Abstract
Chagas disease caused by Trypanosoma cruzi remains an important neglected tropical disease and a cause of significant morbidity and mortality. No longer confined to endemic areas of Latin America, it is now found in non-endemic areas due to immigration. The parasite may persist in any tissue, but in recent years there has been increased recognition of adipose tissue both as an early target of infection and a reservoir of chronic infection. The major complications of this disease are cardiomyopathy and megasyndromes involving the gastrointestinal tract. The pathogenesis of Chagas disease is complex and multifactorial involving many interactive pathways. The significance of innate immunity, including the contributions of cytokines, chemokines, reactive oxygen species, and oxidative stress, has been emphasized. The role of the components of the eicosanoid pathway such as thromboxane A2 and the lipoxins has been demonstrated to have profound effects as both pro-and anti-inflammatory factors. Additionally, we discuss the vasoconstrictive actions of thromboxane A2 and endothelin-1n Chagas disease. Human immunity to T. cruzi infection and its role in pathogen control and disease progression have not been fully investigated. However, recently, it was demonstrated that a reduction in the anti-inflammatory cytokine IL-10 was associated with clinically significant chronic chagasic cardiomyopathy.
Introduction
The genus Trypanosoma encompasses important diseases of humankind. Thus, Trypanosoma brucei gambiense and T.b. rhodesiense cause human African trypanosomiasis (sleeping sickness), and Trypanosoma cruzi is the cause of American trypanosomiasis, also known as Chagas disease. The outcome of Chagas disease in humans is related both to the virulence of the parasite strain as well as the host response to infection. Interestingly, paleo-parasitological studies demonstrating the presence of T. cruzi in tissues of 9,000-year-old mummies from coastal northern regions of Chile have pointed to the possibility that human Chagas disease was present in South America before its “discovery” in 1909 [1,2]. The year 2009 marked the centennial of the discovery of Chagas disease, and, in recognition of this event, many journals published reviews on various aspects of this disease. Our intent here is not to write an exhaustive review of Chagas disease, but rather to focus on individual topics which we believe are of importance in understanding the pathogenesis of this important, but yet neglected, tropical disease.
Life Cycle and Epidemiology of T. cruzi Infection
Epidemiology
Chagas disease has been regarded as an exotic and rare “Latin American” disease. It is endemic in Mexico, Central, and South America, where vector-borne transmission of T. cruzi usually occurs in individuals in rural areas. The insect vectors (triatomines) invade the primitive houses that are common in rural areas, and feed on people often as they sleep; hence the term “assassin bug.” Both domestic and wild mammals can be infected and serve as reservoirs for the parasite. In endemic areas, vector-borne disease has also been observed on the outskirts of large metropolitan areas. Until recently, there were only a handful of indigenous cases in the United States, but recently, 16 cases of autochthonous T. cruzi infection were reported [3], and these were most likely acquired from vectors within the country.
Patterns of emigration from Chagas-endemic areas to other areas of the world have now altered our understanding of the epidemiology of this disease in the United States and other non-endemic areas such as Canada, Europe, Australia and Japan. In a landmark article published in 2009, Bern and Montgomery estimated that 300,000 persons living in the United States were chronically infected with T. cruzi [4]. In addition, there are Latin American immigrants living in Spain, France and Portugal, as well as Brazilian immigrants of Japanese origin in Japan. The vast majority of serologically positive individuals in these non-endemic areas usually have the indeterminate form of cardiac disease. Although these individuals are not aware of their chronic infection, they remain potential sources of transmission via blood transfusion, organ transplantation and mother-to-child vertical transmission (congenital transmission). In fact, congenital Chagas disease in children of mothers who have emigrated from endemic areas has been reported in Europe [5]. Thus, Chagas disease has indeed gone global [6].
Life cycle and infection
T. cruzi has a complex life cycle consisting of four life stages. First, blood from trypomastigotes circulating in the blood of an infected mammalian host is ingested by the feeding vector. The trypomastigotes then transform first into epimastigotes that divide by binary fission and then into non-dividing, infectious metacyclic trypomastigotes in the hindgut of the vector; they are next deposited with the vector feces during subsequent blood meals. Natural transmission to a new mammalian host occurs when the parasite-laden feces contaminate oral or nasal mucous membranes, the conjunctivae, or wounds in the skin, including vector bites. Once in the mammalian host, the trypomastigotes enter host cells and transform into the multiplying intracellular forms or amastigotes, which then transform into blood form trypomastigotes. These forms are released into the bloodstream as the host cell ruptures and are then ready to invade healthy cells.
The molecular mechanism(s) of invasion by this parasite and the associated regulatory pathways have been the subject of intense investigation for many years. T. cruzi interacts with several mammalian host cell receptors, such as toll-like receptors (TLRs), kinins (B1/B2 sub types), receptor tyrosine kinases, TGF- and EGF-receptors, and the activity of these receptors is required for optimal parasite binding and/or invasion (reviewed in [7]). More recently, it was shown that T. cruzi also exploits host cell LDL receptor (LDLr) for their internalization and subsequent fusion of the parasitophorous vacuole with the host cell lysosomal compartment [8]. Parasites directly bind to LDLr, and inhibition or disruption of LDLr resulted in a reduced rate of invasion. Importantly, acutely infected mice displayed a significant decrease in plasma LDL levels and LDL was increased at areas where parasites were present in the heart, which may mean there was an infection-induced increase in phospholipids, triglycerides, and fatty acids that could contribute to the pathogenesis of Chagas disease.
Blood-form trypomastigotes infect adjacent uninfected cells or disseminate via the lymphatics and bloodstream to infect cells at distant sites. Although any nucleated mammalian cell can be parasitized, those commonly infected include cardiac myocytes, peripheral skeletal and smooth muscle cells, endothelial cells, and cells of the nervous and reticulo-endothelial systems and adipose tissue. Besides vectorial transmission, transmission of T. cruzi by transfusion of blood or transplantation of organs from infected donors, congenital transmission, and acquisition of the infection via the oral route have been reported [9].
Clinical Chagas Disease and Pathology
Cardiac involvement
Vector-borne acute Chagas disease is usually mild. After an incubation period of 1 to 3-weeks, a newly infected individual may develop fever, chills, nausea, vomiting, diarrhea, rash, and meningeal irritation. A raised inflammatory lesion at the site of parasite entry (a chagoma), unilateral periorbital edema (Romaña’s sign), conjunctivitis, lymphadenopathy and hepatosplenomegaly are observed in acute infection. Trypomastigotes are observed in wet preparations of blood and cerebrospinal fluid during acute infection [10]. Intracellular amastigotes are also found in the liver Kupffer cells rather than in the hepatocytes. In most patients, an immune response develops, and acute parasitemia and associated symptoms usually resolve within 2 to 4 months. The mortality rate of acutely, naturally infected patients, often children, is less than 2%, and the common mode of death is usually acute myocarditis and/or meningoencephalitis. The presence of arrhythmias is usually considered a poor prognostic finding, and parasite pseudocysts are readily found in cardiac biopsies of such patients, indicating a high level of infection.
At the conclusion of the acute phase, the majority of infected persons enter the indeterminate clinical form of infection, characterized by a positive serology and absence of clinical manifestations. This phase may last months to an entire lifetime; many chronically infected people never develop clinical manifestations. Approximately 30–40% of infected individuals eventually develop symptomatic Chagas disease. Imaging of the heart employing echocardiography, cardiac magnetic resonance imaging and microPET is extremely useful in the evaluation of disease severity. Dilated congestive cardiomyopathy is an important manifestation of chronic Chagas disease and may present insidiously as heart failure or more abruptly with arrhythmia and/or a stroke [11]. Liver pathology may reflect right-sided heart failure. Apical aneurysm of the left ventricle is one of the hallmarks of chronic chagasic cardiomyopathy, observed by cardiac imaging and at autopsy (Fig. 1A). Generally, the therapy of chagasic patients with chronic heart disease is similar to that of other patients with congestive heart failure (CHF). Some patients also exhibit destruction of conduction tissue, most commonly right bundle branch block, that results in atrio-ventricular and intra-ventricular conduction abnormalities, and patients may require pacemaker placement. Patients with end-stage CHF may benefit from heart transplantation [12]. Gastrointestinal (GI) manifestations, such as the mega-syndromes, involving tubular structures of the GI tract are not as common, but may be more frequent in certain geographic areas. In addition, patients may exhibit both cardiac and gastrointestinal manifestations.
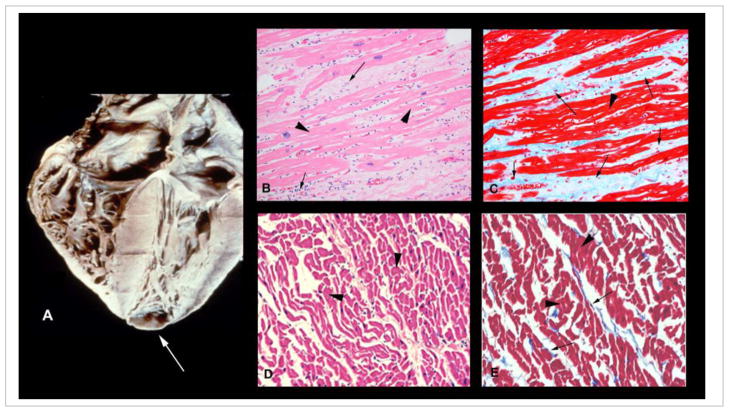
Fig. 1. Heart of a patient with chronic chagasic cardiomyopathy.
(A) Shown is 4-chamber enlargement of the heart in a chagasic patient. Apical aneurysm is marked with an arrow (printed with permission from the Armed Forces Institute of Pathology). (B) H&E-stained myocardium from a patient with chronic chagasic cardiomyopathy showing fibrosis and chronic inflammation (arrow). (C) Myocardium of the same patient sample stained with Masson’s trichrome showing significant fibrosis (blue color, marked by arrow). H&E (D) and Masson’s trichrome (E) staining of the myocardium of an uninfected healthy donor are shown for comparison. Arrow-heads in all panels mark the myocardium.
There are three layers of cardiac myocytes in the heart that are obliquely oriented to each other and then meet at the apex of the heart. Typically, trypomastigotes must pass through the basal laminae and extracellular matrix (ECM) layers of the myocardium and the interstitial matrix layer between the basal laminae layers to reach cardiac myocytes. Studies both in experimental models and chagasic patients demonstrate that myocardial damage results from infection and multiple insults, including ischemia, inflammation, oxidative stress, and necrosis, which contribute to ECM degradation. Indeed, cardiac metalloproteinases are up regulated in the setting of infection, and their inhibition ameliorates myocardial inflammation [13]. ECM degradation gradually results in slippage of the ventricular layers, believed to be the primary event for the formation of the apical aneurysm, which has become the Sine qua non of chronic Chagas heart disease (Fig. 1A). Thus, the remodeling of the heart in chronic Chagas heart disease is a result of reorganization in the heart wall resulting from tissue damage from ischemia, necrosis, inflammation and chronic increase in intra-cavitary pressure associated with hypertrophy and dilation of the ventricles. Histological examination of the heart reveals myocytolysis, myonecrosis and contraction band necrosis resulting from hypoperfusion, followed by periods of reperfusion which may be the result of vasospasm of the branches of the coronary circulation. Eventually bands of fibrous tissue and extracellular collagen replace and engulf hypertrophied cardiac myocytes (Fig. 1C&D).
The virtual absence of parasites in the heart of chronically infected individuals has engendered a discussion in the literature regarding the etiology of chronic chagasic heart disease. It has been accepted that interaction between the parasite and its host, well-adapted for more than 9,000 years, results in a balance that favors chronic disease in the great majority of cases. Different strains of the parasite have been associated with distinct clinical outcomes of infection in experimental models [14,15] and human disease [16]. Host factors such as genetic background of the host, mitochondrial dysfunction, oxidative stress and pathological immunity, have also been associated with distinct clinical outcomes of Chagas disease and are briefly discussed in this review.
