Abstract
Rationale
The motivational process that regulates approach behavior toward salient distal stimuli (i.e., incentive motivation) plays a key role in voluntary behavior and motivational disorders such as addiction. This process may be mediated by many neurotransmitter systems and a network of many brain structures, including the median and dorsal raphe regions (MR and DR, respectively).
Objective
We sought to examine whether the blockade of excitatory amino acid receptors in the MR and DR is rewarding, using intracranial self-administration, and whether the self-administration effect can be explained by drug’s effectiveness to enhance incentive motivation, using a visual sensation seeking procedure.
Results
Rats learned to self-administer the AMPA receptor antagonist ZK 200775 into the vicinity of the MR, DR, or medial oral pontine reticular regions, but not the ventral tegmental area. The NMDA receptor antagonist AP5 was also self-administered into the MR, while it was not readily self-administered into other regions. When ZK 200775 was noncontingently administered into the MR, rats markedly increased approach responses rewarded by brief illumination of a light stimulus. In addition, contingent administration of ZK 200775 into the MR induced a conditioning effect on approach responses.
Conclusions
Rats self-administer excitatory amino acid receptor antagonists into the MR and adjacent regions. Self-administration effect of AMPA receptor antagonists into the MR can be largely explained by the manipulation’s properties to invigorate ongoing approach behavior and induces conditioned approach. Glutamatergic afferents to the median raphe and adjacent regions appear to tonically suppress incentive-motivational processes.
Keywords: Intracranialself-administration, Visualsensation seeking, Reinforcement, Glutamate, Superior central raphe nucleus, Oral pontine reticular nucleus
Introduction
There appear to be multiple-reward systems, and one of them may be involved in the motivational process that regulates approach behavior toward salient distal stimuli. Such process has been referred to in various names: incentive motivation (Bindra 1968; Bolles 1972), SEEKING (Panksepp and Moskal 2008), wanting (Berridge 1996), survival circuit (LeDoux 2012), and approach coordinator (Ikemoto 2010). In particular, this notion (thereafter referred to as incentive motivation) has been helpful in understanding behavioral effects of drugs of abuse in two ways. First, drug administration alters the brain in such a way that distal stimuli (discrete or contextual) associated with drug-taking acquire positive motivational effects of the drugs and subsequently guide and motivate animals (i.e., incentive-motivational learning) (Stewart et al. 1984). In addition, drug administration, especially psychomotor stimulants or nicotine (Chaudhri et al. 2006; Robbins and Everitt 1982), acutely increase ongoing approach behavior guided by salient stimuli (i.e., enhancement of incentive motivation). Thus, the notion of incentive motivation may help to understand how some drug manipulations are rewarding and how they alter motivational state.
Brain mechanisms that regulate incentive motivation may consist of a network of structures localized from the cortex to lower brainstem, including the median raphe (MR) and dorsal raphe (DR) regions (Ikemoto 2010). Past research suggests that the MR and DR play a major role in suppression of voluntary behavior (Cools et al. 2008; Dayan and Huys 2009; Deakin 1983; Lucki 1998; Soubrie 1986; Takahashi et al. 2010), and growing evidence suggests that MR and DR neurons are involved in reward-related processes including incentive motivation. Using intracranial injection procedures as a tool to identify neurotransmitter systems in reward (Ikemoto and Wise 2004; McBride et al. 1999), we recently found that rats learn to self-administer the GABAA receptor agonist muscimol or the GABAB receptor agonist baclofen into these regions (Liu and Ikemoto 2007; Shin and Ikemoto 2010). In addition, pairing a novel place with intra-MR or DR injections of these chemicals induces conditioned place preference (Liu and Ikemoto 2007; Shin and Ikemoto 2010), indicating incentive-motivational learning. Similarly, conditioned place preference is induced by injections of the 5-HT1A receptor agonist 8-OH-DPAT into the MR or DR (Fletcher et al. 1993), a manipulation that inhibits serotonin neurons via stimulation of its autoreceptors.
In addition to GABAergic and serotonergic transmission, functional processes of the MR and DR are markedly modulated by glutamate transmission. Decreased glutamate transmission in the MR or DR has similar arousing effects as increased GABA transmission. Specifically, the blockade of excitatory amino acid receptors, like the stimulation of GABAergic receptors, in the MR or DR increases locomotor activity (Wirtshafter and Klitenick 1989; Wirtshafter et al. 1993; Wirtshafter et al. 1989) and hippocampal theta rhythm (Kinney et al. 1994; Varga et al. 2002). Because the stimulation of GABA receptors in the MR or DR induces reward-related behaviors, we hypothesize that the blockade of excitatory amino acid receptors will also induce reward-related behaviors.
The aim of the present study is threefold. First (experiment 1), we examined whether rats learn to self-administer the AMPA or NMDA receptor antagonists into the MR and DR. To demonstrate anatomical specificity, we also examined adjacent regions including the ventral tegmental area (VTA) into which a previous study (David et al. 1998) found that AMPA or NMDA receptor antagonists are self-administered. Our data suggest that rats readily self-administer the AMPA receptor antagonist ZK 200775 into the MR.
Second, we examined whether intra-MR ZK 200775 enhances incentive motivation. To this end, we used a sensation-seeking procedure (experiments 2 and 3) in which responding was rewarded by brief illumination of a light stimulus (visual sensation or VS) (Kish 1966; Stewart 1960). Previous studies have shown that rewarding drug manipulations readily increase responses toward unconditioned VS, including administration of amphetamine into the ventral striatum (Shin et al. 2010), baclofen into the MR (Vollrath-Smith et al. 2012) and systemic nicotine (Donny et al. 2003; Palmatier et al. 2006; Sorge et al. 2009).
Third, we examined whether intra-MR ZK 200775 induces conditioning (experiment 4). Heightened positive incentive motivation is hypothesized to be positively affective, a state that induces incentive-motivational conditioning (Bindra 1969; Ikemoto 2010). We report here that rats self-administer excitatory amino acid receptor antagonists into the MR, and this manipulation enhances ongoing approach behavior and induce conditioned approach.
Methods
Animals
We used 115 male Wistar rats (Harlan, Dublin, VA) weighing 250–350 g at the time of surgery. The colony room was maintained at constant temperature and humidity on a reverse 12 h dark/12 h light cycle (7:00 AM off). Food and water were freely available except during testing, which was conducted during the dark cycle. All procedures were approved by the Animal Care and Use Committee of the Intramural Research Program of the National Institute on Drug Abuse, and were in accordance with the Guide for the care and use of laboratory animals (National Research Council 2011).
