Abstract
We previously reported that short exposure of tomato (Lycopersicon esculentum L.) fruits to high temperature protects them from chilling injury. To study the involvement of heat-shock proteins (HSPs) in the acquisition of low-temperature tolerance, we cloned two heat-shock-induced genes that are also expressed at low temperatures. The cloned cDNAs belong to the small HSP group. Sequence analyses of the clones showed perfect homology to the tomato-ripening gene tom66 and to the tomato chloroplastic HSP21 gene tom111. The expression of both genes was induced by high temperature in fruits, flowers, leaves, and stems, but not by low or ambient temperatures or by other stresses such as drought and anaerobic conditions. When the heated fruits were transferred to low temperature, tom66 and tom111 mRNA levels first decreased but were then reinduced. Induction was not observed in nonheated fruits at low temperature. Immunodetection of tom111-encoded protein indicated that this protein is present at low temperatures in the heated fruits. The results of this study show that the expression of tom66 and tom111 is correlated with protection against some, but not all, symptoms of chilling injury.
Plants are sensitive to both high and low temperatures. Both extremes inhibit photosynthesis, growth, pollination, fruit set, and fruit development (Vierling, 1991). Plants from temperate zones are less sensitive to low than to high temperatures, whereas the opposite is true for plants originating from tropical or subtropical areas. The latter include tree crops such as mango, avocado, and banana, as well as many vegetables such as cucumber, pepper, squash, and tomato. However, plants from both groups can be damaged by extended exposure to both high and low temperatures (Wang, 1994). Plants can be partially protected against extreme temperatures if the conditions are gradually changed. Gradually raising the temperature to 38°C allows plants to acclimate and tolerate further increases to temperatures that are normally lethal (Vierling, 1991). Conversely, holding plants at 15°C acclimates them to lower temperatures, which would normally cause chilling injuries (Wang, 1994). Since these adaptations can be prevented by cycloheximide, it is reasonable to assume that de novo protein synthesis is required for protection against both high and low temperatures (Vierling, 1991).
All organisms respond to high temperatures by inducing the synthesis of a small group of evolutionarily conserved polypeptides known as HSPs. Some HSPs are required for normal growth at the upper end of their normal growth-temperature range, whereas others help cells withstand the toxic effects of extreme temperatures (Yost et al., 1990). Plants synthesize numerous smHSPs, ranging from 15 to 30 kD, that are related to the smHSPs of other organisms and to the α-crystallins of the vertebrate eye lens (Inoglia and Craig, 1982). In contrast to mammalian smHSPs, those from plants constitute the most abundant and diverse group of proteins synthesized in response to heat stress. At least four classes of plant smHSPs have been identified based on sequence alignments and immunological cross-reactivity. Classes I and II are found in the cytoplasm, class III is localized in the organelles, and class IV is localized in the ER (Vierling, 1991). Little is known about the cellular function of these smHSPs. Recently, it has been shown that both plant and mammalian smHSPs, and the related α-crystallins, possess molecular chaperone activity in vitro (Jacob et al., 1993; Merck et al., 1993). These proteins were able to refold denatured proteins in an ATP-independent manner (Hendrick and Hartl, 1993). SmHSPs form multimeric protein complexes ranging from 200 to 800 kD (Lee et al., 1995). It is these complexes that appear to be physiologically active.
HSPs are also induced by other stresses such as cold, drought, or salinity (Anderson et al., 1994; Coca et al., 1994; Kiyosue et al., 1994). These HSPs are part of a group of proteins induced by environmental stress either to protect the plant from damage caused by the stress or to help repair the damage caused by the stress. There is very likely to be some overlap in function among the different stress proteins. In agreement with this assumption is the observation that one stress can induce protection against another (Lurie et al., 1994; Leshem and Kuiper, 1996).
We have previously shown that mature-green tomatoes (Lycopersicon esculentum L.) held at 38°C can be transferred to 2°C for several weeks without developing symptoms of chilling injury (Lurie and Klein, 1991). These fruits then ripen normally under ambient temperatures. In contrast, nonheated fruits stored at 2°C developed chilling-injury symptoms and did not ripen under ambient conditions. Similar results involving high-temperature protection against low-temperature injury have been found for other plant tissues, such as cotyledons, hypocotyls, and seeds (Lafuente et al., 1991; Jennings and Saltveit, 1994; Collins et al., 1995). Further, we found that high temperatures induce the synthesis of HSPs in tomato fruit (Sabehat et al., 1996). These newly synthesized HSPs were also found in heated fruits that were transferred directly to 2°C and stored for 3 weeks. If heated fruits were first placed at ambient temperature for 4 d and only then stored at 2°C, the level of these HSPs decreased and the fruits lost their resistance to low temperature (Sabehat et al., 1996). These results demonstrated a correlation between HSP expression and chilling tolerance, supporting a role for HSPs in protection against chilling injury.
In the present work we cloned two heat-shock-induced genes and analyzed their expression under low-temperature conditions. The expression of both genes was induced by high temperature. When the heated fruits were transferred to low temperature, the levels of both transcripts were first decreased but then reinduced. The results show a clear correlation between the expression of these genes and the tolerance of the tissue to low temperatures.