Vascular compromise in Chagas disease
The vasculature comprises approximately one-third of the myocardium. Yet, T. cruzi-induced vasculitis, though described in the early years following the recognition of Chagas disease, was not appreciated until the 1980s. In those years, microvascular compromise was demonstrated as an important factor in the pathogenesis of cardiomyopathies of various etiologies [17], and treatment with the calcium-channel blocker (verapamil) was found to improve the coronary blood flow and outcome and to reduce inflammation and fibrosis in the heart [18,19]. Subsequently, alterations in the sub-endocardial microvasculature, aneurysm formation and vasospasm were observed in an experimental model of T. cruzi infection [20], and verapamil was shown to ameliorate reduced blood flow [21] and cardiovascular remodeling [22,23] in infected mice.
In recent studies, Tanowitz and colleagues examined the role of TXA2 (a bioactive lipid eicosanoid) and the 21-amino acid peptide endothelin-1 (ET-1) in Chagas disease. Both of these agents are known to be pro-inflammatory and cause platelet aggregation and vascular spasm. The synthesis of ET-1 is mediated by endothelin-converting enzyme, which converts precursor ET-1 (31 amino acids) to ET-1, noted in endothelial cells. The biological properties of ET-1 are mediated by the G-protein-coupled endothelin receptors, ETA and ETB. It is now appreciated that multiple cell types, such as cardiac myocytes, fibroblasts, astrocytes, and macrophages, can synthesize ET-1. Moreover, T. cruzi infection of cultured endothelial cells resulted in an increase in biologically active ET-1 in studies with T. cruzi-infected mice in which there was an increased expression of ET-1 protein and mRNA in the myocardium and an increase in plasma ET-1 levels [24]. Treatment of infected mice with phosphoramidon, an inhibitor of endothelin converting enzyme, reduced T. cruzi infection-induced right ventricular enlargement [25]. Importantly, mice deficient in ET-1 in either cardiac myocytes or endothelial cells exhibited amelioration in cardiac remodeling that is associated with T. cruzi infection [26]. Hassan et al [27] found increased expression of ET-1 in the carotid arteries of T. cruzi-infected mice, and indicated the importance of ET-1 in the vasculature changes during infection. Elevated plasma levels of ET-1 have also been noted in patients with chronic chagasic cardiomyopathy [28]. However, it is unclear whether this is a result of CHF in general or chagasic cardiomyopathy in particular.
TXA2 serum levels are increased in T. cruzi-infected mice; the majority being parasite-derived [29,30,31]. TXA2-regulated vasospasm, thrombosis, vascular permeability, and endothelial cell dysfunction were observed in acute infection. The TXA2 receptor knockout mice displayed increased tissue parasitism and myocardial inflammation [30]. It is believed that TXA2 signaling acts as a potential quorum-sensing mechanism regulating intracellular amastigote proliferation. This would prevent the overwhelming of the host during acute infection thus enhancing the transition into a chronic persistent state of infection and inflammation.
Involvement of the central nervous system (CNS)
Soon after the description of T. cruzi, CNS involvement in Chagas disease was described. Infants born with congenital Chagas disease may display signs, symptoms and pathological findings of myocarditis and meningoencephalitis [32]. These children may display pathological findings in the brain similar to those observed in other perinatal infections. Trypomastigotes may be present in the cerebral spinal fluid (CSF) during acute infection. In adults, a necrotizing meningoencephalitis is most often observed in the setting of immunosuppression such as that which accompanies HIV/AIDS [33] or the administration of immunosuppressive drugs. As discussed above, stroke is a common finding in patients with chagasic heart disease, especially in those individuals with dilated cardiomyopathy. Neuro-cognitive abnormalities have been reported in experimental Chagas disease [34].
T. cruzi may invade glial cells including astrocytes and microglial cells, while neurons are only infrequently infected [35]. The GI tract is also a target of infection which results in injury to the enteric nervous system. Affected individuals may develop dilation of portions of the GI tract. Although megacolon and megaesophagus are most common, megastomach, megaduodenum, megajejunum, megagallbladder, megacholedochus have all been described. Achalasia, aspiration pneumonia, disturbances of gastric emptying, alterations in intestinal transit, and motility disorders of the colon and gallbladder have also been reported. Also, in one study of colon samples from chagasic patients without megacolon, there was an increase in Foxp3+ T cells compared with those with megacolon. This finding may mean that Foxp3+ T cells reduce inflammatory cells and prevent neuronal destruction [36]. In this regard, parasite-derived neurotropic factor (PDNF) may promote neuronal cell survival [37,38]. Others have suggested gene mutations associated with carcinoma are present in patients with chagasic megaesophagus [39], though further research in this area is needed.
Adipose tissue and the adipocyte: Reservoir tissue for parasite
Until recently, adipose tissue and the adipocyte (fat cell) were underappreciated aspects of Chagas disease pathology and pathogenesis. T. cruzi was first described as parasitizing adipose tissue and adipocytes in the 1970s [40,41] and 1990s [42]. Combs et al in 2005 [43] published the functional consequences of parasitism of adipose tissue and adipocytes in a mouse model. In the same year, Philipp Scherer and colleagues [44] reported that injection of LPS into mice that were rendered fatless (by using the regulated fat apoptosis murine model) did not result in the immediate death of mice as seen in control mice with a normal component of adipose tissue. This seminal observation set the stage for studies by the Tanowitz group exploring the significance of adipose tissue during acute infection. Nagajyothi et al [45] demonstrated that as early as 15 days after infection, when mice exhibited no detectable blood parasitemia, there was an increase in the number of macrophages (Fig. 2) and a greater parasite load in brown and white adipose tissue, obtained from the interscapular and subcutaneous regions, respectively, when compared with those in other organs such as the spleen and heart. The authors also noted a reduction in lipid content, adipocyte size, and adipose tissue mass, accompanied by increased expression of lipolytic enzymes, pro-inflammatory cytokines, chemokines, toll-like receptors and ERK in adipose tissue obtained from infected mice [45]. By day 30 post infection, the tissue and serum levels of adiponectin were significantly reduced and inflammatory mediators were up regulated in the adipose tissue [43]. The reduction in adiponectin expression and local lipolysis has been linked to up-regulation of inflammatory mediators, insulin resistance [46] and increase in the influx of macrophages into adipose tissue [47], as was demonstrated in experimental of Chagas disease. Thus, the authors propose that the persistence of the parasite and macrophages in adipose tissue, well into the chronic phase of infection, creates a “low-level” chronic inflammatory state similar to that observed in morbid obesity. The experimental data as well as recent studies in humans [48] strongly indicate that adipose tissue is an early target of infection, as well as a reservoir from which infection can recrudesce during periods of immune-suppression.
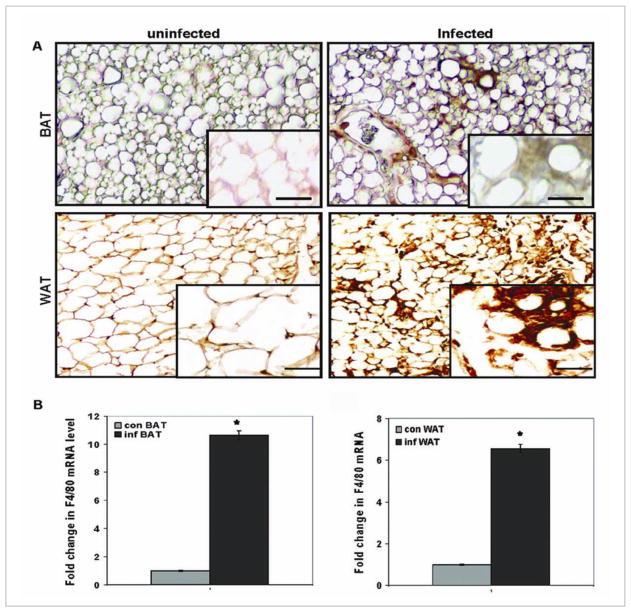
Fig. 2. Macrophages in adipose tissue.
Immunohistochemical analysis using antibody against ionized calcium-binding adaptor molecule 1 (Iba1) of adipose tissue obtained from mice. (A) Brown adipose tissue (BAT), obtained from the interscapluar region and white adipose tissue obtained from the subcutaneous region (WAT). Note the increase in macrophages in the infected adipose tissue. (B) Macrophage-specific F4/80 messenger RNA (mRNA) demonstrating the increase in macrophages in both BAT and WAT, as determined by real-time quantitative polymerase chain reaction. Con=control; Inf=infected (reproduced from Nagajyothi et al. Journal of Infectious Disease [45] with permission from the Journal and the Oxford University Press).
Importantly, Nagajyothi et al [49] found that T. cruzi-infected cultured adipocytes displayed a reduction in levels of adiponectin and PPAR-γ and a concomitant increase in many inflammatory mediators. These observations may mean that the inflammatory phenotype observed in adipose tissue obtained from T. cruzi-infected mice can be attributed to infection of adipocytes per se. Thus, the chronic inflammatory state observed in adipose tissue may lead to insulin resistance and enhance heart disease in patients with Chagas disease. This remains an active area of investigation.
Innate Immunity to T. cruzi Infection: Experimental Studies
Control of T. cruzi depends both on innate and acquired immune responses which are triggered during early infection, critical for host survival, and involve macrophages, natural killer cells, T and B lymphocytes, and the production of pro-inflammatory Th-1 cytokines such as IFN-γ, TNF-α, and IL-12. Excellent recent reviews have discussed in detail the B and T cell immunity to T. cruzi in experimental models (e.g., [50,51,52]). Herein, we focus on innate immune responses to T. cruzi infection that have primarily been studied by using experimental models of infection and have provided important information regarding mechanisms of cell activation and parasite control [53,54].
Macrophage activation and cytokine/chemokine response
Studies in experimental models have shown that macrophages, dendritic cells and natural killer cells play an important role in parasite control via elicitation of an intense inflammatory reaction, accompanied by an up regulation of cytokines and chemokines [55,56,57]. The interaction of T. cruzi with macrophages and other cell types involved in the innate immune response are mediated by pathogen recognition receptors such as Toll-like receptors (TLRs) and a family of type I trans-membrane receptors. Upon recognition of pathogen-associated molecular patterns (PAMPs), TLRs transmit the signal via cytoplasmic Toll/IL-1R domains for the recruitment of cytosolic adaptor molecules including myeloid differentiation primary-response protein 88 (MyD88), and subsequently induce nuclear factor-κB (NFκB) activation, leading to the production of inflammatory cytokines and linking the innate to the adaptive immune response [56]. T. cruzi-derived molecules, such as glycosylphosphatidylinositol (GPIs) and mucins stimulate the synthesis of IL-12 and TNF-α by macrophages [58,59]. The mucin-linked GPI anchors induced TLR2-dependent leukocyte recruitment via CCL2 [58,60]. The parasite also expresses cruzipain, a kinin-releasing cysteine protease, which induces dendritic cell maturation via the activation of bradykinin (BK) B2 receptors (B2R) [59,61]. TLR2 activation by T. cruzi links early-phase inflammation to dendritic cell-driven mechanisms that stimulate Th1 responses via the cruzipain/kinin/B2R pathway [59,62]. Neutrophils expressing TLR2 induce the extravasation of plasma proteins into interstitial spaces, thus allowing for the cruzipain-mediated release of the dendritic cells and the stimulatory peptide BK at the downstream end of the inflammatory process. It has been suggested that T. cruzi evokes interstitial edema through the sequential activation of TLR2/CXCR2/B2R characterized as an innate axis pathway fueled by the cooperative effects of exogenous and endogenous danger-type signals [59]. TLR4 and TLR9 likely recognize parasite-derived GIPLs and DNA, respectively, and cooperate in the activation of host innate immune response leading to infection regulation [58,63]. Other TLRs have been suggested to be activated by as yet unrecognized T. cruzi-derived components [63,64].
T. cruzi-induced up regulation of IL-12 mediates IFN-γ production through activation of NK cells and the induction of the Th1 cells [63]. IFN-γ activates the macrophage expression of iNOS and nitric oxide (NO) production [63,65,66]. TNF-α provides a second signal stimulating NO production and anti-T. cruzi activity in IFN-γ activated macrophages as well as infected cardiac myocytes [56,66,67,68], and thus mediates the trypanocidal function via an autocrine pathway.
