar]
- 7.Feder HH, Resko JA, Goy RW. Progesterone concentrations in the arterial plasma of guinea pigs during the oestrous cycle. J Endocrinol. 1968;40:505–513. doi: 10.1677/joe.0.0400505. [DOI] [PubMed] [Google Scholar]
- 8.Feder HH, Resko JA, Goy RW. Progesterone levels in the arterial plasma of pre-ovulatory and ovariectomized rats. J Endocrinol. 1967;41:563–569. doi: 10.1677/joe.0.0410563. [DOI] [PubMed] [Google Scholar]
- 9.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17b throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 10.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 11.Lisk RD. A comparison of the effectiveness of intravenous, as opposed to subcutaneous, injection of progesterone for the induction of estrous behavior in the rat. Journal of Biochemical Physiology. 1960;38:1381–1383. [PubMed] [Google Scholar]
- 12.Meyerson B. Latency between intravenous injection of progestins and the appearance of estrous behavior in estrogen-treated ovariectomized rats. Horm Behav. 1972;3:1–9. doi: 10.1016/0018-506x(72)90001-3. [DOI] [PubMed] [Google Scholar]
- 13.McGinnis MY, Parsons B, Rainbow TC, Krey LC, McEwen BS. Temporal relationship between cell nuclear progestin receptor levels and sexual receptivity following intravenous progesterone administration. Brain Res. 1981;218:365–371. doi: 10.1016/0006-8993(81)91315-9. [DOI] [PubMed] [Google Scholar]
- 14.Glaser JH, Rubin BS, Barfield RJ. Onset of the receptive and proceptive components of feminine sexual behavior in rats following the intravenous administration of progesterone. Horm Behav. 1983;17:18–27. doi: 10.1016/0018-506x(83)90012-0. [DOI] [PubMed] [Google Scholar]
- 15.Feder HH, Marrone BL. Progesterone: its role in the central nervous system as a facilitator and inhibitor of sexual behavior and gonadotropin release. Annals of the New York Academy of sciences. 1977;286:331–354. doi: 10.1111/j.1749-6632.1977.tb29428.x. [DOI] [PubMed] [Google Scholar]
- 16.Blaustein JD, Wade GN. Progestin binding by brain and pituitary cell nuclei and female rat sexual behavior. Brain Res. 1978;140:360–367. doi: 10.1016/0006-8993(78)90469-9. [DOI] [PubMed] [Google Scholar]
- 17.Moguilewsky M, Raynaud JP. Progestin binding sites in the rat hypothalamus pituitary and uterus. Steroids. 1977;30:99–109. doi: 10.1016/0039-128x(77)90140-4. [DOI] [PubMed] [Google Scholar]
- 18.Kato J. The role of hypothalamic and hypophyseal 5a-dihydrotestosterone, estradiol and progesterone receptors in the mechanism of feedback action. J Ster Biochem. 1975;6:979–987. [PubMed] [Google Scholar]
- 19.Kato J, Onouchi T. Specific progesterone receptors in the hypothalamus and anterior hypophysis of the rat. Endocrinology. 1977;101:920–928. doi: 10.1210/endo-101-3-920. [DOI] [PubMed] [Google Scholar]
- 20.Seiki K, Hattori M. In vivo uptake of progesterone by the hypothalamus and pituitary of the female ovariectomized rat and its relationship to cytoplasmic progesterone-binding protein. Endocrinologica Japonica. 1973;20:111–119. doi: 10.1507/endocrj1954.20.111. [DOI] [PubMed] [Google Scholar]
- 21.Sar M, Stumpf WE. Neurons of the hypothalamus concentrate [3H]progesterone or its metabolites. Science. 1973;183:1266–1268. doi: 10.1126/science.182.4118.1266. [DOI] [PubMed] [Google Scholar]
- 22.Warembourg M. Radioautographic study of the brain and pituitary after [3H]progesterone injection into estrogen-primed ovariectomized guinea pigs. Neuroscience Letts. 1978;7:1–5. [Google Scholar]
- 23.MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- 24.Blaustein JD, Feder HH. Cytoplasmic progestin receptors in guinea pig brain: Characteristics and relationship to the induction of sexual behavior. Brain Res. 1979;169:481–497. doi: 10.1016/0006-8993(79)90398-6. [DOI] [PubMed] [Google Scholar]
