Abstract
Objective
Convergent neuroimaging and neuropsychological research demonstrates disrupted attention and heightened threat sensitivity in PTSD. This might be linked to aberrations in large-scale networks subserving detection of salient stimuli, i.e. the salience network (SN), and stimulus-independent, internally-focused thought, i.e. the default mode network (DMN).
Methods
Resting state brain activity was measured in returning veterans who served in Iraq or Afghanistan with (n=15) and without PTSD (n=15) and in healthy community controls (n=15). Correlation coefficients were calculated between the time course of seed regions in key SN and DMN regions (posterior cingulate, ventromedial prefrontal cortex, and bilateral anterior insula) and all other voxels of the brain.
Results
Compared to control groups, PTSD participants showed reduced functional connectivity within DMN (between DMN seeds and other DMN regions), including rostral ACC/vmPFC (Z=3.31; p=.005, corrected) and hippocampus (Z=2.58; p=.005), and increased connectivity within SN (between insula seeds and other SN regions), including amygdala (Z=3.03; p=.01, corrected). PTSD participants also demonstrated increased cross-network connectivity. DMN seeds exhibited elevated connectivity with SN regions, including insula (Z=3.06; p=.03, corrected), putamen, and supplementary motor area (Z=4.14; Z=4.08; p<.001), and SN seeds exhibited elevated connectivity with DMN regions, including hippocampus (Z=3.10; p=.048, corrected).
Conclusions
During resting state scanning, PTSD participants showed reduced coupling within DMN, greater coupling within SN, and increased coupling between DMN and SN. Our findings suggest a relative dominance of threat-sensitive circuitry in PTSD, even in task-free conditions. Disequilibrium between large-scale networks subserving salience detection versus internally focused thought may be associated with PTSD pathophysiology.
Keywords: PTSD, default mode network, salience network, functional connectivity, resting-state, fMRI
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder characterized by re-experiencing, avoidance and hyperarousal symptoms (1). These symptoms have been linked to deficits in attentional control (2–4), threat sensitivity (5–7), episodic memory (8–10), and fear extinction (11, 12), functions subserved by cortico-subcortical circuits involving amygdala, insula, ventromedial prefrontal cortex (vmPFC) and hippocampus (13, 14). While the activation patterns of these regions have been examined extensively in the past, identifying dysregulated patterns of connectivity between these regions could shed important and unique light on the brain-basis of PTSD (15, 16), and on previously unexplained mechanisms of PTSD symptom development.
Regions implicated in PTSD (e.g., insula, amygdala, vmPFC, hippocampus) are key nodes in several major intrinsic connectivity networks (ICNs). ICNs are large-scale networks identified by connectivity methods that are associated with characteristic functions (17, 18), are stable across tasks (19, 20) and over time (21, 22), correspond to anatomical white matter tracts (23), and demonstrate direct behavioral correlates (24–26). Insula and amygdala, which have been found to be hyperactive in PTSD (15, 16), are involved in the salience network (SN), an ICN responsible for detecting and orienting to salient stimuli (27–30). A second important ICN, the default mode network (DMN) is associated with stimulus-independent, internally focused thought and autobiographical memory (31–33). VMPFC and hippocampus, regions reported to be hypoactive in PTSD during specific tasks (16), along with the posterior cingulate cortex (PCC), are core components of this network (17, 28). A third ICN, the central executive network (CEN), encompasses dlPFC and lateral parietal regions. This network is associated with goal-directed behavior and high level cognitive function, including planning, decision-making, and working memory (27, 30). It has been suggested that SN “arbitrates” between DMN and CEN (34). SN regions assess the significance or salience of stimuli the organism encounters, and help to maintain an adaptive balance between internal mentation and externally-oriented focus and task execution.
Recently, functional connectivity analyses have begun to be used to probe network-level function in PTSD. During task-based studies, PTSD patients show exaggerated connectivity within DMN regions and reduced connectivity within SN and CEN regions (35). These relationships might potentially be better assessed, however, through connectivity analyses at rest, without the confounds of tasks that may be biased to elicit amygdala activity or provoke PTSD symptoms. Resting-state connectivity offers a powerful way to assess intrinsic connections between brain networks (17, 36, 37), which in turn have been linked to important functions such as processing speed (26) and cognitive flexibility (38) in health and in disease. During rest, disrupted connectivity within DMN and SN has been reported in PTSD (39–44) and acute stress disorder (45, 46). However, no research to date has used the ICN framework to investigate the relationship between DMN, SN and CEN at rest in PTSD. Identifying abnormal interrelationships between these networks could illuminate the disruptions in attention regulation and balance of internal/external sensitivity characteristically found in PTSD, including abnormalities underlying symptoms of hyperarousal.
