Abstract
Introduction
Intra-amniotic inflammation is traditionally defined as an elevation of amniotic fluid interleukin (IL)-6. Previous case control studies have suggested an association between an elevated midtrimester amniotic fluid IL-6 and preterm delivery, although such an association has been recently challenged. Intra-amniotic inflammation can also be defined by an elevation of the T-cell chemokine, Interferongamma-inducible protein (IP)-10. An elevation in amniotic fluid IP-10 has been associated with chronic chorioamnionitis, a lesion frequently found in late spontaneous preterm birth and fetal death. In contrast, an elevation in amniotic fluid IL-6 is typically associated with acute chorioamnionitis and funisitis. This study was conducted to examine the relationship between an elevation in amniotic fluid IL-6 in the midtrimester and preterm delivery at or before 32 weeks of gestation, and the amniotic fluid concentration of IP-10 and preterm delivery after 32 weeks of gestation.
Materials and methods
This cohort study included 847 consecutive women undergoing genetic midtrimester amniocentesis; in 796 cases, amniotic fluid and pregnancy outcome was available for study after exclusion of abnormal karyotype and/or fetal congenital anomalies. Spontaneous preterm delivery was defined as early (≤ 32 weeks) or late (after 32 completed weeks of pregnancy). The amniotic fluid and maternal blood concentrations of IL-6 and IP-10 were measured by specific immunoassays.
Results
1) The prevalence of preterm delivery was 8.3% (66/796), while those of early and late spontaneous preterm delivery were 1.5% (n = 12), and 4.5% (n = 36), respectively; 2) patients who had a spontaneous preterm delivery after 32 weeks of gestation had a higher median amniotic fluid IP-10 concentration than those who delivered at term [median 713 pg/mL, inter-quartile range (IQR) 509 – 1427 pg/mL vs. median 589 pg/mL, IQR 402 – 953 pg/mL; P = 0.006] and an elevation of amniotic fluid IP-10 concentration above 502 pg/mL (derived from an ROC curve) was associated with late spontaneous preterm delivery [odds ratio 3.9 (95% CI 1.6 – 9.9)]; 3) patients who had a spontaneous preterm delivery ≤ 32 weeks of gestation had a higher median amniotic fluid IL-6 concentration than those who delivered at term [median 2052 pg/mL, IQR 435 – 3015 pg/mL vs. median 414 pg/mL, IQR 209 – 930 pg/mL; P = 0.006], and an elevated amniotic fluid IL-6 concentration above 1740 pg/mL (derived from an ROC curve) was associated with early spontaneous preterm delivery [odds ratio 9.5 (95% CI 2.9 – 31.1)]; 4) subclinical intra-amniotic inflammation, defined as an elevation of IL-6 (≥ 2.9 ng/mL) or IP-10 (≥ 2.2 ng/mL) concentration above the 95th percentile of patients who had uncomplicated term delivery (n = 652 for IL-6 and n = 633 for IP-10), was observed in 6.3% (50/796) and 5.8% (45/770) of cases, respectively. Although each type of inflammation is a risk factor for spontaneous preterm delivery, many patients had a term delivery without complication; 5) the amniotic fluid in the midtrimester did not contain microorganisms detectable with cultivation techniques.
Conclusions
Intra-amniotic inflammation is heterogeneous. Some patients have elevated amniotic fluid concentrations of IL-6, and are at risk for spontaneous preterm delivery before 32 weeks of gestation, while others have an elevated IP-10 (a chemotactic T-cell chemokine) and such patients are at risk for spontaneous preterm delivery after 32 weeks of gestation. A fraction of patients have subclinical intra-amniotic inflammation and deliver at term. The clinical significance of this condition remains to be determined.
Keywords: Chemokines, chorioamnionitis, chronic chorioamnionitis, cytokines, early preterm labor, late preterm labor, maternal anti-fetal rejection, midtrimester amniocentesis, pregnancy
INTRODUCTION
The amniotic cavity is considered sterile for bacteria during normal pregnancy [15, 147, 150]. The presence of bacteria, or microbial invasion of the amniotic cavity (MIAC), is generally regarded as a pathologic state [16, 50, 61, 70, 94, 119, 124, 154, 159, 172, 176, 190]. MIAC has been reported in approximately 10% of cases with preterm labor and intact membranes [61, 154, 175], 33% of patients with preterm premature rupture of membranes (PROM) [60, 61, 151, 159], approximately 50% of patients with cervical insufficiency [162], 9% of patients with an asymptomatic short cervix [71], 26% of patients with preterm labor and a short cervix (< 15 mm) [59], 18% of patients during term labor [169], 34% of patients with premature rupture of membranes at term [165], 14% of patients with idiopathic vaginal bleeding [58] and 5% of patients with placenta previa [101], using standard cultivation techniques. However, the frequency of MIAC may be even higher if broad primers are used to detect microbial footprints [2, 31, 32, 35, 36, 44, 69, 73, 78, 106, 120, 129, 141, 143], and also if specific primers [80, 131] are used to detect the most common microorganisms, such as genital Mycoplasmas [47, 76, 107, 128, 144, 206]. Moreover, these techniques have detected microbial footprints in the amniotic fluid of patients who developed preeclampsia [34] or delivered a small-for-gestational-age (SGA) neonate [33].
Few studies have examined the microbial state of the amniotic cavity in the midtrimester [13, 19, 53, 65, 74, 104] and the results have varied. Some have reported sterile amniotic fluid [26, 42] while others have found the presence of microorganisms in an unexpectedly high number of cases [13, 65, 104]. The presence of microorganisms in the amniotic cavity elicits, in some cases, an inflammatory response that can be detected by demonstration of an elevation of the amniotic fluid white blood cell count [18, 43, 161, 171], a decrease in amniotic fluid glucose [28, 45, 46, 66, 90, 157, 171], or an elevation in proinflammatory cytokines, such as interleukin-6 (IL-6) [9, 29, 79, 82, 136, 156, 167, 170, 171, 177, 178, 200, 201], MCP-1 [20, 27, 40, 75], and matrix metalloproteinases (MMPs) such as MMP-8 [40, 85, 88, 89, 95, 96, 112, 123, 126, 132, 134, 135].
Such inflammatory response has been extensively studied in preterm labor with intact membranes [6, 10, 11, 21, 25, 50 – 52, 63, 64, 72, 82, 95, 117, 105, 160, 164, 166, 194, 198], preterm PROM [109 – 111, 114, 115, 133, 183], cervical insufficiency [96], and asymptomatic women with a short cervix [81, 187, 188]. In general, the presence of an inflammatory response is a poor prognostic sign and is associated with preterm delivery and neonatal complications [23, 57, 67, 182, 190, 197 – 205, 207, 208, 210]. However, there have been cases in which there is a positive amniotic fluid culture for microorganisms, but no evidence of an inflammatory response. In a few cases we have been successful in eradicating intra-amniotic infection with systemic antibiotic administration to the mother [71, 116, 163]. We have observed many cases in which there is evidence of an intra-amniotic inflammatory response, as determined by an elevation in amniotic fluid cytokines (i.e., IL-6) while there is no evidence of microbial invasion with culture techniques or even broad range polymerase chain reaction (PCR) [95 – 97, 187, 210]. The etiology of these cases of intra-amniotic inflammation remains unknown.
In 1995, we reported a case-control study in which women who had a pregnancy loss after a midtrimester amniocentesis had a higher amniotic fluid IL-6 concentration than patients who delivered at term [173]. Subsequently, several casecontrol studies have demonstrated an association between the amniotic fluid IL-6 concentration at the time of mid-trimester amniocentesis and preterm delivery [186, 191, 193]. The same has been reported for other cytokines [4, 20, 30, 102, 103, 139, 140, 186, 210]. A limitation of case control studies is that they are prone to biases. In order to determine the clinical significance of intra-amniotic inflammation, a cohort study is necessary.
Until recently, the standard definition of intra-amniotic inflammation relied on the demonstration of an elevation of amniotic fluid white blood cell count (largely neutrophils) [161, 171], or proinflammatory cytokines, such as IL-6 [9, 29, 79, 82, 102, 136, 156, 167, 170, 171, 177, 178, 201], IL-1 beta [10, 72, 122, 148, 152, 155, 158, 160, 164, 168, 203], TNF [108, 153, 166, 174, 177, 200 – 202], chemokines [21, 40, 68, 121, 125, 201], or MMP-8 [79, 84, 85, 88, 89, 95, 96, 112, 113, 123, 126, 132, 134, 135]. We have recently identified a different form of intra-amniotic inflammation characterized by an elevation of T-cell chemokines [87, 99, 100, 127, 196], and demonstrated that such inflammatory process is associated with chronic chorioamnionitis, a lesion characterized by the infiltration of maternal lymphocytes into the amnion and chorion [48, 49, 77, 87].