- 25.Blaustein JD, Feder HH. Cytoplasmic progestin receptors in female guinea pig brain and their relationship to refractoriness in expression of female sexual behavior. Brain Res. 1979;177:489–498. doi: 10.1016/0006-8993(79)90466-9. [DOI] [PubMed] [Google Scholar]
- 26.Moguilewsky M, Raynaud JP. The relevance of hypothalamic and hypophyseal progestin receptor regulation in the induction and inhibition of sexual behavior in the female rat. Endocrinology. 1979;105:516–522. doi: 10.1210/endo-105-2-516. [DOI] [PubMed] [Google Scholar]
- 27.Parsons B, McEwen BS, Pfaff DW. A discontinuous schedule of estradiol treatment is sufficient to activate progesterone-facilitated feminine sexual behavior and to increase cytosol receptors for progestins in the hypothalamus of the rat. Endocrinology. 1982;110:613–624. doi: 10.1210/endo-110-2-613. [DOI] [PubMed] [Google Scholar]
- 28.Parsons B, MacLusky NJ, Krey L, Pfaff DW, McEwen BS. The temporal relationship between estrogen-inducible progestin receptors in the female rat brain and the time course of estrogen activation of mating behavior. Endocrinology. 1980;107:774–779. doi: 10.1210/endo-107-3-774. [DOI] [PubMed] [Google Scholar]
- 29.Parsons B, McGinnis MY, McEwen BS. Sequential inhibition by progesterone: Effects on sexual receptivity and associated changes in brain cytosol progestin binding in the female rat. Brain Res. 1981;221:149–160. doi: 10.1016/0006-8993(81)91069-6. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Flores O, Beyer C, Gomora-Arrati P, Garcia-Juarez M, Lima-Hernandez FJ, Soto-Sanchez A, Etgen AM. A role for Src kinase in progestin facilitation of estrous behavior in estradiol-primed female rats. Horm Behav. 2010;58:223–229. doi: 10.1016/j.yhbeh.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 31.King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- 32.Welshons WV, Lieberman ME, Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984;307:747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- 33.Blaustein JD, Feder HH. Nuclear progestin receptors in guinea pig brain measured by and in vitro exchange assay after hormonal treatments that affect lordosis. Endocrinology. 1980;106:1061–1069. doi: 10.1210/endo-106-4-1061. [DOI] [PubMed] [Google Scholar]
- 34.Brown TJ, Blaustein JD. Supplemental progesterone delays heat termination and loss of progestin receptors from hypothalamic cell nuclei in female guinea pigs. Neuroendocrinology. 1984;39:384–391. doi: 10.1159/000124009. [DOI] [PubMed] [Google Scholar]
- 35.Brown TJ, Blaustein JD. Loss of hypothalamic nuclear-bound progestin receptors: Factors involved and the relationship to heat termination in female guinea pigs. Brain Res. 1985;358:180–190. doi: 10.1016/0006-8993(85)90962-x. [DOI] [PubMed] [Google Scholar]
- 36.Brown TJ, Blaustein JD. Abbreviation of the period of sexual behavior in female guinea pigs by the progesterone antagonist, RU 486. Brain Res. 1986;373:103–113. doi: 10.1016/0006-8993(86)90320-3. [DOI] [PubMed] [Google Scholar]
- 37.Warembourg M. Radioautographic study of the rat brain, uterus and vagina after [3H]R-5020 injection. Mol Cell Endocrinol. 1978;12:67–79. doi: 10.1016/0303-7207(78)90102-8. [DOI] [PubMed] [Google Scholar]
- 38.Warembourg M. Uptake of 3H labeled synthetic progestin by rat brain and pituitary. A radioautography study. Neuroscience Letts. 1978;9:329–332. doi: 10.1016/0304-3940(78)90203-3. [DOI] [PubMed] [Google Scholar]
- 39.Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thornton JE, Nock B, McEwen BS, Feder HH. Estrogen induction of progestin receptors in microdissected hypothalamic and limbic nuclei of female guinea pigs. Neuroendocrinology. 1986;43:182–188. doi: 10.1159/000124526. [DOI] [PubMed] [Google Scholar]