Given findings that PTSD patients show alterations in functions linked to DMN and SN, we hypothesized disruptions in the balance between these networks in task-free resting-state, when DMN is typically active and SN and CEN are quiescent. Given aberrations in episodic memory in PTSD, we hypothesized reduced connectivity within DMN. Given heightened threat sensitivity, impaired attentional control, and hyperarousal in PTSD, functions associated with SN connectivity (27, 47, 48), we hypothesized increased connectivity within SN, and reduced segregation (that is, increased cross-network connectivity) between DMN regions and SN regions.
Methods and Materials
Participants
We recruited study participants from among veterans returning from deployments to Afghanistan (Operation Enduring Freedom; OEF) and Iraq (Operation Iraqi Freedom; OIF) with a current PTSD diagnosis and seeking treatment for PTSD at the VA Ann Arbor Healthcare System (n=15), along with veterans without PTSD (combat-exposed controls; n=15), and 15 healthy controls from the community. All procedures took place between August 2008 and July 2010. Healthy controls and combat controls (veterans of OEF and OIF without PTSD) were recruited from the community via advertisement. All participants received comprehensive psychiatric assessment with the Mini-International Neuropsychiatric Interview (M.I.N.I.; 49), which contains a PTSD module, and self-report instruments. Combat veterans also received the Clinician Administered PTSD Scale (CAPS; 50). Clinical interviews were performed by experienced Masters or PhD-level clinicians with extensive training in the CAPS, at a subspecialty clinic specializing in PTSD. PTSD participants were provided with the opportunity to participate in research at the time of their initial VA visit, and all interested eligible participants were included in the study. All participants in the PTSD group met DSM-IV criteria for current (past month) PTSD. All combat exposure (including index trauma for PTSD participants) took place within the past five years. Exclusion criteria were: 1) psychosis, 2) history of traumatic brain injury, 3) alcohol or substance abuse or dependence in the past 3 months, 4) any psychoactive medication other than sleep aids, 5) left-handedness, 6) presence of ferrous-containing metals within the body, and 7) claustrophobia. Control groups included only participants without DSM–IV psychiatric diagnoses, and the healthy community control group included only participants who had not experienced a traumatic event. Demographic and clinical characteristics of the participants are shown in Table 1. All subjects were right-handed males between 21 and 37 years old. Seven participants in the PTSD group also met diagnostic criteria for comorbid depression and one had comorbid panic disorder, however in no case could comorbid diagnosis be considered primary, i.e. preceding PTSD. Two PTSD participants were using low dose trazodone as a sleep aid; no other psychiatric medications were permitted. After a complete description of the study was provided to the participants, written informed consent was obtained. The study was approved by the Institutional Review Boards of the University of Michigan Medical School and the Ann Arbor Veterans Affairs Healthcare System.
Resting State Paradigm
Participants underwent structural (sMRI) and functional (fMRI) scanning that included resting state procedures and emotion regulation and conditioning tasks. Reports on emotion regulation and conditioning tasks are forthcoming; a report of amygdala-seed resting state functional connectivity has been previously published (43). Resting-state scans always occurred prior to tasks. A black fixation cross on a white background was displayed at the center of the screen for 10 minutes. Participants were instructed to relax and keep their eyes open and fixed on the cross. To control for physiological variation, multiple regression was used to remove the effects of cardiac and respiratory signals (51).