Surgery
Each rat was stereotaxically implanted with a permanent single guide cannula (24 gauge) under sodium pentobarbital (31 mg/kg, i.p.) and chloral hydrate (142 mg/kg, i.p.) anesthesia. Each rat’s guide cannula ended 1.0 mm above one of the target regions. All cannulae were inserted into the left hemisphere at a lateral angle (0, 6, 10, or 20°) toward the midline. The incisor bar was set so that the dorsal/ventral coordinate was the same at both lambda and bregma, thereby leveling the skull. Stereotaxic coordinates (in millimeters) were 7.4–8.1 posterior to bregma (P), 1.5–1.6 lateral to the midline (L), and 7.8–8.0 ventral to the skull surface (V) angled at 10° for the MR; P7.2, L2.6, and V5.6 with a 20° for the DR. Additional cannulae placements for control injection sites were aimed at the oral pontine reticular nucleus (PnO) (P7.4–8.0, L0.7–1.5, and V7.8–8.0 with no angle); lateral DR (P7.2, L1.0, and V5.6 with no angle); and posterior VTA (P5.6, L1.3, and V8.0 with a 6° angle). Each cannula was subsequently anchored to the skull with four stainless steel screws and dental acrylic, and was kept patent with a 31-gauge dummy cannula. Rats were housed singly following surgery to prevent other rats from chewing the implant, and were allowed a minimum of five recovery days before the start of experimentation.
Drugs
ZK 200775 (Turski et al. 1998), competitive AMPA receptor antagonist (Tocris Bioscience, MO; molecular weight, 409.3), and D-AP5 (competitive NMDA receptor antagonist; Sigma-Aldrich, MO; molecular weight, 197.1) were dissolved in artificial cerebrospinal fluid consisting of (in millimolars): 148 NaCl, 2.7 KCl, 1.2 CaCl2, and 0.85 MgCl2, pH adjusted to 7.0–7.5.
Experimental apparatuses and general behavioral procedure
Experimental apparatuses are described in our previous papers (Shin et al. 2010; Vollrath-Smith et al. 2012). Briefly, each rat was tested in an operant conditioning chamber equipped with two levers, cue lights, a house light, and a micropump (Ikemoto and Sharpe 2001) that delivered intracranial injections. Locomotor activity was detected by a sensor (Roto-Rat, Med Associates, St. Albans, VT), which quantified rotational activity of the commutator of the micropump. Each session of all experiments lasted 90 min, except in experiment 1 where sessions were terminated early if rats received a total of 60 infusions. This maximum number of infusions was adopted from previous studies (e.g., Ikemoto 2005; Ikemoto et al. 2006), to minimize possible damage to the tissue and diffusion of the drug beyond the target region. Sessions were separated by 24 h, and 1 day before beginning testing, each rat was habituated to the testing chamber for 90 min. Rats used for each experiment were naïve in the sense that they were not trained to lever press for any reward prior to the experiment.
Experiment 1: self-administration of ZK 200775 and AP5 into the MR, DR, and adjacent regions
Each session was started by inserting one lever into the testing chamber; the other lever was not available throughout this experiment. A response on the lever turned on the drug delivery pump, the cue light directly above the lever for 5 s, and turned off the house light for 15 s during which additional lever pressing was recorded but had no consequence. Using this behavioral procedure, self-administration effects of ZK 200775 and AP5 were examined in each rat; that is, each rat received both drugs. The order of testing ZK 200775 and AP5 was counterbalanced among rats. Each rat received the following infusions: vehicle in session 1, 1.0 mM (ZK 200775 or AP5) in sessions 2–4 (acquisition phase), and 0, 0.1, 0.3, and 1.0 mM (ZK 200775 or AP5) in sessions 5–8, respectively (concentration effectiveness test phase). These treatments were delivered into the DR, MR, or adjacent regions including the posterior VTA and PnO.
Experiment 2: effects of noncontingent ZK 200775 into the MR on locomotion and lever pressing rewarded by visual sensation
In this experiment, infusions of ZK 200775 were delivered into the MR in a noncontingent manner. ZK 200775 was delivered in comparable doses into the MR as those self-administered in experiment 1: ZK 200775 or its vehicle (75 nl per infusion) was delivered every 90 s (60 infusions or 4.5 μl per 90-min session) for the next eight sessions. Rats received vehicle in sessions 1 and 8, 0.1 mM (0.45 nmol per session) in sessions 2–3, 0.3 mM (1.5 nmol) in sessions 4–5, and 1.0 mM (4.5 nmol) in session 6–7.
A response on the active lever illuminated the cue light above the lever for 1 s, extinguished the house light for 6 s during which responses on the lever were recorded, but did not deliver drug. Responding on the inactive lever had no programmed consequence throughout session. To enhance discrimination between the two levers and to better detect motivational effects of manipulations, we employed a progressive ratio schedule in which the response requirement on the active lever for VS started with a fixed ratio 1, and progressively increased by 1 every 10 VS earned (this procedure was adopted from our previous study (Shin et al. 2010)). During sessions, locomotor activities were also recorded.
Experiment 3: effects of combining contingent VS and noncontingent intra-MR ZK 200775
Like experiment 2, infusions of ZK 200775 were delivered into the MR in a noncontingent manner. In this experiment, intra-MR infusions were delivered on a fixed-interval of 250 s (23 infusions or 1.7 μl in 90-min session). This procedure was adopted from our previous study (Vollrath-Smith et al. 2012), to minimize total volume of infusions, thereby drug diffusion.
We designed this experiment foremost to examine effects of intra-MR ZK 200775 in the absence of VS, and therefore, its effects were examined in the relatively early phase of the experiment. Levels of lever pressing without drug infusions in the presence and absence of VS were characterized in our previous studies (Shin et al. 2010; Vollrath-Smith et al. 2012), while levels of lever pressing with intra-MR ZK 200775 in the presence of VS were characterized in experiment 2. The experiment was designed to test effects of a combination of VS and ZK 200775 last and to minimize their possible carryover (or conditioning) effects. In sessions 1–2, lever-pressing produced no VS, while rats received intra-MR vehicle. In sessions 3–4, lever pressing produced no VS, while rats received ZK 200775 (0.3 mM or 0.5 nmol per session). In sessions 5–6, rats were rewarded with VS on the same progressive ratio as described in experiment 2, while receiving intra-MR vehicle infusions. Finally, in sessions 7–8, rats were rewarded with VS, while receiving intra-MR ZK 200775.
