Abstract
Bile acids act as signaling molecules and stimulate the G protein coupled receptor, TGR5, in addition to nuclear farnesoid X receptor to regulate lipid, glucose and energy metabolism. Bile acid induced activation of TGR5 in the enteroendocrine cells promotes glucagon like peptide-1 (GLP-1) release, which has insulinotropic effect in the pancreatic β cells. In the present study, we have identified the expression of TGR5 in pancreatic β cell line MIN6 and also in mouse and human pancreatic islets. TGR5 selective ligands, oleanolic acid (OA) and INT-777 selectively activated Gαs and caused an increase in intracellular cAMP and Ca2+. OA and INT-777 also increased phosphoinositide (PI) hydrolysis and the increase was blocked by NF449 (a selective Gαs inhibitor) or U73122 (PI hydrolysis inhibitor). OA, INT-777 and lithocholic acid increased insulin release in MIN6 and human islets and the increase was inhibited by treatment with NF449, U73122 or BAPTA-AM (chelator of calcium), but not with myristoylated PKI (PKA inhibitor), suggesting that the release is dependent on Gs/cAMP/Ca2+ pathway. 8-pCPT-2′-O-Me-cAMP, a cAMP analogue, which activates Epac, but not PKA also stimulated PI hydrolysis. In conclusion, our study demonstrates that the TGR5 expressed in the pancreatic β cells regulates insulin secretion and highlights the importance of ongoing therapeutic strategies targeting TGR5 in the control of glucose homeostasis.
Keywords: Bile salts, Oleanolic acid, TGR5 receptor, G-protein coupled receptor, insulin resistance, incretins
1. Introduction
Bile salts are amphipathic molecules that are derived from cholesterol and are well known for their role in intestinal micelle formation and lipid digestion. It is now known that the range of physiological functions of bile salts extend far beyond lipid digestion and include modulation of cholesterol and triglyceride metabolism, insulin sensitivity, the intestinal endocrine response to meals and energy homeostasis [1]. Several of these functions are mediated via the nuclear receptor farnesoid-X-receptor (FXR) and the membrane receptor TGR5 [2,3]. FXR is widely expressed in liver, adipose tissue and muscle and is the key negative regulator of bile acid synthesis from cholesterol [4,5]. TGR5 (also known as M-BAR, Gpbar1, GPR131 or BG37) is a member of class A, G protein coupled receptor and is expressed ubiquitously in the liver, adipose tissue, gall bladder, skeletal muscle and in various cells of gastrointestinal tract such as smooth muscle cells, enteric neurons and enteroendocrine cells [6,7].
TGR5s have diverse biological functions including regulation of hepatic blood flow, anti-inflammatory responses, intestinal and gallbladder motility and skeletal muscle iodothyronine deiodinase (D2) activity, a key controller of metabolic homeostasis [8–11]. Recently, they have been shown to mediate the release of glucagon-like peptide-1 (GLP-1) from the intestine [12]. GLP-1 and GLP-2 are important mediators of the intestinal endocrine response to meals and promote insulin release from pancreatic β cells [13].
Insulin secretion under basal and post-prandial conditions plays a critical role in energy homeostasis. Circulating glucose has been considered to be the principal stimulus for insulin secretion [14]. Glucose metabolism within the β cell increases the ATP:ADP ratio, which inhibits ATP sensitive K+ channels leading to depolarization of the plasma membrane and opening of voltage-gated Ca2+ channels [15]. The resultant increase in cytosolic Ca2+ initiates exocytosis to mediate insulin release [16,17]. Glucose-dependent insulin secretion pathway is modulated by several paracrine and endocrine mechanisms.
Over the last decade much has been learned about the role of bile salts in metabolism. Bile salts can indirectly affect insulin secretion by TGR5-mediated intestinal release of GLPs. GLP-1 and GLP-2 induce insulin secretion via activation of receptors coupled to Gs/cAMP pathway [18,19]. Recently, FXR has been found in β cells and shown to release insulin [20]. However, it is not known if TGR5s are also present on islet cells and whether they affect insulin secretion. Also, the potential mechanisms involved in such effects are not known. Thus the aims of this study were to determine (1) if TGR5 was present on islet cells, (2) the effects of TGR5 activation on insulin secretion, and (3) the mechanisms underlying such effects.