MATERIALS AND METHODS
Tomato (Lycopersicon esculentum cv Daniella) plants were grown in the greenhouse under normal environmental conditions. Fruits were harvested at the mature-green stage, unless otherwise stated. Heat treatment and fruit storage were carried out as previously described (Sabehat et al., 1996). Chilling injury was determined as the appearance of sunken areas (blemishes) on the fruit surface, the development of fungal rots, and the inhibition of color development (Lurie and Klein, 1991). The appearance of these symptoms caused the fruit to be rated as having chilling injury, but the severity of the injury (whether one or many loci of damage were present on a fruit) was not recorded.
Protein Extraction and Western Analysis
Pericarp tissues were excised, ground in liquid N2, extracted with Tris-buffered phenol, and precipitated by ammonium acetate in methanol according to the method of Hurkman and Tanaka (1986) with some modifications as described previously (Sabehat et al., 1996).
SDS-PAGE was performed according to the method of Laemmli (1970) using 12% (w/v) acrylamide gels. For immunodetection, proteins were transferred from the polyacrylamide gel to a nitrocellulose filter using a gel blotter (Bio-Rad). Protein cross-reaction with the pea HSP21 antibody was detected by alkaline phosphatase reaction. The HSP21 antibody was a gift from E. Vierling's laboratory (University of Arizona, Tucson).
RNA Extraction and Northern Analysis
Total RNA was extracted according to the method of Smith et al. (1986). To extract RNA from tomato fruit pericarp, tissue was first frozen and ground in liquid N2. Two volumes of homogenization buffer (6% [w/v] 4-aminosalicylic acid, 1% [w/v] triisopropylnaphthalene sulfonic acid, and 5% [v/v] phenol mixture [prepared by adding 400 mL of DDW, 140 mL of m-cresol, and 1 g of 8-hydroxyquinoline to 1 kg of phenol]) was added, and after shaking at room temperature the phases were separated by centrifugation. The aqueous layer was re-extracted with phenol-chloroform and RNA was precipitated at −20°C by the addition of 2.5 volumes of ethanol. RNA (10 μg) was size-fractionated on a formaldehyde-denaturing agarose gel (Maniatis et al., 1982) and blotted onto a Hybond-N+ filter (Amersham). Following electrophoresis, the formaldehyde gel was briefly stained with ethidium bromide and photographed before blotting for preliminary assessment of equal loading. The blots were hybridized for 24 h with 32P-labeled full-size cDNA probes for tom66 and tom111. Following hybridization, the blots were washed twice with 0.1× SSC (0.015 m NaCl and 1.5 mm sodium citrate, pH 7.0) and 0.1% SDS at 60°C for 10 min and then autoradiographed. All blots were stripped and re-hybridized with a 1000-bp ribosomal probe, pt3, as an internal standard (Lurie et al., 1996).
Construction of a Tomato cDNA Library
A cDNA library in lambda ZAPII was constructed following the manufacturer's instructions (Stratagene). RNA was isolated from mature-green tomato fruits that were first heated at 38°C for 48 h and then stored for 2 weeks at 2°C. Poly(A+) mRNA was isolated from total RNA using an oligo(dT)-cellulose chromatography column (Sigma), according to the method of Maniatis et al. (1982). A library of 1.3 × 106 plaque-forming units was obtained and used for differential screening.
Differential Screening of a Tomato cDNA Library
The cDNA library was differentially hybridized with 32P-labeled first-strand cDNA probes. For the minus probe, we used a mixture of mRNA from mature-green tomato fruits at harvest and from nonheated, chilled tomato fruits stored at 2°C for 2 weeks. For the plus probe, we used mRNA from tomato fruits heated at 38°C for 2 d and then chilled at 2°C for 2 weeks. For cDNA labeling, 1 to 2 μg of poly(A+) mRNA and 1 μg of oligo(dT) primer were used to synthesize approximately 1.0 × 108 cpm 32P-labeled first-strand cDNA. For differential screening, about 3 × 104 plaques were plated (800 plaques onto 90-mm plates) and duplicate plaque lifts were taken using Hybond-N+ membranes (Amersham) according to the manufacturer's instructions. The filters were hybridized at 60°C for 12 h. After hybridization, the blots were washed twice with 2× SSC and 0.1% SDS at 60°C for 10 min and then autoradiographed. Plaques showing substantially stronger hybridization to the plus probe were picked and retested. Inserts from selected clones were subcloned into phagemid vector pBK-CMV using an in vivo excision kit (Stratagene).
DNA Extraction and Southern Analysis
Total DNA from tomato was isolated from 10 young leaves (Koes et al., 1986), and 15 μg of DNA was digested with BamHI, DraI, HindIII, and EcoRI restriction enzymes (Promega), electrophoresed, and blotted onto a Hybond-N+ filter. The blots were hybridized with 32P-labeled full-size tomato cDNA probes (tom66 and tom111). Hybridization was carried out at 60°C in 0.25 m Na2HPO4, 7% SDS, 1 mm EDTA, and 1% BSA. After hybridization, the blots were washed with 2× SSC and 0.1% SDS at 60°C or with 0.1× SSC and 0.1% SDS at 65°C, and then autoradiographed.