Others have shown that the immuno-regulatory cytokines IL-10 and TGF-β with a susceptibility to acute infection [69,70]. By using genetic knockout mice or antibodies for depletion of specific immune molecules, it was shown that blockage of type 1 cytokines (IFN-γ, TNF-α) correlates with an increased susceptibility to T. cruzi infection [71,72](reviewed in [73]). Neutralization of endogenous IL-10 leads to an increased T. cruzi-induced IFN-γ production and parasite killing, which may point to IL-10 as a potent inhibitor of IFN-γ production during infection in mice, and the resistance to infection is a result of the balance between IFN-γ and IL-10 produced [69]. Indeed, a complete absence of Th2 or anti-inflammatory cytokines had severe negative effects on the infected host; IL-10-deficient mice infected with T. cruzi developed a syndrome similar to that of endotoxic shock due to the enhanced production of TNF-α and IFN-γ [74].
Non-immune cells also respond to T. cruzi infection by cytokine production. For example, infection of endothelial cells with T. cruzi causes the direct induction of IL-1β and IL-6 [75]. Trans-sialidase, a released surface protein of T. cruzi, induced IL-6 production in isolated endothelial cells [76]. Our finding of increased TNF-α and IL-1β mRNAs in infected cardiac myocytes suggested to us that cardiac myocytes also respond to T. cruzi by inflammatory cytokine production [77]. Whether the cytokine response by the non-immune cells is a component of innate immunity or a bystander effect to T. cruzi infection is not known, and it remains to be investigated in future studies. Collectively, these results underscore the importance of both inflammatory and anti-inflammatory responses during T. cruzi infection, and indicate that IL-4 + IL-10/TNF-α + IFN-γ ratio may be an important determinant of desirable outcome.
The cytokines synthesized during T. cruzi infection (IFN-γ, TNF-α, IL-1α TGF-β and IL-10) are capable of inducing or regulating the production of chemokines in infected macrophages and cardiac myocytes [68]. The enhanced expression of CCL3 (MIP-1α), CXCL10 (IP-10), and CCL5 (RANTES) at both the mRNA and protein levels has been noted in macrophages and myocardium of T. cruzi-infected experimental models [78,79,80]. Chemokines stimulated the infected macrophages in an autocrine manner, enhancing NO release and NO-depend killing of the parasites [68]. Chemokines and their receptors also affect T-cells proliferation, Th1/Th2 differentiation [81] and resistance to infection [68,82]. CCR5 and CXC3 are immunological preferential markers of the Th1 response, and CCR3 and CCR4 are preferentially associated with Th2 response [83,84]. Others have suggested that chemokines contribute to the pathogenesis of T. cruzi infection due to their effects on leukocyte migration and activation [82].
Lipid mediators of immune responses
In addition to cytokines and chemokines, eicosanoids, i.e., lipid mediators, also play a role during T. cruzi infection. The arachidonic acid derivatives, including leukotrienes (LTB4) and cysteinyl lymphotoxins (LTC4, LTD4 and LTE4) are produced during experimental T. cruzi infection [85,86,87]. The protein responsible for the synthesis of leukotrienes is the enzyme 5-lipoxigenase (5-LO), which is primarily expressed in macrophages, granulocytes, mast cells, and dendritic cells. Resident and recruited leukocytes are capable of synthesizing LTB4 during infection and activate macrophages to kill intracellular forms of T. cruzi [88]. Deficiency of 5-LO resulted in a dramatic increase in the peak of parasitemia; however, 5-LO knockout mice were eventually able to control parasites and exhibited increased survival and reduced cardiac damage and scarce tissue parasitism [89].
5-LO induces Lipoxin (LX) A4 production that triggers the AhR receptor altering the expression of suppressor of cytokine signaling (SOCS) 2 and serves as an anti-inflammatory eicosanoid. Esper et al [90] recently demonstrated the role of SOCS2 in an experimental model of Chagas disease; T. cruzi infection induced SOCS2 expression in the heart and spleen of mice which was, in part, dependent on the activation of the 5-LO pathway. Moreover, SOCS2 deficiency resulted in a reduction in peripheral parasitemia, but not in heart parasitism, and in the down-modulation of pro-inflammatory cytokines, including TNF-α, IL-12 and IFN-γ, associated with a reduction in myocardial inflammation. Although IFN-γ was reduced, SOCS2-deficient macrophages were hyper-responsive to this cytokine and produced increased levels of NO and dealt with infection more efficiently. In addition, T. cruzi-infected SOCS2 knockout mice displayed an enhanced number of T regulatory cells and levels of LXA4 associated with decreased inflammatory responses and reduced production of pro-inflammatory mediators [90]. In the absence of SOCS2, there was myocardial hypertrophy and cardiac myocyte dysfunction. These results may indicate that production of a 5-LO-derived molecule, presumably LXA4, contributes to SOCS2 expression during experimental T. cruzi infection. It should be noted that LXA4 may function in a SOCS2-dependent and independent manner during infection. As the lipid was enhanced in the absence of SOCS2, it is clear that SOCS2-independent pathways could be operational in the latter animals to mediate any anti-inflammatory actions of LXA4 during infection. Interestingly, the phenotype of 5-LO-deficient mice infected with T. cruzi is, in part, similar to those observed in SOCS2-deficient mice. TXA2, another eicosanoid, has also been shown to participate in regulation of intracellular amastigote proliferation, providing opportunities to survive from infection, but result in transition into a chronic persistent state of disease (discussed above).
Reactive oxygen species
The two major reactive oxygen species (ROS) producers relevant in Chagas disease are NADPH oxidase (gp91phox), renamed NOX2, and mitochondria (Fig. 3). NOX2 catalyzes rapid ROS production by the one-electron reduction of O2, referred to as a respiratory burst that serves as the first line of host defense against microbes. Early studies demonstrated cytochemical detection of NOX2 at the plasma membrane of peritoneal mouse macrophages during interaction with T. cruzi [91]. Others have used in vitro assay systems or animal models and demonstrated that T. cruzi-mediated macrophage activation results in increased levels of O2•− formation, likely by NOX-dependent oxidative burst [92,93,94]. We have extended these observations and shown that splenocytes of infected mice and in vitro cultured macrophages respond to T. cruzi infection by activation of NOX2 and a substantial increase in ROS production (Dhiman & Garg, unpublished data). A robust response of splenocytes of infected mice to T. cruzi antigenic lysate led us to speculate that parasite factors (and not active invasion) are sufficient to activate NOX and ROS generation. Indeed, T. cruzi-derived components are recognized by TLRs and NOD-like receptors, implicated in NOX activation [63]. Yet, further studies are required to identify the T. cruzi-generated stimuli that activate TLR- and NOD-signaling mechanisms and initiate translocation of cytosolic components (p47phox, p67phox, and G protein) and NOX assembly during Chagas disease. In the heart, in response to T. cruzi infection, infiltrating activated neutrophils and macrophages are a major source of NOX- and myeloperoxidase-dependent ROS during the acute stage, though mitochondrial release of electrons and superoxide production in infected cardiac myocytes is also noted [95]. Superoxide and NO promote peroxynitrite-mediated killing of T. cruzi in macrophages [96,97]. The reactive oxygen species (ROS) are also important regulators of parasite control, modulating the cytokine responses and splenic inflammatory cell proliferation during T. cruzi infection, as well as playing an essential role in the formation of acquired immunity for parasite killing [98], discussed below.
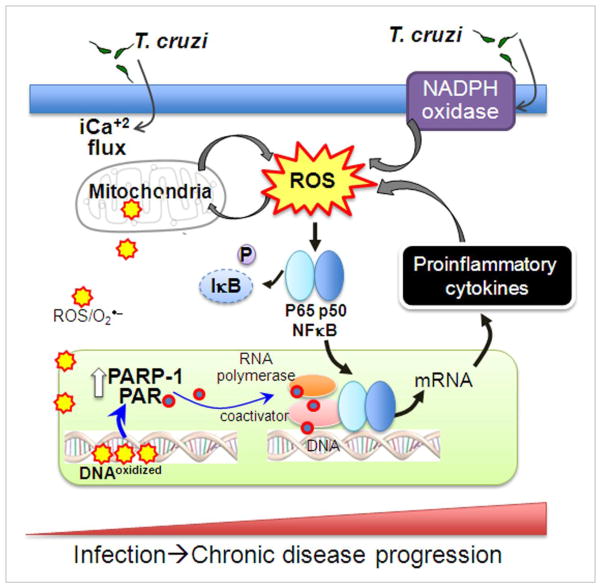
Fig. 3. Oxidative stress in Chagas disease.
T. cruzi infection elicits generation of superoxide (O2•−) through activation of NADPH oxidase in phagocytic cells. T. cruzi invasion of other cells elicits intracellular Ca+2 flux affecting mitochondrial membrane potential and results in increased leakage of electrons from the electron transport chain to molecular oxygen and formation of O2•− and other reactive oxygen species (ROS). Two pathways of ROS signaling of the NFκB pathway are envisioned. One, ROS directly promotes phosphorylation and transport of p65 (RelA) and p50 to nucleus, assembly of transcription complex, and expression of proinflammatory cytokines (e.g. IL-1β, TNF-α). Two, ROS-mediated oxidation of DNA may signal activation of poly (ADP-ribose) polymerase (PARP-1). PARP-1 cleaves NAD+ to form ADP-ribose and polymerizes the latter onto nuclear acceptor proteins (e.g. histones), transcription factors, and PARP itself, and contributes to DNA repair and genomic stability. Binding of poly ADP-ribose (PAR) molecules activates NF-κB transcription complex and inflammatory cytokine gene expression. The feed-back loop of ROS generation and cytokine gene expression contribute to persistence of inflammatory responses and oxidative damage, and progressive evolution of cardiomyopathy in Chagas disease.
ROS signaling of cytokine responses
ROS are critical signaling intermediates linking the innate and adaptive immune systems by triggering the production of proinflammatory cytokines (TNF-α, IL-1β) by macrophages and dendritic cells (DCs) of the innate immune system. Inhibition studies with cultured and primary macrophages showed that NOX/ROS was a critical regulator of cytokine production in response to T. cruzi infection. In vivo studies using splenocytes of T. cruzi-infected mice, with or without in vitro stimulation with parasite antigens, validated the above observations and demonstrated that the inhibition of NOX by apocynin or use of ROS scavengers substantially blocked the activation and proliferation of phagocytes and inflammatory mediators (IL-1, IL-6, IFN-γ, and TNF-α) [99]. Subsequently, inhibition of NOX/ROS resulted in an increased susceptibility to T. cruzi, a finding that suggested that redox status plays an important role in immune activation and control of T. cruzi, also validated by studies in p47phox−/− mice, in which T. cruzi infection, even at a dose 10-fold lower than that used for wild-type mice, was lethal (Dhiman & Garg, unpublished data). Further studies will be required to delineate if NOX/ROS signal the nuclear transport and activation of transcription factors (e.g., NF-κB and AP-1) and promote cytokine gene expression; or if NOX/ROS elicit immune cell proliferation and thereby indirectly alter the cytokine profile in infected mice.
Others have demonstrated that trypomastigotes (or T. cruzi-derived proteins, e.g. trans-sialidase) activate NF-κB in a number of cell types, including epithelial cells, endothelial cells, myocytes, and fibroblasts [100,101,102]. NF-κB activation increased the resistance to infection in many of these cell types. Except for myocytes, these studies, however, did not attempt to determine if ROS signaled the NF-κB-dependent cytokine gene expression in non-phagocytic cells invaded by T. cruzi. Subsequently, the Garg group reported that ROS of mitochondrial origin elicited cytokine gene expression in cardiac myocytes infected by T. cruzi via multiple mechanisms. One, mtROS enhanced the nuclear translocation of RelA (p65), thereby activating NF-κB-dependent gene expression of inflammatory cytokines (e.g. TNF-α, IFN-γ, IL-1β) [77,103]. Two, ROS caused 8-hydroxyguanine (8-oxoG) lesions and DNA fragmentation that signaled polyadenosine ribose polymerase 1 (PARP-1) activation, evidenced by poly-ADP-ribose (PAR) modification of PARP-1 and other proteins in infected cardiac myocytes. PARP-1 signals DNA repair via PARylation of histones; however, its hyper-activation may have pathophysiological effects ranging from the catalytic activation of inflammatory and hypertrophic gene expression, depletion of NAD+ pool, and cell death [104,105]. Inhibition of PARP-1, by using RNAi or a chemical inhibitor (PJ34), or by removing ROS with an antioxidant, was beneficial in blocking mtROS formation and DNA damage [77]. Importantly, we found that PARP-1 inhibition also regulated cytokine gene expression, albeit via a different mechanism. PARP-1 did not directly interact with p65, and it did not signal RelA (p65) translocation to nuclei in infected cardiac myocytes. Instead, PARP-1 contributed to PAR modification of RelA (p65)-interacting nuclear proteins and assembly of an NF-κB transcription complex. These studies pointed to the possibility that the ROS-PARP-1-RelA signaling pathway contributes to inflammatory cytokine production in cardiac myocytes infected by T. cruzi (Fig. 3). It remains to be seen whether mitochondria serve as an activator of an innate defense response by cardiac myocytes upon T. cruzi exposure or if these events are bystander effects of T. cruzi infection of the host cells.