- 41.Rainbow TC, Parsons B, MacLusky NJ, McEwen BS. Estradiol receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1439–1445. doi: 10.1523/JNEUROSCI.02-10-01439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warembourg M, Logeat F, Milgrom E. Immunocytochemical localization of progesterone receptor in the guinea pig central nervous system. Brain Res. 1986;384:121–131. doi: 10.1016/0006-8993(86)91227-8. [DOI] [PubMed] [Google Scholar]
- 43.Blaustein JD, King JC, Toft DO, Turcotte J. Immunocytochemical localization of estrogen-induced progestin receptors in guinea pig brain. Brain Res. 1988;474:1–15. doi: 10.1016/0006-8993(88)90664-6. [DOI] [PubMed] [Google Scholar]
- 44.Blaustein JD, Turcotte JC. Estradiol-induced progestin receptor immunoreactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology. 1989;49:454–461. doi: 10.1159/000125152. [DOI] [PubMed] [Google Scholar]
- 45.Warembourg M, Jolivet A, Milgrom E. Immunohistochemical evidence of the presence of estrogen and progesterone receptors in the same neurons of the guinea pig hypothalamus and preoptic area. Brain Res. 1989;480:1–15. doi: 10.1016/0006-8993(89)91561-8. [DOI] [PubMed] [Google Scholar]
- 46.Turcotte JC, Blaustein JD. Immunocytochemical localization of midbrain estrogen receptor- containing and progestin receptor-containing cells in female guinea pigs. Journal of Comparative Neurology. 1993;328:76–87. doi: 10.1002/cne.903280106. [DOI] [PubMed] [Google Scholar]
- 47.DonCarlos LL, Greene GL, Morrell JI. Estrogen plus progesterone increases progestin receptor immunoreactivity in the brain of ovariectomized guinea pigs. Neuroendocrinology. 1989;50:613–623. doi: 10.1159/000125290. [DOI] [PubMed] [Google Scholar]
- 48.Waters EM, Torres-Reveron A, McEwen BS, Milner TA. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J Comp Neurol. 2008;511:34–46. doi: 10.1002/cne.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delville Y, Blaustein JD. A site for estradiol priming of progesterone-facilitated sexual receptivity in the ventrolateral hypothalamus of female guinea pigs. Brain Res. 1991;559:191–199. doi: 10.1016/0006-8993(91)90002-d. [DOI] [PubMed] [Google Scholar]
- 51.Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience. 2011;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conneely OM, Kettelberger DM, Tsai MJ, Schrader WT, O'Malley BW. The chicken progesterone receptor A and B isoforms are products of an alternate Translation initiation event. J Biol Chem. 1989;264:14062–14064. [PubMed] [Google Scholar]
- 54.Giangrande PH, Kimbrel A, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato J, Hirata S, Nozawa A, Yamadamouri N. Gene expression of progesterone receptor isoforms in the rat brain. Horm Behav. 1994;28:454–463. doi: 10.1006/hbeh.1994.1043. [DOI] [PubMed] [Google Scholar]
- 56.Camacho-Arroyo I, Guerra-Araiza C, Cerbon MA. Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. Neuroreport. 1998;9:3993–3996. doi: 10.1097/00001756-199812210-00001. [DOI] [PubMed] [Google Scholar]
- 57.Guerra-Araiza C, Cerbon MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sciences. 2000;66:1743–1752. doi: 10.1016/s0024-3205(00)00497-5. [DOI] [PubMed] [Google Scholar]
- 58.Guerra-Araiza C, Villamar-Cruz O, Gonzalez-Arenas A, Chavira R, Camacho-Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15:984–990. doi: 10.1046/j.1365-2826.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- 59.Scott EM, Wu-Peng S, Pfaff DW. Regulation and expression of progesterone receptor mRNA isoforms A and B in the male and female rat hypothalamus and pituitary following oestrogen treatment. Journal of Neuroendocrinology. 2002;14:175–183. doi: 10.1046/j.0007-1331.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 60.Mendoza-Garces L, Camacho-Arroyo I, Cerbon MA. Effects of mating on progesterone receptor isoforms in rat hypothalamus. Neuroreport. 2010;21:513–516. doi: 10.1097/WNR.0b013e3283390440. [DOI] [PubMed] [Google Scholar]
