Abstract
We have previously shown the pathophysiological importance of the reactive nitrogen species peroxynitrite (PN) formed from the reaction of nitric oxide (•NO) and superoxide (O2 •−) radicals and its involvement in lipid peroxidation (LP) and protein nitration damage in brain tissue following traumatic brain injury (TBI). Nitric oxide is produced by at least three isoforms of the enzyme nitric oxide synthase (NOS) including: endothelial NOS (eNOS) in the CNS vasculature, neuronal NOS (nNOS), and inducible NOS (iNOS) in macrophages/microglia. In view of the requirement of ●NO synthesis for PN formation, we sought to address the time course of NOS expression (mRNA by real time quantitative PCR and protein by western blot) after TBI in comparison with the time course of PN-mediated protein nitration (3-nitrotyrosine, 3-NT) in ipsilateral cortex (CTX) and hippocampus (HIPP) between 3 hours and 1 week post-injury using a controlled cortical impact (CCI) mouse model of TBI in young adult CF-1 mice. Protein nitration showed a progressive posttraumatic increase that became significant in CTX at 24 hours and then peaked at 72 hours in both CTX and HIPP. During the increase in PN-derived 3-NT, there was no increase in either CTX or HIPP eNOS mRNA levels, whereas eNOS protein levels were significantly (p<0.05) increased at 48 and 72 hours in both brain regions. There was a significant decrease in HIPP, but not CTX nNOS mRNA; however, nNOS protein did not change except for a significant increase in CTX at 1 week. There was significantly increased CTX and HIPP iNOS mRNA levels at 24, 48, and 72 hours (p<.05) post-injury. In contrast, no change was seen in CTX or HIPP iNOS protein at any timepoint. Taken together, eNOS protein expression and iNOS mRNA appear to bear a coincident temporal relationship to the time course of PN-mediated protein nitrative damage after CCI-TBI suggesting that both constitutive and inducible NOS isoforms contribute ●NO for PN formation and 3-NT protein modification after TBI.
Keywords: traumatic brain injury, peroxynitrite, oxidative damage, 3-nitrotyrosine, nitric oxide synthase
Introduction
There is considerable evidence for an important role of peroxynitrite (PN), formed from the diffusion rate-limited reaction of nitric oxide (●NO) and superoxide radical (O2 •−), in the pathophysiology of acute traumatic brain injury (TBI). Peroxynitrite is perhaps the major reactive oxygen species (ROS), often referred to as a reactive nitrogen species (RNS) that is a trigger for the causation of posttraumatic oxidative damage in the injured brain. This includes lipid peroxidation (LP), protein carbonylation and nitration of protein tyrosine residues, the latter being a specific biomarker for PN (Hall, et al., 2010). We have previously demonstrated that increased tyrosine nitration (i.e. 3-nitrotyrosine, 3-NT) is evident in injured mouse brain tissue within the first hours after either diffuse weight drop-induced TBI (Hall, et al., 2004) or controlled cortical impact-induced TBI (CCI-TBI) (Deng, et al., 2007). The nitration of tyrosine is ultimately generated by reactivity with the PN-derived free radical species nitrogen dioxide (●NO2) (Beckman, 1991, Radi, 2004). In both TBI paradigms, the 3-NT time course is nearly identical to that for LP as assessed by the formation and protein binding of the LP-derived aldehydic breakdown product 4-hydroxynonenal (4-HNE). This finding suggests that LP and protein nitration are both caused by elevations in PN. Immunohistochemical studies revealed that the 3-NT and 4-HNE immunostaining was ubiquitous in the injured brain regions being present is cerebral microvessels and throughout the neuropil (Deng, et al., 2007, Hall, et al., 2004).
The belief that 3-NT accumulation in injured brain proteins is functionally deleterious comes from the observation that administration of the nitroxide antioxidant tempol, which catalytically scavenges PN-derived radicals including ●NO2, hydroxyl radical (●OH) and carbonate radical anion (CO3 •−) (Carroll, et al., 2000), is neuroprotective in parallel with a dose-related decrease in 3-NT in the CCI-TBI model (Deng-Bryant, et al., 2008). Furthermore, we have shown that LP plays a role in 3-NT formation since the lipid peroxyl radical (LOO●) scavenging LP inhibitor U-83836E is also able to attenuate post-TBI 3-NT levels.