Data Analysis
Scans were collected on a 3.0 Tesla General Electric Signa® Excite™ scanner (Milwaukee, WI). Further details are provided in Supplemental Digital Content 1. Default mode ROIs (PCC and vmPFC) were 10 mm radius spheres centered at (0,−56,20) for PCC and (−2,48,−4) for vmPFC. These seed regions were adopted from previous studies of the DMN in PTSD (40, 45), schizophrenia (52), and healthy controls (17). Salience network ROIs (bilateral anterior insula) were partitioned from a whole insula mask using linear interpolation based on the location of the middle insular gyrus, following Aupperle and colleagues (53). We extracted spatially averaged time series from each ROI for each participant. Average BOLD time series from subject-specific structural MRI-derived white matter and cerebrospinal fluid masks were added to the model as nuisance covariates to control for non-specific global sources of noise associated with BOLD fMRI scanning. We did not perform global-signal regression, as it had been suggested that this may produce spurious anti-correlation with orthogonal networks that increase in proportion to the size of the those networks (54). Resting state functional connectivity measures low-frequency spontaneous BOLD oscillations (.01 – .10 Hz band) (17), thus, the time-course for each voxel was band-passed filtered in this range. Pearson product-moment correlation coefficients were calculated between average time courses in the seed regions of interest (ROIs) and all other voxels of the brain resulting in a 3-dimensional correlation coefficient image (r-image). Both positive correlations and anti-correlations were computed. These r-images were then transformed to z-scores using a Fisher r-to-z transformation (55).
Z-score images from the individual functional connectivity analyses were entered into second-level random-effects analyses (one-sample and two-sample t-tests) implemented in SPM5. Second-level maps were thresholded at p<.001, uncorrected, extent threshold k=10. In addition, region of interest analysis with small volume correction (SVC) was conducted. A priori ROIs including the amygdala (k=93), hippocampus (k=252), anterior cingulate cortex (ACC; k=838), and insula (k=499) were used as masks, as these regions are of interest in PTSD (15, 16). Images were thresholded using a voxelwise threshold of p<.005 uncorrected with a minimum cluster size of 3 connected voxels (for amygdala clusters), 4 connected voxels (for hippocampus clusters), 6 connected voxels (for insula clusters), and 12 connected voxels (for ACC clusters). These combinations of activation threshold and cluster size were determined using AlphaSim (56) to correspond to a false positive rate of p<.05, corrected for multiple comparisons within ROIs. Using the thresholds and cluster sizes defined above, the corrected voxel-wise probabilities are as follows: amygdala p<.0016, hippocampus p<.00098, insula p<.00045, and ACC p<.00072. Only the activations within the ROIs that survived the volume and voxel correction criteria were extracted and used for further analysis. Connectivity foci were labeled by comparison with the neuroanatomical atlas by Talairach and Tournoux (57). Reported voxel coordinates correspond to standardized Montreal Neurologic Institute (MNI) space. To assess for correlations with symptom severity, CAPS scores were added as regressors in a separate whole brain analysis of connectivity between seed regions and ROIs, corrected for multiple comparisons across four seed regions. Finally, we conducted an exploratory conjunction analysis (58) to determine whether regions of group difference with one ICN seed-region showed overlap with the other ICN (e.g. regions of differential group connectivity with DMN seed regions falling within SN, and vice versa).
Motion parameters (maximum displacement, mean displacement, maximum angle, mean angle) were compared via Independent-Samples Kruskal-Wallis tests.
Results
Participants
While levels of combat exposure were not different in the returning veterans with and without PTSD, PTSD symptoms (CAPS scores) in the healthy combat-exposed control group were low, and significantly lower than the PTSD group. Groups did not differ by age or race, however there was a trend for marital status such that PTSD participants were more likely to be married than healthy community or combat controls (p=.08; see Table 1).
Motion
There were no movements greater than 3 millimeters and no motion differences between groups (in all cases p>.2).
FMRI findings
In the following paragraphs, we focus on comparisons between the PTSD group and Combat-exposed Controls and Healthy Community Controls combined (“Controls”). Of note, single group results and two-group comparisons between the PTSD group and individual control groups are presented in SDC1, Tables S1 through S5.
PTSD versus Controls
PCC
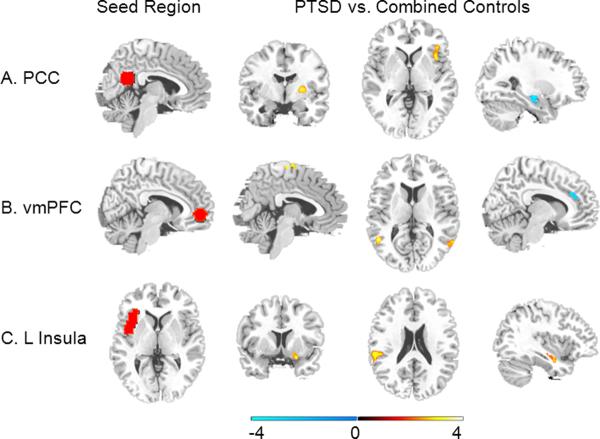
In comparison to controls, PTSD participants showed greater functional connectivity between PCC seed and right putamen ([24,−9,3]; k=30; Z=4.13; p<.001) and right insula ([39,15,−3]; k=6; Z=3.06; p=.03, SVC). Compared to PTSD participants, controls showed greater connectivity between PCC seed and left hippocampus at trend level ([−27,−15,−18]; k=5; Z=2.58; p=.005; see Figure 1 and Figure 2; Table 2).