- 61.Mani SK, Reyna AM, Chen JZ, Mulac-Jericevic B, Conneely OM. Differential response of progesterone receptor isoforms in hormone-dependent and -independent facilitation of female sexual receptivity. Mol Endocrinol. 2006;20:1322–1332. doi: 10.1210/me.2005-0466. [DOI] [PubMed] [Google Scholar]
- 62.O'Malley BW. Coregulators: from whence came these "master genes". Mol Endocrinol. 2007;21:1009–1013. doi: 10.1210/me.2007-0012. [DOI] [PubMed] [Google Scholar]
- 63.Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. 5240, NOV 24. [DOI] [PubMed] [Google Scholar]
- 64.Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 65.Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Horm Behav. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molenda-Figueira HA, Murphy SD, Shea KL, Siegal NK, Zhao Y, Chadwick JG, Jr, Denner LA, Tetel MJ. Steroid receptor coactivator-1 from brain physically interacts differentially with steroid receptor subtypes. Endocrinology. 2008;149:5272–5279. doi: 10.1210/en.2008-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yore MA, Im D, Webb LK, Zhao Y, Chadwick JG, Jr, Molenda-Figueira HA, Haidacher SJ, Denner L, Tetel MJ. Steroid receptor coactivator-2 expression in brain and physical associations with steroid receptors. Neuroscience. 2010;169:1017–1028. doi: 10.1016/j.neuroscience.2010.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tetel MJ, Siegal NK, Murphy SD. Cells in Behaviourally Relevant Brain Regions Coexpress Nuclear Receptor Coactivators and Ovarian Steroid Receptors. J Neuroendocrinol. 2007;19:262–271. doi: 10.1111/j.1365-2826.2007.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- 70.Blaustein JD, Olster DH. Colchicine-induced accumulation of estrogen receptor and progestin receptor immunoreactivity in atypical areas in guinea- pig brain. Journal of Neuroendocrinology. 1993;5:63–70. doi: 10.1111/j.1365-2826.1993.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 71.Debold JF, Frye CA. Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Horm Behav. 1994;28:445–453. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- 72.Moss RL, McCann SM. Induction of mating behavior in rats by luteinizing hormone-releasing factor. Science. 1973;181:177–179. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Sierra JF, Komisaruk BR. Effects of prostaglandin E2 and indomethacin on sexual behavior in the female rat. Horm Behav. 1977;9:281–289. doi: 10.1016/0018-506x(77)90063-0. [DOI] [PubMed] [Google Scholar]
- 74.Beyer C, Gonzalez-Mariscal G. Elevation in hypothalamic cyclic AMP as a common factor in the facilitation of lordosis in rodents: a working hypothesis. Ann NY Acad Sci. 1986;474:270–281. doi: 10.1111/j.1749-6632.1986.tb28018.x. [DOI] [PubMed] [Google Scholar]
- 75.Beyer C, Gonzalez-Flores O, Garcia-Juarez M, Gonzalez-Mariscal G. Non-ligand activation of estrous behavior in rodents: cross-talk at the progesterone receptor. Scand J Psychol. 2003;44:221–229. doi: 10.1111/1467-9450.00339. [DOI] [PubMed] [Google Scholar]
- 76.Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow L-M. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 107–220. [Google Scholar]
