Abstract
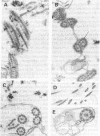
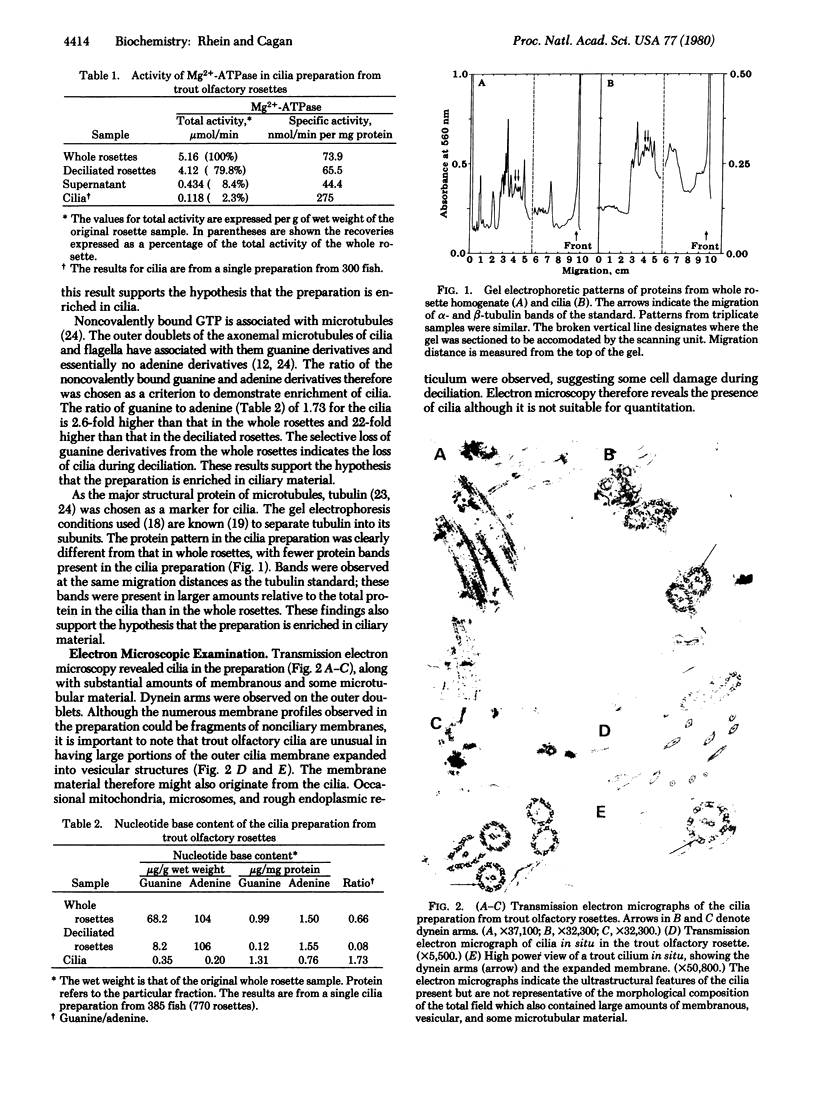
The role of cilia in recognition of olfactory stimuli has been controversial. Cilia from the intact olfactory rosettes of the rainbow trout Salmo gairdneri were isolated, characterized biochemically, and examined by electron microscopy. The markers studied are those associated with cilia in other organisms. Dynein arms contain Mg2+-AtPase; this enzyme was enriched in the isolated cilia preparation. Guanine nucleotides are associated with the outer microtubule doublets of cilia but adenine nucleotides are not; a substantial enrichment in guanine, relative to adenine, was found in the cilia preparation. Tubulin, the structural protein component of microtubules, occurs in large amounts in cilia. Disc gel electrophoresis indicated tubulin in the cilia preparation. Electron microscopy confirmed the presence of cilia in the isolated preparation. Rainbow trout have an acute sense of smell and many amino acids are odorants to this species. Functional activity of the cilia preparation relevant to odorant recognition was assessed by using binding of radioactively labeled odorant amino acids. L-Alanine, L-serine, L-threonine, L-lysine, and D-alanine bound to the cilia preparation. This study provides direct biochemical evidence that olfactory cilia bind odorant molecules and supports the hypothesis that odorant recognition sites are integral parts of the cilia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronshteín A. A., Minor A. V. Regeneratsiia oboniatel'nykh zhgutikov i vosstanovlenie élektrool'faktogrammy posle deistviia tritona X-100 na oboniatel'nuiu vystilku liagushki. Tsitologiia. 1977;19(1):33–39. [PubMed] [Google Scholar]
- Cagan R. H., Zeiger W. N. Biochemical studies of olfaction: binding specificity of radioactively labeled stimuli to an isolated olfactory preparation from rainbow trout (Salmo gairdneri). Proc Natl Acad Sci U S A. 1978 Oct;75(10):4679–4683. doi: 10.1073/pnas.75.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLDGATE D. P., GOODWIN T. W. A RAPID METHOD FOR THE SEPARATION OF NUCLEIC ACID BASES. Biochim Biophys Acta. 1964 Oct 16;91:328–329. doi: 10.1016/0926-6550(64)90258-0. [DOI] [PubMed] [Google Scholar]