Sequence Analyses
Plasmids were isolated according to standard protocols (Maniatis et al., 1982). The sequences of both strands of the isolated clones were determined by a Taq dideoxy-terminator cycle of miniprep plasmid (pBSK+) DNA using synthetic oligonucleotides to the known sequence as primers.
RESULTS
Isolation of smHSP cDNAs from Heated and Chilled Tomato Fruits
Heat-shock-induced genes that are expressed at low temperature were cloned by differential screening of a cDNA library, constructed from mRNA of chilled (for 2 weeks), preheated tomato fruits. For the minus probe, mRNA from mature-green fruits (immediately after harvest) was mixed with mRNA from nonheated fruits stored for 2 weeks at 2°C. For the plus probe, we used mRNA from heated fruits that were stored subsequently for 2 weeks at 2°C. We isolated two positive clones, and their sequence analysis revealed homology to two ripening-related genes previously isolated from tomato. The first clone was identical to the tom66 gene (Slater et al., 1985), which is 77% homologous to the class I smHSP HSP18.1 from pea (DeRocher et al., 1991). The second clone was identical to the class II chloroplastic HSP21-encoding tom111 gene (Lawrence et al., 1997).
Characterization of tom66 and tom111 Gene Expression
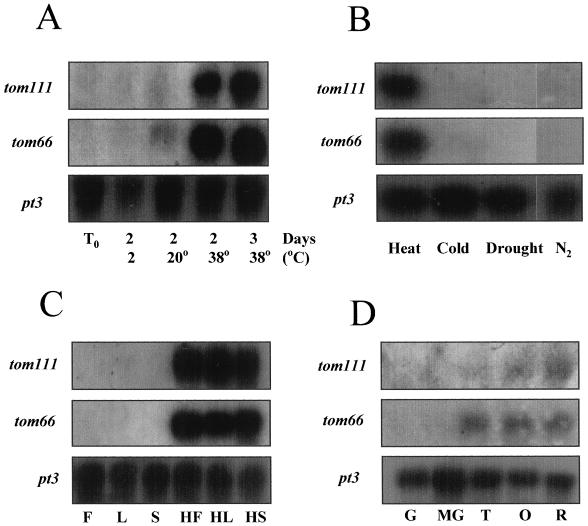
Mature-green fruits were stored for 2 or 3 d at 2, 20, or 38°C, and then analyzed for tom66 and tom111 gene expression. Transcript levels of both genes were low under low and ambient temperatures, but strongly induced by high temperature (Fig. 1A).
Figure 1.
Regulation of tom66 and tom111 mRNA accumulation. A, Mature-green fruits were harvested (T0) and kept for 2 d at 2°C, for 2 d at 20°C, or for 2 or 3 d at 38°C. After each treatment, RNA was extracted and analyzed for tom66 and tom111 expression. B, Tomato fruits were stored for 2 d at 38°C (Heat), for 2 d at 2°C (Cold), for 2 d at 20°C at 15 ± 5% RH (Drought), and for 2 d at 20°C under 100% N2 (N2). RNA was extracted after each treatment and analyzed for tom66 and tom111 expression. C, Tomato flowers (F), leaves (L), and stems (S) were stored in water for 6 h at 20 or at 38°C (H), and then RNA was extracted and analyzed for tom66 and tpm111 expression. D, Young-green (G), mature-green (MG), turning-stage (T), orange (O), and ripe-red (R) fruits were harvested, and RNA was extracted and analyzed for tom66 and tom111 expression. To ensure equal loading of RNA, all of the blots were rehybridized with the ribosomal pt3 probe.
Many HSPs can be induced by stresses other than high temperature. We tested whether exposing fruits to drought conditions, low O2 concentrations, or low temperature would induce tom66 and tom111 expression. Tomato fruits were kept at 20°C at 15 ± 5% RH for 2 d to generate drought conditions. To create anaerobic stress, fruits were kept in chambers supplied with 100% N2 for 2 d. For cold stress, the fruits were stored for 2 d at 2°C. RNA was extracted after each treatment and analyzed for tom66 and tom111 expression. None of these stresses caused induction of these genes, in contrast to the marked response to high temperature (Fig. 1B).
We examined the expression of tom66 and tom111 in various plant organs kept at ambient or high temperatures. Open flowers, leaves, and stems were detached and their bases were placed in glass tubes containing tap water. The detached organs were stored for 6 h at 20 or at 38°C, and then RNA was extracted and analyzed for tom66 and tom111 expression. Both genes were induced by high temperature in all tested organs (Fig. 1C). However, no detectable expression was found at 20°C.
Since tom66 had been previously described as a ripening-related gene (Fray et al., 1990), we tested whether these genes are expressed during fruit development. Fruits at different developmental stages (Fig. 1D) were detached from plants grown at ambient temperature (approximately 20°C) and RNA was extracted and analyzed for tom66 and tom111 expression. Both genes were induced to a low level of expression at late stages of fruit development (Fig. 1D).