The proposed mechanism for this lesion is that an increased concentration of amniotic fluid T-cell chemokines generates a gradient whereby maternal T-cells infiltrate the fetal tissue (chorion and amnion). Therefore, this lesion appears to represent a manifestation of maternal anti-fetal rejection because autoreactive maternal T-cells infiltrate the graft (fetal membranes) and cause apoptosis [87]. This would be equivalent to transplantation rejection, in which there is lymphocyte infiltration and graft dysfunction. Of interest is that this lesion is observed frequently in cases of late preterm delivery and in a very high number of cases of fetal death [98]. CXCL-10 (or IP-10) is a T-cell chemokine which is elevated in the amniotic fluid of patients with chronic chorioamnionitis [87]; however, it is unknown when this inflammatory process begins. It is possible that elevated IP-10 concentrations associated with intra-amniotic inflammation are present in the midtrimester: if so, they could predispose to late preterm delivery.
The purpose of this study was to determine if an elevation of amniotic fluid IL-6 is associated with early preterm delivery (≤ 32 weeks of gestation), and if an elevation of amniotic fluid concentration of IP-10 is associated with preterm delivery after 32 weeks of gestation.
MATERIALS AND METHODS
Study design and participants
This was a prospective cohort study that included 847 women with singleton gestations undergoing midtrimester amniocentesis for clinical indications (advanced maternal age, abnormal quad/triple test, family history of chromosomal abnormalities, suspected fetal anomalies or viral infection and maternal request). Patients were invited to donate amniotic fluid for research purposes, as well as a sample of maternal blood obtained at the time of amniocentesis. The clinical outcome was obtained by chart review or by contacting the referring physician. Exclusion criteria were patients who were lost to follow-up and did not have information about the outcome of pregnancy (n = 25), chromosomal abnormalities, and confirmed fetal anomalies (n = 26). These patients were excluded from the study because the purpose was to determine the relationship between amniotic fluid concentrations of cytokines and microbiologic studies and pregnancy outcome in patients without fetal congenital anomalies. A total of 796 patients with a sample of amniotic fluid were left for analysis. Of these patients, 647 (81.3%) had a sample of maternal plasma. The collection of samples as well as clinical data was approved by the Institutional Review Boards of the participating Institutions (Padova, Azienda Ospedaliera, Treviso Azienda Ospedaliera, Veneto Region, Italy). All patients provided written informed consent. The hospital has a Federal Wide Assurance.
Clinical definitions
Although preterm delivery is traditionally defined as birth before 37 weeks of gestation, we used two different gestational age points to define subgroups of preterm birth. The first was preterm birth at or before 32 weeks of gestation. This gestational age was selected because in these patients, bacterial intra-amniotic infection/inflammation is a frequent mechanism of disease, in which the amniotic fluid concentrations of chemokines (such as IL-8 and MCP-1) [25, 40], cytokines (e.g., IL-6, TNF-α, IL-1) [37, 50, 54 – 56, 152, 179], damage-associated molecular patterns (HMGB-1) [180], heat shock proteins [22], and antimicrobial peptides [17, 38, 39, 184], are elevated at the time of diagnosis of preterm labor. Intra-amniotic infection and acute chorioamnionitis is less frequent in patients who deliver preterm after 32 weeks of gestation [87]. The second subgroup of preterm birth was preterm delivery after 32 weeks of gestation. The rationale for this definition is that after 32 weeks of gestation, chronic chorioamnionitis and vascular lesions emerge as frequent placental lesions. The mechanism of disease responsible for chronic chorioamnionitis is thought to be maternal antifetal rejection. There is also evidence for antibody-mediated fetal rejection; maternal antibodies against paternal human leukocyte antigens (HLA) have been demonstrated in patients with chronic chorioamnionitis [93, 99, 100, 127]. The control group consisted of uncomplicated term deliveries.
Biological samples and analysis
Amniotic fluid was obtained by transabdominal amniocentesis and 4 to 5 mL was collected for research purposes. Amniotic fluid samples were centrifuged at 1300 × g for 10 min and stored at −80 ° C until use. The amniotic fluid underwent Gram stain examination [149], amniotic fluid white blood cell count [161], amniotic fluid glucose [157], culture for aerobic and anaerobic bacteria as well as Mycoplasma species. Plasma was collected immediately in ethylenediaminetetraacetic acid (EDTA) evacuated tubes, centrifuged in a refrigerated centrifuge (2500 g) and stored at −80 ° C until use. Concentrations of IL-6 and IP-10 in amniotic fluid and maternal plasma were measured by a specific immunoassay according to the manufacturers’ instructions (R&D Systems, Minneapolis, MN, USA). For amniotic fluid, the sensitivity of the assay was 2 pg/mL and 4.9 pg/mL; intra-assay coefficient of variation was 4.2% and 2.3%; inter-assay coefficient of variation was 5.2% and 3.9%, respectively. For maternal plasma, the sensitivity of the assay was 0.1 pg/mL and 4.9 pg/mL; intra-assay coefficient of variation was 1.2% and 3.7%; inter-assay coefficient of variation was 3.7% and 3.8%, respectively.
Statistical analysis
Data distribution was tested with Kolmogorov-Smirnov and Shapiro-Wilk tests. Comparisons in the median concentrations of IL-6 and IP-10 among and between groups were tested with Kruskal-Wallis analysis of variance followed by post-hoc Mann-Whitney U-test (adjusted for multiple comparisons with the Bonferroni correction). Comparisons between proportions were performed using contingency table, χ2 or Fisher’s exact tests. Receiver operating characteristic (ROC) curve analysis was employed to determine optimal amniotic fluid concentrations of IL-6 and IP-10 that identified patients who subsequently had an early (≤ 32 weeks) or late (32 weeks) spontaneous preterm birth, respectively. Logistic regression was used to examine the association between elevated concentrations of amniotic fluid IL-6 or IP-10 and spontaneous preterm delivery, adjusting for history of spontaneous preterm delivery (yes/no), parity (nulliparous vs. multiparous), gestational age at amniocentesis (weeks) and duration of sample storage (years). A P-value of < 0.05 was considered significant. The analysis was performed with SPSS, version 15 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics of the study population
Table 1 reports the clinical and obstetrical characteristics of the study population. The prevalence of preterm delivery was 8.3% (66/796), while that of early and late spontaneous preterm births were 1.5% (n = 12) and 4.5% (n = 36), respectively. The remaining preterm deliveries were indicated preterm births (2.3%; n = 18): placenta previa (n = 4), preeclampsia (n = 4), gestational hypertension (n = 3), hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome (n = 2), preeclampsia with SGA (n = 1), gestational diabetes (n = 1), placental abruption (n = 1), Rh isoimmunization (n = 1) and a psychiatric disorder (n = 1). The median gestational age at delivery was 23.2, 36.0 and 35.5 weeks for early spontaneous, late spontaneous and indicated preterm birth, respectively.
Table 1.
Clinical characteristics of the study population (n=796)
| Term deliveries | Early SPTD ≤32 weeks (n=12) | Late SPTD >32 weeks (n=36) | Indicated preterm delivery (n=18) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 36 (35–38) | 38(33–39) | 36 (34–38) | 38 (35–40) | 0.2 |
| Ethnicity | |||||
| Caucasian | 719 (98.5%) | 10 (83.3%) | 35 (97.2%) | 18 (100%) | 0.004 |
| African American | 4 (0.5%) | 1 (8.3%) | 1 (2.8%) | - | |
| Asian | 3 (0.4%) | - | - | - | |
| Others | 4 (0.5%) | 1 (8.3%) | - | - | |
| Nulliparous | 285 (39.0%) | 6 (50%) | 20 (55.6%) | 8 (44.4%) | 0.2 |
| Previous SPTD (among multiparous) | 21/445 (4.7%) | 2/6 (33.3%) | 4/16 (25.0%) | 0/10 | <0.001 |
| GA at amniocentesis (weeks) | 16.3 (15.9–16.9) | 16.5 (15.7–16.9) | 16.3 (15.9–17.1) | 16.1 (15.7–16.8) | 0.9 |
| AF WBC count (cells/mL) | 3 (2–6) | 3 (1–10) | 5 (3–9) | 5 (3–8) | 0.002 |
| AF glucose (mg/dL) | 48 (43–52) | 40 (36–46) | 48 (39–51) | 45 (41–50) | 0.05 |
| Amniocentesis-to-delivery interval (weeks) | 23.2 (22–24) | 5.7 (1.3–13.1) | 19 (17–20) | 17.9 (14.5–19.8) | <0.001 |
| GA at delivery (weeks) | 39.7 (38.9–40.7) | 23.2 (18.1–28.9) | 36.0 (34.5–36.3) | 35.5 (34.5–36.3) | <0.001 |
| Birth weight (g) | 3380 (3120–3620) | 1035 (493–1341) | 2577 (2300–2800) | 2170 (1275–2582) | <0.001 |
Values expressed as median (inter-quartile) or number (percent).
SPTD = spontaneous preterm delivery, GA = gestational age, AF = amniotic fluid.
P = Kruskal-Wallis or Pearson χ2.
There was no significant difference in the median maternal age or gestational age at amniocentesis among groups (Table 1). As expected, patients who subsequently had either early or late spontaneous preterm delivery more frequently had a history of spontaneous preterm birth than those who had a term delivery or indicated preterm birth (P < 0.001). The median duration of sample storage ranged from 3.3 to 3.7 years and there was no significant difference among groups (P = 0.7).