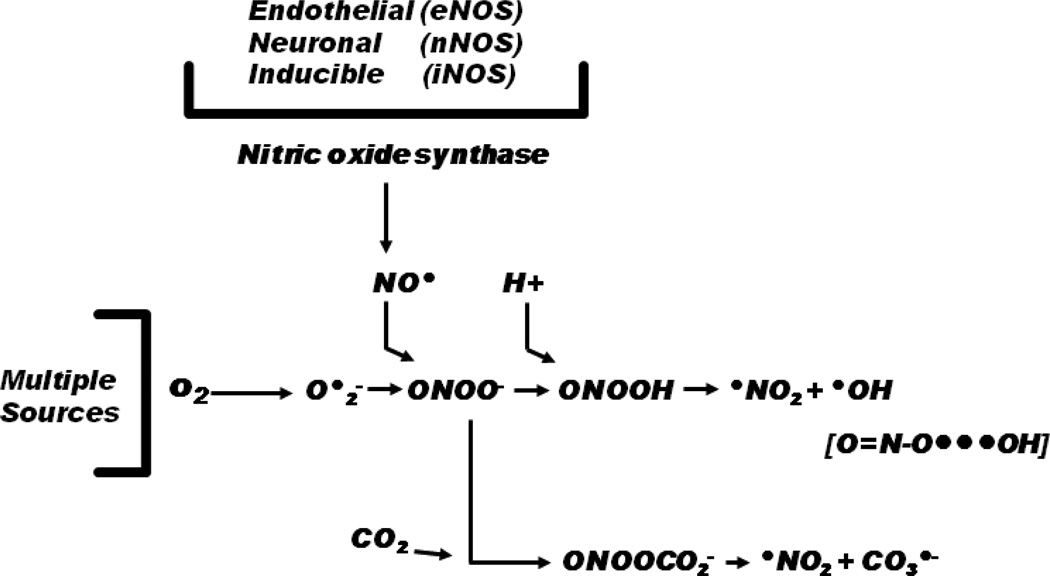
The ●NO that reacts with O2 •− to form PN is synthesized by various isoforms of the enzyme nitric oxide synthase (NOS). These include two constitutive isoforms that require calcium (Ca++)-influx for their activation: endothelial NOS (eNOS) which is predominantly contained in endothelial cells and neuronal NOS (nNOS) which is present in neurons as well as a Ca++-insensitive inducible NOS (iNOS) that has been observed in neutrophils, endothelial cells, neurons, astrocytes and macrophages after contusion TBI in rats (Gahm, et al., 2000) and humans (Gahm, et al., 2002). While each of the NOS isoforms has an important physiological role to play, they can also be pathophysiologically important when their activation and production of ●NO occurs in close proximity to an injury-induced increase in O2 •− which can rapidly react to produce PN (Beckman, 1991, Radi, 2004, Radi, et al., 2001). Figure 1 shows the chemistry of PN formation including the possibility that each of the NOS isoforms could produce ●NO that ends up as PN and contributes to posttraumatic nitrative damage at particular times and cellular compartments in the injured brain.
Figure 1.
Schematic showing the role of nitric oxide synthase (NOS) isoforms in the formation of nitric oxide (NO●) which can rapidly react with superoxide radical (O2•−) with a diffusion rate-limited rate constant to form the reactive nitrogen species peroxynitrite, initially in the form of peroxynitrite anion (ONOO−). The anionic form can then combine with a proton (H+) to form peroxynitrous acid (ONOOH) which can then break down to the highly reactive nitrogen dioxide (●NO2) or hydroxyl (●OH) radicals. Alternatively, ONOO− can combine with carbon dioxide (CO2) to form nitrosoperoxocarbonate anion (ONOOCO2 −) which can break down to ●NO2 radical and carbonate radical anion (CO3 •−).
Therefore, the purpose of this study was to carefully compare the 3 hours to 1 week time courses of the expression of eNOS, nNOS and iNOS mRNA and protein in the area surrounding the contusion site in mice subjected to CCI-TBI, and to compare those to the time course of 3-NT-modified proteins as an index of PN-mediated protein nitration. The NOS mRNA was measured by real-time quantitative polymerase chain reaction (RT-qPCR) and the NOS protein levels were assessed by quantitative western blot. Similarly, 3NT was also measured by quantitative western blot.
Materials and Methods
Animals
The present studies employed young adult male CF-1 mice (Charles River, Portage, MI, USA) weighing 29–32 g at time of surgery. All animals had ad libitum access to food and water and were housed in the Division of Laboratory Animal Resources sector of the University of Kentucky Medical Center, which is fully accredited by AALAC. All procedures herein follow protocols approved by the University of Kentucky’s Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Mouse Model of Controlled Cortical Impact (CCI) TBI
Mice were initially anesthetized in a Plexiglas chamber using 4.0% isoflurane, shaved, weighed and then placed into a stereotaxic frame (David Kopf, Tujunga, CA, USA). Core body temperature was maintained using an underlying heating pad. Throughout the surgical procedure, mice were kept anesthetized by a constant flow of 3% isoflurane and oxygen delivered via nose cone. The head was positioned in the horizontal plane with nose bar set at zero. A 2.0 cm sagittal incision was made in the scalp and the skin retracted using hemostats to expose the skull. After exposing the skull, a 4.0 mm diameter craniotomy was made using a dental bur (SS WHITE, Lakewood, NJ, USA) mounted on a cordless Dremel (Racine, WI, USA) lateral (left) to the sagittal suture, centered between bregma and lambda, leaving the dura mater intact. Sham-operated (control) mice received anesthesia and all surgical procedures (craniotomy) without CCI brain injury. Brain-injured mice received cortical impact injury utilizing an electronically controlled pneumatic impacting device (Precision Systems Instrumentation, TBI-0300 Impactor, Fairfax Station, VA, USA) with a 3.0 mm diameter, beveled (flat) impactor tip. The velocity was held at 3.50 m/sec while the depth of cortical deformation was set at 1.0 mm (severe) as described previously (Hall, et al., 2008). Mortality following this severe CCI brain injury is rare (<5%). After injury, the craniotomy was closed by secure placement of a 6.0 mm diameter disk of dental acrylic cemented in place with cyanoacrylate. The mice were then placed in a Hova-Bator Incubator (model 1583; Randall Burkey Co, Boerne, TX, USA) set at 37°C for at least 20 minutes to prevent post-traumatic hypothermia. Consciousness (i.e. return of right reflex and mobility) was regained within 10 minutes after the surgery. Brain-injured and sham mice typically show no untoward effects after recovering from anesthesia, and resume normal eating, drinking and grooming patterns. In this study, animals survived from 3 hours to 7 days post-injury depending upon group assignment. Additional details on surgical procedure have been previously published by our laboratory.