Kinetics of tom66 and tom111 Expression under Different Temperature Regimes
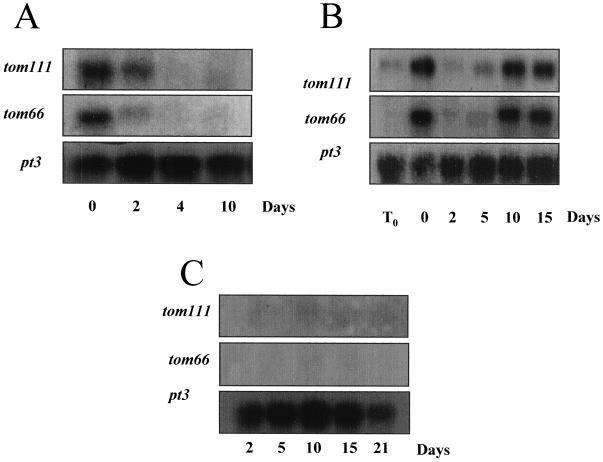
We examined whether tom66 and tom111 genes are expressed at 20°C after induction by high temperature. Mature-green fruits were heated at 38°C for 2 d, then transferred to 20°C for different time periods. RNA analyses of tom66 and tom111 (Fig. 2A) showed that after transferring the heated fruits to 20°C for 4 d, transcript levels were dramatically decreased.
Figure 2.
Kinetics of tom66 and tom111 transcript accumulation under different temperature regimes. A, Mature-green fruits were heated at 38°C for 2 d and then transferred to 20°C for the specified periods of time (0, 2, 4, and 10 d). RNA was extracted and analyzed for tom66 and tom111 expression. B, Mature-green fruits were heated at 38°C for 2 d and then transferred to 2°C for the specified periods of time (0, 2, 4, 10, and 15 d). RNA was extracted and analyzed for tom66 and tom111 expression. C, Mature-green, nonheated fruits were stored for different periods of time (2, 5, 10, 15, and 21 d) at 2°C, and RNA was extracted and analyzed for tom66 and tom111 expression. To ensure equal loading of RNA, all of the blots were rehybridized with the ribosomal pt3 probe. T0, Time of harvest.
We analyzed further whether tom66 and tom111 transcripts are present at low temperature after induction by high temperature. Mature-green fruits were heated at 38°C for 2 d and then transferred to 2°C for different time periods. RNA was extracted at the specified time points and analyzed for tom66 and tom111 transcript accumulation. The levels of mRNA for both genes were low at harvest (T0), induced after 2 d at 38°C (0), and then declined to an almost undetectable level after 2 d at 2°C (Fig. 2B). Surprisingly, however, after further exposure to low temperature (between 5 and 10 d), the expression of both genes was reinduced and transcript levels were similar to those found at the end of the heat treatment.
To examine whether transcript accumulation was induced directly by low temperature, we kept mature-green, nonheated fruits at 2°C for 2, 5, 10, 15, and 21 d, and extracted RNA at each time point for northern analysis. Transcript accumulation was not induced in either gene during the exposure to low temperature (Fig. 2C).
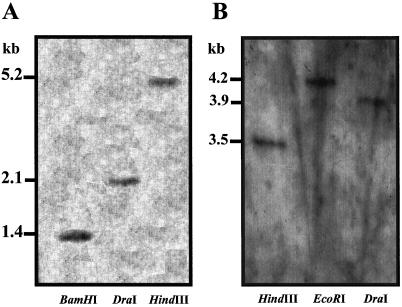
To test the possibility that two different homologous genes are induced, one by high temperature and the other by low temperature, we determined the number of tom66 and tom111 genes present in the tomato genome by DNA-blot analysis. Only one fragment was found to hybridize using either probe (Fig. 3, A and B), suggesting the presence of only one copy of each gene.
Figure 3.
Southern-blot analyses of tom66 and tom111 in tomato. DNA was digested with BamHI, DraI, HindIII, or EcoRI, and hybridyzed with labeled tom66 (A) and tom111 (B) cDNAs.
These results suggest that both genes are induced at low temperatures only if they have first been induced by high temperatures, thus indicating a correlation between the expression of tom66 and tom111 and resistance to chilling injuries.
Ambient Temperature Abolishes Heat-Shock-Induced Protection against Fungal Rot Development
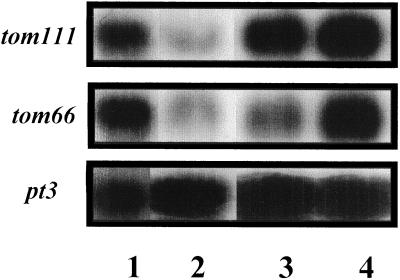
We next examined whether exposure of heated fruits to ambient temperature affects the subsequent accumulation of tom66 and tom111 transcripts under low temperature. Mature-green fruits were exposed to 38°C for 2 d, transferred to 20°C for 4 d, and then transferred to 2°C for 2 or 10 d. Both transcripts accumulated at high temperature and their level was reduced when the fruits were transferred to 20°C for 4 d (Fig. 4). However, once these fruits were placed at 2°C, both mRNAs began to accumulate, and after 10 d the levels of both transcripts were very high.