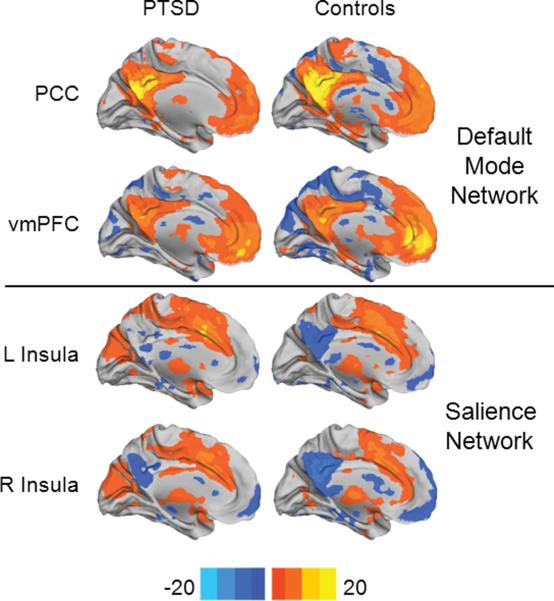
Figure 1. Functional connectivity analysis.
Statistical significance (color-coded t score) of resting-state functional connectivity patterns for Default Mode Network (PCC seed and vmPFC seed) and Salience Network (left anterior insula seed and right anterior insula seed) for 15 PTSD participants and 30 Controls (15 combat-exposed controls and 15 healthy community controls). PCC: posterior cingulate cortex; vmPFC: ventromedial prefrontal cortex.
Figure 2. Group Comparison.
(A) Compared to controls, PTSD participants showed greater connectivity between posterior cingulate cortex (PCC; seed region shown in A) and right putamen (column 2; y = −4) and right insula (column 3; z = 2) and a trend for less connectivity between PCC seed and left hippocampus (column 4; x = −27). (B) Compared to controls, PTSD participants showed greater connectivity between ventromedial prefrontal cortex (vmPFC; seed region shown in B) and supplementary motor area (column 2; x = 5) and bilateral superior temporal sulcus (column 3; z = 11) and less connectivity between vmPFC seed and rostral anterior cingulate cortex (column 4; x = −10). (C) Compared to controls, PTSD participants showed greater connectivity between left anterior insula (=seed region shown in C) and right amygdala (column 2; y = 8), left peri-insula (column 3; z = 21) and right hippocampus (column 4; x = 37).
vmPFC
Compared to controls, PTSD participants showed greater functional connectivity between the vmPFC seed and precentral sulcus/supplementary motor area ([15,−15,72]; k=26; Z=4.08; p<.001), right precentral gyrus ([54,−12,27]; k=12; Z=3.7; p<.001), and bilateral superior temporal sulcus ([−51,−66,12]; k=12; Z=3.51; [48,−57,12]; k=13; Z=4.15; p<.001). Compared to PTSD participants, controls showed greater connectivity between vmPFC seed and rostral anterior cingulate cortex ([−12,27,27]; k=20; Z=3.31; p=.005, SVC; see Figure 2; Table 2).
Left anterior insula
Compared to controls, PTSD participants showed greater functional connectivity between the left anterior insula seed and left peri-insula/superior temporal gyrus ([−48,−33,21]; k=11; Z=3.54; p<.001), right hippocampus ([36,−12,−15]; k=12; Z=3.10; p=.048, SVC), and right amygdala ([24,6,−18]; k=5; Z=3.03; p=.01, SVC; see Figure 2; Table 2). For left anterior insula, controls did not show significantly greater functional connectivity than PTSD participants in any regions.
Right anterior insula
There were no significant group differences in right anterior insula connectivity between the PTSD and combined control group. Please see SDC1 for comparisons to individual control groups.