Quantitative RT-PCR analysis of NOS isoforms
Quantitative real-time PCR (qRT-PCR) was utilized to determine mRNA levels of three NOS isoforms (iNOS, eNOS, and nNOS). Briefly, at a given time point post-injury, mice received an overdose of sodium pentobarbital (200 mg/kg IP). The ipsilateral cortex (penumbra tissue and the injured core) and hippocampus were then rapidly dissected on an ice-chilled stage and immediately transferred to a RNAlater® solution (Ambion) for 24 hours at 4°C to prevent RNase activity and sample degradation. Samples were then placed in a −80°C freezer for storage until further analysis. To isolate total RNA, tissue samples were homogenized in TRIzol® reagent (Ambion) according to manufacturer specifications. Isolated total RNA was precipitated out using isopropanol, washed with ethanol, and then decontaminated of residual genomic DNA by DNase I treatment per manufacturer specifications. Total RNA concentrations were determined using a Nanodrop®, with 260/280 ratios of 1.8–2.2 considered acceptable. Purified total RNA (1µg) was then reverse transcribed (RTed) to acquire complementary total DNA (cDNA). Final cDNA samples were then used for quantitative real-time PCR assay. In this study, qRT-PCR was performed using the StepOne-Plus real-Time PCR System (Applied Biosystems, CA) in conjunction with Taqman® primer-probe reagent-based chemistry. Commercial, inventoried Taqman® gene expression assays consisting of a gene specific set of primers and a fluorogenic internal probe were used (Applied Biosystems, CA). The mouse GAPDH endogenous control was used for normalization purposes of all target gene analysis as previously validated in our laboratory. PCR reactions were run in triplicate in a 96 well format using a standard (~2.5 hours) amplification protocol. Each reaction well of the plate contained a total of 25µl. The PCR reaction for a specific gene (e.g. iNOS, eNOS, or nNOS) contained 10µl of 1:10 diluted total cDNA and a total of 15µl of a Taqman® PCR master mix and gene specific primers and probe. The PCR reaction for GAPDH gene expression assay contained 2µl of 1:10 diluted total cDNA and 23µl of a TaqMan® PCR master mix and control gene primers and probe. Following PCR reaction, the resulting amplification curves were then further analyzed by the established ΔΔCt method wherein GAPDH was used as the reference gene and the sham group as a control.
Western blotting of 3-nitrotyrosine and NOS isoforms
Western blotting technique was utilized to detect levels of 3-nitrotyrosine (3-NT) adducts of nitrated proteins and the protein levels of three NOS isoforms (eNOS, nNOS, and iNOS). Briefly, at a given time point post-injury, mice received an overdose of sodium pentobarbital (200 mg/kg IP). The ipsilateral cortex (penumbra tissue and the injured core) and hippocampus were then rapidly dissected on an ice-chilled stage and immediately transferred to Triton lysis buffer (1% Triton, 20mM Tris HCL, 150mM NaCl, 5mM EGTA, 10mM EDTA, and 10% glycerol) containing protease inhibitors (Complete Mini Protease Inhibitor Cocktail; Roche Diagnostics, Indianapolis, IN). Samples were then sonicated and centrifuged for 30 minutes (15,000rpm at 4°C). The supernatants were collected and the remaining pellet was discarded. Protein concentrations were determined using the BioRad DC Protein Assay. An aliquot of each protein sample (15µg for 3-NT; 20µg for NOS isoforms) was separated on an SDS–PAGE precast gel (12% Bis-Tris w/v acrylamide; Criterion XT, Bio-Rad) using a XT-MES running buffer system and then transferred to nitrocellulose membranes using a semi-dry electro-transferring unit at 15 V for 30 minutes. Membranes were incubated for 1 hour at room temperature in 5% milk/TBS blocking solution. The membranes were then incubated overnight at 4°C in blocking solution with 0.5 mM Tween-20 (TBST) containing the appropriate dilution of primary antibody. A mouse monoclonal primary antibody was used for detecting 3-NT bands (#20-321-175259, Genway; San Diego, CA). For detection of NOS isoforms, the following primary antibodies were used: anti-iNOS (1:100; SC-650, Santa Cruz Biotechnology, CA), anti-eNOS (1:100; SC-654, Santa Cruz Biotechnology, CA), and anti-nNOS (1:100; SC-648, Santa Cruz Biotechnology, CA). A goat anti-mouse secondary antibody (2 hour incubation at room temperature) conjugated to an infrared dye (1:5000, IRdye800CW, Rockland) was used for detection of the primary labeled bands. Dry membranes were imaged and quantified using Li-Cor Odyssey InfraRed Imaging System (Li-Cor Biosciences, Lincoln, NE). For 3-NT blot analysis, all bands ranging from 250 kD to 50 kD were quantified for each lane, representing the smear of 3-NT labeled proteins for each sample. This was then analyzed as percent of control samples.