2. Materials and methods
2.1. Materials
INT-777 (S-EMCA: 6α-Ethyl-23(S)-methylcholic Acid) was a generous gift from Intercept Pharmaceuticals. Antibody to TGR5 was purchased from Abcam (Cambridge, MA), antibodies to Gαs, Gαq, Gαi1, Gαi2 and Gαi3, and NF449 were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), ultrasensitive mouse insulin ELISA kit from Crystal Chem. Inc. (Downers Grove, IL), [35S] GTPγS, myo-[3H] inositol and [125I]cAMP from Perkin Elmer (Boston, MA), Fura 2-AM from Invitrogen (Eugene, OR), U73122 from Calbiochem (La Jolla, CA). Oleanolic acid (OA), lithocholic acid (LCA), Dulbecco’s Modified Eagle Medium (DMEM), 2-mercaptoethanol, 8-pCPT-2′-O-Me-cAMP, BAPTA-AM and all other reagents were obtained from Sigma (St Louis, MO).
2.2. Cell culture
The insulin secreting pancreatic β cell line, MIN6, was cultured in DMEM containing 25 mM glucose supplemented with 10% fetal bovine serum, L-glutamine, sodium carbonate, 2.5 mM 2-mercaptoethanol and 100U/ml pencillin-streptomycin and incubated at 37°C in 5% CO2.
2.3. Isolation and maintenance of mouse islets
Pancreatic islets were isolated from C57BL/6J mice as previously described [21]. The isolated islets were maintained in RPMI-1640 medium supplemented with 10% FBS and 100U/ml pencillin-streptomycin and incubated at 37°C in 5% CO2.
Human Islets were obtained from the National Human Tissue Resource Center, Philadelphia.
2.4. RNA isolation and RT-PCR analysis
Total RNA from MIN6 cells and islets was isolated using Ambion RNA isolation kit following the manufacture’s instruction and the purified RNA was reverse transcribed to single-stranded cDNA using high capacity reverse transcription kit. The conventional PCR and real-time PCR (TGR5 primers: forward 5′-GAGCGTCGCCCACCACTAGG-3′ and reverse 5′-CGCTGATCACCCAGCCCCATG-3′) analysis was carried out as described previously [22]. Mouse GAPDH primers (forward 5′-AGAAACCTGCCAAGTATGATG and reverse 5′-GGAGTTGCTGTTGAAGTCG-3′) were used as endogenous controls. Cycle threshold (Ct) values were obtained and the relative fold change in gene expression was calculated as 2−ΔΔCt.
2.5. Western blot analysis
Equivalent amounts of protein were resolved by SDS-PAGE and transferred onto a nitrocellulose membrane. Blots were blocked in 5% nonfat dry milk for 1 h followed by immunoblotting with anti-TGR5 antibody and anti-rabbit IgG horseradish peroxidase secondary antibody. Blots were visualized using advance ECL western blotting detection reagents.
2.6. Identification of activated G proteins
Membranes isolated from MIN6 cells were incubated with 60 nM [35S] GTPγS containing 10 mM Hepes (pH 7.4), 0.1 mM EDTA and 10 mM MgCl2 for 30 min at 37°C in the presence or absence of OA (10 μM) and INT-777 (25 μM). The reaction was stopped by adding 10 volumes of 100 mM Tris/HCl (pH 8) containing 10 mM MgCl2, 100 mM NaCl and 20 μM GTP and was then incubated in wells precoated with specific antibodies to Gαs, Gαq, Gαi1, Gαi2 and Gαi3 for 2 h in ice. This was then followed by washing with PBS containing 0.05% Tween and solubilization with 0.1N NaOH. The radioactivity in each well was counted by liquid scintillation and expressed in cpm/mg protein [23].
2.7. Phosphoinositide (PI) hydrolysis assay
MIN6 cells were labeled with myo-[3H] inositol (0.5 μCi/ml) in DMEM medium for 24 h. After 24 h, cells were washed with PBS and treated with OA, INT-777, or a selective Epac ligand, 8-pCPT-2′-O-Me-cAMP in the presence or absence of NF449 (a selective Gαs inhibitor), or U73122 (PI hydrolysis inhibitor) for 1 min. The reaction was terminated using 940 μl of chloroform-methanol-HCl (50:100:1 v/v) as described previously [24]. The upper aqueous phase was applied to the column prepared with Dowex AG-50W-X8 resin and water (1:1) and the [3H] inositol triphosphate was eluted with 0.8 M ammonium formate plus 0.1 M formic acid. Radioactivity was determined by liquid-scintillation counting and the results expressed as percent increase above basal.