Oxidative stress in Chagas disease
In addition to pro-inflammatory cytokines, pro-oxidants also affect cardiac function in chagasic conditions. Studies in experimental models and infected humans demonstrate that an infected host sustains oxidative stress due to T. cruzi-elicited, splenic NOX/ROS and the enhanced mitochondrial release of ROS in the myocardium [95]. Our studies demonstrate that the host responds to acute T. cruzi infection by up regulating its glutathione antioxidant defense constituted by GPx, GSR, and GSH. However, in the chronic phase, the pro-oxidant milieu of the heart was evidenced by a) increased ROS levels, b) decreased activity of MnSOD, c) insensitivity of glutathione defense to oxidative stress, and d) increased GSSG, and lipid (MDA) and protein (carbonyl) oxidation products [106]. A similar pro-oxidant status in seropositive humans has been reported and demonstrated by a) increased GSSG and MDA contents; b) decreased MnSOD, GPX activity and GSH contents [107,108]; and c) inhibition of CIII activity [109]. Moreover, the treatment of T. cruzi-infected animals with an antioxidant tipped the balance in favor of preserving mitochondrial and cardiac function. T. cruzi-infected mice and rats, treated with an antioxidant, exhibited a significant decline in the myocardial accumulation of oxidative adducts concurrent with improved mitochondrial function as evidenced by increased ATP synthesis and decreased ROS production [110]. Importantly, preventing the oxidative injuries during the chronic stage preserved the cardiac hemodynamic state that otherwise was compromised in chagasic rats [111]. Others have shown a decline in oxidative stress in human chagasic patients given Vitamin A [112]. All of these observations support the idea that antioxidant depletion and inefficient scavenging of ROS, resulting in sustained oxidative stress, are of pathological importance in human chagasic cardiomyopathy progression (Fig. 3).
The re-expression of fetal genes (ANP, BNP, αsk-Actin and β-MHC) is a hallmark of hypertrophic remodeling, and a considerable body of evidence shows the redox regulation of various signaling cascades and remodeling responses in cardiac diseases of various etiologies [113]. Current research findings support the involvement of the following pathways: (i) ERK-1/2 [114] and the small GTPase Ras [115] in response to α-adrenergic agonist stimulation and angiotensin II [116]; (ii) MAPKs in pressure-overload hypertrophy [117]; and (iii) NF-κB and apoptosis-signal-regulated kinase 1 (ASK-1) in response to angiotensin II infusion [118]. ASK-1 is upstream of p38MAPK and JNK in the MAPK-signaling cascade, and both of these have been shown to be activated by NOX/ROS [119]. The inhibition or scavenging of free radicals has been shown to modulate ERK signaling and hypertrophic responses in neonatal and adult cardiac myocytes [120,121].
Mice and cultured myoblasts infected with T. cruzi display increased expression of ERK and cyclin D1 [122,123], as well as of ERK activator protein 1 (AP-1) and NFκB, and a reduced expression of Cav-1 and Cav-3 [124,125]. The protein caveolin (Cav) is a negative regulator of both ERK and cyclin D1 [126]. Cav-3 is expressed only in cardiac myocytes, while Cav-1 is expressed in cells other than cardiac myocytes. Cav-1 and Cav-3 null mice and Cav-1/Cav-3 double null mice display a cardiomyopathic phenotype associated with cardiac myocyte hypertrophy and interstitial fibrosis [127,128,129]. These observations, along with the knowledge of a decline in the expression of hypertrophic markers and collagen deposition in response to antioxidant treatment, suggest that ROS signals pathological hypertrophic remodeling in chagasic myocardium, likely through ERK/AP-1 signaling. The role of ROS from a mitochondrial, but not inflammatory, origin in signaling hypertrophy in chagasic hearts was evidenced by the observation that NOX and MPO, the classical mediators of inflammatory ROS, were equally depressed upon treatment of infected rodents with an anti-parasite drug (benznidazole) and ROS scavenger (PBN), while a hypertrophic phenotype was depressed in PBN-treated rodents only [111]. Inflammatory cytokines (e.g., TNF-α, IL-1β, and MCP-1) have been shown to also promote myocardial hypertrophy and contribute to the development and progression of heart failure [130]. Further studies are required to identify whether inflammatory cytokines, noted to be enhanced in chagasic experimental animals and human patients (reviewed in [73,131]), synergistically enhance the ROS-mediated signaling cascades involved in the activation of hypertrophic responses in chagasic hearts.
Human Immunity To T. cruzi Infection
The contact between T. cruzi and cells from the human host triggers an immune reaction that leads to the control of parasite levels. Yet, chronic persistence of parasites, albeit at low levels, and inflammation are hallmarks of Chagas disease in humans. In this section, we focus on human responses to T. cruzi (Fig. 4) and briefly present studies in experimental animals.
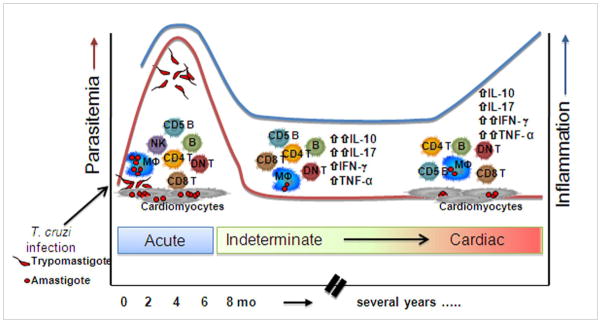
Fig. 4. Progression of human Chagas disease.
Following infection with T. cruzi the majority of individuals develop parasitemia associated with a robust cellular and humoral immune response involving cells of the innate and adaptive immune response. This stage can present with severe clinical features ranging from high fever to arrhythmia and death. Upon successful control of the initial infection by the immune response, parasitemia wanes and symptoms subside, as the individual enters the chronic phase of Chagas disease. Here the majority of individuals enter the indeterminate (asymptomatic) clinical form, associated with a balanced inflammatory and regulatory immune response (low inflammatory index). Approximately 30% of the individuals will progress to the cardiac clinical form of disease associated with a higher inflammatory state. Cell types referred in the figure: CD4, CD8 and double negative (DN) T cells, macrophages (MΦ), Natural killer (NK) cells, CD5 (IgM-secreting) B cells. The increased inflammation refers to data demonstrating an up regulation of the inflammatory response (cytokines, chemokines).
Two main strategies have been employed to understand the immunological mechanisms triggered by the infection of human cells with T. cruzi: (1) in vitro studies of infection of different human cell types with trypomastigote forms of the parasite, and (2) analysis of the immune response in acutely infected patients. That acute infection is not always clinically apparent presents a challenge when performing studies during this phase of the disease. Despite this difficulty, a few studies were recently performed and the findings seem to indicate that innate immune cells, such as natural killer cells (NK) and macrophages, mediate the control of parasite replication in the early stages of human infection. In addition to the role of these cells, antibody production seems to also play an important part in the early control of parasitemia.
Humoral responses
Two types of antibodies are produced during infection: diagnostic antibodies, which form the basis for the serological diagnosis of Chagas disease, and lytic antibodies, which have been defined as antibodies that mediate lysis of the infective forms of the parasite [132,133]. Regardless of the type of antibody produced, it is clear that T. cruzi triggers a vigorous B cell response in acutely infected individuals [134]. Lytic antibodies were first identified in mice and demonstrated to correlate with protection to infection. Later studies showed that humans with apparent or in-apparent, acute Chagas disease had similar levels of complement-fixing antibodies [135]. IgM and IgG anti-galactose antibodies against glycoylated epitopes of parasite surface proteins has been a useful tool to discriminate between early, indeterminate and late stages of acute infection in humans due to changing ratios and specificities of the two types as disease progresses [136]. More recently, an association between the presence of lytic antibodies and a protective response in chronic patients was described and strongly indicated that patients in the indeterminate phase displayed higher levels of lytic antibodies compared with those in patients with chagasic heart disease. These data pointed to a possibly protective role for these antibodies [137]. Lytic antibodies have also been brought to attention as a possible marker of parasitological cure, since treated patients that displayed negative hemo-cultures for over ten years did not have circulating lytic antibodies, despite occasionally testing positive by conventional serology [138]. While lytic antibody production depends on the presence of active infection, the loss of such markers does not occur immediately after effective treatment. Thus, they cannot be used as markers for parasitological cure immediately after treatment, but may be useful years after treatment when clearance of preformed antibodies has been achieved.
In addition to their possible role in parasite control and maintenance of protective responses in chagasic patients, antibodies may also contribute to the pathologic alterations observed in this disease. Several studies have shown that individuals within the chronic phase of Chagas disease produce antibodies capable of recognizing several host proteins, which may lead to tissue damage. Among these self-reactive antibodies, the anti-beta adrenergic receptor and the anti-myosin antibodies have been associated with auto-reactive responses [139,140]. The mechanisms by which such antibodies are produced have not been completely clarified, though evidence of T. cruzi-derived cross-reactive molecules exists [141]. A recent study proposed that cardiac antigens exposed to T. cruzi-induced oxidative stress produce neo-antigens eliciting self-directed antibody response [142]. Others have shown that anti-epimastigote antibodies purified from the sera of chronically infected patients are capable of triggering proliferative responses in peripheral blood mononuclear cells from Chagas patients [143,144,145]. CD5+ (B1) cells, the main cell type that proliferate upon stimulation with such anti-epimastigote antibodies [146], have been demonstrated to correlate with pathology development in experimental models of T. cruzi infection [147]. This finding may mean that anti-parasite antibodies or antibodies against host-derived neo-antigens could serve as a constant source of stimulation, providing a mechanism that would perpetuate cellular activation during the chronic phase when parasitemia is particularly low. The antigens responsible for differential B cell activation, the mechanisms that lead to the production of lytic versus conventional antibodies, and the role of antibodies in the cross talk between different cell types are questions that require further investigation.
Natural killer cells
Recent observations point to NK cells as important in the regulation of parasitemia. For example, Sathler-Avelar et al. analyzed patients classified as being in the early acute, late acute and early chronic phases of Chagas disease, based upon the expression of anti-gal IgM and IgG antibodies [148]. These authors reported that patients in the early stages of the acute disease displayed an expansion of B cells, but no significant changes in the frequency of NK cell population. However, during the late acute stage, patients displayed a selective increase in a distinct lineage of NK cells (CD16+CD56−), as well as a persistent expansion of B cells, possibly indicative of a relationship between B cell activation and a subset of NK cells. Recent studies demonstrated that NKT cells provide help for the development and response of lipid-specific B cells in the mouse [149]. While the response to lipid antigens remains poorly understood in Chagas disease, the relationship between lipid metabolism and inflammatory response in T. cruzi infection has been demonstrated, as discussed above, and is an area that merits greater investigation.
T cell responses
There are few studies characterizing the T cell responses during acute infection in humans (Fig. 5). Early investigations suggested that T. cruzi infection leads to inhibition of T cell responses, since acutely infected patients failed to mount a delayed type response to a series of non-related antigens [135]. The mechanism of this impaired response was later associated with a low expression of IL-2 receptor, induced by T. cruzi [150]. A study of the T cell repertoire in acutely infected patients from Bolivia showed a marked decrease in the frequency of T cells expressing the variable region 5 (Vβ5) of the T cell receptor (TCR) [151] perhaps indicating that a vigorous T cell response, followed by clonal exhaustion, could lead to the decrease in the frequency of Vβ5+ T cells. The impaired T cell response observed in acutely infected patients could reflect a temporarily suppressed or exhausted response rather than the lack of a response. Interestingly, Vβ5+ TCR-expressing T cells are highly expanded in chronic chagasic patients, observed in both freshly isolated PBMCs as well as after in vitro stimulation with parasite antigens [151,152], possibly indicative of the pathologic significance of vβ5 containing TCR. Finally, the involvement of T cells in the acute phase may be signaled by the identification of T cells in the inflammatory infiltrate in the hearts of acutely infected patients. These patients presented with a myocarditis associated with the presence of CD4+ and CD8+ T cells, as well as parasite antigens [153], which may indicate the pathologic significance of T cells in acute myocarditis. Results in studies in the murine model of T. cruzi infection could point to a protective role for CD8+ T cells in parasite control during acute infection [52,154]. To our knowledge, there is little evidence to support this finding in human disease.