Sample Size & Data Analysis
The sample sizes for this study were 3 per group (qRT-PCR analysis) and 6 per group (Western blot analysis). All data were expressed as the mean ± standard deviation (SD) and were analyzed using GraphPad PRISM v. 5.0. The 3-NT, NOS mRNA and NOS protein levels across time points after TBI were compared to Sham using a one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc comparisons. A value of p<0.05 was considered statistically significant.
Results
Time course of posttraumatic peroxynitrite-mediated oxidative damage
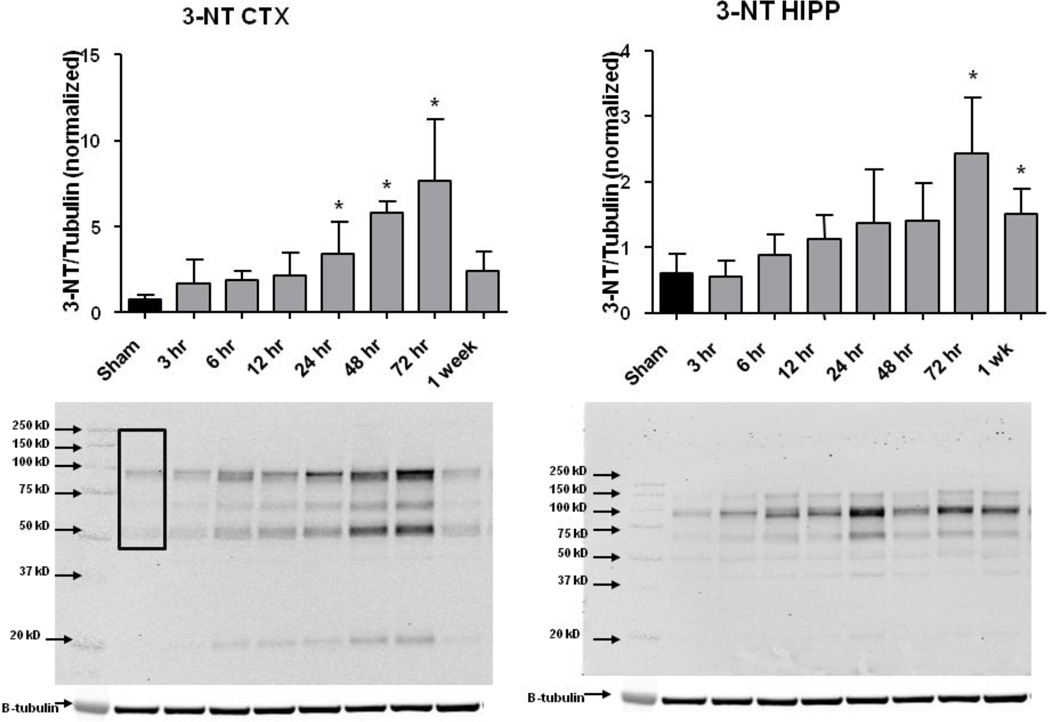
As shown in Figure 2, CCI-TBI produced a progressive increase in ipsilateral cortical (CTX) and hippocampal (HIPP) levels of 3-NT-modified proteins. In CTX, the increase was significantly higher than that in the Sham, non-injured mice at 24, 48 and 72 hours with the largest elevation in 3-NT occurring at 72 hours at which time the quantitation of the 3-NT stained protein bands between 50 and 250 kD in the western blots, and expression of intensity of staining as a ratio to β-tubulin, showed that the increase in 3-NT was 10-fold higher than in Sham cortices. Between 72 hours and 1 week after injury, the 3-NT levels decreased back toward the Sham level. In the hippocampus a similar time course is seen except that the increase in 3-NT did not become significant compared to Sham until 72 hours. In viewing the entire time course it is apparent that for both the CTX and the HIPP the elevation in 3-NT begins as early as 6 hours post-TBI although with 7 post-injury time points and the built in compensation for multiple comparisons with the Dunnett’s test significance was not statistically achieved until at least 24 hours.
Figure 2.
Time course of 3-NT following CCI-TBI. Western blot analysis of 3-NT in ipsilateral cortex (CTX) and hippocampus (HIPP) revealed a significant increase in 3-NT at 48 and 72 hours (in CTX) and 72 hours and 1 week (in HIPP) after TBI as compared to sham controls by One-way ANOVA followed by Dunnett’s post-hoc test. * = p<.05. Error bars represent +/− SD. Box in left most lane indicates the molecular weight range (250 to 50 kD) that was included in the analysis.
It must be noted that the time course of posttraumatic 3-NT after CCI-TBI in mice differs from that which we published previously (Deng, et al., 2007). In the earlier study, we employed a polyclonal antibody for 3-NT modified proteins that showed that the injury-induced increase occurred as early as 30 minutes post-injury, peaked at 1–6 hours and by 24 hours had lost statistical significance. The largest increase in 3-NT, compared to Sham was only 2-fold. In the current study, we used a monoclonal antibody that appeared to replicate the early 2-fold increase in 3-NT during the first 12 hours, but most strikingly revealed the much larger increase in 3-NT between 24 and 72 hours after CCI-TBI. We believe that the difference in the time course may represent the fact that across the post-injury time course different proteins are being oxidatively damaged by PN and the polyclonal anti-3-NT antibody employed in the earlier study does not react with those nitrated proteins that predominate at 24 hours and beyond in contrast to the monoclonal anti-3-NT antibody which does. In any case, we are convinced that the present time course of post-CCI-TBI is the more accurate representation of the full time course of PN-induced protein nitration in the model.