2.8. Measurement of intracellular calcium
MIN6 cells cultured on glass cover slips, and mouse and human islets were washed with PBS and then loaded with 5 μM fura2-AM in HBSS buffer containing 3 mM glucose for 2 h at room temperature. Clusters of cells were selected for imaging and visualized as described previously [25]. The cells were alternatively excited at 340 nm and 380 nm and the increase in intracellular calcium by the TGR5 ligands (OA or LCA) was measured by determining the ratio of the fluorescence of fura-2 at 340 and 380 nm excitation.
2.9. Measurement of insulin release
MIN6 cells were incubated in HBSS (114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.16 mM MgSO4, 20 mM HEPES, 2.5 mM CaCl2, 25.5 mM NaHCO3, pH 7.2, 0.1% BSA) containing 3 mM glucose for 2 h at 37°C. Cells were then treated for 30 min in HBSS containing 3 mM (basal) or 25 mM glucose (stimulated) with or without OA, INT-777 or LCA. The supernatants were collected and assayed for insulin using ELISA kit. For insulin secretion from human islets, 10 islets/condition were incubated at 37°C for 2 h in HBSS with 3mM glucose. The same procedure as described above was followed.
2.10. Statistical analysis
Results were calculated as mean ± SEM and the experiments were performed at least three times. Statistical significance was analyzed using Student’s t-test and differences were considered significant at P < 0.05.
3. Results
3.1. Expression of TGR5 in human and murine pancreatic islets and in pancreatic β cells
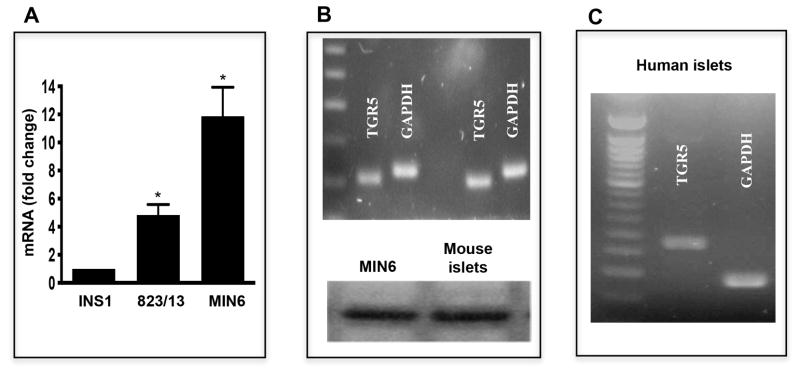
Expression of TGR5 mRNA was detected in all three pancreatic β cell lines (MIN6, INS1, and 823/13), however, expression was nearly 11 fold higher in MIN6 cells compared to INS1 and 2.5 fold higher compared to 823/13 cells (Fig. 1A). Analysis of TGR5 mRNA expression in islets from mouse and human demonstrated amplification of a product of predicted size (104 bp and 277 bp, respectively) (Figs. 1B and 1C). Analysis of TGR5 protein expression by western blot using a selective TGR5 antibody also demonstrated a band of expected size (35 kDa) in homogenates isolated from both MIN6 and mouse islets (Fig. 1B lower panel).
Figure 1. Expression of TGR5 in human and murine pancreatic islets and MIN6 cells.