In patients progressing to the chronic phase, a robust expansion of T cell response to parasite and host-derived antigens has been clearly demonstrated [146,155,156,157]. A high frequency of activated T cells was found in peripheral blood of indeterminate and cardiac patients [157,158] and in inflammatory infiltrate observed in the hearts of cardiac patients [159]. Detection of high levels of ICAM-1 expression by endothelial cells in the heart [160] and of the chemokine receptors CCR5 and CCR7 by infiltrating inflammatory cells in the myocardium [161,162] may mean that these molecules play a role in the mechanisms of T cell recruitment to the heart of chagasic patients. The CD8+ granzyme+ T cells were the main cell type found in infiltrating infiltrate in the myocardium. Based upon these observations, it is currently believed that CD8+ T cells play an important role in tissue destruction in Chagas disease [163,164]. However, others have suggested that CD8+ T cells undergo immunological exhaustion in chronic patients and, thus, their lack of activity could contribute to the establishment of pathology [165]. In summary, it is difficult to define a single role for CD8+ T cells in human Chagas disease, and the evidence points to the involvement of these cells in several distinct processes during disease progression including; a) playing a role in parasite control during acute infection, b) mediating pathology in the chronic phase, and c) a possible lack of activity aiding in the establishment of pathology. The body of work already generated concerning CD8+ T cells in human Chagas disease leads to speculation that distinct populations of CD8+ T cells may co-exist during the course of Chagas disease and play different functions. Future studies would be required to test this hypothesis and further our understanding of the role of T cells in Chagas disease.
CD4+ T cells and monocytes/macrophages act as key orchestrators of the cellular response during chronic Chagas disease. These cells are capable of producing inflammatory and anti-inflammatory cytokines after stimulation with parasite-derived antigens or upon in vitro infection with trypomastigote forms of T. cruzi. Several studies have demonstrated a correlation between the production of inflammatory cytokines by CD4+ T cells and monocytes of patients with the cardiac form of the disease, and the production of IL-10 by the same cells of clinically asymptomatic patients [166,167]. For example, peripheral blood mononuclear cells from chronic chagasic patients produce more IFN-γ and less IL-10 than do those from indeterminate patients [167]. Accordingly, chagasic patients exhibit a Th1 type (IFN-γ) cytokine profile with suppression of Th2 type cytokines (IL-4, IL-10), and elevated plasma levels of TNF-α in the chronic stage [168,169].
Increased myocardial expression of the adhesion molecules MCP-1, IP-10 and MIG, their receptors CCR2 and CXCR3, and cytokines IFN-γ, TNF-α, IL-15, IL-6 and IL-4 has been reported in chagasic patients by several researchers [167,170]. High levels of TNF-α have been associated with worse cardiac function. Gene expression profiling of myocardial tissue from chagasic experimental animals and human patients showed that 15% of the genes known to be selectively up regulated are IFN-γ-inducible [171]. These observations point to the pathologic significance of IFN-γ and TNF-α in chagasic cardiomyopathy. Notably, the regulatory FoxP3+IL-10+ T cells are predominantly found in indeterminate patients, providing evidence for the importance of IL-10 producing cells in the regulation of pathogenic responses in asymptomatic patients [172].
It has been demonstrated that T cells that do not express the co-receptors CD4 and CD8 (referred to as double negative T cells) have emerged as abundant producers of immunoregulatory cytokines in chagasic patients [173]. The double-negative T cells appear at a low frequency in the peripheral blood of chagasic patients, but are highly expanded by exposure to T. cruzi trypomastigotes and express preferentially inflammatory and anti-inflammatory cytokines in cardiac and indeterminate patients, respectively. This finding relates to the possible role of lipid antigens in the T. cruzi – host interaction, given that many double-negative T cell populations are preferentially activated by lipid antigens. Given the particular characteristics of these cells to mount fast responses and to tolerate chronic stimulation, it is possible that double-negative T cells also represent a link between innate and adaptive responses in Chagas disease [174]. Thus, the regulatory networks established during the chronic phase of Chagas disease are critical for the clinical evolution of patients into indeterminate or cardiac disease stage. The indeterminate patients are capable of producing inflammatory and anti-inflammatory cytokines; the balance between these molecules in chronic phase may ultimately determine disease pathogenesis [163].
A critical question is what drives the anti-inflammatory balance observed in indeterminate patients towards an inflammatory environment observed in cardiac patients? (Fig. 5). It is possible that cardiac patients display a genetic predisposition towards the establishment of inflammatory responses. Supporting this hypothesis are several studies that have shown polymorphism of genes encoding cytokines, which affect protein expression levels, is associated with the indeterminate and cardiac forms of the disease [167]. Of note, a functional polymorphism in the promoter region of IL-10, which leads to the low expression of this cytokine, is associated with the cardiac form of Chagas disease [175]. Additionally, the occurrence of a promoter region polymorphism in the TNF-α gene leading to high expression of this cytokine is associated with worse prognosis and progression to death for cardiac chagasic patients who undergo a heart transplant [175]. However, it is important to consider that, even patients who display genetic susceptibility profiles to develop cardiac disease still go through the indeterminate form. Thus, it is possible that external factors will combine with genetic risk factors to trigger the shift in cytokine balance from the controlled, regulatory profile observed in indeterminate patients to the exacerbated inflammatory profile observed in cardiac patients. Epigenetic mechanisms and gene regulation studies may help clarify this question.
Summary and Future Perspectives
The literature strongly suggests that infected host responds to T. cruzi infection by eliciting inflammatory cytokines (TNF-α, IFN-γ) and ROS production. A sustained ROS generation of inflammatory and mitochondrial origin, coupled with an inadequate antioxidant response, results in the inefficient scavenging of ROS in the heart and leads to long-term oxidative stress. Thus, while ROS are essential for activation of inflammatory responses and pathogen control at an acute stage, the persistent oxidative stress denies the control of the inflammatory state. Further, intracellular T. cruzi or Tc-antigens that persist during late or chronic infection might interact with the immune and non-immune cells in the myocardium and subsequently activate signaling cascades (e.g NF-κB pathway) that trigger the production of inflammatory cytokines (TNF-α, IL-1β), ROS induced-DNA damage, and hypertrophy in cardiac myocytes. Importantly inflammatory cytokines and ROS create a complex feedback mechanism that can positively sustain stress responses and, thus, play an important role in cardiac remodeling and the evolution of chronic Chagas disease. We propose that a substantial effort should be made in delineating the complex interrelationships between oxidative stress and inflammatory mediators, wherein the promise of antioxidant and anti-inflammatory therapies in controlling progressive chagasic cardiomyopathy can be realized.
As noted, there remains a dearth of data on the immune response in human Chagas disease, and these data are required to understand the pathogenesis of this important disease. In addition, these data are needed for vaccine development as well as for the development of new therapeutic agents. The current therapies are prolonged and have unacceptable side-effects. We do not know after years of efforts whether these agents actually prevent the evolution to chronic disease in humans.
Acknowledgments
The work in authors’ laboratories is supported in part by National Institutes of Health grants AI076248, HL73732, AI06538 (HBT) and AI054578, HL088230, HL094802 (NJG), CNPq and FAPEMIG grants (FSM, WOD, KJG), INCT-DT (WOD and KJG) and the Einstein Diabetes Research and Treatment Center Grant (HBT).
References
- 1.Aufderheide AC, Salo W, Madden M, Streitz J, Buikstra J, et al. A 9,000-year record of Chagas’ disease. Proc Natl Acad Sci U S A. 2004;101:2034–2039. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo A, Jansen AM, Reinhard K, Ferreira LF. Paleoparasitology of Chagas disease--a review. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):9–16. doi: 10.1590/s0074-02762009000900004. [DOI] [PubMed] [Google Scholar]
- 3.Cantey PT, Stramer SL, Townsend RL, Kamel H, Ofafa K, et al. The United States Trypanosoma cruzi Infection Study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion. 2012;52:1922–1930. doi: 10.1111/j.1537-2995.2012.03581.x. [DOI] [PubMed] [Google Scholar]
- 4.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49:e52–54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 5.Basile L, Jansa JM, Carlier Y, Salamanca DD, Angheben A, et al. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 6.Tanowitz HB, Weiss LM, Montgomery SP. Chagas disease has now gone global. PLoS Negl Trop Dis. 2011;5:e1136. doi: 10.1371/journal.pntd.0001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caradonna KL, Burleigh BA. Mechanisms of host cell invasion by Trypanosoma cruzi. Adv Parasitol. 2011;76:33–61. doi: 10.1016/B978-0-12-385895-5.00002-5. [DOI] [PubMed] [Google Scholar]
- 8.Nagajyothi F, Weiss LM, Silver DL, Desruisseaux MS, Scherer PE, et al. Trypanosoma cruzi utilizes the host low density lipoprotein receptor in invasion. PLoS Negl Trop Dis. 2011;5:e953. doi: 10.1371/journal.pntd.0000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shikanai-Yasuda MA, Carvalho NB. Oral transmission of chagas disease. Clin Infect Dis. 2012;54:845–852. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- 10.Hoff R, Teixeira RS, Carvalho JS, Mott KE. Trypanosoma cruzi in the cerebrospinal fluid during the acute stage of Chagas’ disease. N Engl J Med. 1978;298:604–606. doi: 10.1056/NEJM197803162981106. [DOI] [PubMed] [Google Scholar]
- 11.Carod-Artal FJ, Vargas AP, Falcao T. Stroke in asymptomatic Trypanosoma cruzi-infected patients. Cerebrovasc Dis. 2011;31:24–28. doi: 10.1159/000320248. [DOI] [PubMed] [Google Scholar]
- 12.Fiorelli AI, Santos RH, Oliveira JL, Jr, Lourenco-Filho DD, Dias RR, et al. Heart transplantation in 107 cases of Chagas’ disease. Transplant Proc. 2011;43:220–224. doi: 10.1016/j.transproceed.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez FR, Lalu MM, Mariano FS, Milanezi CM, Cena J, et al. Increased activities of cardiac matrix metalloproteinases matrix metalloproteinase (MMP)-2 and MMP-9 are associated with mortality during the acute phase of experimental Trypanosoma cruzi infection. J Infect Dis. 2008;197:1468–1476. doi: 10.1086/587487. [DOI] [PubMed] [Google Scholar]
- 14.Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol Biochem Parasitol. 1999;100:163–172. doi: 10.1016/s0166-6851(99)90035-x. [DOI] [PubMed] [Google Scholar]
- 15.dos Santos DM, Talvani A, Guedes PM, Machado-Coelho GL, de Lana M, et al. Trypanosoma cruzi: Genetic diversity influences the profile of immunoglobulins during experimental infection. Exp Parasitol. 2009;121:8–14. doi: 10.1016/j.exppara.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Vago AR, Andrade LO, Leite AA, d’Avila Reis D, Macedo AM, et al. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol. 2000;156:1805–1809. doi: 10.1016/s0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Factor SM, Sonnenblick EH. The pathogenesis of clinical and experimental congestive cardiomyopathies: recent concepts. Prog Cardiovasc Dis. 1985;27:395–420. doi: 10.1016/0033-0620(85)90002-7. [DOI] [PubMed] [Google Scholar]
