Abstract
Background
Delirium commonly occurs in patients with dementia. Though several tools for detecting delirium exist, it is unclear which are valid in patients with delirium superimposed on dementia.
Objectives
Identify valid tools to diagnose delirium superimposed on dementia
Design
We performed a systematic review of studies of delirium tools, which explicitly included patients with dementia.
Setting
In-hospital patients
Participants
Studies were included if delirium assessment tools were validated against standard criteria, and the presence of dementia was assessed according to standard criteria that used validated instruments.
Measurements
PubMed, Embase, and Web of Science databases were searched for articles in English published between January 1960 and January 2012.
Results
Nine studies fulfilled the selection criteria. Of the total of 1569 patients, 401 had dementia, and 50 had delirium superimposed on dementia. Six delirium tools were evaluated. One studyusing the Confusion Assessment Method (CAM) with 85% patients with dementia showed a high specificity (96–100%) and moderate sensitivity (77%).Two intensive care unit studies that used the CAM for the Intensive Care Unit (CAM-ICU) ICU reported 100% sensitivity and specificity for delirium among 23 dementia patients. One study using electroencephalography reported a sensitivity of 67% and a specificity of 91% among a population with 100% prevalence of dementia. No studies examined potential effects of dementia severity or subtype upon diagnostic accuracy.
Conclusions
The evidence base on tools for detection of delirium superimposed on dementia is limited, although some existing tools show promise. Further studies of existing or refined tools with larger samples and more detailed characterization of dementia are now required to address the identification of delirium superimposed on dementia.
Keywords: delirium, dementia, delirium superimposed on dementia, delirium tools
BACKGROUND
Delirium is a common geriatric syndrome characterized by acute and fluctuating disturbance of consciousness, inattention, and deficits in arousal and cognition. Delirium that occurs in patients with dementia is referred to as delirium superimposed on dementia (DSD). The prevalence of DSD in community and hospital setting ranges from 22 to 89%,1 and is greater than described in patients without dementia. By 2050, the number of individuals aged 65 and older in the United States with Alzheimer’s disease, the most common form of dementia, is projected to number between 11 and16 million.2 By extrapolation based on the expected proportion of patients with dementia, up to 14 million patients will potentially experience DSD, representing a massive health care challenge.3
DSD is associated with adverse outcomes that include accelerated cognitive and functional decline, rehospitalization, institutionalization and mortality.4 Delirium has been proposed as an additional vital sign5 and its presence is often the first sign of a change in clinical condition, especially in older persons and those with dementia. For instance DSD might be the harbinger of an undiscovered infection or a recent change of a medication with psychoactive effects. Recognition of DSD should prompt an urgent and thorough clinical evaluation of the patient and subsequent therapeutic actions.
The diagnosis of DSD, however, is often challenging because signs of delirium might be mistaken for the fluctuation of cognitive function, or psychological symptoms of patients with dementia. Aside from the low levels of delirium detection in general6–8 practitioners may not assess for DSD due to the perception that it cannot be readily distinguished from dementia. There may also be a belief that current delirium detection instruments lack adequate measurement properties in the context of dementia.
Although multiple tools have been developed and validated to diagnose delirium, it is currently unclear which tools, if any, can be used in dementia patients and how accurately such tools perform in this growing population. This is surprising, given that a substantial number of patients with delirium also have dementia. A recent systematic review of instruments to detect delirium9 reported detection instrument test characteristics in hospitalized non-critically ill patients, but did not comment specifically on the performance of these tools in patients with dementia.
The purpose of this systematic review is to summarize the available literature on the performance characteristics of delirium screening instruments in samples explicitly containing patients with dementia.
METHODS
Literature search strategy
This systematic review was registered on the Prospero systematic review website (PROSPERO 2011: CRD42011001271). Searches of PUBMED, EMBASE, and WEB of SCIENCE were conducted for articles published between January 1960 and January 22nd, 2012. The search terms were: delirium tools, delirium assessments combined with delirium, confusion, acute confusional state, acute brain failure, acute confusion, dementia, cognitive impairment, and Alzheimer’s disease. A complete search strategy can be found in Appendix 1,available in the online version.