- 77.Kimura F, Kawakami M, Nakano H, McCann SM. Changes in adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate concentrations in the anterior pituitary and hypothalamus during the rat estrous cycle and effects of administration of sodium pentobarbital in proestrus. Endocrinology. 1980;106:631–635. doi: 10.1210/endo-106-2-631. [DOI] [PubMed] [Google Scholar]
- 78.Zubin P, Taleisnik S. Hypothalamic cyclic-AMP throughout the 4-day estrous cycle of the female rat. Brain Res. 1983;271:273–277. doi: 10.1016/0006-8993(83)90289-5. [DOI] [PubMed] [Google Scholar]
- 79.Brown CG, Poyser NL. Studies on the control of prostaglandin production by the hypothalamus in relation to LH release in the rat. J Endocrinol. 1984;103:155–164. doi: 10.1677/joe.0.1030155. [DOI] [PubMed] [Google Scholar]
- 80.Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press, Ltd.; 1994. pp. 613–658. [Google Scholar]
- 81.Etgen AM, Morales JC. Somatosensory stimuli evoke norepinephrine release in the anterior ventromedial hypothalamus of sexually receptive female rats. J Neuroendocrinol. 2002;14:213–218. doi: 10.1046/j.0007-1331.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- 82.Mani S, Portillo W. Activation of progestin receptors in female reproductive behavior: Interactions with neurotransmitters. Front Neuroendocrinol. 2010;31:157–171. doi: 10.1016/j.yfrne.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meisel RL, Pfaff DW. RNA and protein synthesis inhibitors: Effects on sexual behavior in female rats. Brain Research Bulletin. 1984;12:187–193. doi: 10.1016/0361-9230(84)90188-6. [DOI] [PubMed] [Google Scholar]
- 84.Meisel RL, Pfaff DW. Specificity and neural sites of action of anisomycin in the reduction or facilitation of female sexual behavior in rats. Horm Behav. 1985;19:237–251. doi: 10.1016/0018-506x(85)90024-8. [DOI] [PubMed] [Google Scholar]
- 85.Rainbow TC, Davis PG, McEwen BS. Anisomycin inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1980;194:548–555. doi: 10.1016/0006-8993(80)91240-8. [DOI] [PubMed] [Google Scholar]
- 86.Whalen RE, Gorzalka BB, Debold JF, Quadagno DM, Kan-wha Ho G, Hough JC. Studies on the effects of intracerebral actinomycin D implants on estrogen-induced receptivity in rats. Horm Behav. 1974;5:337–343. doi: 10.1016/0018-506x(74)90019-1. [DOI] [PubMed] [Google Scholar]
- 87.Mani SK, Blaustein JD, Allen JM, Law SW, O'Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- 88.Pollio G, Xue P, Zanisi M, Nicolin A, Maggi A. Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Molecular Brain Research. 1993;19:135–139. doi: 10.1016/0169-328x(93)90158-l. [DOI] [PubMed] [Google Scholar]
- 89.Ogawa S, Olazabal UE, Parhar IS, Pfaff DW. Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mrna on reproductive behavior and progesterone receptor immunoreactivity in female rat. J Neurosci. 1994;14:1766–1774. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mani SK, Allen JMC, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely O, O'Malley BW. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10:1728–1737. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- 91.Foreman MM, Moss RL. Effects of subcutaneous injection and intrahypothalamic infusion of releasing hormones upon lordotic response to repetitive coital stimulation. Horm Behav. 1977;8:219–234. doi: 10.1016/0018-506x(77)90039-3. [DOI] [PubMed] [Google Scholar]
- 92.Mani SK, Allen JMC, Clark JH, Blaustein JD, O'Malley BW. Convergent pathways for steroid hormone- and neurotransmitter- induced rat sexual behavior. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 93.Mani SK, Fienberg AA, Ocallaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O'Malley BW. Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- 94.Rowan BG, Garrison N, Weigel NL, O'Malley BW. 8-bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol Cell Biol. 2000;20:8720–8730. doi: 10.1128/mcb.20.23.8720-8730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lange CA, Gioeli D, Hammes SR, Marker PC. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol. 2007;69:171–199. doi: 10.1146/annurev.physiol.69.031905.160319. [DOI] [PubMed] [Google Scholar]