The indications for amniocentesis were: 1) advanced maternal age (n = 475; 59.7%); 2) abnormal serum screening (n = 230; 28.9%); 3) suspected congenital anomaly (n = 47; 5.9%); 4) family history (n = 14; 1.8%); 5) suspected viral infection (n = 11; 1.4%); 6) maternal request (n = 11; 1.4%); and 7) other (n = 8; 1%).
Amniotic fluid Gram stain, white blood cell count, glucose, and culture results
The amniotic fluid Gram stain was negative in all cases; however, two patients had a positive amniotic fluid culture for bacteria (the organisms isolated were Pseudomonas aeruginosa and Staphylococcus auricularis). In both cases, the indication for performing amniocentesis was advanced maternal age (> 35 years) and the amniotic fluid concentrations of glucose, IL-6 and IP-10 were within normal range. Both patients delivered a term neonate with a normal karyotype and appropriate weight for gestational age [41]. Newborns had no evidence of infection at discharge.
Amniotic fluid IL-6 and IP-10
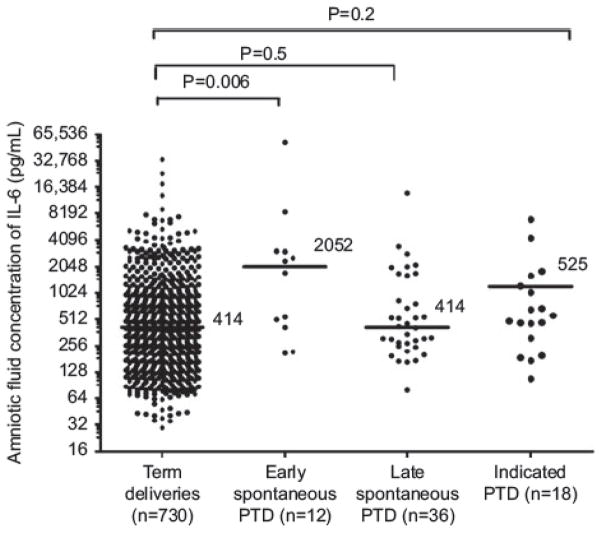
The median amniotic fluid IL-6 concentration was significantly higher in patients who had a preterm delivery ≤ 32 weeks than in those who had a term delivery (median 2052 pg/mL, IQR 435 – 3015 pg/mL vs. median 414 pg/mL, IQR 209 – 930 pg/mL; P = 0.006). In contrast, the median amniotic fluid IL-6 concentration in patients who had a preterm delivery >32 weeks was not significantly different from that of the patients who delivered at term (median 414, IQR 259 – 2348 pg/mL vs. median 414 pg/mL, IQR 209 – 930 pg/mL; P = 0.5) (Table 2, Figure 1).
Table 2.
Amniotic fluid and maternal blood IL-6 and IP-10 concentrations by types of preterm delivery.
| Term deliveries (n=730) | Early SPTD ≤32 weeks (n=12) | Late SPTD >32 weeks (n=36) | Indicated preterm delivery (n=18) | P-value | |
|---|---|---|---|---|---|
| AF interleukin-6 (pg/mL) | 414 (209–930) | 2052.5 (435–3015) | 414.9 (259–2348) | 525.1 (283–1316) | 0.02 |
| AF interferon-gamma-inducible protein-10 (pg/mL) | 583 (403–954) | 823.6 (342–2253) | 713.7 (509–1427) | 591.5 (414–911) | 0.04 |
| Duration of sample storage (years) | 3.47 (3.0–3.8) | 3.76 (2.5–4.3) | 3.36 (2.4–3.9) | 3.46 (2.6–3.8) | 0.7 |
| MB interleukin-6 (pg/mL) | 0.71 (0.55–0.93) | 0.89 (0.79–1.04) | 0.72 (0.54–0.95) | 0.86 (0.67–1.46) | 0.04 |
| MB interferon-gamma-inducible protein-10 (pg/mL) | 86.6 (68.0–113.1) | 90.8 (72.9–126.8) | 78.4 (64.9–104.3) | 79.5 (52.4–101.7) | 0.4 |
P = Kruskal-Wallis test, AF = amniotic fluid, MB = maternal blood.
AF IL-6: early spontaneous preterm delivery (SPTD) vs. uncomplicated term deliveries: P = 0.006; late SPTD vs. uncomplicated term deliveries: P = 0.5; indicated preterm deliveries vs. uncomplicated term deliveries: P = 0.2.
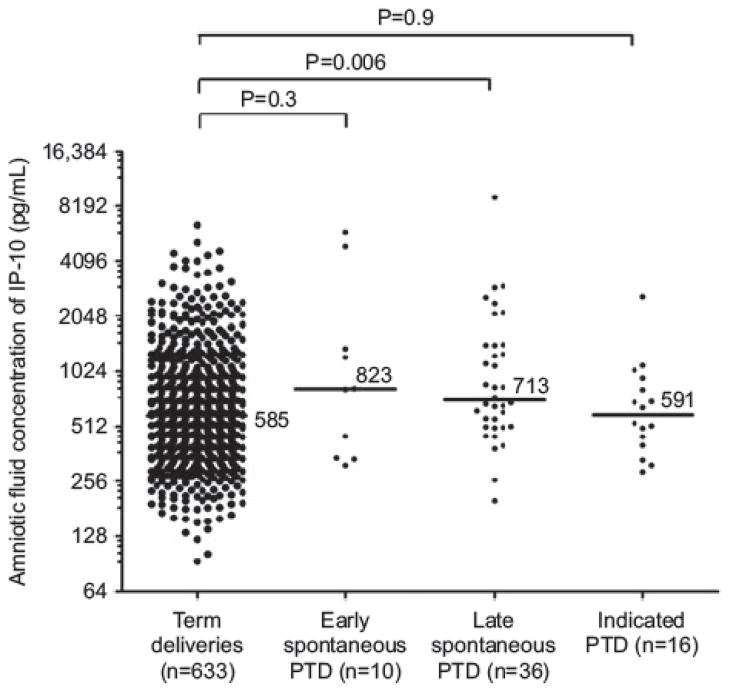
AF IP-10: early SPTD vs. uncomplicated term deliveries: P = 0.3; late SPTD vs. uncomplicated term deliveries: P = 0.006; indicated preterm deliveries vs. uncomplicated term deliveries: P = 0.9.
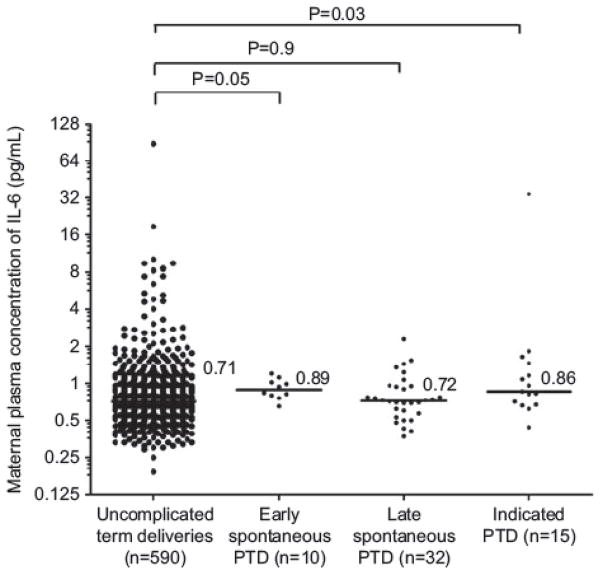
MB IL-6: early SPTD vs. uncomplicated term deliveries: P = 0.05; late SPTD vs. uncomplicated term deliveries: P = 0.9; indicated preterm deliveries vs. uncomplicated term deliveries: P = 0.03 (P = 0.09 after adjusting for multiple comparisons).
MB IP-10: early SPTD vs. uncomplicated term deliveries: P = 0.5; late SPTD vs. uncomplicated term deliveries: P = 0.3; indicated preterm deliveries vs. uncomplicated term deliveries: P = 0.3.
Figure 1.
Median amniotic fluid IL-6 concentration in the controls, spontaneous preterm delivery ≤ 32 weeks, spontaneous preterm delivery > 32 weeks and indicated preterm group (preterm ≤ 32 weeks vs. controls P = 0.006, preterm > 32 weeks vs. controls P = 0.5).
The median amniotic fluid IP-10 concentration was significantly higher in patients who had a preterm delivery > 32 weeks than in those who had a term delivery (median 713.7, IQR 509 – 1427 pg/mL vs. median 583, IQR 403 – 954 pg/mL, P = 0.006). In contrast, the median amniotic fluid IP-10 concentration was not significantly different in patients with a preterm delivery ≤ 32 weeks of gestation from that of patients who delivered at term (median 823, IQR 342 – 2253 pg/mL vs. median 583, IQR 403 – 954 pg/mL, P = 0.3) (Table 2, Figure 2).
Figure 2.
Median amniotic fluid IP-10 concentration in the controls, spontaneous preterm deliveries ≤ 32 weeks, spontaneous preterm deliveries > 32 weeks and indicated preterm group (preterm group > 32 weeks vs. controls P = 0.006, preterm group ≤ 32 weeks vs. controls P = 0.3).