Study selection and Data Extraction
We included validation studies which had evaluated delirium with tools using the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV)10or DSM-III11criteria as a gold standard and included patients with dementia (diagnosed by neuropsychological battery or using DSM-III, DSM-III-R, DSM-IV, DSM-IV-TR;10–12 or National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association [NINCDS-ADRDA];13 or Blessed dementia rating scale score [BDRS];14Informant Questionnaire on Cognitive Decline in the Elderly [IQCODE];15or Clinical Dementia Rating Scale [CDR]16) or severe dementia (diagnosed by neuropsychological battery using DSM-III, DSM-III-R, DSM-IV, DSM-IV-TR; or NINCDS-ADRDA; or BDRS score ≥12; or CDR=3). Admissible study designs were randomized controlled trials or observational studies with longitudinal or cross-sectional designs.
We excluded studies that assessed solely alcohol-related delirium, had a study population with age <18 years, did not apply DSM-IV or DSM-III criteria as a gold standard delirium assessment, and did not assess dementia with validated measures, as described above. We also excluded review articles, case series, duplicates, studies in which the index and reference tests were administered by the same individual, and studies in which the index and reference tests were administered on different days.
Each abstract was independently reviewed by 2 reviewers (A.M. and J.M.; E.V. and D.F; G.B and J.C.J) to identify publications which met inclusion and exclusion criteria, and studies included and excluded were reported using the PRISMA systematic review protocol.17 During the screening process, full texts were retrieved when information in the abstract was not available or insufficient. In cases of disagreement between the 2 reviewers, inclusion decisions were resolved by discussion and consensus with a third reviewer. This procedure was required for 5 articles (5%) over the 100 selected.
Data were extracted independently by the reviewers cited above using the Standard for Reporting of Diagnostic Accuracy (STARD)18and the Assessment of Methodological Quality (QUADAS).19The following data were extracted if available: sensitivity, specificity and likelihood ratios (LR) of the tools which included patients with dementia, sensitivity, specificity, number of patients with dementia, number of patients with delirium and DSD, presence of confounding psychiatric illnesses (i.e., depression), time interval between assessment for delirium, definition of dementia, rater for delirium and dementia.