(A) TGR5 mRNA level was measured in pancreatic β cell lines- INS1, 823/13 and MIN6 by qRT-PCR. (B) Expression of TGR5 mRNA (104 bp) and TGR5 protein (35 kDa) in MIN6 and mouse pancreatic islets measured by RT-PCR using specific primers (upper panel) and immunoblotting using TGR selective antibody (lower panel). (C) Expression of TGR5 mRNA (277 bp) in human pancreatic islets. Values are expressed as mean ± SEM of 3 experiments. *p<0.05 vs. INS1
3.2. Stimulation of insulin secretion by TGR5 ligands
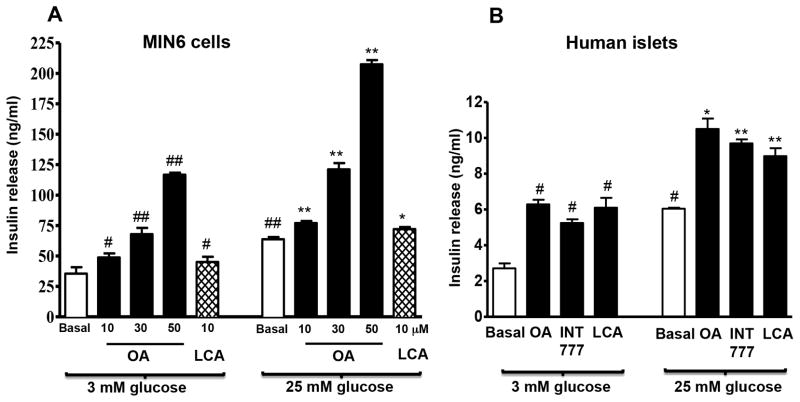
To investigate the role of TGR5 in insulin release, we examined whether basal (3 mM) and stimulated (25 mM glucose) insulin release would be modulated by activation of TGR5 receptors. The selective TGR5 ligand OA dose-dependently (10–50 μM) augmented both basal and stimulated insulin secretion (Fig. 2A). LCA (10 μM) also caused an increase in basal and glucose-mediated insulin secretion in MIN6 cells (Fig. 2A). Treatment of cells with the TGR5 specific ligand INT-777 (25 μM) or the FXR specific ligand INT-747 (25 μM) augmented both basal (33–34% increase) and stimulated insulin release (27–30% increase). However, treatment of cells with both ligands had no significant additive effect (basal: 30% increase; stimulated: 27% increase).
Figure 2. TGR5 regulates insulin secretion in pancreatic β cells.
(A) MIN6 cells were treated with different concentrations of OA (10, 30 or 50 μM) or LCA (10 μM) for 30 min in the presence of 3 mM or 25 mM glucose and insulin secretion was measured by ELISA as described in the methods. (B) Human pancreatic islets were incubated for 30 min with OA (25 μM), INT-777 (25 μM) or LCA (25 μM) in the presence of 3 mM or 25 mM glucose and insulin secretion was measured. Values are expressed as mean ± SEM of 3 experiments. ##p<0.01 or #p<0.05 vs. 3 mM glucose basal; **p<0.01 or *p<0.05 vs. 25 mM glucose basal.
Both basal and stimulated insulin release from human islets were also significantly augmented by OA (25 μM), LCA (25 μM) or INT-777 (25 μM) and the extent of increase was similar with all three ligands (Fig. 2B).
3.3. Mechanisms of TGR5-mediated insulin secretion
A: Activation of Gs and adenylyl cyclase by TGR5 selective ligands
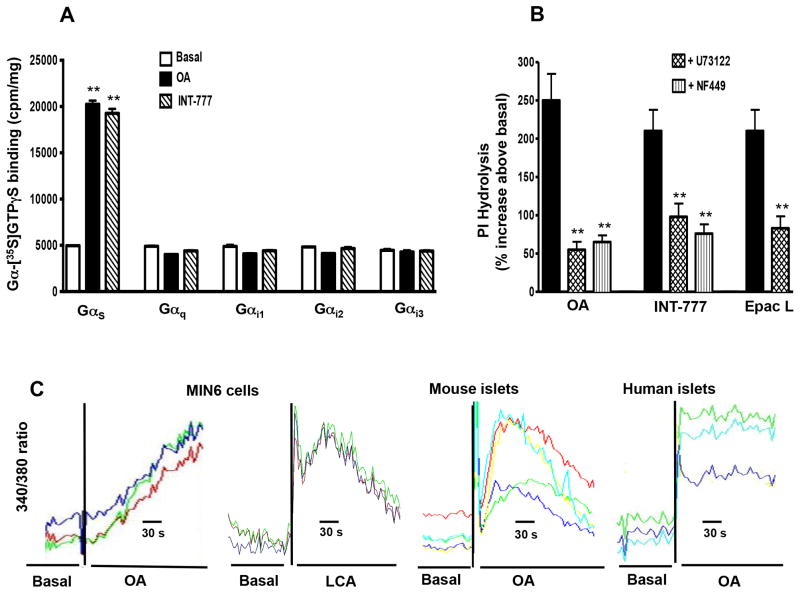
Incubation of cell membranes with OA or INT-777 caused a significant increase in the binding of [35S] GTPγS selectively to Gαs (300% increase above basal levels), but not to Gαq, Gαi1, Gαi2 or Gαi3 (Fig. 3A). These results suggest that TGR5 receptors are preferentially coupled to activation of Gαs in pancreatic β cells. The results are consistent with the selective activation of Gαs by OA in enteroendocrine cells and gastric smooth muscle cells [26]. Consistent with the activation of Gαs proteins, incubation of MIN6 cells with OA (25 μM) or INT-777 (25μM) stimulated adenylyl cyclase activity (56% and 75% increase in cAMP levels above basal levels, respectively).