ROC curve analysis indicated that an amniotic fluid concentration of IL-6 ≥ 1740 pg/mL had an area under the curve of 0.73 (95% CI 0.6 – 0.9; P = 0.007), a sensitivity of 58% (7/12), a specificity of 88% (688/784), a positive predictive value of 7% (7/103), and a negative predictive value of 99% (688/693) for the identification of patients who subsequently delivered ≤ 32 weeks of gestation. The corresponding indices for amniotic fluid concentration of IP-10 > 502 pg/mL were 0.64 (95% CI 0.5 – 0.7; P = 0.007), 83% (30/36), 40% (295/734), 6% (30/469) and 98% (295/301), respectively, for the identification of patients who subsequently delivered > 32 weeks of gestation.
An elevation of amniotic fluid IL-6 concentration above 1740 pg/mL was associated with early (≤ 32 weeks) spontaneous preterm delivery with an odds ratio of 9.5 (95% CI 2.9 – 31.1) after adjusting for history of spontaneous preterm delivery, parity, gestational age at amniocentesis and duration of sample storage. Similarly, an elevation of amniotic fluid IP-10 concentration above 502 pg/mL was associated with late (> 32 weeks) spontaneous preterm delivery with an odds ratio of 3.9 (95% CI 1.6 – 9.9) after adjusting for similar potential confounders.
There was no significant difference in the median amniotic fluid concentration of either IL-6 or IP-10 between patients who delivered because of maternal or fetal indications and women who delivered at term [IL-6; unadjusted P = 0.03, adjusted P = 0.09 (Bonferroni correction) and IP-10; P = 0.9].
When the analysis in the control group was restricted to women who delivered at term (n = 652 and n = 633 for IL-6 and IP-10, respectively) without obstetrical complications (i.e., abruption, SGA, fetal death, gestational hypertension and preeclampsia), the 95th percentile cut-off for amniotic fluid IL-6 and IP-10 were 2935 pg/mL and 2200 pg/mL, respectively. There were 6.3% (50/796) and 5.8% (45/770) of patients who had amniotic fluid IL-6 and IP-10 above these cut-offs.
Among patients (n = 50) who had amniotic fluid IL-6 above this extremely high concentration, only 8% (n = 4) delivered before 32 weeks of gestation and the majority delivered at term (84%; 42/50). Similarly, among patients who had amniotic fluid IP-10 above this extremely high concentration (n = 45), only 11% (n = 5) delivered after 32 weeks of gestation and the majority delivered at term (82%; 37/45). Among the patients who delivered at term, women with extremely high IL-6 concentrations had a significantly higher rate of pregnancy complications (gestational hypertension, preeclampsia, SGA, placental abruption and placental previa) than those with lower IL-6 concentrations [extremely high IL-6 23.8% (10/42) vs. normal/low IL-6 9.9% (68/688), P = 0.009]. In contrast, this difference was not significant in women with extremely high IP-10 who delivered at term [extremely high IP – 10 13.2% (6/37) vs. normal/low IP-10 10.3% (69/671), P = 0.27].
Maternal plasma IL-6 and IP-10
Neither the maternal plasma IL-6 or IP-10 concentrations in the midtrimester were associated with the occurrence of spontaneous preterm delivery ≤32 weeks or > 32 weeks of gestation. There was no association between maternal plasma IL-6 or IP-10 concentrations and the occurrence of an indicated preterm birth (Table 2, Figures 3 and 4).
Figure 3.
Median maternal plasma IL-6 concentration in the controls, spontaneous delivery ≤ 32 weeks, spontaneous preterm delivery > 32 weeks and indicated preterm group (preterm group ≤ 32 weeks vs. controls P = 0.05, preterm group > 32 weeks vs. controls P = 0.9).
Figure 4.
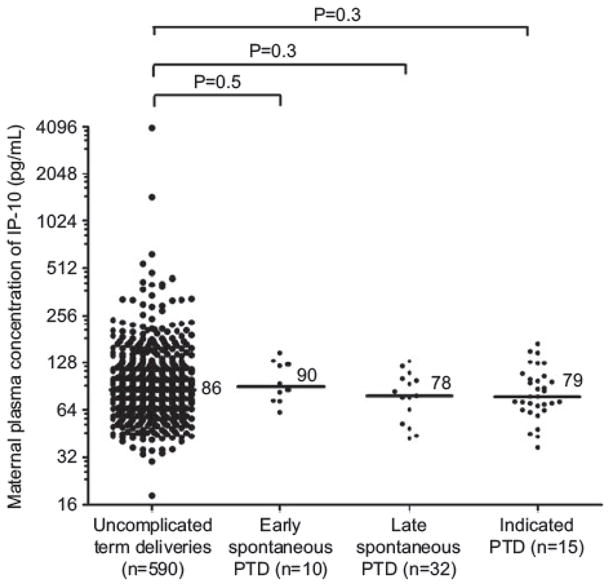
Median maternal plasma IP-10 concentration in the controls (uncomplicated term deliveries), spontaneous preterm deliveries ≤ 32 weeks, spontaneous preterm deliveries > 32 weeks and indicated preterm group (preterm group > 32 weeks vs. controls P = 0.3, preterm group ≤ 32 weeks vs. controls P = 0.5).
DISCUSSION
Principal findings of the study
Among patients who underwent a mid-trimester amniocentesis for clinical indications, the following observations were made: 1) patients who subsequently had a spontaneous preterm delivery at or before 32 weeks had a higher median amniotic fluid IL-6 concentration than those who delivered at term or > 32 weeks of gestation; 2) patients who had a spontaneous preterm delivery > 32 weeks had a higher median amniotic fluid IP-10 than those who delivered at term; 3) there were no significant differences in median midtrimester amniotic fluid concentrations of IL-6 and IP-10 between women who had an indicated preterm birth and those who delivered at term; and 4) standard cultivation techniques for aerobic and anaerobic bacteria as well as Mycoplasma species yielded negative results for the majority (99.8%) of patients. A positive amniotic fluid culture was found in two cases (2/796; 0.2%). However, these patients had a term delivery and they had no evidence of intra-amniotic inflammation (by amniotic fluid white blood cell count, glucose concentration, or the amniotic fluid levels of IL-6 or IP-10). Therefore, these cases are likely to represent contamination of the specimens.
Amniotic fluid IP-10 and preterm delivery after 32 weeks of gestation
This study is the first to demonstrate an association between amniotic fluid IP-10 concentrations and the risk for spontaneous preterm delivery after 32 weeks of gestation. Chemokines of the CXC family share the expression of their specific receptors on both leukocytes and endothelial cells [181], and have a role in the control of inflammation and angiogenesis [12, 14, 145, 181]. IP-10 has been implicated in preeclampsia because of its proinflammatory and anti-angiogenic properties. Indeed, Gotsch et al. [62] reported a higher median maternal plasma IP-10 concentration in patients with preeclampsia than in controls.
ROC curve analysis indicated that an amniotic fluid concentration of IP-10 > 502 pg/mL in the mid-trimester had a sensitivity of 83%, a specificity of 40%, a positive predictive value of 6% (7/103), and a negative predictive value of 98% for the identification of patients who subsequently delivered after 32 weeks of gestation. IP-10 has been implicated in the mechanism responsible for organ rejection [130, 138, 185, 195]. We have previously demonstrated an association between an elevation of IP-10 in the amniotic fluid, maternal blood, and umbilical cord blood and the diagnosis of chronic chorioamnionitis and villitis of unknown etiology in patients with preterm labor, and in particular, late preterm labor [86]. We have proposed that a fraction of late preterm labor is a result of maternal-fetal rejection [86, 87], and that IP-10 elevations in amniotic fluid act as a T-cell chemotactic cytokine responsible for the recruitment of T-cells from the maternal decidua into the chorioamniotic membranes.
When intra-amniotic inflammation was defined as an amniotic fluid concentration of IP-10 above 2200 pg/mL (which corresponds to the 95th percentile of patients who delivered at term without any complications), the prevalence of intra-amniotic inflammation among patients who underwent midtrimester amniocentesis was 5.8% (45/770).
In this study, placental pathology was not available in the majority of cases, because ours is a referral center for prenatal diagnosis, so patients often delivered at other institutions. It would be important to determine if there is an association between an elevation of amniotic fluid IP-10 in the midtrimester and the subsequent diagnosis of chronic chorioamnionitis and/or villitis of unknown etiology.
Indicated preterm deliveries
A subset of patients in our study was delivered preterm due to maternal or fetal indications. There were no significant differences in the median midtrimester amniotic fluid and maternal plasma concentrations of IL-6 and IP-10 between women who had an indicated preterm delivery and those who delivered at term. Therefore, biomarkers used in this study do not seem to be associated with the subsequent development of indicated preterm delivery, which is largely due to SGA and preeclampsia.