Experiment 4: does intra-MR ZK 200775 reinforce behavior
Two cue lights located above the levers differed between the active and inactive levers with respect to their orientation of a shape (25×3 mm)—either a horizontal or vertical stripe. The assignments of cue orientation and ZK 200775 to left and right levers were counterbalanced among rats, while the assignments of cue orientations to the levers and the drug to lever locations remained the same for each rat throughout the experiment.
During sessions 1–4, rats were trained on a fixed ratio 1 where a response on the active lever delivered an infusion of 0.3 mM ZK 200775 or vehicle, illuminated the cue light above the lever for 1 s, turned off the house light for 10 s, and retracted both active and inactive levers for 40 s. Responding on the inactive lever did the same, except without drug infusions. During sessions 5–7, rats received ZK 200775 or vehicle infusions on a progressive ratio schedule where response requirement to produce a drug infusion was a fixed ratio 1 and progressively increased by 1 after every eight infusions earned. Responding on the active lever delivered an infusion, illuminated the cue light for 1 s, turned off the house light for 5 s, and retracted both active and inactive levers for 30 s, while responding on the inactive lever did the same, except infusions. No programmed cue was provided until rats pressed the required number of responses. When response requirement changed for the active lever, the same requirement controlled cue presentation upon inactive lever presses.
Histology
Following completion of the experiments, rats were perfused under sodium pentobarbital (31 mg/kg, i.p.) and chloral hydrate (142 mg/kg, i.p.) anesthesia. Brains were removed and placed in a 10 % formalin solution for a minimum of 2 days prior to sectioning on a cryostat. Frozen coronal sections (40 μm thick) were stained with cresyl violet.
Statistical analyses
All data on self-administration rates, lever responses, locomotor activity, and infusions are square-root transformed, to minimize heterogeneous variances for parametric statistical tests (McDonald 2009). Square-root transformed data were analyzed with the ANOVA module of Statistica (version 6.1, StatSoft, Inc., Tulsa, OK). Significant effects were further analyzed by the Newman–Keuls post hoc test, which executes all possible comparisons.
Results
Experiment 1: rats self-administered excitatory amino acid receptor antagonists into the MR, DR, and adjacent regions
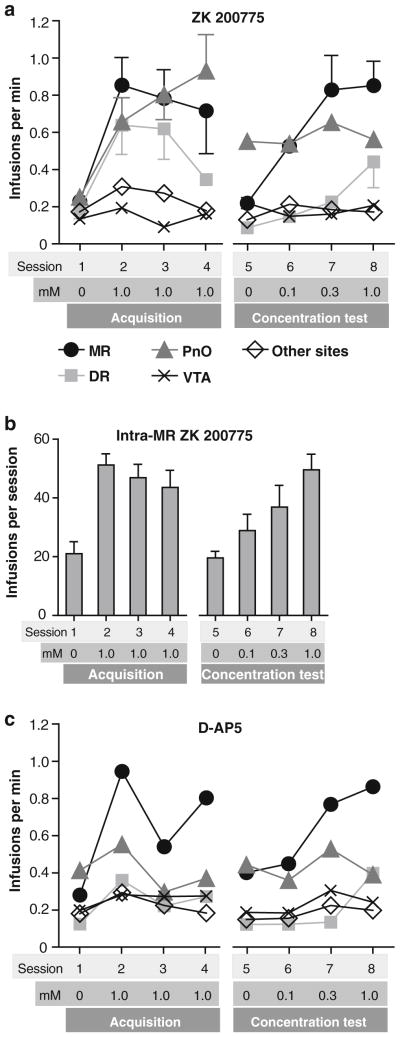
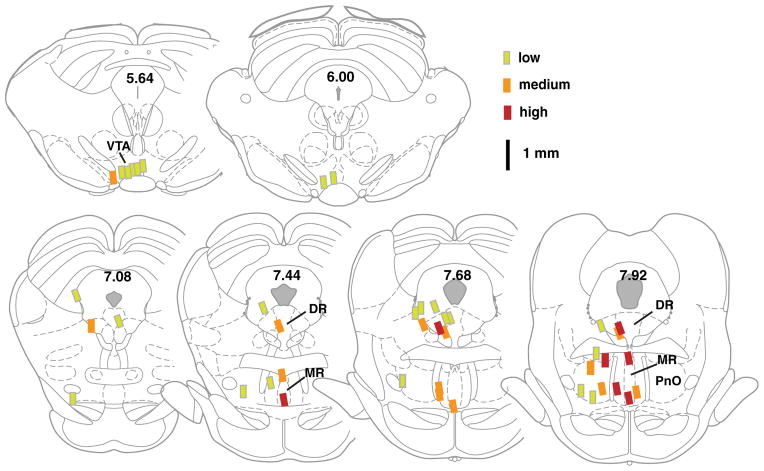
We examined whether rats learn to self-administer either the AMPA receptor antagonist ZK 200775 or the NMDA receptor antagonist AP5 into the MR, DR, or adjacent regions. It should be noted that we included injection tips found in both the median and paramedian raphe nuclei for the MR group, because it was difficult to distinguish the drugs’ actions between these closely adjacent structures. Our data collected during the acquisition phase (sessions 1–4; N = 58) suggest that rats reliably self-administered ZK 200775 into the MR (N = 9), DR (N = 8), or PnO (N = 12), but not the VTA (N = 9) or control sites (N = 20) (Fig. 1a). These observations are supported by a significant interaction between region and session (F12,159 = 2.49, P<0.01 found with a 5×4 (region×session) mixed ANOVA on infusion rates), followed by a Newman–Keuls test comparing the vehicle value of session 1 against values of sessions 2–4 for each region. Figure 2 depicts photomicrographs of typical injection sites. Figure 3 depicts each injection site and its effectiveness of ZK 200775 self-administration.
Fig. 1.
Self-administration rates. Data are means and S.E.M. a ZK 200775 was self-administered into the MR, DR or PnO. Filled symbols with error bars indicate significantly greater rates than vehicle rates (P<0.05). Although individual self-administration rates of intra-PnO ZK 200775 during the concentration test did not differ from each other, overall self-administration rate for the PnO was greater than that for the DR or VTA. b Mean number of infusions earned in each session is shown for intra-MR ZK 200775. c Rats self-administered AP-5 into the MR at higher rates than other regions excluding the PnO for both acquisition and concentration test phases
Fig. 2.
Photomicrographs showing representative sites of injections into the MR, DR, and PnO. The arrowheads indicate the tips of injection cannula
Fig. 3.