Figure 4.
Reinduction of tom66 and tom111 mRNA accumulation at low temperature after incubation of heated fruits at ambient temperature. Mature-green fruits were exposed to 38°C for 2 d (lane 1), transferred to 20°C for 4 d (lane 2), and then transferred to 2°C for 2 d (lane 3) or for 10 d (lane 4). RNA was extracted and analyzed for tom66 and tom111 expression. To ensure equal loading of RNA, all of the blots were rehybridized with the ribosomal pt3 probe.
Chilling injury appears in tomato fruits in the form of a number of symptoms. Chilled fruits lose their ability to develop full color, show increased susceptibility to decay, and develop sunken areas on the fruit (blemishes). Fruits that had first been heated, exposed to 20°C, and then stored at low temperature were examined for the appearance of each of these symptoms. Mature-green fruits were exposed to 38°C for 2 d and transferred directly to 2°C for 3 weeks, or were first transferred to 20°C for 2 or 4 d and then to 2°C for 3 weeks. All fruits were then transferred to 20°C and chilling injuries were examined. As can be seen in Table I, nonheated control fruits that were stored for 3 weeks at 2°C showed blemishes, development of decay, and lack of normal pigmentation. As previously described (Lurie and Klein, 1991), heated fruits that were transferred directly to low temperature barely developed any chilling injuries. If the heated fruits were kept for 4 d at ambient temperature before exposure to the low temperature, they decayed. However, they did not develop sunken areas (blemishes) and color developed normally, suggesting that the expression of tom66 and tom111 is correlated with protection against some, but not all, symptoms of chilling injury.
Table I.
Development of chilling-injury symptoms in tomato fruits exposed to different temperature regimes
| Treatment | Chilling-Injury Symptoms
|
||
|---|---|---|---|
| Rot | Blemishes | Inhibition of color development | |
| % of total | |||
| Control (nonheated) | 70 | 60 | 95 |
| 2 d at 38°C | 10 | 8 | 0 |
| 2 d at 38°C + 2 d at 20°C | 10 | 10 | 0 |
| 2 d at 38°C + 4 d at 20°C | 65 | 10 | 0 |
Mature-green fruits were exposed to 38°C for 2 d and transferred directly to 2°C for 3 weeks, or first transferred to 20°C for 2 or 4 d and then to 2°C for 3 weeks. As a control, nonheated fruits were stored for 3 weeks at 2°C. All fruits were then transferred to 20°C and chilling injuries were examined. The experiment was repeated four times with 20 fruits per treatment.
Immunodetection of tom111-Encoded Proteins
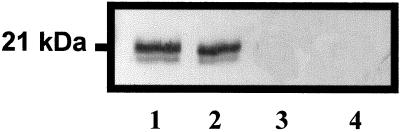
In our previous study we used an antibody to pea HSP18.1 (a pea class I smHSP, a homolog to tom66) to show the presence of a cross-reacting protein in heated fruits that have been stored at low temperature (Sabehat et al., 1996). To test whether the tom111-encoded protein accumulates at low temperature we used an antibody for its pea homolog, HSP21 (Vierling and Sun, 1989). Proteins were extracted from fruits that had been heated for 2 d, from heated and nonheated fruits that had been stored at 2°C for 2 weeks, and from fruits that were kept for 2 d at 20°C. Immunodetection using the pea HSP21 antibody (Fig. 5) showed high levels of protein with the expected molecular mass of approximately 21 kD in heated fruit and in heated fruit stored for 2 weeks at 2°C. This protein was not detected in nonheated, chilled fruits or in fruits kept at 20°C.
Figure 5.
Immunodetection of tom111-encoded protein in tomato fruits subjected to different temperature regimes. Proteins were extracted from fruits that had been heated for 2 d at 38°C (lane 1), from heated (lane 2) and nonheated (lane 3) fruits stored at 2°C for 2 weeks, and from fruits that were kept for 2 d at 20°C (lane 4). Western-blot analysis was performed using the pea HSP21 antibody.
DISCUSSION
Exposing plants to moderately high temperatures for short periods often induces thermotolerance, which allows them to survive under higher, normally lethal temperatures (Vierling, 1991). During moderate heat stress, HSPs are produced and are thought to contribute to the acquisition of thermotolerance (Vierling, 1991). A number of studies have shown the existence of cross-tolerance, i.e. exposing a plant to moderate stress conditions induces tolerance to other stresses (Lurie et al., 1994; Leshem and Kuiper, 1996). High-temperature stress has been found to protect against chilling injury in a number of fruits and vegetables, including avocado (Woolf et al., 1995), cucumber (McCollum et al., 1995), pepper (Mencarelli et al., 1993), and tomato (Lurie and Klein, 1991). The broad range of plants that show this cross-tolerance suggests that it may be a general response.