Amniotic fluid IL-6 and pregnancy outcomes
We reported IL-6 in amniotic fluid in patients with preterm labor and subsequently in preterm PROM, and demonstrated an association with microbiologically proven intra-amniotic infection using cultivation techniques [9, 29, 79, 82, 136, 156, 167, 170, 171, 177, 178, 200, 201]. However, an observation that has been consistent across studies was that a subgroup of patients with a high concentration of IL-6 in amniotic fluid but negative amniotic fluid culture for bacteria, are at high risk for adverse pregnancy outcome including preterm delivery, a short amniocentesis-to-delivery interval, and neonatal complications [3, 37, 96, 158, 191, 193, 210]. We reported that among patients undergoing midtrimester amniocentesis, those who lost their pregnancy had a higher median amniotic fluid concentration of IL-6 than of those patients who delivered at term [173].
Subsequently, a large number of studies have confirmed these observations. Indeed, amniotic fluid IL-6 is widely considered a sensitive and specific parameter for the detection of intra-amniotic infection [9, 29, 79, 82, 136, 156, 167, 170, 171, 177, 178, 200, 201]. Consequently, an elevated IL-6 has become synonymous with the presence of intra-amniotic inflammation [9, 29, 79, 82, 136, 156, 167, 170, 171, 177, 178, 200, 201, 210].
Most previous studies examining the relationship between amniotic fluid IL-6 and pregnancy outcome have been case control studies. Such studies have been largely consistent in demonstrating a higher median concentration of IL-6 in patients who delivered preterm [9, 29, 79, 82, 136, 156, 167, 170, 171, 177, 178, 200, 201]; however, some investigators have not found this association [7]. The advantages of a cohort study over case-control studies are well known.
The main finding of this study was that, among patients who had a preterm delivery (< 37 weeks of gestation), only those with early preterm birth (≤ 32 weeks of gestation) had a significantly higher median midtrimester IL-6 amniotic fluid concentration than women delivering at term. The cut-off for analysis ≤ 32 weeks and between 32 (1/7) and 36 (6/7) weeks of gestation were selected pre-hoc. We did so because of previous observations that patients with an elevated amniotic fluid IL-6 tended to have spontaneous rupture of membranes, spontaneous abortion after a midtrimester amniocentesis, or preterm delivery shortly after the procedure or early preterm deliveries (defined as ≤ 32 weeks of gestation) [191, 193]. We also made a similar observation using other biological markers of intra-amniotic inflammation, such as matrix metalloproteinase (MMP)-8 [209], and chemokines, such as monocyte chemotactic protein (MCP)-1 [40]. Therefore, we reasoned that an intra-amniotic inflammatory process characterized by an elevation of amniotic fluid IL-6 would be associated with early preterm delivery, but not necessarily with a late preterm delivery. Our data suggest that this is indeed the case. The most frequent cause of intra-amniotic inflammation is microbial invasion of the amniotic cavity with genital Mycoplasmas [61, 147, 154, 159, 206, 210]. However, in this cohort study that included 847 patients, most amniotic fluid specimens were sterile using cultivation techniques. Therefore, the cause of intra-amniotic inflammation has not been identified. It is possible that a fraction of these patients had subclinical bacterial infections which escaped detection with cultivation techniques and which may have been identified using molecular microbiologic techniques. Future studies are necessary to address this question. Another possibility is that the inflammatory process is caused by viruses [5, 24, 92]. Such microorganisms have been found in the amniotic cavity in other studies [8, 118, 142, 189, 192]. It is also possible that the inflammatory process does not have an infectious origin. Indeed, inflammation is a non-specific host response to tissue injury, and can be induced under sterile conditions. Recently, we have reported the presence of damage-associated molecular patterns (DAMPs) in amniotic fluid [180]. These molecules can recognize “danger signals” that may lead to inflammation by mechanisms which involve a different set of pattern recognition receptors from those involved in the detection of microorganisms, although there may be some overlap [91, 137]. For example, some Toll-like receptors can recognize endogenous ligands released during tissue damage [1, 83, 84].
This is the first study to allow a rigorous calculation of the diagnostic indices and predictive values of elevated amniotic fluid IL-6, because previous studies were of a case-control nature, and therefore, have limitations in the generation of positive and negative predictive values. ROC curve analysis indicated that an amniotic fluid concentration of IL-6 ≥ 1740 pg/mL. This cutoff had a sensitivity of 58%, a specificity of 88%, a positive predictive value of 7% (7/103), and a negative predictive value of 99% for the identification of patients who subsequently delivered ≤ 32 weeks of gestation.
When intra-amniotic inflammation was defined as an amniotic fluid concentration of IL-6 above 2935 pg/mL (which corresponds to the 95th percentile of patients who delivered at term without any complications), the prevalence of intraamniotic inflammation was 6% (50/796) among patients who underwent mid-trimester amniocentesis. These observations are noteworthy for several reasons. First, they indicate that an inflammatory process defined as an elevation of amniotic fluid IL-6 in the midtrimester would only account for approximately half of all preterm deliveries before 32 weeks of gestation. Second, our findings indicate that many patients have an elevation of amniotic fluid IL-6 in the midtrimester, and this is not followed by early spontaneous preterm delivery or even preterm delivery (< 37 weeks). Most patients with an elevated amniotic fluid IL-6 had an uncomplicated delivery at term. It is possible that a combination of cytokines, chemokines, and growth factors may identify patients at risk for the subsequent development of different obstetrical syndromes (e.g., preterm labor, preterm prelabor rupture of membrane, fetal death, SGA, preeclampsia, etc.) [146].
Maternal plasma concentrations of IP-10 and IL-6
Of interest, the median maternal plasma IP-10 concentration was not significantly different between patients who delivered preterm and those who delivered at term. This indicates that the inflammatory process is confined to the amniotic cavity and cannot be detected by measuring IP-10 in the maternal circulation. This was also the case for IL-6.
Clinical significance of the observations
This study confirms that there are at least two distinct types of intra-amniotic inflammatory processes in the midtrimester of pregnancy; one that is characterized by an elevation of amniotic fluid IL-6 and a second one, by elevations of the T-cell chemokine, IP-10. The former is a risk factor for early preterm delivery (< 32 weeks), while the latter is a risk factor for late preterm delivery (defined for the purposes of this study as > 32 weeks). Of interest is that we have identified a subgroup of patients that have either form of intra-amniotic inflammation but have an apparently normal pregnancy outcome. The significance, etiology, natural history, and long-term consequences of these inflammatory processes remain to be established. Moreover, we have defined these two types of inflammation by using one cytokine (IL-6) and one chemokine (IP-10). These two types of intra-amniotic inflammation may represent two extreme phenotypes, and some patients have an elevation (above the 95th percentile for both) of both IL-6 and IP-10, which may constitute the evolution of one type of inflammation into a different type. It is noteworthy that the cytokine and chemokine network in the amniotic cavity is complex, and we anticipate that there would be changes in the profile of other cytokines and chemokines, which have not been studied yet. Such studies are needed to characterize not only the intra-amniotic inflammatory response, but also what such profile will tell us about the consequences of the inflammatory process on the fetus and pregnancy.
Strengths and limitations of the study
The major strength of this study is the cohort design and the ascertainment of a relatively large number of patients. Also, the definition of preterm delivery at < 32 weeks of gestation and after 32 weeks of gestation was decided pre-hoc, based upon previous studies [87, 147, 150, 154]. Future studies are required to examine whether microbial invasion of the amniotic cavity can be detected in cases with the different types of inflammation using molecular techniques, such as broad range PCR and specific primers. It would also be interesting to determine if viral invasion of the amniotic cavity can account for some cases of intra-amniotic inflammation and if so, what type.
Another limitation is that placental pathology was not available for examination. Unfortunately the majority of the study population did not deliver in the same institution where the amniotic fluid had been collected and, as a consequence, it was not possible to study an adequate number of placentas for a correlation with acute and chronic chorioamnionitis.