Correlations with Symptom Severity
PCC
Within the PTSD group, functional connectivity between PCC seed and precentral sulcus was positively correlated with symptom severity as measured by the CAPS ([−9,−6,72]; k=7; Z=3.79; r=.88, p<.001; corrected for comparisons at multiple seed regions), as well as CAPS subscales (Intrusive subscale: r=.72, p=.009, Avoidance subscale: r=.64, p=.03, Hyperarousal subscale: r=.69, p=.01).
vmPFC
Within the PTSD group, functional connectivity between vmPFC seed and left hippocampus was negatively correlated with total CAPS score ([−33,−21,−12]; k=12; Z=3.9; r=−.89, p<.001; corrected for multiple comparisons), as well as CAPS subscales (Intrusive subscale: r=−.71, p=.004, Avoidance subscale: r=−.68, p=.007, Hyperarousal subscale: r=−.75, p=.002).
Left anterior insula
There were no significant correlations between left anterior insula connectivity and total CAPS score in the PTSD group.
Right anterior insula
Within the PTSD group, functional connectivity between right anterior insula seed and right peri-insula/rolandic operculum was positively correlated with symptom severity as measured by the CAPS ([57,−6,18]; k=6; Z=4.47; r=.67, p<.001; corrected for multiple comparisons), as well as CAPS subscales (Intrusive subscale: r=.6, p=.02, Avoidance subscale: r=.56, p=.04).
Conjunction Analysis
A conjunction analysis was performed comparing the map of functional connectivity with a PCC seed, PTSD participants > controls, and control group connectivity with an anterior insula seed. Results showed overlap in right putamen ([27,−6,3]; k=30) and right thalamus ([9,−21,6]; k=18).
Functional connectivity with a vmPFC seed, PTSD participants > controls, was compared with a map of control group connectivity with an anterior insula seed. Results showed overlap in precentral sulcus ([18,−12,72]; k=8), right superior temporal sulcus ([51,−54,9]; k=7), and left superior temporal sulcus ([−51,−60,9]; k=8).
Discussion
In this study, we investigated patterns of resting-state default and salience network functional connectivity, comparing military combat veterans deployed to Iraq or Afghanistan (OEF/OIF) with PTSD versus OEF/OIF combat-exposed healthy veterans and healthy community controls. To our knowledge, this is the first study to systematically examine the relationship between the SN and DMN ICNs in PTSD during rest, and the first to examine resting-state DMN functional connectivity in combat veterans. Overall, we found reduced connectivity within DMN, increased connectivity within SN, and increased connectivity between DMN regions and SN regions in PTSD subjects. The results suggest a possible brain basis for exaggerated attention to external stimuli, potentially contributing to hypervigilance and hyperarousal symptoms of PTSD.
Our within group findings (presented in SDC1) are highly consistent with existing models of DMN and SN functional connectivity (17, 31, 32, 37). We found strong functional connectivity in healthy control groups (i.e., combat controls and community controls) between PCC and vmPFC seeds and other DMN regions, including angular gyrus, lateral temporal lobe, and hippocampus. The DMN is associated with stimulus-independent thought, including spontaneous cognition, autobiographical memory, and mind-wandering (59), thus this network is active during non-structured tasks. DMN seed regions also showed expected anti-correlation with regions of the SN (including insula, inferior frontal gyrus, and supplementary motor area) (27). Our analysis also revealed high functional connectivity between SN seeds (bilateral anterior insula) and other SN regions, including dorsal ACC/supplementary motor area, amygdala, and striatum, along with anti-correlation between SN seeds and DMN regions including PCC, vmPFC, and hippocampus. The SN is associated with attention to external stimuli, and though SN is co-active with DMN during certain tasks (60), it typically anti-correlates with DMN during rest (17, 61, 62). Our results thus confirm existing models that suggest that rest and other non-structured activities require deactivation of attentional and executive processes reflected by anti-correlation between DMN and SN.