- 18.Factor SM, Cho SH, Scheuer J, Sonnenblick EH, Malhotra A. Prevention of hereditary cardiomyopathy in the Syrian hamster with chronic verapamil therapy. J Am Coll Cardiol. 1988;12:1599–1604. doi: 10.1016/s0735-1097(88)80031-7. [DOI] [PubMed] [Google Scholar]
- 19.Sonnenblick EH, Fein F, Capasso JM, Factor SM. Microvascular spasm as a cause of cardiomyopathies and the calcium-blocking agent verapamil as potential primary therapy. Am J Cardiol. 1985;55:179B–184B. doi: 10.1016/0002-9149(85)90629-0. [DOI] [PubMed] [Google Scholar]
- 20.Factor SM, Cho S, Wittner M, Tanowitz H. Abnormalities of the coronary microcirculation in acute murine Chagas’ disease. Am J Trop Med Hyg. 1985;34:246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- 21.Tanowitz HB, Kaul DK, Chen B, Morris SA, Factor SM, et al. Compromised microcirculation in acute murine Trypanosoma cruzi infection. J Parasitol. 1996;82:124–130. [PubMed] [Google Scholar]
- 22.Chandra M, Shirani J, Shtutin V, Weiss LM, Factor SM, et al. Cardioprotective effects of verapamil on myocardial structure and function in a murine model of chronic Trypanosoma cruzi infection (Brazil Strain): an echocardiographic study. Int J Parasitol. 2002;32:207–215. doi: 10.1016/s0020-7519(01)00320-4. [DOI] [PubMed] [Google Scholar]
- 23.De Souza AP, Tanowitz HB, Chandra M, Shtutin V, Weiss LM, et al. Effects of early and late verapamil administration on the development of cardiomyopathy in experimental chronic Trypanosoma cruzi (Brazil strain) infection. Parasitol Res. 2004;92:496–501. doi: 10.1007/s00436-004-1080-1. [DOI] [PubMed] [Google Scholar]
- 24.Petkova SB, Tanowitz HB, Magazine HI, Factor SM, Chan J, et al. Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc Pathol. 2000;9:257–265. doi: 10.1016/s1054-8807(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 25.Jelicks LA, Chandra M, Shirani J, Shtutin V, Tang B, et al. Cardioprotective effects of phosphoramidon on myocardial structure and function in murine Chagas’ disease. Int J Parasitol. 2002;32:1497–1506. doi: 10.1016/s0020-7519(02)00136-4. [DOI] [PubMed] [Google Scholar]
- 26.Tanowitz HB, Huang H, Jelicks LA, Chandra M, Loredo ML, et al. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect Immun. 2005;73:2496–2503. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan GS, Mukherjee S, Nagajyothi F, Weiss LM, Petkova SB, et al. Trypanosoma cruzi infection induces proliferation of vascular smooth muscle cells. Infect Immun. 2006;74:152–159. doi: 10.1128/IAI.74.1.152-159.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salomone OA, Caeiro TF, Madoery RJ, Amuchastegui M, Omelinauk M, et al. High plasma immunoreactive endothelin levels in patients with Chagas’ cardiomyopathy. Am J Cardiol. 2001;87:1217–1220. A1217. doi: 10.1016/s0002-9149(01)01502-8. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee S, Machado FS, Huang H, Oz HS, Jelicks LA, et al. Aspirin treatment of mice infected with Trypanosoma cruzi and implications for the pathogenesis of Chagas disease. PLoS One. 2011;6:e16959. doi: 10.1371/journal.pone.0016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashton AW, Mukherjee S, Nagajyothi FN, Huang H, Braunstein VL, et al. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exp Med. 2007;204:929–940. doi: 10.1084/jem.20062432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanowitz HB, Burns ER, Sinha AK, Kahn NN, Morris SA, et al. Enhanced platelet adherence and aggregation in Chagas’ disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990;43:274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- 32.Bern C, Martin DL, Gilman RH. Acute and congenital Chagas disease. Adv Parasitol. 2011;75:19–47. doi: 10.1016/B978-0-12-385863-4.00002-2. [DOI] [PubMed] [Google Scholar]
- 33.Diazgranados CA, Saavedra-Trujillo CH, Mantilla M, Valderrama SL, Alquichire C, et al. Chagasic encephalitis in HIV patients: common presentation of an evolving epidemiological and clinical association. Lancet Infect Dis. 2009;9:324–330. doi: 10.1016/S1473-3099(09)70088-X. [DOI] [PubMed] [Google Scholar]
- 34.da Silva AA, Pereira GV, de Souza AS, Silva RR, Rocha MS, et al. Trypanosma cruzi-induced central nervous system alterations: from entry of inflammatory cells to potential cognitive and psychiatric abnormalities. J Neuroparasitol. 2010 article ID: N100901. [Google Scholar]
- 35.Tanowitz HB, Brosnan C, Guastamacchio D, Baron G, Raventos-Suarez C, et al. Infection of organotypic cultures of spinal cord and dorsal root ganglia with Trypanosoma cruzi. Am J Trop Med Hyg. 1982;31:1090–1097. doi: 10.4269/ajtmh.1982.31.1090. [DOI] [PubMed] [Google Scholar]
- 36.da Silveira AB, de Araujo FF, Freitas MA, Gomes JA, Chaves AT, et al. Characterization of the presence and distribution of Foxp3(+) cells in chagasic patients with and without megacolon. Hum Immunol. 2009;70:65–67. doi: 10.1016/j.humimm.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Chuenkova MV, Pereiraperrin M. Trypanosoma cruzi-Derived Neurotrophic Factor: Role in Neural Repair and Neuroprotection. J Neuroparasitology. 2010;1:55–60. doi: 10.4303/jnp/N100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuenkova MV, PereiraPerrin M. A synthetic peptide modeled on PDNF, Chagas’ disease parasite neurotrophic factor, promotes survival and differentiation of neuronal cells through TrkA receptor. Biochemistry. 2005;44:15685–15694. doi: 10.1021/bi0512039. [DOI] [PubMed] [Google Scholar]
- 39.FDASM-C, Silveira AF, Silva AE. Gene mutations in esophageal mucosa of chagas disease patients. Anticancer Res. 2009;29:1243–1247. [PubMed] [Google Scholar]
- 40.Shoemaker JP, Hoffman RV., Jr Trypanosoma cruzi: possible stimulatory factor(s) on brown adipose tissue of mice. Exp Parasitol. 1974;35:272–274. doi: 10.1016/0014-4894(74)90033-2. [DOI] [PubMed] [Google Scholar]
- 41.Shoemaker JP, Hoffman RV, Jr, Huffman DG. Trypanosoma cruzi: preference for brown adipose tissue in mice by the Tulahuen strain. Exp Parasitol. 1970;27:403–407. doi: 10.1016/0014-4894(70)90045-7. [DOI] [PubMed] [Google Scholar]
- 42.Andrade ZA, Silva HR. Parasitism of adipocytes by Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1995;90:521–522. doi: 10.1590/s0074-02761995000400018. [DOI] [PubMed] [Google Scholar]
- 43.Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 44.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 45.Nagajyothi F, Machado FS, Burleigh BA, Jelicks LA, Scherer PE, et al. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol. 2012 doi: 10.1111/j.1462-5822.2012.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira AV, Segatto M, Menezes Z, Macedo AM, Gelape C, et al. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect. 2011;13:1002–1005. doi: 10.1016/j.micinf.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagajyothi F, Desruisseaux MS, Thiruvur N, Weiss LM, Braunstein VL, et al. Trypanosoma cruzi infection of cultured adipocytes results in an inflammatory phenotype. Obesity (Silver Spring) 2008;16:1992–1997. doi: 10.1038/oby.2008.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krautz GM, Kissinger JC, Krettli AU. The targets of the lytic antibody response against Trypanosoma cruzi. Parasitol Today. 2000;16:31–34. doi: 10.1016/s0169-4758(99)01581-1. [DOI] [PubMed] [Google Scholar]
- 51.Tarleton RL. Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol. 2007;19:430–434. doi: 10.1016/j.coi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Padilla AM, Bustamante JM, Tarleton RL. CD8+ T cells in Trypanosoma cruzi infection. Curr Opin Immunol. 2009;21:385–390. doi: 10.1016/j.coi.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto M, Takeda K. Current views of toll-like receptor signaling pathways. Gastroenterol Res Pract. 2010;2010:240365. doi: 10.1155/2010/240365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang H, Calderon TM, Berman JW, Braunstein VL, Weiss LM, et al. Infection of endothelial cells with Trypanosoma cruzi activates NF-kappaB and induces vascular adhesion molecule expression. Infect Immun. 1999;67:5434–5440. doi: 10.1128/iai.67.10.5434-5440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Machado FS, Tyler KM, Brant F, Esper L, Teixeira MM, et al. Pathogenesis of Chagas disease: time to move on. Front Biosci (Elite Ed) 2012;4:1743–1758. doi: 10.2741/495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Machado FS, Souto JT, Rossi MA, Esper L, Tanowitz HB, et al. Nitric oxide synthase-2 modulates chemokine production by Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 2008;10:1558–1566. doi: 10.1016/j.micinf.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coelho PS, Klein A, Talvani A, Coutinho SF, Takeuchi O, et al. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes induce in vivo leukocyte recruitment dependent on MCP-1 production by IFN-gamma-primed-macrophages. J Leukoc Biol. 2002;71:837–844. [PubMed] [Google Scholar]
- 59.Schmitz V, Svensjo E, Serra RR, Teixeira MM, Scharfstein J. Proteolytic generation of kinins in tissues infected by Trypanosoma cruzi depends on CXC chemokine secretion by macrophages activated via Toll-like 2 receptors. J Leukoc Biol. 2009;85:1005–1014. doi: 10.1189/jlb.1108693. [DOI] [PubMed] [Google Scholar]
- 60.Camargo MM, Almedia IC, Pereira MES, Ferguson MAJ, Travassos LR, et al. Glycosylphosphatidylinositol anchored mucin like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 61.Monteiro AC, Schmitz V, Morrot A, de Arruda LB, Nagajyothi F, et al. Bradykinin B2 Receptors of dendritic cells, acting as sensors of kinins proteolytically released by Trypanosoma cruzi, are critical for the development of protective type-1 responses. PLoS Pathog. 2007;3:e185. doi: 10.1371/journal.ppat.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monteiro AC, Schmitz V, Svensjo E, Gazzinelli RT, Almeida IC, et al. Cooperative activation of TLR2 and bradykinin B2 receptor is required for induction of type 1 immunity in a mouse model of subcutaneous infection by Trypanosoma cruzi. J Immunol. 2006;177:6325–6335. doi: 10.4049/jimmunol.177.9.6325. [DOI] [PubMed] [Google Scholar]
- 63.Kayama H, Takeda K. The innate immune response to Trypanosoma cruzi infection. Microbes Infect. 2010;12:511–517. doi: 10.1016/j.micinf.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 65.Plata F, Wietzerbin J, Pons FG, Falcoff E, Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984;14:930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- 66.Vespa GN, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 68.Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, et al. Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation. 2000;102:3003–3008. doi: 10.1161/01.cir.102.24.3003. [DOI] [PubMed] [Google Scholar]
- 69.Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silva JS, Twardzik DR, Reed SG. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta) J Exp Med. 1991;174:539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reed SG. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988;140:4342–4347. [PubMed] [Google Scholar]
- 72.Miller MJ, Wrightsman RA, Stryker GA, Manning JE. Protection of mice against Trypanosoma cruzi by immunization with paraflagellar rod proteins requires T cell, but not B cell, function. J Immunol. 1997;158:5330–5337. [PubMed] [Google Scholar]
- 73.Zacks MA, Wen JJ, Vyatkina G, Bhatia V, Garg N. An overview of chagasic cardiomyopathy: pathogenic importance of oxidative stress. An Acad Bras Cienc. 2005;77:695–715. doi: 10.1590/s0001-37652005000400009. [DOI] [PubMed] [Google Scholar]
- 74.Holscher C, Mohrs M, Dai WJ, Kohler G, Ryffel B, et al. Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect Immun. 2000;68:4075–4083. doi: 10.1128/iai.68.7.4075-4083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanowitz HB, Gumprecht JP, Spurr D, Calderon TM, Ventura MC, et al. Cytokine gene expression of endothelial cells infected with Trypanosoma cruzi. J Infect Dis. 1992;166:598–603. doi: 10.1093/infdis/166.3.598. [DOI] [PubMed] [Google Scholar]