Time course of posttraumatic NOS isoform induction
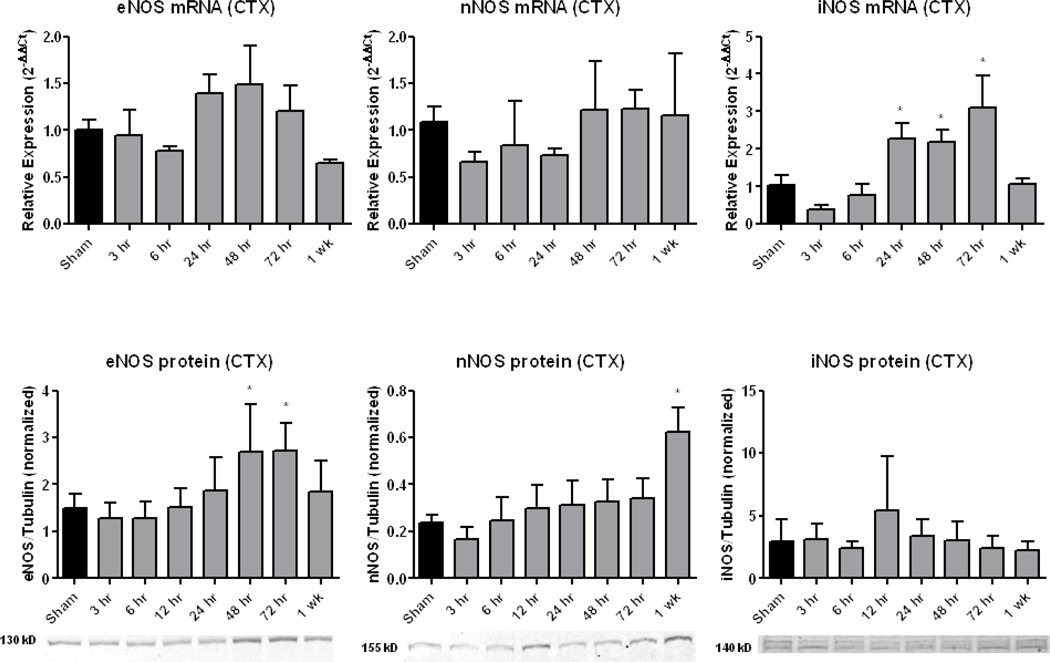
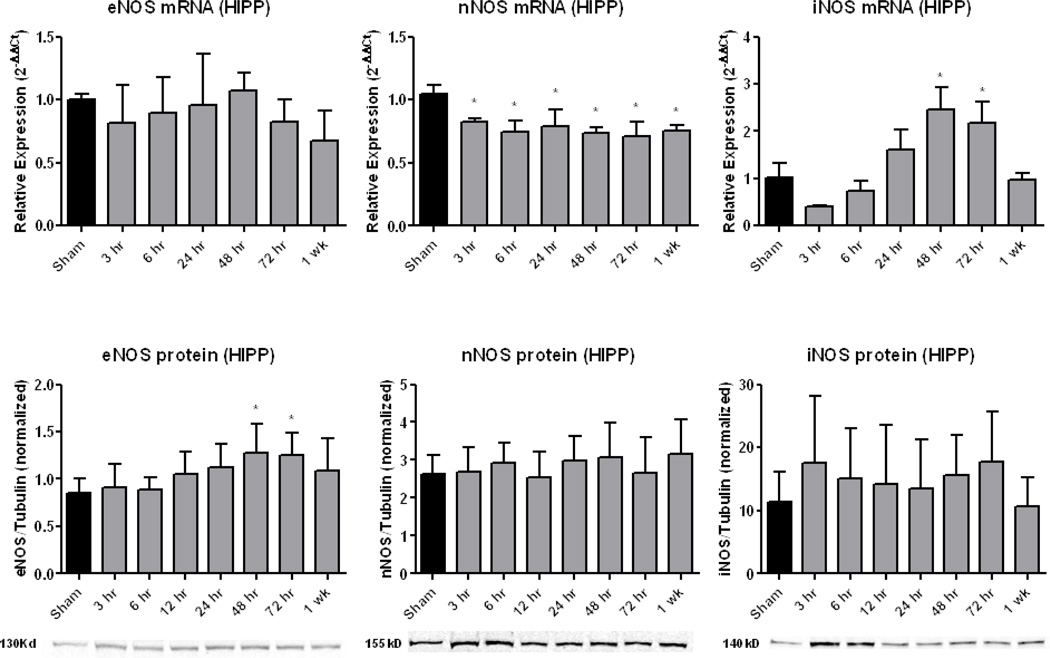
Figures 3 and 4 display the time courses of NOS activation in terms of mRNA and protein levels for CTX and HIPP, respectively. During the increase in PN-derived 3-NT (Figure 2), there was no increase in either CTX or HIPP eNOS mRNA levels, whereas eNOS protein levels were significantly (p<0.05) increased at 48 and 72 hours in both brain regions. There was a significant decrease in HIPP, but not CTX nNOS mRNA; however, nNOS protein did not change except for a significant increase in CTX at 1 week. There was significantly increased CTX and HIPP iNOS mRNA levels at 24, 48, and 72 hours (p<.05) post-injury. In contrast, no change was seen in CTX or HIPP iNOS protein at any timepoint.