Injection sites of ZK 200775 and their effectiveness in self-administration. Injection sites of rats (N = 58) from experiment 1 are shown by rectangles depicting the 0.3-mm tip of injection cannulas. Each site’s effectiveness on self-administration is indicated by its color. Median lever presses per minute among ZK 200775 sessions of each rat were classified into one of three levels: red greater than 0.700; orange between 0.701 and 0.350; green less than 0.350. The numbers indicate distances from bregma. Brain sections are adopted from Paxinos and Watson (2005)
When effects of concentration were examined in sessions 5–8 (Fig. 1a; N = 58), concentration-dependent effects of ZK 200775 were found for the MR and DR, but not for the PnO (session 5 vs. sessions 6–8 for each region separately found with a Newman–Keuls test, following a significant region× concentration interaction, F12,135 = 2.47, P<0.01 found with a 5×4 (region×concentration) ANOVA). The lack of concentration effect for the PnO appears to be due to elevated self-administration rates with vehicle injections (session 5). PnO rats, like MR rats, earned drug infusions at greater rates over sessions 5–8 than rats receiving infusions into the VTA or other sites (Newman–Keuls test following a significant site effect, F4,53 = 4.85, P<0.01). Figure 1b depicts numbers of infusions of ZK 200775 that MR rats self-administered; these figures are given to facilitate comparison with results from subsequent experiments 2, 3, and 5. Some of the MR rats obtained the maximum infusions of 60: Among the nine rats, three, four, and four rats obtained 60 infusions in sessions 2, 3, and 4, respectively, and one, four, and five rats obtained 60 in sessions 6, 7, and 8, respectively. It should be also noted that 60 infusions of 0.1, 0.3, and 1 mM delivered 0.45, 1.3, and 4.5 nmol of ZK 200775, respectively.
In contrast to ZK 200775, the NMDA antagonist AP5 was reliably self-administered only into the MR, and not other regions, for both the acquisition (sessions 1–4) and concentration (sessions 5–8) test phases (Fig. 1c). These observations are supported by Newman–Keuls tests following 5×4 (region×session or region×concentration) mixed ANOVAs on self-administration rates revealing significant region effects for the acquisition phase (F4,52 = 4.04, P<0.01; N = 57) and for the concentration test phase (F4,50 = 4.92, P <0.005; N = 55), but not region xsession interaction or region×concentration interaction. It should be noted that slightly fewer rats were included for data analyses of AP5 than ZK 200775. Three rats either lost cannula implants or became sick before the completion of AP5 experiments: one rat (with control site infusions) was not included in either the acquisition or concentration test phase; and two rats (with one control site and one VTA infusions) were included for the acquisition, but not concentration test phase with AP5.
Experiment 2: noncontingent administration of ZK 200775 increased locomotion and lever presses rewarded by VS in a dose-dependent manner
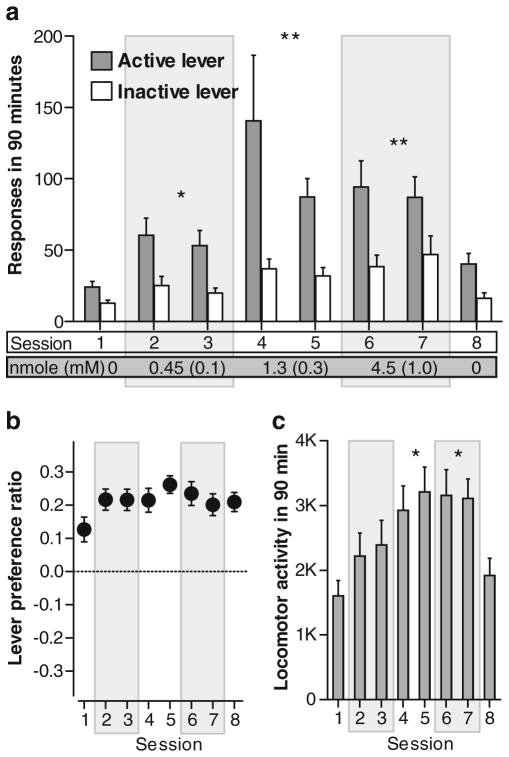
In this experiment, we used comparable drug concentrations/doses to those used in experiment 1. In the absence of drug manipulation, rats tended to respond more on the active lever than the inactive lever in session 1 (Fig. 4a, b), an effect that is consistent with a weak rewarding property of VS. Noncontingent injections of ZK 200775 into the MR increased lever presses rewarded by VS in a dose-related manner, and they proportionally increased both active and inactive lever-presses (Fig. 4a). A 4×2×2 (dose×lever×repeated session) ANOVA on lever responses revealed significant dose (F3,72 = 19.79, P<0.001), and lever (F1,24 = 55.91, P<0.001) effects and significant dose×lever interaction (F3,72 = 5.27, P <0.01); no reliable effect was found for repeated session or its interaction with dose or lever. For both active and inactive lever presses, the 0.45 nmol dose (0.1 mM) produced more responses than vehicle, and the 1.3 or 4.5 nmol dose (0.3 or 1 mM, respectively) produced more responses than vehicle or 0.45 nmol. A 4×2 ANOVA on lever preference ratio indicated no reliable effect of dose (Fig. 4b). Similar to lever presses, we found a dose-dependent effect of ZK 200775 on locomotor activity (Fig. 4c; F3,72 = 15.13, P <0.001 found with a 4×2 ANOVA on locomotor activity). It should be noted that we did not observe reliable differences in lever pressing or locomotor activity between sessions 1 and 8 when the rats only received vehicle infusions, suggesting that increased lever pressing or locomotor activity when the rats received ZK 200775 infusions largely indicate primary (acute) effects of the drug, but not its conditioning effects from previous sessions. Figure 5 depicts injection sites and their effectiveness. Injection sites at the levels of 6.36, 6.72, and 7.08 were not included for statistical analysis since they are localized areas anterior to the MR.
Fig. 4.
Noncontingent administration of ZK 200775 into the MR increases locomotor activity and lever presses rewarded by visual sensation. Data are means with SEM. Doses of 0.45, 1.3, and 4.5 nmol were infused in the span of 90 min in sessions 2–3, 4–5, and 6–7, respectively, using the concentrations of 0.1, 0.3, and 1.0 mM. a Both active and inactive lever presses increased in dose-dependent manners (N = 25). *P<0.05, different from vehicle values. **P<0.01, different from vehicle or 0.45 nmol values. b Active lever preference did not significantly change over eight sessions. c Locomotor activity increased in a dose-dependent manner. *P<0.01, different from vehicle or 0.45 nmol values
Fig. 5.