CONCLUSION
This is the first cohort study to report the relationship between midtrimester amniotic fluid IP-10 and IL-6 concentrations and pregnancy outcome. We report an association between elevated amniotic fluid IP-10 concentrations and the subsequent development of spontaneous preterm delivery after 32 weeks of gestation. We also provide evidence for an association between an elevated amniotic fluid IL-6 concentration and spontaneous preterm delivery before 32 weeks of gestation. Importantly, we have identified that a fraction of patients who undergo a midtrimester amniocentesis have a subclinical inflammatory process, defined either as an elevated IL-6 or IP-10. Further studies are required to determine the short- and long-term clinical significance for mother and infant of such subclinical processes.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- 1.Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, et al. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180:6035– 43. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alanen A. Polymerase chain reaction in the detection of microbes in amniotic fluid. Ann Med. 1998;30:288– 95. doi: 10.3109/07853899809005857. [DOI] [PubMed] [Google Scholar]
- 3.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606– 12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 4.Apuzzio J, Chan Y, Al-Khan A, Illsley N, Kim PL, Vonhaggen S. Second-trimester amniotic fluid interleukin-10 concentration predicts preterm delivery. J Matern Fetal Neonatal Med. 2004;15:313– 7. doi: 10.1080/14767050410001702186. [DOI] [PubMed] [Google Scholar]
- 5.Arechavaleta-Velasco F, Koi H, Strauss JF, III, Parry S. Viral infection of the trophoblast: time to take a serious look at its role in abnormal implantation and placentation? J Reprod Immunol. 2002;55:113– 21. doi: 10.1016/s0165-0378(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 6.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3:121– 6. doi: 10.1177/107155769600300304. [DOI] [PubMed] [Google Scholar]
- 7.Bamberg C, Fotopoulou C, Thiem D, Roehr CC, Dudenhausen JW, Kalache KD. Correlation of midtrimester amniotic fluid cytokine concentrations with adverse pregnancy outcome in terms of spontaneous Abortion, preterm birth, and preeclampsia. J Matern Fetal Neonatal Med. 2011 Jul 5; doi: 10.3109/14767058.2011.587918. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Baschat AA, Towbin J, Bowles NE, Harman CR, Weiner CP. Prevalence of viral DNA in amniotic fluid of low-risk pregnancies in the second trimester. J Matern Fetal Neonatal Med. 2003;13:381– 4. doi: 10.1080/jmf.13.6.381.384. [DOI] [PubMed] [Google Scholar]
- 9.Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intraamniotic infection with Ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. 1999;78:379– 82. [PubMed] [Google Scholar]
- 10.Baud O, Emilie D, Pelletier E, Lacaze-Masmonteil T, Zupan V, Fernandez H, et al. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol. 1999;106:72– 7. doi: 10.1111/j.1471-0528.1999.tb08088.x. [DOI] [PubMed] [Google Scholar]
- 11.Baumann P, Romero R, Berry S, Gomez R, McFarlin B, Araneda H, et al. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am J Reprod Immunol. 1993;30:184– 93. doi: 10.1111/j.1600-0897.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 12.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1– 8. [PubMed] [Google Scholar]
- 13.Berg TG, Philpot KL, Welsh MS, Sanger WG, Smith CV. Ureaplasma/Mycoplasma-infected amniotic fluid: pregnancy outcome in treated and nontreated patients. J Perinatol. 1999;19:275– 7. doi: 10.1038/sj.jp.7200185. [DOI] [PubMed] [Google Scholar]
- 14.Bernardini G, Ribatti D, Spinetti G, Morbidelli L, Ziche M, Santoni A, et al. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83– 101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 15.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140:947– 52. doi: 10.1016/0002-9378(81)90090-9. [DOI] [PubMed] [Google Scholar]
- 16.Brocklehurst P. Infection and preterm delivery. Br Med J. 1999;318:548– 9. doi: 10.1136/bmj.318.7183.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buhimschi I, Christner R, Buhimschi C, Chaiworapongsa T, Romero R. Proteomic analysis of preterm parturition: a novel method of identifying the patient at risk for preterm delivery. Am J Obstet Gynecol. 2002;187:S55. [Google Scholar]
- 18.Carroll SG, Philpott-Howard J, Nicolaides KH. Amniotic fluid gram stain and leukocyte count in the prediction of intrauterine infection in preterm prelabour amniorrhexis. Fetal Diagn Ther. 1996;11:1– 5. doi: 10.1159/000264270. [DOI] [PubMed] [Google Scholar]
- 19.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16 – 20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis. 1983;10:294– 302. [PubMed] [Google Scholar]
- 20.Chaiworapongsa T, Romero R, Tolosa JE, Yoshimatsu J, Espinoza J, Kim YM, et al. Elevated monocyte chemotactic protein-1 in amniotic fluid is a risk factor for pregnancy loss. J Matern Fetal Neonatal Med. 2002;12:159– 64. doi: 10.1080/jmf.12.3.159.164. [DOI] [PubMed] [Google Scholar]
- 21.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405– 16. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008;21:449– 61. doi: 10.1080/14767050802054550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaiworapongsa T, Romero R, Berry SM, Hassan SS, Yoon BH, Edwin S, et al. The role of granulocyte colony-stimulating factor in the neutrophilia observed in the fetal inflamatory response syndrome. J Perinat Med. 2011;39:653– 66. doi: 10.1515/JPM.2011.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan G, Hemmings DG, Yurochko AD, Guilbert LJ. Human cytomegalovirus-caused damage to placental trophoblasts mediated by immediate-early gene-induced tumor necrosis factor-alpha. Am J Pathol. 2002;161:1371– 81. doi: 10.1016/s0002-9440(10)64413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299– 303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 26.Cherouny PH, Pankuch GA, Botti JJ. Occult intraamniotic infection at the time of midtrimester genetic amniocentesis: a reassessment. Infect Dis Obstet Gynecol. 1994;2:136– 9. doi: 10.1155/S1064744994000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow SS, Craig ME, Jones CA, Hall B, Catteau J, Lloyd AR, et al. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine. 2008;44:78– 84. doi: 10.1016/j.cyto.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Coultrip LL, Grossman JH. Evaluation of rapid diagnostic tests in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1992;167:1231– 42. doi: 10.1016/s0002-9378(11)91694-9. [DOI] [PubMed] [Google Scholar]
- 29.Coultrip LL, Lien JM, Gomez R, Kapernick P, Khoury A, Grossman JH. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1994;171:901– 11. doi: 10.1016/s0002-9378(94)70057-5. [DOI] [PubMed] [Google Scholar]
- 30.Daskalakis G, Thomakos N, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-18 at mid – trimester genetic amniocentesis: relationship to intraamniotic microbial invasion and preterm delivery. Br J Obstet Gynaecol. 2009;116:1743– 8. doi: 10.1111/j.1471-0528.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 31.DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 2012;17:2– 11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 32.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiGiulio DB, Gervasi MT, Romero R, Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med. 2010;38:495– 502. doi: 10.1515/JPM.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiGiulio DB, Gervasi M, Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med. 2010;38:503– 13. doi: 10.1515/JPM.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38– 57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debieve F, Ska S, Williams O, Hutchings G, Bernard P, Grandjean P, et al. Evaluation of a universal real-time polymerase chain reaction for detection of amniotic fluid infection in premature rupture of membranes. Am J Perinatol. 2011;28:501– 8. doi: 10.1055/s-0030-1271212. [DOI] [PubMed] [Google Scholar]
- 37.El-Bastawissi AY, Williams MA, Riley DE, Hitti J, Krieger JN. Amniotic fluid interleukin-6 and preterm delivery: a review. Obstet Gynecol. 2000;95:1056– 64. [PubMed] [Google Scholar]
- 38.Erez O, Romero R, Tarca AL, Chaiworapongsa T, Kim YM, Than NG, et al. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2009;22:1103– 15. doi: 10.3109/14767050902994796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2– 21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 40.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365– 73. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 41.Fenton TR. A new growth chart for perterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;16:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez H, Montuclard B, Guibert M. Does intraamniotic infection in the early phase of the second trimester really exist? Am J Obstet Gynecol. 1996;175:1077– 8. doi: 10.1016/s0002-9378(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 43.Foulon W, Van LD, Demanet C, Decatte L, Dewaele M, Naessens A. Markers of infection and their relationship to preterm delivery. Am J Perinatol. 1995;12:208– 11. doi: 10.1055/s-2007-994454. [DOI] [PubMed] [Google Scholar]
- 44.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culturenegative amniotic fluid from women in premature labor. Am J Perinatol. 2004;21:319– 23. doi: 10.1055/s-2004-831884. [DOI] [PubMed] [Google Scholar]
- 45.Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol. 1992;167:1092– 5. doi: 10.1016/s0002-9378(12)80044-5. [DOI] [PubMed] [Google Scholar]
- 46.Gauthier DW, Meyer WJ, Bieniarz A. Correlation of amniotic fluid glucose concentration and intraamniotic infection in patients with preterm labor or premature rupture of membranes. Am J Obstet Gynecol. 1991;165:1105– 10. doi: 10.1016/0002-9378(91)90480-f. [DOI] [PubMed] [Google Scholar]
- 47.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518– 21. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 48.Gersell DJ. Chronic villitis, chronic chorioamnionitis, and maternal floor infarction. Semin Diagn Pathol. 1993;10:251– 66. [PubMed] [Google Scholar]
- 49.Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol. 1991;10:217– 29. [PubMed] [Google Scholar]
- 50.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515– 28. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 51.Goldenberg RL, Andrews WW, Mercer BM, Moawad AH, Meis PJ, Iams JD, et al. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182:625– 30. doi: 10.1067/mob.2000.104210. [DOI] [PubMed] [Google Scholar]
- 52.Goldenberg R, Andrews W, Caritis S, Goepfert A, Ramsey PS, Rouse D, et al. Steering Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD) Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2008;199:e14– 15. doi: 10.1016/j.ajog.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein I, Zimmer EZ, Merzbach D, Peretz BA, Paldi E. Intraamniotic infection in the very early phase of the second trimester. Am J Obstet Gynecol. 1990;163:1261– 3. doi: 10.1016/0002-9378(90)90703-a. [DOI] [PubMed] [Google Scholar]
- 54.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and Gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32:200– 10. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 55.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol. 1995;22:281– 342. [PubMed] [Google Scholar]
- 56.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135– 76. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 57.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194– 202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 58.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31– 7. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 59.Gomez R, Romero R, Nien JK, Chaiworapongsa T, Medina L, Kim YM, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192:678– 89. doi: 10.1016/j.ajog.2004.10.624. [DOI] [PubMed] [Google Scholar]
- 60.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med. 2007;20:167– 73. doi: 10.1080/14767050601135485. [DOI] [PubMed] [Google Scholar]