Figure 3.
Time course of NOS isoform expression in ipsilateral (injured) cortex (CTX) following CCI-TBI. qRT-PCR analysis of eNOS mRNA expression revealed no significant changes. For nNOS, no changes were observed for either mRNA or protein, except for the latter, there was a significant increase at 1 week. For iNOS, mRNA was significantly increased at 24, 48 and 72 hours, but there was no increase in iNOS protein. Sample western blots are shown below the protein graphs. One-way ANOVA followed by Dunnett’s post-hoc test. * = p<.05. Error bars represent +/− SD.
Figure 4.
Time course of NOS isoform expression in ipsilateral (injured) hippocampus (HIPP) following CCI-TBI. Similar to CTX (Fig. 4), qRT-PCR analysis of eNOS expression revealed no change in eNOS mRNA while western blot analysis of eNOS protein levels revealed significant increases at 48 and 72 hours post-injury. For nNOS, there was a significant decrease in mRNA at all time points post-injury, but no significant changes in protein levels at any time. For iNOS, there was a significant increase in mRNA at 48 and 72 hours, but no change in protein levels at any time. Sample western blots are shown below the protein graphs. One-way ANOVA followed by Dunnett’s post-hoc test. * = p<.05. Error bars represent +/− SD.
Discussion
Nitric oxide synthesis has been known to be an important player in posttraumatic secondary brain injury for many years dating back to 1998 when it was first demonstrated to be generated in the rat lateral fluid percussion TBI model as early as 5 minutes after injury and to remain increased for at least a week (Wada, et al., 1998). Pretreatment with the 7-nitroindazole, a selective inhibitor of nNOS, reduced contusion volume (Wada, et al., 1998) and improved behavioral recovery (Wada, et al., 1999), However, the therapeutic window for the neuroprotective effects of selective nNOS inhibition was very short since early post-treatment with 7-nitroindazole was ineffective (Wada, et al., 1998). The same authors also observed an increase in iNOS activation at 3 days post-injury and that the selective iNOS inhibitor aminoguanidine was able to reduce histological damage (Wada, et al., 1998). The expression of iNOS, measured immunohistochemically, has also been reported in surgically excised human brain contusions as early as 6 hours post-injury with an apparent peak occurring between 8 and 23 hours (Gahm, et al., 2002). The occurrence of iNOS immunostaining was equally present in neurons (31%), neutrophils 39%) and macrophages (29%) from 8 to 12 hours after TBI, but from 23 to 120 hours, was mainly present in neurons (85%) and macrophages (13%). No significant differences in either eNOS or nNOS were seen between control and contused brain tissue. Disappointingly, none of these early studies measured PN-induced 3-NT in the injured tissue.
The first study to document that 3-NT was increased in the injured brain reported a progressive increase in 3-NT between 4 and 24 hrs after diffuse TBI in mice, and this was attenuated by treatment with the non-selective NOS inhibitor L-nitroarginine methyl ester (L-NAME (Mesenge, et al., 1998). The same investigators demonstrated an improvement in 24 hour motor recovery in mice treated with either L-NAME or the nNOS selective 7-nitroindazole as long as the NOS inhibitors were administered within 1 hour post-TBI (Mesenge, et al., 1996) confirming the fact that NOS inhibitors have a short therapeutic efficacy window. Similarly, our previous studies have shown that the nitroxide antioxidant tempol, which catalytically scavenges PN-derived free radicals (Carroll, et al., 2000), can decrease brain 3-NT after CCI-TBI, but only if administered during the first hour (Deng-Bryant, et al., 2008). Thus, attempting to block PN formation by either inhibiting ●NO synthesis or scavenging PN radicals does not appear to be a practical therapeutic approach to reducing early PN-mediated oxidative damage from a therapeutic window point of view.
A very important aspect of ●NO and PN-related TBI pathophysiology that is manifest during the first 12 hours after injury concerns the existence of a constitutive Ca++-activated mitochondrial NOS that is present in the inner mitochondrial membrane that normally helps to regulate a number of mitochondrial functions (Ghafourifar and Cadenas, 2005). Whether mtNOS is a distinct isoform of constitutive NOS has been debated for the past decade and is still unresolved. However, it is known that mitochondrial NOS (mtNOS) in mouse brain is a 147kD protein that cross reacts with nNOS antibodies and is Ca++-activated. The mitochondrial NOS may also be similar to eNOS since it has been shown that there is a strong correlation between eNOS expression and mitochondrial content in skeletal muscle fibers (Kobzik, et al., 1995). Additionally, eNOS and mtNOS have other similarities. For instance, both isoforms depend on acylation to enable them to bind to endothelial and mitochondrial membranes, respectively, and both have their ●NO-generational activities enhanced by serine phosphorylation (Elfering, et al., 2002). Furthermore, low levels of eNOS protein have been reported in mitochondria (Lacza, et al., 2003). Thus, some of the eNOS protein we see increasing as early as 12 hrs may be mitochondrial.