Injection sites of ZK 200775 and their effectiveness in VS seeking. Injection sites of rats (N = 30) from experiment 3 are shown by rectangles depicting the 0.3-mm tip of injection cannulas. Each site’s effectiveness on VS seeking is indicated by its color. Each rat’s second highest active lever press per session among all sessions was classified into one of three levels: red greater than 100; orange greater than 50 less than 101; green less than 51. The numbers indicate distances from bregma
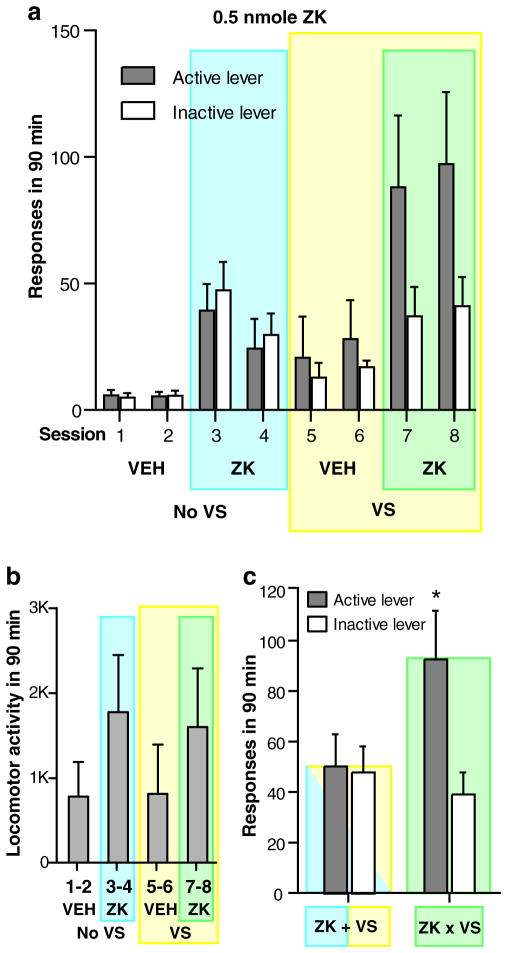
Experiment 3: noncontingent intra-MR ZK 200775 synergistically increased approach rewarded by VS
To determine whether noncontingent administration of intra-MR ZK 200775 increases approach-related motivation, we examined effects of intra-MR ZK 200775 and VS separately and together using a within-subjects design. A 2×2×2×2 (VS×ZK×repeated session×lever) ANOVA on lever pressing revealed a significant VS×ZK×lever interaction (F1,10 = 9.81, P<0.05). Active lever pressing with the presence of both VS and ZK (i.e., sessions 7–8) was significantly greater than pressing in any other conditions (Fig. 6a). Importantly, inactive lever pressing with the presence of both VS and ZK did not reliably differ from inactive lever pressing with ZK in the absence of VS (sessions 3–4), suggesting that the rats selectively increased their responding rewarded by VS. When ZK and VS were presented in separate sessions (i.e., either sessions 3–4 or 5–6), each reliably increased lever presses more than those when sessions had neither ZK nor VS (i.e., sessions 1–2). The ANOVA on the low-dose data detected no effect of repeated session. Intra-MR ZK 200775 also increased locomotor activity (Fig. 6b). The main effect on ZK was significant (F1,10 = 72.08, P<0.001), while VS or ZK×VS interaction was not, revealed by a 2×2×2 (VS× ZK×repeated session) ANOVA.
Fig. 6.
Intra-MR ZK 200775 enhances approach-related motivation, but not mere hyperactivity. Data are means with SEM (N = 11). a Lever responses markedly increased when contingent presentations of VS were available and when 0.5 nmol ZK was noncontingently infused into the MR in the span of 90 min. b, c Data are combined between two repeated sessions: 1–2, 3–4, 5–6, and 7–8. b ZK administration significantly increased locomotor activity. *P<0.001, significantly greater than the values of sessions 1–2. c Lever presses of the ZK×VS condition (i.e., sessions 7–8) were compared with those of additive condition (ZK+VS; see text for detail). *P<0.005, significantly different from any other value
Although the above analysis shows a reliable interaction between VS, ZK, and lever, it is unclear whether the interaction was merely additive or synergistic (see the Discussion section for the significance of synergy). Therefore, we compared the lever press counts of sessions 7–8 with lever press counts that reflect addition of those of the ZK alone and VS alone. To obtain additive counts, we added presses of sessions 3–4 with those of sessions 5–6, and then, lever presses of sessions 1–2 were subtracted from them (Fig. 6c). A 2×2×2 (condition×repeated session×lever) ANOVA revealed a significant interaction between condition and lever, F1,10 = 14.18, P<0.005. The active lever presses of sessions 7–8 were greater than the active lever presses of the additive condition (P<0.005), suggesting a synergistic interaction between ZK and VS when both are present. In addition, the inactive presses of sessions 7–8 did not differ from the inactive presses of the additive condition, suggesting that the synergistic interaction is selective to active lever presses.
Experiment 4: contingent administration of intra-MR ZK 200775 induced a conditioning effect on lever pressing
The results of experiments 2–4 suggest that intra-MR ZK 200775 invigorates ongoing approach behavior. This effect may be sufficient to explain self-administration of ZK 200775 into the MR. However, this manipulation may also induce positive conditioning effects, which contribute to its self-administration. Therefore, we examined whether intra-MR ZK induces conditioning, using a self-administration procedure. Specifically, responding on the active lever was followed by an infusion of 0.3 mM ZK 200775 or vehicle accompanied by a visual cue and the retraction of both levers, while responding on the inactive lever had the same consequences except without drug infusions. It should be emphasized that the “inactive” lever in this experiment did produce a visual stimulus, unlike the inactive lever in experiments 1–4, which did not deliver visual stimuli.