- 97.Lange CA. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol. 2004;18:269–278. doi: 10.1210/me.2003-0331. [DOI] [PubMed] [Google Scholar]
- 98.Auger AP, Moffatt CA, Blaustein JD. Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinology. 1997;138:511–514. doi: 10.1210/endo.138.1.4986. [DOI] [PubMed] [Google Scholar]
- 99.Auger AP, LaRiccia LM, Moffatt CA, Blaustein JD. Progesterone, but not progesterone-independent activation of progestin receptors by a mating stimulus, rapidly decreases progestin receptor immunoreactivity in female rat brain. Horm Behav. 2000;37:135–144. doi: 10.1006/hbeh.1999.1565. [DOI] [PubMed] [Google Scholar]
- 100.Auger AP. Ligand-independent activation of progestin receptors: relevance for female sexual behaviour. Reproduction. 2001;122:847–855. doi: 10.1530/rep.0.1220847. [DOI] [PubMed] [Google Scholar]
- 101.Bennett AL, Blasberg ME, Blaustein JD. Mating stimulation required for mating-induced estrous abbreviation in female rats: Effects of repeated testing. Horm Behav. 2002;42:206–211. doi: 10.1006/hbeh.2002.1809. [DOI] [PubMed] [Google Scholar]
- 102.Kohlert JG, Rowe RK, Meisel RL. Intromissive stimulation from the male increases extracellular dopamine release from fluoro-gold-identified neurons within the midbrain of female hamsters. Horm Behav. 1997;32:143–154. doi: 10.1006/hbeh.1997.1415. [DOI] [PubMed] [Google Scholar]
- 103.Matuszewich L, Lorrain DS, Hull EM. Dopamine release in the medial preoptic area of female rats in response to hormonal manipulation and sexual activity. Behavioral Neuroscience. 2000;114:772–782. doi: 10.1037//0735-7044.114.4.772. [DOI] [PubMed] [Google Scholar]
- 104.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behavioral Neuroscience. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- 105.Vathy I, Etgen AM. Hormonal activation of female sexual behavior is accompanied by hypothalamic norepinephrine release. J Neuroendocrinol. 1989;1:383–388. doi: 10.1111/j.1365-2826.1989.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 106.Auger AP, Moffatt CA, Blaustein JD. Reproductively-relevant stimuli induce Fos-immunoreactivity within progestin receptor-containing neurons in localized regions of female rat forebrain. J Neuroendocrinol. 1996;8:831–838. doi: 10.1046/j.1365-2826.1996.02684.x. [DOI] [PubMed] [Google Scholar]
- 107.Beyer C, Gonzalez-Flores O, Gonzalez-Mariscal G. Progesterone receptor participates in the stimulatory effect of LHRH, prostaglandin E2, and cyclic AMP on lordosis and proceptive behaviours in rats. J Neuroendocrinol. 1997;9:609–614. doi: 10.1046/j.1365-2826.1997.00617.x. [DOI] [PubMed] [Google Scholar]
- 108.Acosta-Martinez M, Gonzalez-Flores O, Etgen AM. The role of progestin receptors and the mitogen-activated protein kinase pathway in delta opioid receptor facilitation of female reproductive behaviors. Horm Behav. 2006;49:458–462. doi: 10.1016/j.yhbeh.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 109.Chu HP, Etgen AM. A potential role of cyclic GMP in the regulation of lordosis behavior of female rats. Horm Behav. 1997;32:125–132. doi: 10.1006/hbeh.1997.1413. [DOI] [PubMed] [Google Scholar]