To study the mechanism involved in the acquisition of tolerance to chilling injuries by heat shock, we cloned two genes that are induced by heat shock and that are also expressed under low temperatures and found that they belong to the smHSP group. The first isolated cDNA was identical to the smHSP tom66 gene previously cloned from ripened tomato fruits and was defined as a ripening-related gene (Fray et al., 1990). The second clone showed perfect homology to the recently isolated smHSP gene, tom111 (Lawrence et al., 1997). The mRNA levels of both genes was induced by high temperature, and transcripts could be detected after the heated fruits were stored for 3 weeks at 2°C. Other studies have shown similar results: a class II smHSP gene from heated tomato fruit was induced by high temperature and expressed when the heated fruits were transferred to low temperature (D. Dilley, personal communication). Lurie et al. (1996) used the hsp17 cDNA from soybean as a probe for tomato RNA and showed expression at low temperature in preheated fruits. SmHSPs may play a specific role in the acquisition of tolerance against chilling stress.
The expression of tom66 and tom111 in heated fruits under low temperature does not necessarily indicate that the transcripts are also translated into proteins under these conditions; in fact, transcript is not associated with polysomes in carrot (Apuya and Zimmerman, 1992). Our results using antibody to the TOM111 homolog from pea (Fig. 5) show that this protein (or other similar smHSPs that cross-react with this antibody) is present at low temperatures. Similar results were found in our previous work (Sabehat et al., 1996) using the antibody to the TOM66 homolog from pea (HSP18.1).
Both tom66 and tom111 genes were specifically induced in mature-green fruits by high temperature, and expression was not detected in nonheated fruits under ambient or low temperatures (Fig. 1A). Furthermore, the expression of these genes was not induced by other stresses such as drought or low O2 (Fig. 1B), although many other smHSPs have been found to be induced by these stresses in other systems (Heikkila et al., 1984; Coca et al., 1994; Anderson et al., 1994).
Although we suggest that the TOM66 and TOM111 proteins are involved in the protection against low-temperature stress in tomato fruits, these proteins may have the same function in other organs. The expression of tom66 and tom111 was induced by high temperature in flowers, stems, and leaves (Fig. 1C), and mRNA could be detected after the heated organs were transferred to low temperatures (data not shown). A similar mechanism of heat-shock-induced tolerance to chilling injury may exist in all plant organs. Protection against chilling injury by high-temperature treatment has been found in mung bean hypocotyls (Collins et al., 1995) and cucumber cotyledons and seeds (Lafuente et al., 1991; Jennings and Saltveit, 1994). In these studies loss of protection was correlated with the disappearance of HSPs from the tissue (Lafuente et al., 1991; Collins et al., 1995).
In a number of plants the expression of smHSPs is induced during specific developmental stages. Several class II smHSP mRNAs are expressed during microspore meiosis in both lily (Bouchard, 1990) and maize (Dietrich et al., 1991). Class I cytoplasmic smHSP mRNAs are expressed during embryogenesis in carrot (Zimmerman et al., 1989) and alfalfa (Gyorgyey et al., 1991). Class I smHSP mRNAs have also been detected in pea seeds (Vierling and Sun, 1989) and in a number of germinating and young seedlings of sorghum (Howarth, 1990), sunflower (Coca et al., 1994), and wheat (Helm and Abernethy, 1990). Hernandez and Vierling (1993) have detected class I smHSPs in nonheated developing legume seedlings. We found that the expression of tom66 and tom111 genes is regulated during tomato fruit ripening (Fig. 1D). Thus, smHSPs may also play a role in the regulation of specific developmental processes under normal growth conditions.
The kinetic study of the accumulation of tom66 and tom111 mRNAs following heat treatment and during storage at low temperature revealed an interesting phenomenon: transcript accumulation was induced by high temperature, was reduced after the transition to low temperature, but was reinduced after further exposure to low temperature (Fig. 2B). Expression was not induced at low temperature if the fruits had not been first exposed to high temperature. To the best of our knowledge, there have been no previous reports of such a phenomenon in plants or other organisms. The possibility that two different homologous genes are induced, one by high and the other by low temperatures, is unlikely, since DNA-blot analysis indicated only one copy of each gene (Fig. 3), even under relatively low-stringency washing conditions (data not shown). The reinduction of mRNA accumulation at low temperature may be caused by the reinduction of gene transcription. It is possible that the activation of these genes by high temperature requires the production and binding of several heat-shock transcription factors (Nover et al., 1996). Transition to low temperature may inhibit the expression of one of the factors and, therefore, cause a decrease in tom66 and tom111 expression. Further exposure to low temperature may activate new cold-induced transcription factors that replace the missing heat-shock factor to reactivate gene transcription. At this stage, however, we do not have evidence that can support this hypothesis.