With regard to its role in post-TBI pathophysiology, the constitutively expressed mtNOS is activated during the early Ca++ influx that is triggered almost immediately after mechanical CNS trauma due to entry of Ca++ through voltage-gated and glutamate-operated N-methyl-D-aspartate receptor-operated channels. Together with the mtNOS-mediated production of ●NO, complex I of the electron transport chain begins to increase its leakage of O2 •− which reacts with the ●NO to form PN that rapidly modifies susceptible mitochondrial targets. In the present model, an increase in 3-NT (and 4-HNE) modified proteins is apparent within cortical mitochondria within the first 3 hours after TBI at the time when complex I respiratory function and Ca++ buffering becomes compromised. The decrement in mitochondrial functions is progressive and the occurrence of mitochondrial permeability transition and intra-mitochondrial Ca++ release peaks at 12 hrs after CCI-TBI (Singh, et al., 2006). However, we have also seen evidence that there may be second wave of mitochondrial respiratory failure in the CCI-TBI model that is apparent at 48 and 72 hours after injury (Singh, et al., 2006).
Others have reported that nNOS, which may be indistinguishable from mtNOS (Elfering, et al., 2002, Ghafourifar and Cadenas, 2005), is responsible for nitrating and inactivating the mitochondrial Mn++ superoxide dismutase (MnSOD) enzyme during the first 24 hrs after experimental or human TBI. The MnSOD normally scavenges O2•− radicals as an antioxidant protective mechanism within mitochondria (Bayir, et al., 2007). These investigators also showed that the PN-mediated MnSOD nitration and loss of activity is reduced by genetic deficiency or pharmacological inhibition of nNOS (aka. mtNOS). Moreover, they concluded that nitration and inactivation of MnSOD leads to an amplification of oxidative stress in the brain which progressively enhances PN production and secondary damage Inducible NOS has been associated with neuroprotective antioxidant effects as well as neuropathological oxidant actions. The former is due to the potent LOO● scavenging effects of ●NO (LOO● + ●NO → LOONO) which serve to inhibit LP reactions when the concentration of ●NO is in the nanomolar range. Consistent with this, an enhanced oxidative stress has been reported in iNOS-deficient mice due to the loss of this antioxidant effect (Bayir, et al., 2005). However, if iNOS induction is sufficiently increased and ●NO generation reaches the micromolar range, and a source of O2 •− is present to react with it to form PN, then the oxidant effects of ●NO begin to overwhelm the antioxidant effects (Hummel, et al., 2006). Our finding that iNOS mRNA expression is increased coincident with the peaking of 3-NT levels between 24 and 72 hrs supports the idea that as iNOS activity is increased, the role of basal iNOS activity shifts from antioxidant neuroprotection to becoming a source of oxidative damage when iNOS activity is increased to pathological levels. This phase has been shown to be associated with neutrophil and macrophage influx at 72 hours after CCI-TBI and the pro-oxidant action is reflected in the observation that these inflammatory cells display 3-NT immunostaining themselves (Bayir, et al., 2005). Finally, in the same report, it was also observed that this PN generation and oxidative damage is augmented by neutrophil invasion of the injured tissue which peaks at 24 hours after injury and macrophage influx which predominates in the injured brain at 72 hours after TBI (Bayir, et al., 2007). Both of these cell types are also highly reactive for iNOS after human contusive TBI (Gahm, et al., 2002).
In contrast to this human TBI report, we can only speculate as to why we failed to see that the increase in iNOS mRNA was translated into a measurable net increase in iNOS protein. There are several possible explanations for this iNOS mRNA vs. iNOS protein dichotomy. Most relevant to the present study of the relationship of NOS expression to post-TBI increase in protein nitrative damage, an increased turnover of iNOS protein may be occurring. For instance, the increased oxidative environment that exists within the invading neutrophils and macrophages is most likely causing some lipid peroxidative or nitrative modification of some of the iNOS protein as fast as it is formed. At some point the modified iNOS protein might be so altered that it would not be recognized by the iNOS antibody during the western blot analysis. In other words, there may be either a net increase in iNOS protein or an increased turnover that we are unable to see, but which is nevertheless contributing to the increase in 3-NT in the injured brain. We will test this hypothesis in future studies.
Conclusions
The present study has extended our understanding of the relationship of increased NOS expression to PN-mediated oxidative damage (i.e. protein nitration) in the injured brain, using CCI-TBI in mice as a paradigm. While we have evidence from our prior work of an increased activity of mtNOS during the first hours after injury along with an increase in PN-derived 3-NT damage to mitochondrial proteins within 30 min. post-TBI (Singh, et al., 2006), this is not visible in regards to a significant increase in 3-NT in whole cortical tissue before 24 hrs post-TBI. Similarly, there was no increase in mRNA or protein levels of the three canonical NOS isoforms prior to 24 hrs. However, the 24 to 72 hour time course of protein nitrative damage which peaks at 72 hours in the ipsilateral CTX and HIPP is paralleled in both brain regions by a significant increase in eNOS protein and iNOS mRNA. The increase in eNOS protein may represent either a protective or a reparative response since it has been reported that eNOS is necessary to counteract posttraumatic cerebral hypoperfusion at 24 hours after CCI-TBI in mice. This is based on the observation that eNOS knockout mice have lower cerebral blood flow at that timepoint compared to wild-type mice (Lundblad, et al., 2009). Whether eNOS also contributes to 3-NT damage is uncertain. Lastly, the increase in iNOS expression probably reflects an increase in neutrophil and macrophage influx which peaks at 72 hours after CCI-TBI in mice and is associated with the posttraumatic maximum in protein nitration (Bayir, et al., 2005, Bayir, et al., 2007). Finally, the delayed peak in 3-NT secondary to increased NOS activity suggests that attenuation of PN-dependent nitrative damage in the injured brain may be pharmacologically modifiable for as long as 24 to 48 hrs after TBI.