- 61.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3– 13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 62.Gotsch F, Romero R, Friel L, Kusanovic JP, Espinoza J, Erez O, et al. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia ? J Matern Fetal Neonatal Med. 2007;20:777– 92. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529– 47. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660– 7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 65.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111– 7. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 66.Greig PC, Ernest JM, Teot L. Low amniotic fluid glucose levels are a specific but not a sensitive marker for subclinical intrauterine infections in patients in preterm labor with intact membranes. Am J Obstet Gynecol. 1994;171:365– 70. doi: 10.1016/s0002-9378(94)70036-2. [DOI] [PubMed] [Google Scholar]
- 67.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. Br J Obstet Gynaecol. 2005;112:16– 8. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 68.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36:217– 27. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38– 47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harwick HJ, Iuppa JB, Fekety FR., Jr Microorganisms and amniotic fluid. Obstet Gynecol. 1969;33:256– 9. [PubMed] [Google Scholar]
- 71.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13– 9. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941– 8. [PubMed] [Google Scholar]
- 73.Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228– 32. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 74.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med. 1995;40:375– 9. [PubMed] [Google Scholar]
- 75.Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H, Hagberg H. Monocyte chemotactic protein-1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra-amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189:1161– 7. doi: 10.1067/s0002-9378(03)00594-5. [DOI] [PubMed] [Google Scholar]
- 76.Jacobsson B, Aaltonen R, Rantakokko-Jalava K, Morken NH, Alanen A. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta Obstet Gynecol Scand. 2009;88:63– 70. doi: 10.1080/00016340802572646. [DOI] [PubMed] [Google Scholar]
- 77.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:1457– 61. doi: 10.1016/s0046-8177(98)90016-8. [DOI] [PubMed] [Google Scholar]
- 78.Jalava J, Mantymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103:664– 9. doi: 10.1111/j.1471-0528.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 79.Kasper DC, Mechtler TP, Reischer GH, Witt A, Langgartner M, Pollak A, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis. 2010;67:117– 21. doi: 10.1016/j.diagmicrobio.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 80.Kay ID, Palladino S, Alexander R, Leahy BJ, Pearman JW. Evaluation of a commercial polymerase chain reaction assay for the detection of Chlamydia trachomatis. Diagn Microbiol Infect Dis. 1997;28:75– 9. doi: 10.1016/s0732-8893(97)00003-5. [DOI] [PubMed] [Google Scholar]
- 81.Keeler SM, Kiefer DG, Rust OA, Vintzileos A, Atlas RO, Bornstein E, et al. Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome ? Am J Obstet Gynecol. 2009;201:276. doi: 10.1016/j.ajog.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 82.Kim M, Kim G, Romero R, Shim SS, Kim EC, Yoon BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31:146– 52. doi: 10.1515/JPM.2003.020. [DOI] [PubMed] [Google Scholar]
- 83.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346– 55. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 84.Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia ? Am J Obstet Gynecol. 2005;193:921– 7. doi: 10.1016/j.ajog.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 85.Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292– 5. doi: 10.1016/j.ajog.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 86.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919– 27. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000– 11. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim BJ, Romero R, Mi LS, Park CW, Shin PJ, Jun JK, et al. Clinical significance of oligohydramnios in patients with preterm labor and intact membranes. J Perinat Med. 2011;39:131– 6. doi: 10.1515/JPM.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim SM, Romero R, Lee J, Lee SM, Park C, Park JS, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed ? J Matern Fetal Neonatal Med. 2011 Oct 14; doi: 10.3109/14767058.2011.629256. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kirshon B, Rosenfeld B, Mari G, Belfort M. Amniotic fluid glucose and intraamniotic infection. Am J Obstet Gynecol. 1991;164:818– 20. doi: 10.1016/0002-9378(91)90522-s. [DOI] [PubMed] [Google Scholar]
- 91.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476– 84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koi H, Zhang J, Parry S. The mechanisms of placental viral infection. Ann N Y Acad Sci. 2001;943:148– 56. doi: 10.1111/j.1749-6632.2001.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 93.Kusanovic JP, Romero R, Jodicke C, Mazaki-Tovi S, Vaisbuch E, Erez O, et al. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2009;22:1151– 66. doi: 10.3109/14767050903019684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ledger WJ. Infection and premature labor. Am J Perinatol. 1989;6:234– 6. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 95.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294– 6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 96.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633– 8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 97.Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra – amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med. 2010;38:39– 44. doi: 10.1515/JPM.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee J, Romero R, Dong Z, Xu Y, Qureshi F, Jacques S, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59:928– 38. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510– 26. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti - human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Madan I, Romero R, Kusanovic JP, Mittal P, Chaiworapongsa T, Dong Z, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010;38:275– 9. doi: 10.1515/JPM.2010.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malamitsi-Puchner A, Vrachnis N, Samoli E, Baka S, Hassiakos D, Creatsas G. Elevated second trimester amniotic fluid interferon gamma-inducible T-cell alpha chemoattractant concentrations as a possible predictor of preterm birth. J Soc Gynecol Investig. 2006;13:25– 9. doi: 10.1016/j.jsgi.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Malamitsi-Puchner A, Vrachnis N, Samoli E, Baka S, Iliodromiti Z, Puchner KP, et al. Possible early prediction of preterm birth by determination of novel proinflammatory factors in midtrimester amniotic fluid. Ann N Y Acad Sci. 2006;1092:440– 9. doi: 10.1196/annals.1365.043. [DOI] [PubMed] [Google Scholar]
- 104.Mandar R, Livukene K, Ehrenberg A, Smidt I, Raukas E, Kask V, et al. Amniotic fluid microflora in asymptomatic women at mid-gestation. Scand J Infect Dis. 2001;33:60– 2. doi: 10.1080/003655401750064095. [DOI] [PubMed] [Google Scholar]
- 105.Marconi C, de Andrade Ramos BR, Peracoli JC, Donders GG, da Silva MG. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol. 2011;65:549– 56. doi: 10.1111/j.1600-0897.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 106.Markenson GR, Martin RK, Tillotson-Criss M, Foley KS, Stewart RS, Jr, Yancey M. The use of the polymerase chain reaction to detect bacteria in amniotic fluid in pregnancies complicated by preterm labor. Am J Obstet Gynecol. 1997;177:1471– 7. doi: 10.1016/s0002-9378(97)70093-0. [DOI] [PubMed] [Google Scholar]
- 107.Markenson GR, Adams LA, Hoffman DE, Reece MT. Prevalence of Mycoplasma bacteria in amniotic fluid at the time of genetic amniocentesis using the polymerase chain reaction. J Reprod Med. 2003;48:775– 9. [PubMed] [Google Scholar]
- 108.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142– 8. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 109.Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914– 20. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 110.Maymon E, Romero R, Pacora P, Gervasi MT, Edwin SS, Gomez R, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1545– 53. doi: 10.1067/mob.2000.107652. [DOI] [PubMed] [Google Scholar]
- 111.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000;183:887– 94. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 112.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94– 9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 113.Maymon E, Romero R, Chaiworapongsa T, Berman S, Conoscenti G, Gomez R, et al. Amniotic fluid matrix metalloproteinase-8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149– 55. doi: 10.1067/mob.2001.118165. [DOI] [PubMed] [Google Scholar]
- 114.Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143– 8. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 115.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308– 16. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 116.Mazor M, Chaim W, Horowitz S, Leiberman JR, Glezerman M. Successful treatment of preterm labour by eradication of Ureaplasma urealyticum with erythromycin. Arch Gynecol Obstet. 1993;253:215– 8. doi: 10.1007/BF02766648. [DOI] [PubMed] [Google Scholar]
- 117.McDuffie RS, Jr, Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol. 1992;167:1583– 8. doi: 10.1016/0002-9378(92)91745-v. [DOI] [PubMed] [Google Scholar]
- 118.McLean LK, Chehab FF, Goldberg JD. Detection of viral deoxyribonucleic acid in the amniotic fluid of low-risk pregnancies by polymerase chain reaction. Am J Obstet Gynecol. 1995;173:1282– 6. doi: 10.1016/0002-9378(95)91371-8. [DOI] [PubMed] [Google Scholar]
- 119.Minkoff H. Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62:137– 44. [PubMed] [Google Scholar]
- 120.Miralles R, Hodge R, McParland PC, Field DJ, Bell SC, Taylor DJ, et al. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res. 2005;57:570– 7. doi: 10.1203/01.PDR.0000155944.48195.97. [DOI] [PubMed] [Google Scholar]
- 121.Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;60:246– 57. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Molnar M, Romero R, Hertelendy F. Interleukin-1 and tumor necrosis factor stimulate arachidonic acid release and phospholipid metabolism in human myometrial cells. Am J Obstet Gynecol. 1993;169:825– 9. doi: 10.1016/0002-9378(93)90011-7. [DOI] [PubMed] [Google Scholar]
- 123.Moon JB, Kim JC, Yoon BH, Romero R, Kim G, Oh SY, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med. 2002;30:301– 6. doi: 10.1515/JPM.2002.044. [DOI] [PubMed] [Google Scholar]
- 124.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin Obstet Gynaecol. 1982;9:593– 607. [PubMed] [Google Scholar]
- 125.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, et al. A role for CXCL13 (BCA-1) in pregnancy and intra – amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21:763– 75. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nien JK, Yoon BH, Espinoza J, Kusanovic JP, Erez O, Soto E, et al. A rapid MMP-8 bedside test for the detection of intraamniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195:1025– 30. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 127.Ogge G, Romero R, Lee DC, Gotsch F, Than NG, Lee J, et al. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol. 2011;223:553– 65. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261– 8. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oyarzun E, Yamamoto M, Kato S, Gomez R, Lizama L, Moenne A. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol. 1998;179:1115– 9. doi: 10.1016/s0002-9378(98)70115-2. [DOI] [PubMed] [Google Scholar]
- 130.Panzer U, Reinking RR, Steinmetz OM, Zahner G, Sudbeck U, Fehr S, et al. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341– 50. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 131.Pao CC, Kao SM, Wang HC, Lee CC. Intraamniotic detection of Chlamydia trachomatis deoxyribonucleic acid sequences by polymerase chain reaction. Am J Obstet Gynecol. 1991;164:1295– 9. doi: 10.1016/0002-9378(91)90702-s. [DOI] [PubMed] [Google Scholar]