- Hara T. J. Olfactory responses to amino acids in rainbow trout, Salmo gairdneri. Comp Biochem Physiol A Comp Physiol. 1973 Feb 1;44(2):407–416. doi: 10.1016/0300-9629(73)90493-3. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D. The central tubuli in distal segments of olfactory cilia lack dynein arms. Experientia. 1976 Nov 15;32(11):1459–1460. doi: 10.1007/BF01937433. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W., Suter M., Weingarten M., Littman D. The role of rings in the assembly of microtubules in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:90–106. doi: 10.1111/j.1749-6632.1975.tb19195.x. [DOI] [PubMed] [Google Scholar]
- Koch R. B., Gilliland T. I. Responses of Na+-K+ ATPase activities from dog olfactory tissue to selected odorants. Life Sci. 1977 Mar 15;20(6):1051–1061. doi: 10.1016/0024-3205(77)90293-4. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Cagan R. H. Biochemical studies of tast sensation. Binding of L-[3H]alanine to a sedimentable fraction from catfish barbel epithelium. J Biol Chem. 1976 Jan 10;251(1):88–97. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linck R. W. Comparative isolation of cilia and flagella from the lamellibranch mollusc, Aequipecten irradians. J Cell Sci. 1973 Mar;12(2):345–367. doi: 10.1242/jcs.12.2.345. [DOI] [PubMed] [Google Scholar]
- Moulton D. G. Spatial patterning of response to odors in the peripheral olfactory system. Physiol Rev. 1976 Jul;56(3):578–593. doi: 10.1152/physrev.1976.56.3.578. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- RANDERATH K. TWO-DIMENSIONAL SEPARATION OF NUCLEIC ACID BASES ON CELLULOSE LAYERS. Nature. 1965 Feb 27;205:908–908. doi: 10.1038/205908a0. [DOI] [PubMed] [Google Scholar]
- Shibuya T. Dissociation of Olfactory Neural Response and Mucosal Potential. Science. 1964 Mar 20;143(3612):1338–1339. doi: 10.1126/science.143.3612.1338. [DOI] [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Biochemistry and physiology of microtubules. Annu Rev Biochem. 1976;45:699–720. doi: 10.1146/annurev.bi.45.070176.003411. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Renaud F. L., Gibbons I. R., Stevens R. E. Guanine nucleotide associated with the protein of the outer fibers of flagella and cilia. Science. 1967 Jun 23;156(3782):1606–1608. doi: 10.1126/science.156.3782.1606. [DOI] [PubMed] [Google Scholar]
- WATSON M. R., HOPKINS J. M. Isolated cilia from Tetrahymena pyriformis. Exp Cell Res. 1962 Nov;28:280–295. doi: 10.1016/0014-4827(62)90284-7. [DOI] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]