In our previous paper we showed that exposure of heated fruits to a period of 4 d at ambient temperature before transfer to low temperature abolishes tolerance to low temperature (Sabehat et al., 1996). However, in this study exposure of heated fruits for 4 d to 20°C did not prevent tom66 and tom111 reinduction at low temperature. Closer examination of the chilling-injury symptoms on fruits that had been heated, held at 20°C, and then stored at 2°C revealed that they developed only decay, whereas nonheated fruits developed decay, blemishes, and the inability to redden (Table I). It is therefore possible that tom66 and tom111 gene products are involved in the promotion of color development and protection against blemishes, but not against decay. It is also possible that these proteins can protect against the invasion of the pathogen, but the fungi develop to an irreversible stage during the time the fruit is held at 20°C, when gene expression decreases (Fig. 4).
In conclusion, in this study we show that the expression of two smHSP genes, tom66 and tom111, is induced at low temperature only if it is first induced by high temperature. We found a correlation between the expression of these genes and the acquisition of tolerance to low temperatures. Such a correlation suggests an involvement in a protective mechanism against chilling injury. Heat-shock genes that are involved in this process may be used for molecular breeding to generate low- and high-temperature-resistant transgenic plants.
ACKNOWLEDGMENTS
We would like to thank Dr. I. Wilson and Prof. D. Grierson, Nottingham University, for their help in constructing the cDNA library, and Prof. E. Vierling, University of Arizona, who kindly provided us with the pea HSP21 antibody.
Abbreviations:
- HSP
heat-shock protein
- smHSP
small HSP
Footnotes
This research was supported by the U.S.-Israel Binational Agricultural Research and Development Fund (grant no. IS-2179) and by a grant from the Israeli Ministry of Science and Fine Arts.
LITERATURE CITED
- Anderson JV, Li QB, Haskell DW, Guy CL. Structural organization of the spinach endoplasmic reticulum-luminal 70-kilodalton heat-shock cognate gene and expression of 70-heat-shock genes during cold acclimation. Plant Physiol. 1994;104:1359–1370. doi: 10.1104/pp.104.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apuya NR, Zimmerman JL. Heat shock gene expression is controlled primarily at the translational level in carrot cells and somatic embryos. Plant Cell. 1992;4:657–665. doi: 10.1105/tpc.4.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard RA. Characterization of expressed meiotic prophase repeat transcript clones of Lilium: meiosis-specific expression, relatedness, and affinities to small heat shock protein genes. Genome. 1990;33:68–79. doi: 10.1139/g90-012. [DOI] [PubMed] [Google Scholar]
- Coca MA, Almoguera C, Jordano J. Expression of sunflower low-molecular-weight heat-shock proteins during embryogenesis and persistence after germination: localization and possible functional implications. Plant Mol Biol. 1994;25:479–492. doi: 10.1007/BF00043876. [DOI] [PubMed] [Google Scholar]
- Collins G, Nie XL, Saltveit M. Heat shock proteins and chilling sensitivity of mung bean hypocotyls. J Exp Biol. 1995;46:479–492. [Google Scholar]
- DeRocher AE, Helm KW, Lauzon LM, Vierling E. Expression of a conserved family of cytoplasmic low molecular weight heat shock proteins during heat stress and recovery. Plant Physiol. 1991;96:1038–1047. doi: 10.1104/pp.96.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich PS, Bouchard RA, Casey ES, Sinibaldi RM. Isolation and characterization of a small heat shock protein gene from maize. Plant Physiol. 1991;96:1268–1276. doi: 10.1104/pp.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray RG, Lycett GW, Grierson D. Nucleotide sequence of a heat-shock and ripening-related cDNA from tomato. Nucleic Acids Res. 1990;18:7148. doi: 10.1093/nar/18.23.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgyey J, Gartner A, Nemeth K, Magyar Z, Hirt H, Heberle-Bors E, Dudits D. Alfalfa heat shock genes are differentially expressed during somatic embryogenesis. Plant Mol Biol. 1991;16:999–1007. doi: 10.1007/BF00016072. [DOI] [PubMed] [Google Scholar]
- Heikkila JJ, Papp JET, Schutz GA, Brewley JD. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic acid, and wounding. Plant Physiol. 1984;76:270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KW, Abernethy RH. Heat shock proteins and their mRNAs in dry and early imbibing embryos of wheat. Plant Physiol. 1990;93:1626–1633. doi: 10.1104/pp.93.4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick JP, Hartl FU. Molecular chaperone functions of heat shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Hernandez LD, Vierling E. Expression of low molecular weight heat shock proteins in plants under field conditions. Plant Physiol. 1993;101:1209–1216. doi: 10.1104/pp.101.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C. Heat shock proteins in Sorghum bicolor and Pennisetum americanum. II. Stored RNA in sorghum seed and its relationship to heat shock protein synthesis during germination. Plant Cell Environ. 1990;13:57–64. [Google Scholar]