During sessions 1–4, with a fixed ratio 1, the rats receiving ZK responded on the levers and obtained infusions more than the rats receiving vehicle (Fig. 7a, lever: F1,14 = 21.00, P<0.001 found with a 2×2×4 (group×lever×session) ANOVA; Fig. 7b, infusion: F1,14 = 11.39, P <0.005 found with a 2×4 (group×session) ANOVA). However, ZK rats, like control rats, failed to discriminate between the two levers. Locomotor activity between the two groups did not reliably differ although ZK rats tended to have greater activity (Fig. 7c, found with a 2 × 4 (drug × session) ANOVA). During sessions 5–7, with a progressive ratio schedule, the rats receiving ZK gradually increased responses on both levers and earned more infusions over the three sessions (Fig. 7a: a significant group×session interaction on lever, F2,28 = 3.89, P<0.05 found with a 2×2×3 (group×lever×session) ANOVA; Fig. 7b: a significant group×session interaction on infusion, F2,28 = 4.56, P<0.05 found with a 2×3 (group×session) ANOVA). Again, ZK rats, like control rats, failed to discriminate between the two levers. A reliable group effect was also found on locomotor activity (F2,28 = 9.32, P <0.01), but not a group×session interaction. Although the rats failed to discriminate the two levers, the increases in lever presses and infusions over sessions 5–7 suggest that intra-MR ZK 200775 had a positive conditioning effect on approach behavior.
Fig. 7.
Contingent administration of intra-MR ZK 200775 induced a conditioning effect on lever pressing, but not lever discrimination. Data are means with SEM. a The rats receiving ZK (N = 9) responded on levers more than the rats receiving vehicle (VEH, N = 7). In particular, with a progressive ratio schedule, ZK rats increased lever presses gradually over sessions 5–7. However, they failed to discriminate the active lever (AL) from the inactive lever (IL). *P<0.05, session 7 lever presses of ZK group differed from those of session 5 of ZK group or session 7 of VEH group. b Infusions that the rats earned differed between ZK and VEH groups for sessions 1–4 and 5–7. A significant group×session interaction was found during sessions 5–7. **P<0.01, session 7 infusions of ZK group differed from those of session 5 of ZK group or session 7 of VEH group; *P<0.05, different from session 6 of VEH group. c Locomotor activity counts reliably differed between ZK and VEH groups during sessions 5–7, but not sessions 1–4
Discussion
Self-administration effects
Rats reliably self-administered AP5 into the MR, while they self-administered ZK 200775 into a widespread area of the mesopontine regions (experiment 1). Our observation generates a reasonable hypothesis that in addition to the MR and DR, regions just outside the MR such as the oral pontine reticular nucleus mediate reward-related effects of ZK 200775. It should be noted that we examined effects of drugs in various regions in the same analysis, a procedure that tends to capture large effects, but not small effects; we may detect reliable self-administration of AP5 into the vicinity of the PnO if we design a procedure to focus on this effect alone. The possible role of the PnO in reward should be considered in light of drug diffusion. Our previous studies suggest that a distance of 0.3 mm makes a significant difference in intracranial self-administration of a variety of drugs (e.g., Ikemoto 2005; Ikemoto et al. 2006; Ikemoto and Wise 2002). Some effective sites for ZK 200775 were found more than 0.3 mm away from the MR. In addition, effects of ZK 200775 are relatively unique to the PnO: Rats did not reliably self-administer AP5, muscimol, or baclofen into the PnO, whereas these drugs were self-administered into the MR (Liu and Ikemoto 2007; Shin and Ikemoto 2010; present study). These findings suggest blockade of AMPA transmission is uniquely important to regions adjacent but outside of the MR. The notion that the PnO region is involved in reward-related processes has some support. Large lesions including the medial PnO decrease responding rewarded by lateral hypothalamic stimulation (Ikemoto and Panksepp 1994). Injections of the cholinergic muscarinic receptor antagonist atropine into the vicinity of the PnO disrupt responding rewarded by sucrose solutions (Ikemoto and Panksepp 1996).
Our rats did not learn to self-administer the AMPA or NMDA receptor antagonists into the posterior VTA. This finding seems to contradict previous findings that mice learn to self-administer AMPA or NMDA receptor antagonists into the VTA (David et al. 1998). This discrepancy may be resolved by considering possible differences in injection site within the VTA. The VTA consists of structurally and functionally heterogeneous elements (Ikemoto 2007). The present study delivered the glutamate receptor antagonists into a posterior VTA region centered on the paranigral nucleus, which mediates rewarding effects of many drugs (Ikemoto 2007), while David et al. (1998) appear to have delivered glutamate antagonists into a region slightly more posteriorly. Just posterior to the paranigral nucleus, resides a recently identified structure consisting predominantly of GABAergic neurons called the “GABAergic tail of the VTA” or “rostromedial tegmental nucleus” (Jhou 2005; Jhou et al. 2009a; Jhou et al. 2009b; Kaufling et al. 2009; Perrotti et al. 2005), which inhibits midbrain dopamine neurons. It is possible that selective blockade of glutamate inputs to this cluster of neurons disinhibit midbrain dopamine neurons and, thereby, induces reward-related effects. However, the rostromedial tegmental nucleus was not studied in the present study other than to note that injections in the current study did not reside in this region.
Motivational effects on approach
Our data from experiments 2–3 suggest that the administration of ZK 200775 into the MR increases approach responses toward salient distal stimuli, i.e., enhancement of incentive motivation. We observed a dose-dependent effect of intra-MR ZK on VS seeking behavior (experiment 2). In addition, we found a significant interaction between intra-MR ZK and VS on the active lever, but not on the inactive lever. Their interaction was synergistic rather than additive. The distinction between synergistic and additive interactions is important for understanding the nature of action of intra-MR ZK. While additive effects can be explained by two independent processes occurring at the same time, synergistic effects depends on an integrator. Incentive motivation is postulated to arise from the interaction between internal state and external stimuli integrated by a central motive state or approach coordinator module (Bindra 1969; Ikemoto 2010). Thus, the data suggest that intra-MR ZK 200775 alters integrative process involved in approach behavior. Similarly, we previously found that the GABAB receptor agonist baclofen administered into the MR synergistically interacts with VS in approach responding (Vollrath-Smith et al. 2012). Therefore, accumulating evidence suggests that the MR participates in the integration process of incentive motivation.
It is likely that the MR is involved in motivational processes of salient distal stimuli unconditioned or conditioned with a variety of rewards. Previous studies showed that selective destruction of MR and DR serotonin neurons by 5,7-dihydroxytryptamine increases responding rewarded by conditioned sensory stimuli previously paired with water reward (Fletcher et al. 1999). Similarly, muscimol injections into the MR reinstate previously extinguished alcohol-seeking responses in the same context (Le et al. 2008). Moreover, intra-MR 8-OH-DPAT facilitates responses rewarded by lateral hypothalamic stimulation (Fletcher et al. 1995). Therefore, the MR likely plays a critical role in incentive motivation guided by salient distal stimuli unconditioned or conditioned with a variety of rewards. We should note that although we focused on VS as a salient stimulus in the test box, other physical features (i.e., stimuli) of the test box may have had varying degrees of salience, which may have contributed to the effects of intra-MR ZK. We made no attempt to identify and control them.