- 76.Saavedra E, Herrera M, Gao W, Uemura H, Pereira MA. The Trypanosoma cruzi trans-sialidase, through its COOH-terminal tandem repeat, upregulates interleukin 6 secretion in normal human intestinal microvascular endothelial cells and peripheral blood mononuclear cells. J Exp Med. 1999;190:1825–1836. doi: 10.1084/jem.190.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ba X, Gupta S, Davidson M, Garg NJ. Trypanosoma cruzi induces ROS-PARP-1-RelA pathway for up regulation of cytokine expression in cardiomyocytes. J Biol Chem. 2010;285:11596–11606. doi: 10.1074/jbc.M109.076984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hardison JL, Kuziel WA, Manning JE, Lane TE. Chemokine CC receptor 2 is important for acute control of cardiac parasitism but does not contribute to cardiac inflammation after infection with Trypanosoma cruzi. J Infect Dis. 2006;193:1584–1588. doi: 10.1086/503812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardison JL, Wrightsman RA, Carpenter PM, Kuziel WA, Lane TE, et al. The CC chemokine receptor 5 is important in control of parasite replication and acute cardiac inflammation following infection with Trypanosoma cruzi. Infect Immun. 2006;74:135–143. doi: 10.1128/IAI.74.1.135-143.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hardison JL, Wrightsman RA, Carpenter PM, Lane TE, Manning JE. The chemokines CXCL9 and CXCL10 promote a protective immune response but do not contribute to cardiac inflammation following infection with Trypanosoma cruzi. Infect Immun. 2006;74:125–134. doi: 10.1128/IAI.74.1.125-134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao JL, Wynn TA, Chang Y, Lee EJ, Broxmeyer HE, et al. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teixeira MM, Gazzinelli RT, Silva JS. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18:262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- 83.Gutierrez FR, Guedes PM, Gazzinelli RT, Silva JS. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol. 2009;31:673–685. doi: 10.1111/j.1365-3024.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 84.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 86.Goulet JL, Griffiths RC, Ruiz P, Spurney RF, Pisetsky DS, et al. Deficiency of 5-lipoxygenase abolishes sex-related survival differences in MRL-lpr/lpr mice. J Immunol. 1999;163:359–366. [PubMed] [Google Scholar]
- 87.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Rola-Pleszczynski M. Differential effects of leukotriene B4 on T4+ and T8+ lymphocyte phenotype and immunoregulatory functions. J Immunol. 1985;135:1357–1360. [PubMed] [Google Scholar]
- 89.Pavanelli WR, Gutierrez FR, Mariano FS, Prado CM, Ferreira BR, et al. 5-lipoxygenase is a key determinant of acute myocardial inflammation and mortality during Trypanosoma cruzi infection. Microbes Infect. 2010;12:587–597. doi: 10.1016/j.micinf.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 90.Esper L, Roman-Campos D, Lara A, Brant F, Castro LL, et al. Role of SOCS2 in modulating heart damage and function in a murine model of acute Chagas disease. Am J Pathol. 2012;181:130–140. doi: 10.1016/j.ajpath.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cardoni RL, Antunez MI, Morales C, Nantes IR. Release of reactive oxygen species by phagocytic cells in response to live parasites in mice infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1997;56:329–334. doi: 10.4269/ajtmh.1997.56.329. [DOI] [PubMed] [Google Scholar]
- 92.Alvarez MN, Piacenza L, Irigoin F, Peluffo G, Radi R. Macrophage-derived peroxynitrite diffusion and toxicity to Trypanosoma cruzi. Arch Biochem Biophys. 2004;432:222–232. doi: 10.1016/j.abb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 93.Munoz-Fernandez MA, Fernandez MA, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- 94.Melo RC, Fabrino DL, D’Avila H, Teixeira HC, Ferreira AP. Production of hydrogen peroxide by peripheral blood monocytes and specific macrophages during experimental infection with Trypanosoma cruzi in vivo. Cell Biol Int. 2003;27:853–861. doi: 10.1016/s1065-6995(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 95.Gupta S, Dhiman M, Wen JJ, Garg NJ. ROS Signalling of Inflammatory Cytokines During Trypanosoma cruzi Infection. Adv Parasitol. 2011;76:153–170. doi: 10.1016/B978-0-12-385895-5.00007-4. [DOI] [PubMed] [Google Scholar]
- 96.Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J Biol Chem. 2011;286:6627–6640. doi: 10.1074/jbc.M110.167247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piacenza L, Peluffo G, Alvarez MN, Kelly JM, Wilkinson SR, et al. Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage- and endogenously-derived peroxynitrite. Biochem J. 2008;410:359–368. doi: 10.1042/BJ20071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dhiman M, Garg NJ. NADPH oxidase inhibition ameliorates Trypanosoma cruzi-induced myocarditis during Chagas disease. J Pathol. 2011;225:583–596. doi: 10.1002/path.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dhiman M, Garg NJ. NADPH oxidase inhibition ameliorates Trypanosoma cruzi-induced myocarditis during Chagas disease. J Pathol. 2011;225:583–596. doi: 10.1002/path.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang H, Chan J, Wittner M, Jelicks LA, Morris SA, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 101.Hall BS, Tam W, Sen R, Pereira ME. Cell-specific activation of nuclear factor-kappaB by the parasite Trypanosoma cruzi promotes resistance to intracellular infection. Mol Biol Cell. 2000;11:153–160. doi: 10.1091/mbc.11.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang H, Petkova SB, Cohen AW, Bouzahzah B, Chan J, et al. Activation of transcription factors AP-1 and NF-kappa B in murine chagasic myocarditis. Infect Immun. 2003;71:2859–2867. doi: 10.1128/IAI.71.5.2859-2867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta S, Bhatia V, Wen J-J, Wu Y, Huang M-H, et al. Trypanosoma cruzi infection disturbs mitochondrial membrane potential and ROS production rate in cardiomyocytes. Free Radic Biol Med. 2009;47:1414–1421. doi: 10.1016/j.freeradbiomed.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balakumar P, Singh M. Possible role of poly(ADP-ribose) polymerase in pathological and physiological cardiac hypertrophy. Methods Find Exp Clin Pharmacol. 2006;28:683–689. doi: 10.1358/mf.2006.28.10.1037495. [DOI] [PubMed] [Google Scholar]
- 105.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wen J-J, Vyatkina G, Garg N. Oxidative damage during chagasic cardiomyopathy development: Role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med. 2004;37:1821–1833. doi: 10.1016/j.freeradbiomed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 107.Perez-Fuentes R, Guegan JF, Barnabe C, Lopez-Colombo A, Salgado-Rosas H, et al. Severity of chronic Chagas disease is associated with cytokine/antioxidant imbalance in chronically infected individuals. Int J Parasitol. 2003;33:293–299. doi: 10.1016/s0020-7519(02)00283-7. [DOI] [PubMed] [Google Scholar]
- 108.de Oliveira TB, Pedrosa RC, Filho DW. Oxidative stress in chronic cardiopathy associated with Chagas disease. Int J Cardiol. 2007;116:357–363. doi: 10.1016/j.ijcard.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 109.Wen J-J, Bhatia V, Popov VL, Garg NJ. Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas disease. Am J Pathol. 2006;169:1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wen J-J, Yachelini PC, Sembaj A, Manzur RE, Garg N. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Rad Biol Med. 2006;41:270–276. doi: 10.1016/j.freeradbiomed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 111.Wen J-J, Gupta S, Guan Z, Dhiman M, Condon D, et al. Phenyl-alpha-tert-butyl-nitrone and benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic rats. J Am Coll Cardiol. 2010;55:2499–2508. doi: 10.1016/j.jacc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Macao LB, Filho DW, Pedrosa RC, Pereira A, Backes P, et al. Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas’ disease. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 113.Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci. 2009;14:4809–4814. doi: 10.2741/3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB, et al. MEK1/2-ERK1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol. 2001;33:779–787. doi: 10.1006/jmcc.2001.1348. [DOI] [PubMed] [Google Scholar]
- 115.Kuster GM, Pimentel DR, Adachi T, Ido Y, Brenner DA, et al. Alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1-sensitive oxidative modification of thiols on Ras. Circulation. 2005;111:1192–1198. doi: 10.1161/01.CIR.0000157148.59308.F5. [DOI] [PubMed] [Google Scholar]
- 116.Satoh S, Tanaka H, Ueda Y, Oyama J, Sugano M, et al. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol Cell Biochem. 2007;294:205–215. doi: 10.1007/s11010-006-9261-0. [DOI] [PubMed] [Google Scholar]
- 117.Dingar D, Merlen C, Grandy S, Gillis MA, Villeneuve LR, et al. Effect of pressure overload-induced hypertrophy on the expression and localization of p38 MAP kinase isoforms in the mouse heart. Cell Signal. 2010;22:1634–1644. doi: 10.1016/j.cellsig.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pan J, Chang Q, Wang X, Son Y, Zhang Z, et al. Reactive oxygen species-activated Akt/ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS 2B cells. Chem Res Toxicol. 2010;23:568–577. doi: 10.1021/tx9003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, et al. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 121.Tanaka K, Honda M, Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol. 2001;37:676–685. doi: 10.1016/s0735-1097(00)01123-2. [DOI] [PubMed] [Google Scholar]
- 122.Bouzahzah B, Yurchenko V, Nagajyothi F, Hulit J, Sadofsky M, et al. Regulation of host cell cyclin D1 by Trypanosoma cruzi in myoblasts. Cell Cycle. 2008;7:500–503. doi: 10.4161/cc.7.4.5327. [DOI] [PubMed] [Google Scholar]
- 123.Huang YF, Gong KZ, Zhang ZG. Different roles of ERK(1/2) and p38 MAPK(alpha/beta) in cellular signaling during cardiomyocyte anoxia preconditioning. Sheng Li Xue Bao. 2003;55:454–458. [PubMed] [Google Scholar]
- 124.Adesse D, Lisanti MP, Spray DC, Machado FS, de Meirelles MN, et al. Trypanosoma cruzi infection results in the reduced expression of caveolin-3 in the heart. Cell Cycle. 2010;9:1639–1646. doi: 10.4161/cc.9.8.11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagajyothi F, Desruisseaux M, Bouzahzah B, Weiss LM, dos Andrade DS, et al. Cyclin and caveolin expression in an acute model of murine chagasic myocarditis. Cell Cycle. 2006;5:107–112. doi: 10.4161/cc.5.1.2284. [DOI] [PubMed] [Google Scholar]
- 126.Hulit J, Bash T, Fu M, Galbiati F, Albanese C, et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 2000;275:21203–21209. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- 127.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 128.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 129.Park DS, Woodman SE, Schubert W, Cohen AW, Frank PG, et al. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160:2207–2217. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gullestad L, Aukrust P. Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am J Cardiol. 2005;95:17C–23C. doi: 10.1016/j.amjcard.2005.03.008. discussion 38C–40C. [DOI] [PubMed] [Google Scholar]
- 131.Cunha-Neto E, Rizzo LV, Albuquerque F, Abel L, Guilherme L, et al. Cytokine production profile of heart-infiltrating T cells in Chagas’ disease cardiomyopathy. Braz J Med Biol Res. 1998;31:133–137. doi: 10.1590/s0100-879x1998000100018. [DOI] [PubMed] [Google Scholar]
- 132.Krettli AU, Brener Z. Resistance against Trypanosoma cruzi associated to anti-living trypomastigote antibodies. J Immunol. 1982;128:2009–2012. [PubMed] [Google Scholar]