Figure 3. (A) Selective activation of Gs by OA and INT-777.
Membranes from MIN6 cells were treated with OA (10 μM) or INT-777 (25 μM) in the presence of [35S] GTPγS for 30 min at 37 °C. Aliquots were added to wells precoated with specific antibodies to Gαs, Gαq, Gαi1, Gαi2 or Gαi3 and incubated for 2 h. The bound radioactivity was measured. Values are expressed as mean ± SEM of 4 experiments. **p<0.01 vs. basal. (B) Activation of phosphoinositide (PI) hydrolysis by TGR5 ligands. MIN6 cells were labeled with myo-[3H] inositol for 24 h and then treated with OA (10 μM), INT-777 (25 μM) or Epac ligand (10 μM) with or without inhibitors of Gαs (NF449, 10 μM) or PI hydrolysis (U73122, 10 μM). PI hydrolysis was measured by ion exchange chromatography and the results are expressed as percent increase above basal levels (274±20 cpm/mg protein). Values are expressed as mean ± SEM of 3 experiments. **p<0.01 significant inhibition in PI hydrolysis compared to TGR5 ligands. (C) Release of Ca2+ by OA. MIN6 cells, mouse islets and human islets were loaded with fura-2AM for 2 h at room temperature and then stimulated with OA or LCA. The change in cytosolic Ca2+ was measured by monitoring the 340/380 nm fluorescence ratio. Representative traces with each ligand is shown.
B: Activation of PI hydrolysis by TGR5 selective ligands
Previous studies in enteroendocrine cells have shown that GLP-1 release by OA was mediated via PKA-independent mechanism involving sequential activation of Epac (cAMP-dependent exchange factor), Epac-dependent PLC-ε and release of Ca2+ [26]. Activation of a similar pathway was examined in pancreatic β cells by measurements of PI hydrolysis in response to TGR5 selective ligands, OA and INT-777 and an Epac selective ligand, 8-pCPT-2′-O-Me-cAMP. Both OA and INT-777 caused a significant and similar increase in PI hydrolysis (250% and 210% increase above basal levels of 274±20 cpm/mg protein) (Fig. 3B). The increase in PI hydrolysis was blocked by the selective PI hydrolysis inhibitor, U73122 and by a selective Gαs protein inhibitor, NF449. These results suggest that activation of PI hydrolysis by OA and INT-777 was mediated via activation of Gαs proteins, probably involving activation of Epac and Epac-dependent PLC-ε. In support to this notion, the selective Epac ligand also stimulated PI hydrolysis and the extent of stimulation (210% increase above basal level) was similar to OA and INT-777 (Fig. 3B).
C: Release of Ca2+ by TGR5 specific ligands
Consistent with the activation of PI hydrolysis, addition of OA or LCA to MIN6 cells resulted in a rapid increase in cytosolic Ca2+ (Fig. 3C). A similar increase in cytosolic Ca2+ in response to OA was demonstrated in islets isolated from mouse and human (Fig. 3C).