Conditioning effects on approach
We examined whether contingent administration of intra-MR ZK selectively increased responses on the active lever more than those on the inactive lever. Both active and inactive levers were associated with the presentation of VS in experiment 4. During the progressive ratio schedule, rats gradually increased lever presses over three sessions. These results suggest that intra-MR ZK induced a conditioning effect on lever responses. However, it is not clear why intra-MR ZK did not induce selective conditioning with the active lever. An important factor seems that the difference between the stimuli was so subtle that it was overridden by the salience of stimuli for generalization. In addition, our injection procedure may have made it difficult for rats to attribute effects of the drug to selective responding and cue. Even though the drug was administered relatively discretely over a 5-s period, the infused drug most likely exerted its effects over a few minutes rather than seconds. Indeed, it was shown that a single infusion of excitatory amino acid antagonists can increase locomotor activity over 60 min (Wirtshafter et al. 1989). Consistently, discriminative learning is thought to depend on various factors including the salience of unconditioned stimuli, salience of conditioned stimuli, and similarity of discriminating stimuli (Trevino et al. 2011). In any case, the conditioning effects are consistent with our theoretical view that the incentive motivation enhanced by intra-MR ZK is affective, a process that recruits memory processes to induce conditioning.
Possible involvement of serotonergic neurons and the mesolimbic dopamine system
The administration of AMPA or NMDA receptor antagonists into the MR or DR likely inhibits serotonin neurons by tilting the tonically maintained balance between excitatory and inhibitory afferents toward inhibition. However, supportive evidence is circumstantial and needs to be substantiated: Administration of NMDA receptor antagonists into the MR decreases serotonin metabolism (i.e., 5-HIAA/5-HT) in the hippocampus (Wirtshafter et al. 1989). Similarly, administration of the excitatory amino acid receptor antagonist kynurenic acid into the DR decreases serotonin levels in the DR (Tao and Auerbach 2000), suggesting the inhibition of serotonin neurons, while administration of excitatory amino acids into the MR or DR increases serotonin levels at the perfusion sites and in the nucleus accumbens (Mokler et al. 2009; Tao and Auerbach 2000; Tao et al. 1997). Although it is likely that AMPA or NMDA receptor antagonists can inhibit serotonin neurons, their actions may not be selective since they likely have similar effects on non-serotonergic neurons in the MR and DR.
Incentive motivation enhanced by intra-MR administration of excitatory amino acid receptor antagonists may be mediated by MR’s interaction with the mesolimbic dopamine system. Indeed, an interaction between the serotonergic and dopaminergic systems has long been suggested (Daw et al. 2002; Deakin 1983; Kapur and Remington 1996). Consistently, NMDA receptor antagonist administration into the MR appears to increase dopamine metabolism in the ventral striatum (Wirtshafter et al. 1989). Moreover, enhanced dopamine transmission in the ventral striatum has been shown to invigorate VS seeking in a VS-dependent manner (Shin et al. 2010). In addition, dopaminergic drugs are self-administered into the ventral striatum (Ikemoto et al. 1997; Ikemoto et al. 2005; Shin et al. 2008). It seems worth examining how dopaminergic and serotonergic systems interact with each other.
In summary, we found that rats self-administered excitatory amino acid receptor antagonists into the MR and adjacent regions, and the pharmacological manipulations also increased approach responses toward salient stimuli and induced conditioned approach. The present study showcases a reward phenomenon in which the rewarding effects of a central manipulation may arise from its action on incentive motivational process; hence the incentive motivation concept helps to understand how intra-MR ZK 200775 is rewarding. The central system of incentive motivation, in which the MR participates, may play a role in rewarding effects of many drugs of abuse. We propose that at a gross functional level, a role of glutamate transmission in the MR or DR is to tonically suppress incentive motivation.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. We would like to thank Drs. Anton Ilango and Dong Wang for their comments on earlier versions of the manuscript.
Footnotes
Disclosure/conflict of interest We do not have any conflict of interest or financial compensation to disclose.
References
- Berridge KC. Food reward: brain substrate of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Bindra D. Neuropsychological interpretation of the effects of drive and incentive-motivation on general activity and instrumental behavior. Psychol Rev. 1968;75:1–22. [Google Scholar]
- Bindra D. The interrelated mechanisms of reinforcement and motivation, and the nature of their influence on response. In: Arnold WJ, Levine D, editors. Nebraska Symposium on Motivation. University of Nebraska Press; Lincoln: 1969. pp. 1–33. [Google Scholar]
- Bolles RC. Reinforcement, expectancy, and learning. Psychol Rev. 1972;79:394–409. [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends in cognitive sciences. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. Rewarding effects elicited by the microinjection of either AMPA or NMDA glutamatergic antagonists into the ventral tegmental area revealed by an intracranial self-administration paradigm in mice. Eur J Neurosci. 1998;10:1394–1402. doi: 10.1046/j.1460-9568.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys QJM. Serotonin in affective control. Annu Rev Neurosci. 2009;32:95–126. doi: 10.1146/annurev.neuro.051508.135607. [DOI] [PubMed] [Google Scholar]
- Deakin JFW. Roles of serotonergic systems in escape, avoidance and other behaviour. In: Cooper SJ, editor. Theory in psychopharmacology. Academic Press; London: 1983. pp. 149–193. [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Ming Z-H, Higgins GA. Conditioned place preference induced by microinjection of 8-OH-DPAT into the dorsal or median raphe nucleus. Psychopharmacology. 1993;113:31–36. doi: 10.1007/BF02244330. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Yeomans JS. Median raphe injections of 8-OH-DPAT lower frequency thresholds for lateral hypothalamic self-stimulation. Pharmacol Biochem Behav. 1995;52:65–71. doi: 10.1016/0091-3057(94)00441-k. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Chambers JW. Selective destruction of brain serotonin neurons by 5,7-dihydroxytryptamine increases responding for a conditioned reward. Psychopharmacology (Berl) 1999;147:291–299. doi: 10.1007/s002130051170. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. The supramammillary nucleus mediates primary reinforcement via GABA(A) receptors. Neuropsychopharmacology. 