Compared to control groups, PTSD participants showed reduced functional connectivity between DMN seeds and other DMN regions, including rostral ACC/vmPFC and hippocampus, consistent with a previous study in PTSD (40), as well as another study in OCD (63). These regions are also hypoactive in PTSD during tasks (16) including script-driven imagery, trauma-relevant and trauma-irrelevant negative stimuli (15). Thus, our results suggest that regions underactive in PTSD in a variety of task-based contexts also fail to incorporate into resting-state networks, potentially indicating more general disruptions in self-referential thought in PTSD. Our findings add to a growing body of evidence suggesting that in PTSD there is weakened DMN functional integration during resting-state (40, 41, 46), and alterations in DMN function more generally (35, 39). Though the implications of this dysfunction remain to be further investigated, it may suggest that default mode functioning is hampered by hyperarousal and avoidance, or that abnormal interconnectedness between DMN and SN contributes to these symptoms. Indeed, within our PTSD sample, less DMN connectivity to hippocampus was associated with higher CAPS score (total score, as well as reexperiencing, avoidance and hyperarousal subscales). Thus, reduced cohesiveness of DMN may be associated with greater symptom severity in PTSD.
PTSD participants also demonstrated greater functional connectivity between SN seeds and other SN regions, including insula, peri-insula, and amygdala. The SN is associated with homeostatic regulation, interoceptive, autonomic, and reward processing (27, 28, 34, 64), functions known to be dysregulated in anxiety disorders (65, 66). Greater within-SN connectivity may reflect exaggerated sustained attention to extraneous stimuli. In activation studies, co-occurring increases in insula and amygdala activity are greater in PTSD than in control groups during trauma reminders (67) and fear acquisition (68), and a recent meta-analysis confirms patterns of disrupted DMN and SN in PTSD during structured tasks (69). Recently we have reported that individuals with PTSD show greater intrinsic connectivity between amygdala and insula at rest (43), potentially reflecting a tighter functional link between visceral perception and emotional response. Our findings complement those of Daniels and colleagues (35), who report ICN alterations in PTSD during a working memory task. However, while Daniels et al. reported decreased within-network SN connectivity and increased within-network DMN connectivity during task, we found increased within-network SN connectivity and decreased within-network DMN connectivity during rest. This discrepancy might reflect differences in the balance between ICNs during cognitively demanding tasks (where CEN and SN are predominant) versus rest (where DMN is predominant). Thus Daniels and colleagues' study and our own both suggest aberrant ICN activity in PTSD. In our analysis, greater functional connectivity between SN seed regions and peri-insula/operculum was associated with higher CAPS score, further supporting the hypothesis that pervasive SN activity during task-free states may be involved in PTSD psychopathology.
In addition to weakened within-network DMN connectivity and exaggerated within-network SN connectivity, PTSD participants also demonstrated increased cross-network connectivity between DMN regions and SN regions. In PTSD, as compared to controls, SN seed regions showed increased connectivity to left hippocampus, part of DMN. In turn, DMN seed regions showed increased connectivity to SN regions of insula, putamen, and supplementary motor area, as well as posterior superior temporal sulcus. The superior temporal sulcus, though not previously implicated in the SN, is an associative area that shows strong resting-state functional connectivity with SN regions including supplementary motor area, insula, and striatum (70), thus it may contribute to SN function. A conjunction analysis confirmed that regions showing exaggerated connectivity with DMN in PTSD were constituents of the SN in control groups. Enhanced cross-network connectivity in PTSD between DMN and regions of SN has also been shown in prior studies. For example, elevated connectivity in PTSD has been observed between thalamus (a key SN node) and DMN (71). Additionally, enhanced connectivity between DMN and amygdala predicted the development of PTSD symptoms in acutely traumatized individuals (45), and enhanced connectivity between DMN and insula was associated with higher self-report anxiety (72).
The SN supports monitoring of salient cognitive, homeostatic, or emotional events, and is typically anti-correlated with DMN during rest (27, 73). Thus our results indicate a disruption of equilibrium between these networks, or diminished network segregation. In particular, the co-activation of SN with DMN might reflect sustained and likely inappropriate activation of SN during periods of rest, when this network is typically quiescent. This finding may reflect or help to explain sustained hypervigilance and hyperarousal in PTSD patients. Indeed, in our sample, greater functional connectivity between DMN seeds and supplementary motor area, an important node in the SN network, was correlated with enhanced hyperarousal symptoms (and total PTSD symptoms). Our results are thus consistent with previous findings of disrupted cortico-subcortical connectivity in PTSD (45, 71, 72), and extend these findings by revealing an underlying dysfunction in the balance between ICNs.