- 110.Chu HP, Morales JC, Etgen AM. Cyclic GMP may potentiate lordosis behaviour by progesterone receptor activation. Journal of Neuroendocrinology. 1999;11:107–113. doi: 10.1046/j.1365-2826.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 111.Gonzalez-Flores O, Etgen AM, Komisaruk BK, Gomora-Arrati P, Macias-Jimenez A, Lima-Hernandez FJ, Garcia-Juarez M, Beyer C. Antagonists of the protein kinase A and mitogen-activated protein kinase systems and of the progestin receptor block the ability of vaginocervical/flank-perineal stimulation to induce female rat sexual behaviour. J Neuroendocrinol. 2008;20:1361–1367. doi: 10.1111/j.1365-2826.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 112.Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73:922–928. doi: 10.1016/j.steroids.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 113.Schumacher M, Coirini H, Robert F, Guennoun R, Eletr M. Genomic and membrane actions of progesterone: implications for reproductive physiology and behavior. Behav Brain Res. 1999;105:37–52. doi: 10.1016/s0166-4328(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 114.Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein-25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci U S A. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Intlekofer KA, Petersen SL. 17beta-estradiol and progesterone regulate multiple progestin signaling molecules in the anteroventral periventricular nucleus, ventromedial nucleus and sexually dimorphic nucleus of the preoptic area in female rats. Neuroscience. 2011;176:86–92. doi: 10.1016/j.neuroscience.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu B, Arbogast LA. Gene expression profiles of intracellular and membrane progesterone receptor isoforms in the mediobasal hypothalamus during pro-oestrus. J Neuroendocrinol. 2009;21:993–1000. doi: 10.1111/j.1365-2826.2009.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Y, Rice CD, Pang YF, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Falkenstein E, Meyer C, Eisen C, Scriba PC, Wehling M. Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1996;229:86–89. doi: 10.1006/bbrc.1996.1761. [DOI] [PubMed] [Google Scholar]
- 121.Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein-25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. PNAS. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beyer C, Gonzalez-Mariscal G, Eguibar JR, Gomora P. Lordosis facilitation in estrogen primed rats by intrabrain injection of pregnanes. Pharmacol Biochem Behav. 1988;31:919–926. doi: 10.1016/0091-3057(88)90405-4. [DOI] [PubMed] [Google Scholar]
- 123.Beyer C, Gonzalez-Flores O, Gonzalez-Mariscal G. Ring a reduced progestins potently stimulate estrous behavior in rats: paradoxical effect through the progesterone receptor. Physiology & Behavior. 1995;58:985–993. doi: 10.1016/0031-9384(95)00141-5. 5, NOV. [DOI] [PubMed] [Google Scholar]
- 124.Glaser JH, Etgen AM, Barfield RJ. Intrahypothalamic effects of progestin agonists on estrous behavior and progestin receptor binding. Physiol Behav. 1985;34:871–877. doi: 10.1016/0031-9384(85)90006-x. [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez-Flores O, Ramirez-Orduna JM, Lima-Hernandez FJ, Garcia-Juarez M, Beyer C. Differential effect of kinase A and C blockers on lordosis facilitation by progesterone and its metabolites in ovariectomized estrogen-primed rats. Horm Behav. 2006;49:398–404. doi: 10.1016/j.yhbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 126.Gonzalez-Mariscal G, Gonzalez-Flores O, Beyer C. Intrahypothalamic injection of RU 486 antagonizes the lordosis induced by ring A-reduced progestins. Physiol Behav. 1989;46(3):435–438. doi: 10.1016/0031-9384(89)90016-4. [DOI] [PubMed] [Google Scholar]