D: Pathways involved in TGR5-mediated insulin secretion
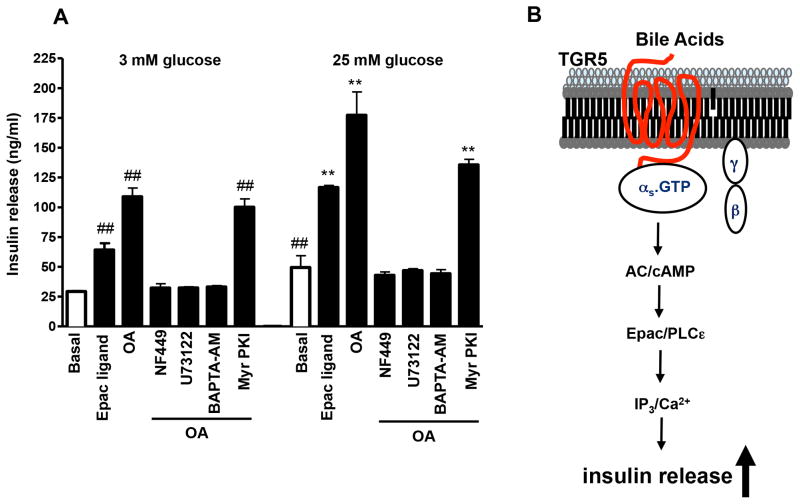
To examine the mechanism involved in OA-induced insulin secretion, MIN6 cells were preincubated with inhibitors of Gαs (NF449, 10 μM), PI hydrolysis (U73122, 10 μM), or PKA (myristolylated PKI, 1μM), or with Ca2+ chelator BAPTA-AM (10 μM), and then the insulin response to OA was measured. OA-induced increase in both basal and stimulated insulin secretion was abolished by NF449, U73122 and BAPTA-AM (Fig. 4A), but not by myristoylated PKI (Fig. 4A). These results suggest that insulin release in response to OA was mediated via activation of Gαs and Epac/PLC-ε/Ca2+ pathway (Fig. 4B). Consistent with this notion and with the increase in PI hydrolysis, the selective Epac ligand, 8-pCPT-2′-O-Me-cAMP (1 μM) also increased both basal and stimulated insulin release (Fig. 4A).
Figure 4. (A) Effect of Gs and PI hydrolysis inhibitors on OA-induced insulin release.
MIN6 cells were treated with the Epac ligand, 8-pCPT-2′-O-Me-cAMP (1 μM), or OA (50 μM) in the presence or absence of NF449 (10 μM), U73122 (10 μM), BAPTA-AM (10 μM) or myristolylated PKI (1 μM). After 30 min, the supernatant was collected and used to measure insulin levels by ELISA. Values are expressed as mean ± SEM of 3 experiments. ##p<0.01 vs. 3 mM glucose basal; **p<0.01 vs. 25 mM glucose basal. (B) Schematic representation of the signaling pathway coupled to TGR5 receptors to mediate insulin release from pancreatic β cells.
4. Discussion
The regulated synthesis and secretion of insulin is one of the center-pieces of the body’s strategy to maintain metabolic homeostasis. It is therefore not surprising that pancreatic islet β cell function is modulated by neural, endocrine and paracrine factors and cell-cell interactions [27–29]. A wide variety of ligands thus bind their specific receptors on islet cells, which integrates these signals to modulate its synthetic and secretory functions. Bile salts have recently been shown to be one of the ligands that affect islet function. Specifically, via binding to FXR, bile salts have been shown to affect insulin secretion. This study demonstrates that islet β cells also express TGR5 receptors, which upon activation enhance glucose-mediated insulin secretion. This discovery further supports a role for bile salts, the endogenous ligands for TGR5, in the regulation of metabolism.
Bile salts may impact islet function in both direct and indirect ways. At the level of the intestine, activation of TGR5 by bile salts enhance GLP-1 and GLP-2 release [30]. GLP-1 delays gastric emptying and thus the absorption of glucose thereby flattening the post-prandial glycemic response [31]. GLP-1 also directly functions as a trophic factor for islet cells and promotes insulin secretion following a glucose challenge [19,32]. The current study demonstrates that bile salts promote glucose-mediated insulin release via TGR5 and also confirm prior observations that FXR activation can enhance glucose-mediated insulin secretion [20].
At a cellular level, TGR5 ligands lithocholic acid, OA and INT 777 all increased intracellular Ca2+ and calcium chelation blunted the insulin secretory response indicating that the final exocytosis of insulin via TGR5 involved classical Ca2+-dependent pathways used by other stimulators of insulin secretion. We further showed that bile acid induced TGR5 receptors in the pancreatic β cells is Gαs coupled and activation of these receptors leads to stimulation of adenylyl cyclase activity generating cAMP which in turn leads to the activation of cAMP dependent Epac/PLC-ε pathway stimulating insulin secretion. This mechanism was found to be similar to that used by the TGR5 expressed in the enteroendocrine cells which stimulated GLP1 release [26, 30].