The functional impact of disrupted resting-state connectivity has been debated. Disrupted ICN segregation could be related to the development of other symptoms or physiological abnormalities in PTSD. For instance, greater intrinsic connectivity in the SN is associated with higher self-report anxiety (27) and greater HPA axis responsivity to aversive stimuli (47). Disruptions in the equilibrium between ICNs may affect regulatory efficiency, particularly alterations in SN, which plays a role in switching between other large-scale networks (34). Finally, weaker anti-correlation between DMN and its anti-correlated networks (both at task and at rest) is associated with diminished performance in a cognitive interference task (26) and a psychomotor vigilance task (48). Taken together, these findings suggest that disequilibrium between ICNs is a pervasive phenomenon that may affect PTSD patients across a variety of contexts.
This study has several limitations. First, our sample size was small, thus our results should be considered preliminary. Second, seed voxel-based resting state connectivity provides an important method to assess connectivity within specific networks, but it is essentially correlational and thus does not allow for inferences about causal relationships. Activation differences and connectivity differences cannot be disentangled using seed-based connectivity measures. Since we did not collect measures of mental activity during the resting state scan, we cannot discern whether differential engagement in various cognitive processes could be driving the observed group differences. Third, since only male veterans with combat exposure were included in this study, generalizations to women or to non-combat related PTSD should be made with caution. Fourth, our PTSD sample included participants with co-morbid major depression. Though depression commonly co-occurs with PTSD in veteran populations (up to 80% by some estimates: 74), inclusion of depressed participants may render some of the variance attributable to the presence of depression. However, our results were not affected by the removal of participants with current comorbid depression (see Supplemental Results in SDC1). Therefore, we retained depressed participants in our final analysis. In conclusion, this study found reduced coupling within DMN, increased coupling within SN, and increased coupling between DMN and SN in PTSD participants during resting-state. These findings may reflect a dominance of threat-sensitive circuitry in PTSD, even in task-free conditions. Our results suggest that disequilibrium between large-scale networks subserving salience detection versus internally focused thought may be associated with PTSD pathophysiology.
Supplementary Material
Acknowledgments
The research reported in this article was supported by grants from the National Institute of Mental Health (R24 MH075999) to IL, from the Telemedicine and Advanced Technology Research Center (W81XWH-08-2-0208) to IL and AK, from the Michigan Institute for Clinical & Health Research (U028028) to SG and from the University of Michigan Center for Computational Medicine and Bioinformatics Pilot Grant Program to AK.
Abbreviations
- PTSD
posttraumatic stress disorder
- vmPFC
ventromedial prefrontal cortex
- ICN
intrinsic connectivity networks
- DMN
default mode network
- PCC
posterior cingulate cortex
- CEN
central executive network
- SN
salience network
- OEF
Operation Enduring Freedom
- OIF
Operation Iraqi Freedom
- MINI
Mini-International Neuropsychiatric Interview
- CAPS
Clinician Administered PTSD Scale
- sMRI
structural MRI
- fMRI
functional MRI
Footnotes
Financial Disclosures All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.APA . Diagnostic and statistical manual of mental disorders (4th ed., text rev.) 4th ed American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- 2.Marx BP, Brailey K, Proctor SP, Macdonald HZ, Graefe AC, Amoroso P, Heeren T, Vasterling JJ. Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following Iraq war deployment. Arch Gen Psychiatry. 2009;66:996–1004. doi: 10.1001/archgenpsychiatry.2009.109. [DOI] [PubMed] [Google Scholar]
- 3.Bardeen JR, Orcutt HK. Attentional control as a moderator of the relationship between posttraumatic stress symptoms and attentional threat bias. J Anxiety Disord. 2011;25:1008–18. doi: 10.1016/j.janxdis.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–30. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cisler JM, Wolitzky-Taylor KB, Adams TG, Jr, Babson KA, Badour CL, Willems JL. The emotional Stroop task and posttraumatic stress disorder: a meta-analysis. Clin Psychol Rev. 2011;31:817–28. doi: 10.1016/j.cpr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pineles SL, Shipherd JC, Mostoufi SM, Abramovitz SM, Yovel I. Attentional biases in PTSD: More evidence for interference. Behav Res Ther. 2009;47:1050–7. doi: 10.1016/j.brat.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clin Psychol Rev. 2006;26:939–55. doi: 10.1016/j.cpr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behav Brain Res. 2010;215:162–71. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Guez J, Naveh-Benjamin M, Yankovsky Y, Cohen J, Shiber A, Shalev H. Traumatic stress is linked to a deficit in associative episodic memory. J Trauma Stress. 2011;24:260–7. doi: 10.1002/jts.20635. [DOI] [PubMed] [Google Scholar]