- Hurkman WJ, Tanaka CK. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986;81:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoglia T, Craig E. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Jennings P, Saltveit ME. Temperature and chemical shocks induce chilling tolerance in germinating Cucumis sativus (cv. Poinsett 76) seeds. Physiol Plant. 1994;91:703–707. [Google Scholar]
- Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K. Cloning of cDNAs for genes that are early responsive to dehydration stress in Arabidopsis thaliana L.: identification of three ERDs as HSP cognate genes. Plant Mol Biol. 1994;25:791–798. doi: 10.1007/BF00028874. [DOI] [PubMed] [Google Scholar]
- Koes RE, Spelt CE, Reif HJ, van der Elsen PJM, Veltkamp E, Mol JNM. Floral tissue of Petunia hybrida (V30) expresses only one member of the chalcone synthase multigene family. Nucleic Acids Res. 1986;14:5229–5239. doi: 10.1093/nar/14.13.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafuente MT, Belver A, Guye MG, Saltveit M. Effect of temperature conditioning on chilling injury of cucumber cotyledons. Plant Physiol. 1991;95:443–449. doi: 10.1104/pp.95.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence SD, Cline K, Moore GA. Chloroplast development in ripening tomato fruit: identification of cDNAs for chromoplast-targeted proteins and characterization of a cDNA encoding a plastid-localized low molecular weight heat shock protein. Plant Mol Biol. 1997;33:483–492. doi: 10.1023/a:1005785321165. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Pokala N, Vierling E. Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J Biol Chem. 1995;270:10432–10438. doi: 10.1074/jbc.270.18.10432. [DOI] [PubMed] [Google Scholar]
- Leshem Y, Kuiper P. Is there a GAS (general adaption syndrome) response to various types of environmental stress? Biol Plant. 1996;38:1–18. [Google Scholar]
- Lurie S, Handros A, Fallik E, Shapira R. Reversible inhibition of tomato fruit gene expression at high temperature. Effects on tomato fruit ripening. Plant Physiol. 1996;110:1207–1214. doi: 10.1104/pp.110.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie S, Klein JD. Acquisition of low-temperature tolerance in tomatoes by exposure to high temperature stress. J Am Soc Hortic Sci. 1991;116:1007–1012. [Google Scholar]
- Lurie S, Klein JD, Fallik E. Cross protection of one stress by another. In: Cherry J, editor. Biochemical and Cellular Mechanisms of Stress Tolerance of Plants. Berlin: Springer Verlag; 1994. pp. 201–212. [Google Scholar]
- Maniatis T, Fritch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- McCollum TG, Doostdar H, Mayer RT, McDonald RE. Immersion of cucumber fruit in heated water alters chilling-induced physiological changes. Postharv Biol Technol. 1995;6:55–64. [Google Scholar]
- Mencarelli F, Ceccantoni B, Bolini A, Anelli G. Influence of heat treatment on the physiological response of sweet pepper kept at chilling temperature. Acta Hortic. 1993;343:238–243. [Google Scholar]
- Merck K, Groenon D, Voorter C, de Haard-Hoekman W, Horwitz J, Bloemendal H, de Jong W. Structure and functional similarities of bovine α-crystallin and mouse small heat shock protein. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- Nover L, Scharf KD, Gagliardi D, Vergne P, Czarnecka-Verner E, Gurley WB. The hsf world: classification and properties of plant heat stress transcription factors. Cell Stress and Chaperones. 1996;1:215–223. doi: 10.1379/1466-1268(1996)001<0215:thwcap>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabehat A, Weiss D, Lurie S. The correlation between heat- shock protein accumulation and persistence and chilling tolerance in tomato fruit. Plant Physiol. 1996;110:531–547. doi: 10.1104/pp.110.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater A, Maunders MJ, Edwards K, Schuch W, Grierson D. Isolation and characterization of cDNA clones for tomato polygalacturonase and other ripening related proteins. Plant Mol Biol. 1985;5:137–147. doi: 10.1007/BF00015677. [DOI] [PubMed] [Google Scholar]
- Smith CJS, Slater A, Grierson D. Rapid appearance of an mRNA correlated with ethylene synthesis encoding a protein of molecular weight 35000. Planta. 1986;168:94–100. doi: 10.1007/BF00407014. [DOI] [PubMed] [Google Scholar]
- Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- Vierling E, Sun A. Developmental expression of heat shock proteins in higher plants. In: Cherry J, editor. Environmental Stress in Plants. Berlin: Springer-Verlag; 1989. pp. 343–354. [Google Scholar]
- Wang CY. Chilling injury in horticultural commodities. HortScience. 1994;29:986–988. [Google Scholar]
- Woolf AB, Watkins CB, Bowen JH, Lay-Yee M, Maindonals JH, Ferguson IB. Reducing external chilling injury in stored ‘Hass’ avocados with dry heat treatments. J Am Soc Hortic Sci. 1995;120:1050–1056. [Google Scholar]
- Yost HJ, Petersen RB, Lindquist S (1990) Posttranslational regulation of heat shock protein synthesis in Drosophila. In RI Morimoto, A Tissieres, C Georgopolus, eds, Stress Proteins in Biology and Medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 379–409
- Zimmerman JL, Apuya N, Darwish K, O'Carroll C. Novel regulation of heat shock genes during carrot somatic embryo development. Plant Cell. 1989;1:1137–1146. doi: 10.1105/tpc.1.12.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]