Normally, circulating glucose is the key driver of insulin secretion; islet cells sense ambient glucose levels along with fatty acids and various amino acids and activate proinsulin synthesis and insulin secretory mechanisms in response to the metabolic coupling factors [33–35]. The observed enhancement of insulin secretion by TGR5 ligands under low and high glucose conditions suggests that the known insulin-sensitizing effects of bile salts may be partly due to improved insulin secretory responses to a glucose load. Interestingly, the simultaneous use of a FXR agonist (INT-747) and a TGR5 agonist (INT-777) did not lead to an additive response although both INT-747 and INT-777 stimulated glucose mediated insulin secretion to a similar degree when used alone. This suggests a degree of antagonism between the intracellular pathways activated by FXR and TGR5 respectively that converge on insulin secretion. This however remains to be experimentally elucidated.
Under fasting conditions, circulating bile salt concentrations are generally less than 5 μM. Under post-prandial conditions, these concentrations may rise to 15 μM [36,37]. The precise effect of bile salts in vivo is likely to be not only a function of its concentration but also the nature of the circulating bile salts. For example, it is well known that tauro-ursodeoxycholic acid is only a weak ligand for TGR5 and FXR whereas chenodeoxycholic acid and lithocholic acid are more potent ligands. In chronic liver disease, particularly with cholestasis, fasting levels of bile salts are elevated and there is a loss of secondary bile salts [38]. There is also a decrease in intestinal exposure to bile salts, which is expected to dampen the incretin response to meals. The contribution of these findings to the well-known susceptibility of individuals with cirrhosis to develop type 2 diabetes now need to be considered in the context of the increasing recognition of the effects of bile salts on both insulin secretion and also on insulin sensitivity [39].
From a public health point of view, type 2 diabetes is a leading threat to the health of the human race. Fasting hyperinsulinemia and eventual β cell exhaustion are hallmarks of the insulin resistance syndrome and development of type 2 diabetes [40]. It is generally believed that a combination of increased exposure to inflammatory cytokines, fatty acids and other products of lipolysis along with increased blood sugar drive increased insulin synthesis and secretion in such cases [41]. The potential role of bile salts in modulating the insulin secretory response to these factors and as a disease modifier in the genesis of type 2 diabetes awaits elucidation.
In summary, the current study adds to the growing body of evidence supporting a key role for bile salts as a regulator of nutritional and metabolic homeostasis. It activates the release of GLP-1, a known stimulator of insulin secretion, in response to a meal in the intestine by activation of TGR5 receptors [26, 30]. We now demonstrate that TGR5 receptors are present in pancreatic islet β cells and that activation of TGR5 increases insulin secretion under both low and high ambient glucose levels.
Highlights.
G protein coupled receptor TGR5 is expressed in mouse and human islets
TGR5 is coupled to activation of Gs and Ca2+ release via cAMP/Epac/PLC-ε pathway
Activation of TGR5 by bile salts and selective ligands causes insulin secretion
TGR5 could be a potential therapeutic target to treat diabetes
Acknowledgments
This work is supported by grants from the National Institutes of Health to Dr. Arun J. Sanyal RO1 DK 081410 and to Dr. Karnam S. Murthy RO1 DK 28300. We thank Dr. V. Lyall and S. Mummalaneni for their excellent support with Ca2+ measurements, and Intercept Pharmaceuticals, New York, for the generous gift of INT-777. The work has been presented in partial form at the annual meeting of the American Gastroenterological Association in San Diego, CA (2012) and Gordon Research Conference at the University of New England, ME (2012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pols TW, Noriega LG, Nomura M, et al. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol. 2011;54:1263–1272. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Lou G, Meng Z, Huang W. TGR5: a novel target for weight maintenance and glucose metabolism. Exp Diabetes Res. 2011;2011:1–5. doi: 10.1155/2011/853501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 5.Chiang JY, Kimmel R, Weinberger C, Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem. 2000;275:10918–10924. doi: 10.1074/jbc.275.15.10918. [DOI] [PubMed] [Google Scholar]