- 11.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–41. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–37. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–29. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–77. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–41. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–84. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–33. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–37. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 30.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 32.Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–9. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Daniels JK, McFarlane AC, Bluhm RL, Moores KA, Clark CR, Shaw ME, Williamson PC, Densmore M, Lanius RA. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35:258–66. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 38.Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. J Neurophysiol. 2009;101:2632–48. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu RZ, Zhang JR, Qiu CJ, Meng YJ, Zhu HR, Gong QY, Huang XQ, Zhang W. [Study on resting-state default mode network in patients with posttraumatic stress disorder after the earthquake] Sichuan Da Xue Xue Bao Yi Xue Ban. 2011;42:397–400. [PubMed] [Google Scholar]
- 40.Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Neufeld RW, Theberge J, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34:187–94. [PMC free article] [PubMed] [Google Scholar]
- 41.Daniels JK, Frewen P, McKinnon MC, Lanius RA. Default mode alterations in posttraumatic stress disorder related to early-life trauma: a developmental perspective. J Psychiatry Neurosci. 2011;36:56–9. doi: 10.1503/jpn.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP, Goetz JM, Fischman AJ, Rauch SL, Pitman RK. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–9. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, Neufeld RW, Williamson PC, Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 46.Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, Li D, Kuang W, Chan RC, Mechelli A, Sweeney JA, Gong Q. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci U S A. 2009;106:15412–7. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernandez G. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–3. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- 48.Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan WJ, McKinley A, Tripp LD, Schumacher EH, Keilholz SD. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum Brain Mapp. doi: 10.1002/hbm.22140. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 50.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 51.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 52.Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–12. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aupperle RL, Ravindran L, Tankersley D, Flagan T, Stein NR, Simmons AN, Stein MB, Paulus MP. Pregabalin influences insula and amygdala activation during anticipation of emotional images. Neuropsychopharmacology. 2011;36:1466–77. doi: 10.1038/npp.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 2011;32:919–34. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher RA. Frequency distribution of the values of the correlation coefficient in samples of an indefinitely large population. Biometrika. 1915;10:507–21. [Google Scholar]
- 56.Ward B. Simultaneous inference for fMRI data. 2000 [Google Scholar]
- 57.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme. 1988 [Google Scholar]
- 58.Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–70. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 59.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 60.Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. 2012;33:192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jang JH, Kim JH, Jung WH, Choi JS, Jung MH, Lee JM, Choi CH, Kang DH, Kwon JS. Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder. Neurosci Lett. 2010;474:158–62. doi: 10.1016/j.neulet.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 64.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–63. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–6. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- 67.Vermetten E, Schmahl C, Southwick SM, Bremner JD. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull. 2007;40:8–30. [PMC free article] [PubMed] [Google Scholar]
- 68.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2012.06.003. in press. [DOI] [PubMed] [Google Scholar]
- 70.Habas C, Guillevin R, Abanou A. Functional connectivity of the superior human temporal sulcus in the brain resting state at 3T. Neuroradiology. 2011;53:129–40. doi: 10.1007/s00234-010-0775-5. [DOI] [PubMed] [Google Scholar]
- 71.Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, Chen H, Feng B, Jiang T, Jin H, Wong C, Gong Q, Li L. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging study. Brain Res. 2011;1411:98–107. doi: 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 72.Dennis EL, Gotlib IH, Thompson PM, Thomason ME. Anxiety modulates insula recruitment in resting-state functional magnetic resonance imaging in youth and adults. Brain Connectivity. 2011;1:245–54. doi: 10.1089/brain.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krebs RM, Boehler CN, Roberts KC, Song AW, Woldorff MG. The Involvement of the Dopaminergic Midbrain and Cortico-Striatal-Thalamic Circuits in the Integration of Reward Prospect and Attentional Task Demands. Cereb Cortex. 2012;22:607–15. doi: 10.1093/cercor/bhr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hankin CS, Spiro A, 3rd, Miller DR, Kazis L. Mental disorders and mental health treatment among U.S. Department of Veterans Affairs outpatients: the Veterans Health Study. Am J Psychiatry. 1999;156:1924–30. doi: 10.1176/ajp.156.12.1924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.