- 127.Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: I. Protein kinase C activation in the hypothalamus of female rats. Endocrinology. 2008;149:5509–5517. doi: 10.1210/en.2008-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: II. Role of calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinology. 2008;149:5518–5526. doi: 10.1210/en.2008-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chu HP, Morales JC, Etgen AM. Cyclic GMP may potentiate lordosis behaviour by progesterone receptor activation. J Neuroendocrinol. 1999;11:107–113. doi: 10.1046/j.1365-2826.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 130.Chu HP, Etgen AM. Ovarian hormone dependence of alpha(1)-adrenoceptor activation of the nitric oxide-cGMP pathway: relevance for hormonal facilitation of lordosis behavior. J Neurosci. 1999;19:7191–7197. doi: 10.1523/JNEUROSCI.19-16-07191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kow LM, Mobbs CV, Pfaff DW. Roles of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides, and estrogen: a review. Neurosci Biobehav Rev. 1994;18:251–268. doi: 10.1016/0149-7634(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 132.Petitti N, Etgen AM. Progesterone depression of norepinephrine-stimulated cAMP accumulation in hypothalamic slices. Mol Brain Res. 1989;5:109–119. doi: 10.1016/0169-328x(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 133.Petitti N, Etgen AM. Alpha-1-Adrenoceptor Augmentation of Beta-Stimulated cAMP Formation Is Enhanced by Estrogen and Reduced by Progesterone in Rat Hypothalamic Slices. J Neurosci. 1990;10:2842–2849. doi: 10.1523/JNEUROSCI.10-08-02842.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- 135.Gonzalez-Flores O, Shu J, Camacho-Arroyo I, Etgen AM. Regulation of lordosis by cyclic 3',5'-guanosine monophosphate, progesterone, and its 5-alpha-reduced metabolites involves mitogen-activated protein kinase. Endocrinology. 2004;145:5560–5567. doi: 10.1210/en.2004-0823. [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez-Flores O, Guerra-Araiza C, Cerbon M, Camacho-Arroyo I, Etgen AM. The 26S proteasome participates in the sequential inhibition of estrous behavior induced by progesterone in rats. Endocrinology. 2004;145:2328–2336. doi: 10.1210/en.2003-1162. [DOI] [PubMed] [Google Scholar]
- 137.Gonzalez-Flores O, Gomora-Arrati P, Garcia-Juarez M, Gomez-Camarillo MA, Lima-Hernandez FJ, Beyer C, Etgen AM. Nitric oxide and ERK/MAPK mediation of estrous behavior induced by GnRH, PGE2 and db-cAMP in rats. Physiol Behav. 2009;96:606–612. doi: 10.1016/j.physbeh.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hagan CR, Daniel AR, Dressing GE, Lange CA. Role of phosphorylation in progesterone receptor signaling and specificity. Mol Cell Endocrinol. 2011 doi: 10.1016/j.mce.2011.09.017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Proietti C, Salatino M, Rosemblit C, Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau EH, Schillaci R, Elizalde PV. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol Cell Biol. 2005;25:4826–4840. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 141.Rowan BG, Weigel NL, O'Malley BW. Phosphorylation of steroid receptor coactivator-1 - Identification of the phosphorylation sites and phosphorylation through the mitogen- activated protein kinase pathway. J Biol Chem. 2000;275:4475–4483. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 142.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c- fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 143.Watters JJ, Dorsa DM. Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci. 1998;18:6672–6680. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Auger AP, Blaustein JD. Progesterone treatment increases Fos-immunoreactivity within some progestin receptor-containing neurons in localized regions of female rat forebrain. Brain Res. 1997;746:164–170. doi: 10.1016/s0006-8993(96)01190-0. [DOI] [PubMed] [Google Scholar]