- 132.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156– 61. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 133.Park KH, Chaiworapongsa T, Kim YM, Espinoza J, Yoshimatsu J, Edwin S, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31:12– 22. doi: 10.1515/JPM.2003.002. [DOI] [PubMed] [Google Scholar]
- 134.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36:497– 502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park CW, Moon KC, Park JS, Jun JK, Romero R, Yoon BH. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta. 2009;30:56– 61. doi: 10.1016/j.placenta.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382– 6. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 137.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signaling. Mediators Inflamm. 2010 Jul 13; doi: 10.1155/2010/672395. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Piper KP, Horlock C, Curnow SJ, Arrazi J, Nicholls S, Mahendra P, et al. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827– 32. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 139.Pressman EK, Thornburg LL, Glantz JC, Earhart A, Wall PD, Ashraf M, et al. Inflammatory cytokines and antioxidants in midtrimester amniotic fluid: correlation with pregnancy outcome. Am J Obstet Gynecol. 2011;204:155– 7. doi: 10.1016/j.ajog.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 140.Puchner K, Iavazzo C, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D, et al. Mid-trimester amniotic fluid interleukins (IL-1beta, IL-10 and IL-18) as possible predictors of preterm delivery. In Vivo. 2011;25:141– 8. [PubMed] [Google Scholar]
- 141.Rallu F, Morency AM, Laferriere C, Bujold E. Invasion of the amniotic cavity by an uncultured bacterium, a gram-positive coccus. J Matern Fetal Neonatal Med. 2007;20:185– 7. doi: 10.1080/14767050601135212. [DOI] [PubMed] [Google Scholar]
- 142.Reddy UM, Baschat AA, Zlatnik MG, Towbin JA, Harman CR, Weiner CP. Detection of viral deoxyribonucleic acid in amniotic fluid: association with fetal malformation and pregnancy abnormalities. Fetal Diagn Ther. 2005;20:203– 7. doi: 10.1159/000083906. [DOI] [PubMed] [Google Scholar]
- 143.Relman DA. The search for unrecognized pathogens. Science. 1999;284:1308– 10. doi: 10.1126/science.284.5418.1308. [DOI] [PubMed] [Google Scholar]
- 144.Rodriguez N, Fernandez C, Zamora Y, Berdasquera D, Rivera JA. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:47– 50. doi: 10.3109/14767058.2010.482609. [DOI] [PubMed] [Google Scholar]
- 145.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201– 9. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 146.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636– 9. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 147.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553– 84. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 148.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041– 5. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- 149.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114– 9. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 150.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262– 79. [PubMed] [Google Scholar]
- 151.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661– 6. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 152.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117– 23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 153.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336– 41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 154.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817– 24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 155.Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet Gynecol. 1989;73:31– 4. [PubMed] [Google Scholar]
- 156.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392– 400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968– 74. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 158.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235– 8. [PubMed] [Google Scholar]
- 159.Romero R, Ghidini A, Mazor M, Behnke E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34:769– 78. doi: 10.1097/00003081-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 160.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969– 71. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 161.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821– 30. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 162.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086– 91. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 163.Romero R, Hagay Z, Nores J, Sepulveda W, Mazor M. Eradication of Ureaplasma urealyticum from the amniotic fluid with transplacental antibiotic treatment. Am J Obstet Gynecol. 1992;166:618– 20. doi: 10.1016/0002-9378(92)91686-5. [DOI] [PubMed] [Google Scholar]
- 164.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 a and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117– 23. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 165.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129– 33. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 166.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576– 87. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- 167.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–20. doi: 10.1002/9780470514269.ch13. discussion 220 – 3.:205 – 20. [DOI] [PubMed] [Google Scholar]
- 168.Romero R, Sepulveda W, Mazor M, Brandt F, Cotton DB, Dinarello CA, et al. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992;167:863– 72. doi: 10.1016/s0002-9378(12)80003-2. [DOI] [PubMed] [Google Scholar]
- 169.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543– 8. [PubMed] [Google Scholar]
- 170.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167– 83. doi: 10.1111/j.1600-0897.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 171.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805– 16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 172.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414– 29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 173.Romero R, Munoz H, Gomez R. Two-thirds of spontaneous abortion/fetal deaths after midtrimester amniocentesis are the result of a pre-existing subclinical imflammatory process of the amniotic cavity. Am J Obstet Gynecol. 1995;40:375– 9. [Google Scholar]
- 174.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH, et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol. 2000;183:1070– 7. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 175.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15:41– 56. doi: 10.1046/j.1365-3016.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 176.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259– 74. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 177.Romero R, Erez O, Espinoza J. Intrauterine infection, preterm labor, and cytokines. J Soc Gynecol Investig. 2005;12:463– 5. doi: 10.1016/j.jsgi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 178.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317– 26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Romero R, Kusanovic JP, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:261– 80. doi: 10.3109/14767050903067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Romero R, Chaiworapongsa T, Alpay SZ, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444– 55. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rosenkilde MM, Schwartz TW. The chemokine system: a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481– 95. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- 182.Seo K, McGregor JA, French JI. Preterm birth is associated with increased risk of maternal and neonatal infection. Obstet Gynecol. 1992;79:75– 80. [PubMed] [Google Scholar]
- 183.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339– 45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 184.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15– 22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tan J, Zhou G. Chemokine receptors and transplantation. Cell Mol Immunol. 2005;2:343– 9. [PubMed] [Google Scholar]
- 186.Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2010;148:147– 51. doi: 10.1016/j.ejogrb.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 187.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, et al. Patients with an asymptomatic short cervix (< or = 15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433– 8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vaisbuch E, Romero R, Erez O, Kusanovic JP, Mazaki-Tovi S, Gotsch F, et al. Clinical significance of early (< 20 weeks) vs. late (20 – 24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36:471– 81. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Van den Veyver I, Ni J, Bowles N, Carpenter RJ, Jr, Weiner CP, Yankowitz J, et al. Detection of intrauterine viral infection using the polymerase chain reaction. Mol Genet Metab. 1998;63:85– 95. doi: 10.1006/mgme.1997.2651. [DOI] [PubMed] [Google Scholar]
- 190.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351– 7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 191.Wenstrom KD, Andrews WW, Tamura T, DuBard MB, Johnston KE, Hemstreet GP. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am J Obstet Gynecol. 1996;175:830– 3. doi: 10.1016/s0002-9378(96)80007-x. [DOI] [PubMed] [Google Scholar]
- 192.Wenstrom KD, Andrews WW, Bowles NE, Towbin JA, Hauth JC, Goldenberg RL. Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstet Gynecol. 1998;92:420– 4. doi: 10.1016/s0029-7844(98)00210-5. [DOI] [PubMed] [Google Scholar]
- 193.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546– 50. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 194.Witkin SS, Gravett MG, Haluska GJ, Novy MJ. Induction of interleukin-1 receptor antagonist in rhesus monkeys after intraamniotic infection with group B streptococci or interleukin-1 infusion. Am J Obstet Gynecol. 1994;171:1668– 72. doi: 10.1016/0002-9378(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 195.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191– 9. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, et al. Peripheral CD300a + CD8 + T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol. 2012;67:184– 97. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res. 2002;51:310– 6. doi: 10.1203/00006450-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 198.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960– 70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 199.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433– 40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 200.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19– 26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 201.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-α, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825– 30. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 202.Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406– 11. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- 203.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254– 60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 204.Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1999;181:784– 8. doi: 10.1016/s0002-9378(99)70301-7. [DOI] [PubMed] [Google Scholar]
- 205.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773– 9. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 206.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130– 7. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 207.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675– 81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 208.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124– 9. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 209.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS, et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol. 2001;185:1162– 7. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 210.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130– 6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]