Highlights.
Peroxnitrite plays a role in oxidative damage after traumatic brain injury (TBI)
Peroxynitrite is linked to protein nitration that peaks at 72 hours
Increased expression of eNOS and iNOS matches the nitration time course
Acknowledgements
This work was supported by 1R01 NS046566, 2P01 NS058484, 5P30 NS051220 and the Kentucky Spinal Cord & Head Injury Research Trust. Darren M. Miller was supported by fellowship funds from the NIH Blueprint Translational Neuroscience Training Grant 1T32 DA022738.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Juan A. Wang, Email: jawang@uky.edu.
Darren M. Miller, Email: darren.miller@uky.edu.
References
- Alvarez B, Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, Billiar TR, Williams DL, Kochanek PM. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J Cereb Blood Flow Metab. 2005;25:673–684. doi: 10.1038/sj.jcbfm.9600068. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- Beckman JS. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991;15:53–59. [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Carroll RT, Galatsis P, Borosky S, Kopec KK, Kumar V, Althaus JS, Hall ED. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chem Res Toxicol. 2000;13:294–300. doi: 10.1021/tx990159t. [DOI] [PubMed] [Google Scholar]
- Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Deng Y, Thompson BM, Gao X, Hall ED. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp Neurol. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denicola A, Freeman BA, Trujillo M, Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- Elfering SL, Sarkela TM, Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. J Biol Chem. 2002;277:38079–38086. doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- Elfering SL, Sarkela TM, Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. J Biol Chem. 2002 doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- Gahm C, Holmin S, Mathiesen T. Temporal profiles and cellular sources of three nitric oxide synthase isoforms in the brain after experimental contusion. Neurosurgery. 2000;46:169–177. [PubMed] [Google Scholar]
- Gahm C, Holmin S, Mathiesen T. Nitric oxide synthase expression after human brain contusion. Neurosurgery. 2002;50:1319–1326. doi: 10.1097/00006123-200206000-00024. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci. 2005;26:190–195. doi: 10.1016/j.tips.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Hall ED, Bryant YD, Cho W, Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25:235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel SG, Fischer AJ, Martin SM, Schafer FQ, Buettner GR. Nitric oxide as a cellular antioxidant: a little goes a long way. Free Radic Biol Med. 2006;40:501–506. doi: 10.1016/j.freeradbiomed.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun. 1995;211:375–381. doi: 10.1006/bbrc.1995.1824. [DOI] [PubMed] [Google Scholar]
- Lacza Z, Snipes JA, Zhang J, Horvath EM, Figueroa JP, Szabo C, Busija DW. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic Biol Med. 2003;35:1217–1228. doi: 10.1016/s0891-5849(03)00510-0. [DOI] [PubMed] [Google Scholar]
- Lundblad C, Grande PO, Bentzer P. Hemodynamic and histological effects of traumatic brain injury in eNOS-deficient mice. J Neurotrauma. 2009;26:1953–1962. doi: 10.1089/neu.2009.0955. [DOI] [PubMed] [Google Scholar]
- Mesenge C, Charriaut-Marlangue C, Verrecchia C, Allix M, Boulu RR, Plotkine M. Reduction of tyrosine nitration after N(omega)-nitro-L-arginine-methylester treatment of mice with traumatic brain injury. Eur J Pharmacol. 1998;353:53–57. doi: 10.1016/s0014-2999(98)00432-4. [DOI] [PubMed] [Google Scholar]
- Mesenge C, Verrecchia C, Allix M, Boulu RR, Plotkine M. Reduction of the neurological deficit in mice with traumatic brain injury by nitric oxide synthase inhibitors. PG-209-14. J Neurotrauma. 1996:13. [PubMed] [Google Scholar]
- Radi R. Peroxynitrite reactions and diffusion in biology. Chem Res Toxicol. 1998;11:720–721. doi: 10.1021/tx980096z. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of posttraumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Wada K, Chatzipanteli K, Busto R, Dietrich WD. Role of nitric oxide in traumatic brain injury in the rat. J Neurosurg. 1998;89:807–818. doi: 10.3171/jns.1998.89.5.0807. [DOI] [PubMed] [Google Scholar]
- Wada K, Chatzipanteli K, Busto R, Dietrich WD. Effects of L-NAME and 7-NI on NOS catalytic activity and behavioral outcome after traumatic brain injury in the rat. J Neurotrauma. 1999;16:203–212. doi: 10.1089/neu.1999.16.203. [DOI] [PubMed] [Google Scholar]
- Wada K, Chatzipanteli K, Kraydieh S, Busto R, Dietrich WD. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery. 1998;43:1427–1436. doi: 10.1097/00006123-199812000-00096. [DOI] [PubMed] [Google Scholar]