- 133.Gazzinelli RT, Pereira ME, Romanha A, Gazzinelli G, Brener Z. Direct lysis of Trypanosoma cruzi: a novel effector mechanism of protection mediated by human anti-gal antibodies. Parasite Immunol. 1991;13:345–356. doi: 10.1111/j.1365-3024.1991.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 134.Grauert MR, Houdayer M, Hontebeyrie-Joskowciz M. Trypanosoma cruzi infection enhances polyreactive antibody response in an acute case of human Chagas’ disease. Clin Exp Immunol. 1993;93:85–92. doi: 10.1111/j.1365-2249.1993.tb06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teixeira AR, Teixeira G, Macedo V, Prata A. Acquired cell-mediated immunodepression in acute Chagas’ disease. J Clin Invest. 1978;62:1132–1141. doi: 10.1172/JCI109232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Antas PR, Medrano-Mercado N, Torrico F, Ugarte-Fernandez R, Gomez F, et al. Early, intermediate, and late acute stages in Chagas’ disease: a study combining anti-galactose IgG, specific serodiagnosis, and polymerase chain reaction analysis. Am J Trop Med Hyg. 1999;61:308–314. doi: 10.4269/ajtmh.1999.61.308. [DOI] [PubMed] [Google Scholar]
- 137.Cordeiro FD, Martins-Filho OA, Da Costa Rocha MO, Adad SJ, Correa-Oliveira R, et al. Anti-Trypanosoma cruzi immunoglobulin G1 can be a useful tool for diagnosis and prognosis of human Chagas’ disease. Clin Diagn Lab Immunol. 2001;8:112–118. doi: 10.1128/CDLI.8.1.112-118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Galvao LM, Nunes RM, Cancado JR, Brener Z, Krettli AU. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R Soc Trop Med Hyg. 1993;87:220–223. doi: 10.1016/0035-9203(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 139.Cunha-Neto E, Duranti M, Gruber A, Zingales B, De Messias I, et al. Autoimmunity in Chagas disease cardiopathy: biological relevance of a cardiac myosin-specific epitope crossreactive to an immunodominant Trypanosoma cruzi antigen. Proc Natl Acad Sci U S A. 1995;92:3541–3545. doi: 10.1073/pnas.92.8.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Talvani A, Rocha MO, Ribeiro AL, Borda E, Sterin-Borda L, et al. Levels of anti-M2 and anti-beta1 autoantibodies do not correlate with the degree of heart dysfunction in Chagas’ heart disease. Microbes Infect. 2006;8:2459–2464. doi: 10.1016/j.micinf.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 141.Cunha-Neto E, Teixeira PC, Fonseca SG, Bilate AM, Kalil J. Myocardial gene and protein expression profiles after autoimmune injury in Chagas’ disease cardiomyopathy. Autoimmun Rev. 2011;10:163–165. doi: 10.1016/j.autrev.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 142.Dhiman M, Zago MP, Nunez S, Nunez-Burgio F, Garg NJ. Cardiac oxidized antigens are targets of immune recognition by antibodies and potential molecular determinants in Chagas disease pathogenesis. Plos One In press. 2011 doi: 10.1371/journal.pone.0028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reis DD, Gazzinelli RT, Gazzinelli G, Colley DG. Antibodies to Trypanosoma cruzi express idiotypic patterns that can differentiate between patients with asymptomatic or severe Chagas’ disease. J Immunol. 1993;150:1611–1618. [PubMed] [Google Scholar]
- 144.Gazzinelli RT, Leme VM, Cancado JR, Gazzinelli G, Scharfstein J. Identification and partial characterization of Trypanosoma cruzi antigens recognized by T cells and immune sera from patients with Chagas’ disease. Infect Immun. 1990;58:1437–1444. doi: 10.1128/iai.58.5.1437-1444.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gazzinelli RT, Leme VM, Cancado JR, Gazzinelli G, Scharfstein J. Identification of Trypanosoma cruzi antigens recognized by T cells and immune sera from chagasic patients. Mem Inst Oswaldo Cruz. 1988;83(Suppl 1):272–279. doi: 10.1590/s0074-02761988000500010. [DOI] [PubMed] [Google Scholar]
- 146.Dutra WO, Colley DG, Pinto-Dias JC, Gazzinelli G, Brener Z, et al. Self and nonself stimulatory molecules induce preferential expansion of CD5+ B cells or activated T cells of chagasic patients, respectively. Scand J Immunol. 2000;51:91–97. doi: 10.1046/j.1365-3083.2000.00648.x. [DOI] [PubMed] [Google Scholar]
- 147.el Cheikh MC, Hontebeyrie-Joskowicz M, Coutinho A, Minoprio P. CD5 B cells. Potential role in the (auto)immune responses to Trypanosoma cruzi infection. Ann N Y Acad Sci. 1992;651:557–563. doi: 10.1111/j.1749-6632.1992.tb24662.x. [DOI] [PubMed] [Google Scholar]
- 148.Sathler-Avelar R, Lemos EM, Reis DD, Medrano-Mercado N, Araujo-Jorge TC, et al. Phenotypic features of peripheral blood leucocytes during early stages of human infection with Trypanosoma cruzi. Scand J Immunol. 2003;58:655–663. doi: 10.1111/j.1365-3083.2003.01340.x. [DOI] [PubMed] [Google Scholar]
- 149.King IL, Fortier A, Tighe M, Dibble J, Watts GF, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2012;13:44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kierszenbaum F, Cuna WR, Beltz LA, Sztein MB. Trypanosomal immunosuppressive factor: a secretion product(s) of Trypanosoma cruzi that inhibits proliferation and IL-2 receptor expression by activated human peripheral blood mononuclear cells. J Immunol. 1990;144:4000–4004. [PubMed] [Google Scholar]
- 151.Costa RP, Gollob KJ, Fonseca LL, Rocha MO, Chaves AC, et al. T-cell repertoire analysis in acute and chronic human Chagas’ disease: differential frequencies of Vbeta5 expressing T cells. Scand J Immunol. 2000;51:511–519. doi: 10.1046/j.1365-3083.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 152.Menezes CA, Rocha MO, Souza PE, Chaves AC, Gollob KJ, et al. Phenotypic and functional characteristics of CD28+ and CD28- cells from chagasic patients: distinct repertoire and cytokine expression. Clin Exp Immunol. 2004;137:129–138. doi: 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fuenmayor C, Higuchi ML, Carrasco H, Parada H, Gutierrez P, et al. Acute Chagas’ disease: immunohistochemical characteristics of T cell infiltrate and its relationship with T. cruzi parasitic antigens. Acta Cardiol. 2005;60:33–37. doi: 10.2143/AC.60.1.2005046. [DOI] [PubMed] [Google Scholar]
- 154.Tarleton RL. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol. 1990;144:717–724. [PubMed] [Google Scholar]
- 155.Mosca W, Briceno L, Hernandez MI. Cell mediated immunity in Chagas’ disease. Trypanosoma cruzi antigens induce suppression of the in vitro proliferative response of mononuclear cells. Mem Inst Oswaldo Cruz. 1991;86:147–152. doi: 10.1590/s0074-02761991000200002. [DOI] [PubMed] [Google Scholar]
- 156.Morato MJ, Brener Z, Cancado JR, Nunes RM, Chiari E, et al. Cellular immune responses of chagasic patients to antigens derived from different Trypanosoma cruzi strains and clones. Am J Trop Med Hyg. 1986;35:505–511. doi: 10.4269/ajtmh.1986.35.505. [DOI] [PubMed] [Google Scholar]
- 157.Dutra WO, da Luz ZM, Cancado JR, Pereira ME, Brigido-Nunes RM, et al. Influence of parasite presence on the immunologic profile of peripheral blood mononuclear cells from chagasic patients after specific drug therapy. Parasite Immunol. 1996;18:579–585. doi: 10.1046/j.1365-3024.1996.d01-29.x. [DOI] [PubMed] [Google Scholar]
- 158.Dutra WO, Martins-Filho OA, Cancado JR, Pinto-Dias JC, Brener Z, et al. Activated T and B lymphocytes in peripheral blood of patients with Chagas’ disease. Int Immunol. 1994;6:499–506. doi: 10.1093/intimm/6.4.499. [DOI] [PubMed] [Google Scholar]
- 159.Reis DD, Jones EM, Tostes S, Jr, Lopes ER, Gazzinelli G, et al. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-alpha+ cells and dominance of granzyme A+, CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 160.Reis DD, Jones EM, Tostes S, Lopes ER, Chapadeiro E, et al. Expression of major histocompatibility complex antigens and adhesion molecules in hearts of patients with chronic Chagas’ disease. Am J Trop Med Hyg. 1993;49:192–200. doi: 10.4269/ajtmh.1993.49.192. [DOI] [PubMed] [Google Scholar]
- 161.Gomes JA, Bahia-Oliveira LM, Rocha MO, Busek SC, Teixeira MM, et al. Type 1 chemokine receptor expression in Chagas’ disease correlates with morbidity in cardiac patients. Infect Immun. 2005;73:7960–7966. doi: 10.1128/IAI.73.12.7960-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Talvani A, Rocha MO, Cogan J, Maewal P, de Lemos J, et al. Brain natriuretic peptide and left ventricular dysfunction in chagasic cardiomyopathy. Mem Inst Oswaldo Cruz. 2004;99:645–649. doi: 10.1590/s0074-02762004000600020. [DOI] [PubMed] [Google Scholar]
- 163.Dutra WO, Menezes CA, Villani FN, da Costa GC, da Silveira AB, et al. Cellular and genetic mechanisms involved in the generation of protective and pathogenic immune responses in human Chagas disease. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):208–218. doi: 10.1590/s0074-02762009000900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Higuchi MD, Benvenuti LA, Martins Reis M, Metzger M. Pathophysiology of the heart in Chagas’ disease: current status and new developments. Cardiovasc Res. 2003;60:96–107. doi: 10.1016/s0008-6363(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 165.Albareda MC, Laucella SA, Alvarez MG, Armenti AH, Bertochi G, et al. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas’ disease patients. Int Immunol. 2006;18:465–471. doi: 10.1093/intimm/dxh387. [DOI] [PubMed] [Google Scholar]
- 166.Souza PE, Rocha MO, Menezes CA, Coelho JS, Chaves AC, et al. Trypanosoma cruzi infection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas’ disease. Infect Immun. 2007;75:1886–1894. doi: 10.1128/IAI.01931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Souza PE, Rocha MO, Rocha-Vieira E, Menezes CA, Chaves AC, et al. Monocytes from patients with indeterminate and cardiac forms of Chagas’ disease display distinct phenotypic and functional characteristics associated with morbidity. Infect Immun. 2004;72:5283–5291. doi: 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cuellar A, Santander SP, del Thomas MC, Guzman F, Gomez A, et al. Monocyte-derived dendritic cells from chagasic patients vs healthy donors secrete differential levels of IL-10 and IL-12 when stimulated with a protein fragment of Trypanosoma cruzi heat-shock protein-70. Immunol Cell Biol. 2008;86:255–260. doi: 10.1038/sj.icb.7100146. [DOI] [PubMed] [Google Scholar]
- 169.Dutra WO, Gollob KJ, Pinto-Dias JC, Gazzinelli G, Correa-Oliveira R, et al. Cytokine mRNA profile of peripheral blood mononuclear cells isolated from individuals with Trypanosoma cruzi chronic infection. Scand J Immunol. 1997;45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- 170.Benvenuti LA, Higuchi ML, Reis MM. Upregulation of adhesion molecules and class I HLA in the myocardium of chronic chagasic cardiomyopathy and heart allograft rejection, but not in dilated cardiomyopathy. Cardiovasc Pathol. 2000;9:111–117. doi: 10.1016/s1054-8807(00)00027-2. [DOI] [PubMed] [Google Scholar]
- 171.Cunha-Neto E, Dzau VJ, Allen PD, Stamatiou D, Benvenutti L, et al. Cardiac gene expression profiling provides evidence for cytokinopathy as a molecular mechanism in Chagas’ disease cardiomyopathy. Am J Pathol. 2005;167:305–313. doi: 10.1016/S0002-9440(10)62976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Araujo FF, Gomes JA, Rocha MO, Williams-Blangero S, Pinheiro VM, et al. Potential role of CD4+CD25HIGH regulatory T cells in morbidity in Chagas disease. Front Biosci. 2007;12:2797–2806. doi: 10.2741/2273. [DOI] [PubMed] [Google Scholar]
- 173.Villani FN, Rocha MO, do Nunes MC, Antonelli LR, Magalhaes LM, et al. Trypanosoma cruzi-induced activation of functionally distinct alphabeta and gammadelta CD4- CD8- T cells in individuals with polar forms of Chagas’ disease. Infect Immun. 2010;78:4421–4430. doi: 10.1128/IAI.00179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dutra WO, Gollob KJ. Current concepts in immunoregulation and pathology of human Chagas disease. Curr Opin Infect Dis. 2008;21:287–292. doi: 10.1097/QCO.0b013e3282f88b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Drigo SA, Cunha-Neto E, Ianni B, Cardoso MR, Braga PE, et al. TNF gene polymorphisms are associated with reduced survival in severe Chagas’ disease cardiomyopathy patients. Microbes Infect. 2006;8:598–603. doi: 10.1016/j.micinf.2005.08.009. [DOI] [PubMed] [Google Scholar]