- 6.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:435–440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 7.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 9.Keitel V, Donner M, Winandy S, et al. Expression and function of the bile acid receptor TGR5 in kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Holmstrom SR, Kir S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. 2011;25:1066–1071. doi: 10.1210/me.2010-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 12.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- 14.Grodsky GM, Batts AA, Bennett LL, et al. Effects of carbohydrates on secretion of insulin from isolated rat pancreas. Am J Physiol. 1963;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- 15.Rorsman P, Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985;405:305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- 16.Rorsman P. The pancreatic beta-cell as a fuel sensor: an electrophysiologist’s viewpoint. Diabetologia. 1997;40:487–495. doi: 10.1007/s001250050706. [DOI] [PubMed] [Google Scholar]
- 17.Henquin JC. Triggering and Amplifying Pathways of Regulation of Insulin Secretion by Glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 18.Drucker DJ, Philippe J, Mojsov S, et al. Glucagon-like peptide 1 stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahren B. Glucagon-like peptide-1 (GLP-1): a gut hormone of potential interest in the treatment of diabetes. Bioessays. 1998;20:642–651. doi: 10.1002/(SICI)1521-1878(199808)20:8<642::AID-BIES7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Renga B, Mencarelli A, Vavassori P, et al. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–372. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Carter JD, Dula SB, Corbin KL, et al. A practical guide to rodent islet isolation and assessment. Biol Proced. 2009;11:3–31. doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu W, Li F, Mahavadi S, Murthy KS. Upregulation of RGS4 expression by IL-1beta in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3beta pathway. Am J Physiol Cell Physiol. 2009;296:C1310–C1320. doi: 10.1152/ajpcell.00573.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy KS, Makhlouf GM. Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-beta 3 and inhibition of adenylyl cyclase is mediated by Gi2 and G(o) in smooth muscle. Mol Pharmacol. 1996;50:870–877. [PubMed] [Google Scholar]
- 24.Murthy KS, Makhlouf GM. Phosphoinositide metabolism in intestinal smooth muscle: preferential production of Ins (1,4,5) P3 in circular muscle cells. Am J Physiol. 1991;261:G945–951. doi: 10.1152/ajpgi.1991.261.6.G945. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Zhou H, Mahavadi S, et al. Signaling pathway mediating gastrointestinal smooth muscle contraction and MLC20 phosphorylation by motilin receptors. Am J Physiol Gastrointest Liver Physiol. 2005;288:G23–31. doi: 10.1152/ajpgi.00305.2004. [DOI] [PubMed] [Google Scholar]
- 26.Bala V, Mahavadi S, Rajagopal S, Zhou R, Kuemmerle JF, Sanyal AJ, Murthy KS. Bile Acid-Induced Stimulation of ERK1/2 Activity, GLP-1 and PYY Release in Enteroendocrine Cells is Mediated by the Activation of Epac/PLC-ε Signaling Pathway via GS-Coupled TGR5. Gastroenterology. 2011;40(5):S–147. [Google Scholar]
- 27.Ahren B, Veith RC, Taborsky GJ. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology. 1987;121:323–331. doi: 10.1210/endo-121-1-323. [DOI] [PubMed] [Google Scholar]
- 28.Cabrera O, Berman DM, Kenyon NS, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Best CH, Haist RE, Wrenshall GA. The pancreas, insulin, and glucagon. Annu Rev Physiol. 1955;17:393–416. doi: 10.1146/annurev.ph.17.030155.002141. [DOI] [PubMed] [Google Scholar]
- 30.Parker HE, Wallis K, Le Roux CW, et al. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schirra J, Katschinski M, Weidmann C, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreymann B, Ghatei MA, Williams G, Bloom SR. Glucagon-like peptide-1 7–36: A Physiological incretin in man. The Lancet. 1987;330:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 33.Oya M, Suzuki H, Watanabe Y, et al. Amino acid taste receptor regulates insulin secretion in pancreatic β-cell line MIN6 cells. Genes Cells. 2011;16:608–616. doi: 10.1111/j.1365-2443.2011.01509.x. [DOI] [PubMed] [Google Scholar]
- 34.Itoh Y, Kawamata Y, Harada M, et al. Free fatty acids regulates insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 35.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 36.Everson GT. Steady-state kinetics of serum bile acids in healthy human subjects: single and dual isotope techniques using stable isotopes and mass spectrometry. J Lipid Res. 1987;28:238–252. [PubMed] [Google Scholar]
- 37.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angelico M, Attili AF, Capocaccia L. Fasting and postprandial serum bile acids as a screening test for hepatocellular disease. Am J Dig Dis. 1977;22:941–946. doi: 10.1007/BF01076191. [DOI] [PubMed] [Google Scholar]
- 39.Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med. 2007;120:829–834. doi: 10.1016/j.amjmed.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 41.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]