2005;30:1088–1095. doi: 10.1038/sj.npp.1300660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The relationship between self-stimulation and sniffing in rats: does a common brain system mediate these behaviors? Behav Brain Res. 1994;61:143–162. doi: 10.1016/0166-4328(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci. 1996;110:331–345. doi: 10.1037//0735-7044.110.2.331. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Sharpe LG. A head-attachable device for injecting nanoliter volumes of drug solutions into brain sites of freely moving rats. J Neurosci Methods. 2001;110:135–140. doi: 10.1016/s0165-0270(01)00428-9. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci. 2002;22:9895–9904. doi: 10.1523/JNEUROSCI.22-22-09895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Mapping of chemical trigger zones for reward. Neuropharmacology. 2004;47:190–201. doi: 10.1016/j.neuropharm.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell and olfactory tubercle valid? J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou T. Neural mechanisms of freezing and passive aversive behaviors. J Comp Neurol. 2005;493:111–114. doi: 10.1002/cne.20734. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier M-J, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Kocsis B, Vertes RP. Injections of excitatory amino acid antagonists into the median raphe nucleus produce hippocampal theta rhythm in the urethane-anesthetized rat. Brain Res. 1994;654:96–104. doi: 10.1016/0006-8993(94)91575-x. [DOI] [PubMed] [Google Scholar]
- Kish GB. Studies of sensory reinforcement. In: Honing WK, editor. Operant behavior: areas of research and application. Appleton-Century-Crofts; New York: 1966. pp. 109–159. [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Li Z, Fletcher PJ. Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and reward. Psychopharmacology (Berl) 2008;195:605–615. doi: 10.1007/s00213-007-0943-4. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Ikemoto S. The midbrain raphe nuclei mediate primary reinforcement via GABA(A) receptors. Eur J Neurosci. 2007;25:735–743. doi: 10.1111/j.1460-9568.2007.05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- McDonald JH. Handbook of biological statistics. 2. Sparky House; Baltimore: 2009. [Google Scholar]
- Mokler DJ, Dugal JR, Hoffman JM, Morgane PJ. Functional interrelations between nucleus raphé dorsalis and nucleus raphé medianus: a dual probe microdialysis study of glutamate-stimulated serotonin release. Brain Res Bull. 2009;78:132–138. doi: 10.1016/j.brainresbull.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. The National Academies Press; Washington, D.C: 2011. [PubMed] [Google Scholar]
- Palmatier M, Evans-Martin F, Hoffman A, Caggiula A, Chaudhri N, Donny E, Liu X, Booth S, Gharib M, Craven L, Sved A. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Moskal J. Dopamine and SEEKING: subcortical “reward” systems and appetitive urges. In: Elliot A, editor. Handbook of approach and avoidance motivation. Taylor & Francis Group, LLC; New York: 2008. pp. 67–87. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Elsevier; Burlington: 2005. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Functional studies of the central catecholamines. Int Rev Neurobiol. 1982;23:303–365. doi: 10.1016/s0074-7742(08)60628-5. [DOI] [PubMed] [Google Scholar]
- Shin R, Ikemoto S. The GABA(B) receptor agonist baclofen administered into the median and dorsal raphe nuclei is rewarding as shown by intracranial self-administration and conditioned place preference in rats. Psychopharmacology. 2010;208:545–554. doi: 10.1007/s00213-009-1757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Qin M, Liu Z-H, Ikemoto S. Intracranial self-administration of MDMA into the ventral striatum of the rat: differential roles of the nucleus accumbens shell, core and olfactory tubercle. Psychopharmacology. 2008;198:261–270. doi: 10.1007/s00213-008-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Cao J, Webb SM, Ikemoto S. Amphetamine administration into the ventral striatum facilitates behavioral interaction with unconditioned visual signals in rats. PLoS One. 2010;5:e8741. doi: 10.1371/journal.pone.0008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R, Pierre V, Clarke P. Facilitation of intravenous nicotine self-administration in rats by a motivationally neutral sensory stimulus. Psychopharmacology. 2009;207:191–200. doi: 10.1007/s00213-009-1647-8. [DOI] [PubMed] [Google Scholar]
- Soubrie P. Reconciling the role of central serotonin neurons in human and animal behavior. Behav Brain Sci. 1986;9:319–364. [Google Scholar]
- Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol. 1960;53:187–193. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABAB receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci. 2010;30:11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Regulation of serotonin release by GABA and excitatory amino acids. J Psychopharmacol. 2000;14:100–113. doi: 10.1177/026988110001400201. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Influence of AMPA/kainate receptors on extracellular 5-hydroxytryptamine in rat midbrain raphe and forebrain. Br J Pharmacol. 1997;121:1707–1715. doi: 10.1038/sj.bjp.0701292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino M, Aguilar-Garnica E, Jendritza P, Li S-B, Oviedo T, Khr G, De Marco R. Discrimination learning with variable stimulus ‘salience’. International Archives of Medicine. 2011;4:26–26. doi: 10.1186/1755-7682-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, Huth A, Sheardown M, McDonald F, Neuhaus R, Schneider HH, Dirnagl U, Wiegand F, Jacobsen P, Ottow E. ZK200775: a phosphonate quinoxalinedione AMPA antagonist for neuroprotection in stroke and trauma. Proc Natl Acad Sci. 1998;95:10960–10965. doi: 10.1073/pnas.95.18.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Sik A, Freund TF, Kocsis B. GABA(B) receptors in the median raphe nucleus: distribution and role in the serotonergic control of hippocampal activity. Neuroscience. 2002;109:119–132. doi: 10.1016/s0306-4522(01)00448-1. [DOI] [PubMed] [Google Scholar]
- Vollrath-Smith FR, Shin R, Ikemoto S. Synergistic interaction between baclofen administration into the median raphe nucleus and inconsequential visual stimuli on investigatory behavior of rats. Psychopharmacology. 2012;220:15–25. doi: 10.1007/s00213-011-2450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtshafter D, Klitenick MA. Comparative studies of locomotor behavior following microinjections of muscimol into various sites in the paramedian tegmentum. Pharmacol Biochem Behav. 1989;32:625–628. doi: 10.1016/0091-3057(89)90008-7. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Trifunovic R, Krebs JC. Behavioral and biochemical evidence for a functional role of excitatory amino acids in the median raphe nucleus. Brain research. 1989;482:225–234. doi: 10.1016/0006-8993(89)91185-2. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Stratford TR, Pitzer MR. Studies on the behavioral activation produced by stimulation of GABAB receptors in the median raphe nucleus. Behav Brain Res. 1993;59:83–93. doi: 10.1016/0166-4328(93)90154-i. [DOI] [PubMed] [Google Scholar]