Assessment of Quality and Biases
Outcome reporting bias was evaluated comparing the methods section of each article with the results. Unreported outcomes were those mentioned in the “Methods” section of the study but not in the “Result” section. Publication bias was evaluated by reviewing reference lists in each included study for abstracts, which had not been formally published in full manuscript format. Quality of data reporting was evaluated with the Assessment of Quality of Reporting (STARD criteria: score range 1–25 (higher=better).18 Quality of study methodology was assessed with the Assessment of Methodological Quality (QUADAS tool): score range 1–14 (higher=better).19
RESULTS
Search Results
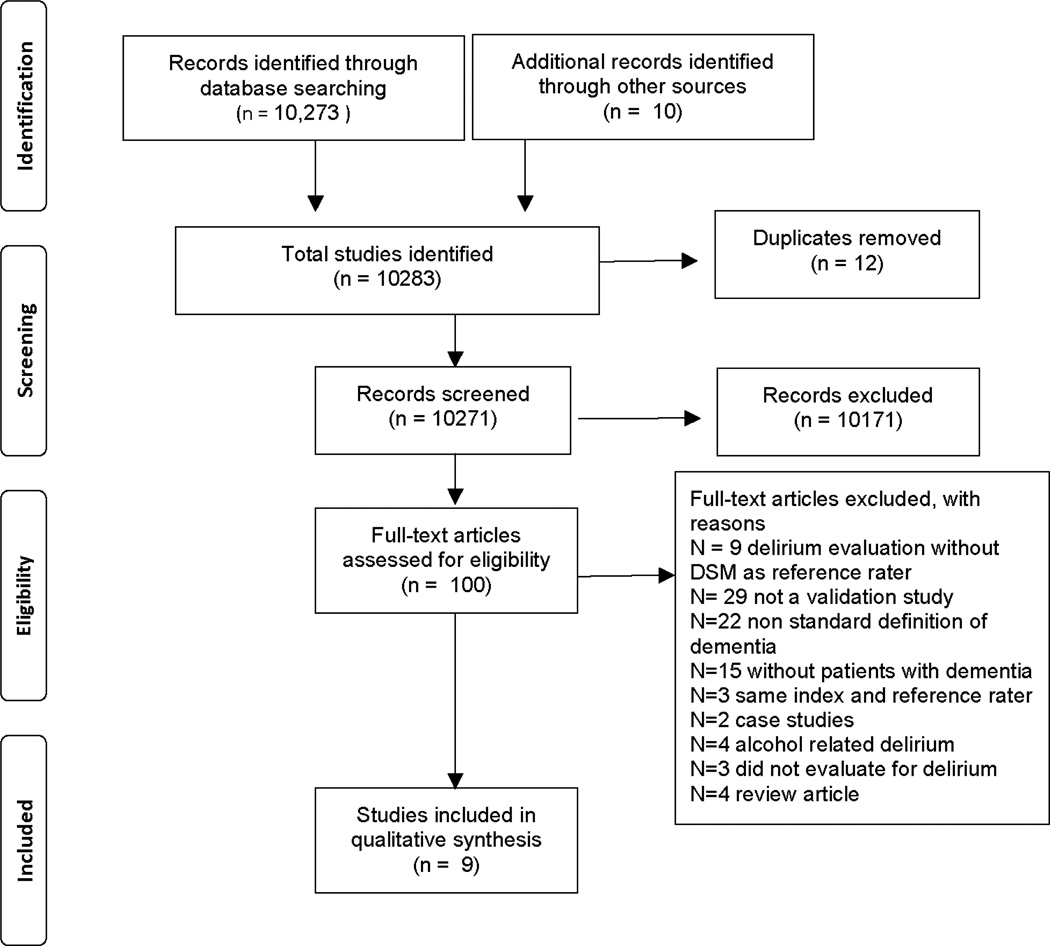
A total of 10,273 citations were identified in the original literature search (Figure 1). An additional 10 articles were identified through hand searching and 12 duplicates were removed, resulting in 10,271 records that were screened for inclusion. 10,171 abstracts met initial exclusion criteria and 100 full text articles were assessed for inclusion eligibility; 91of those were excluded, with 9 included in the final review (Table 1).
Figure.
PRISMA flow diagram.
Table 1.
Characteristics of included studies.
| Tools (Author, Year) | Age (mean±SD) |
Setting | Total sample (N) |
Sample with delirium (N, %) |
Sample without delirium or dementia (N,%) |
Sample with dementia (N, %) |
Sample with DSD (N,%) |
Sample with depression (N,%) |
Sensitivity of the tool in the entire sample |
Specificity of the tool in the entire sample |
|---|---|---|---|---|---|---|---|---|---|---|
| SPMSQ (Erkinjuntti1987)22 | 75.2 ± 7.2 | Geriatrics | 282 | 58 (20.5) | 197 (70) | 34 (12) | 7 (2) | NA | 7.3%–98%% | 82%–100% |
| CAM (Inouye 1990)24 | 77 to 81 ± 5.4 to 7.9 | Internal Medicine Service | 56 | 26 (46) | 27 (48) | 12 (21) | 9 (16) | 9 | 94%–100% | 90%–95% |
| DRS (Rosen 1994)26 | 72.6 ± 9.4 | Geriatrics | 791 | 70 (9) | 524 (66) | 197 (27) | NA | NA | 94% | 82% |
| CTD (Hart 1996)23 | 34.4 to 64.9 ± 12.3 to 14.6 | ICU | 103 | 22 (21) | 55 (53) | 26 (25) | NA | 224 (28), major depression | 100% | 95% |
| CAM-ICU (Ely 2001)20 | 55.3 ± 17.4 | ICU | 96 | 80 (83) | 15 (16) | 12 (15) | 11(11) | 30 (29) | 93%–100% | 98% –100% |
| CAM-ICU (Ely 2001)21 | 60 ± 19 | ICU | 38 | 33 (89) | 5 (13) | 11 (29) | NA | NA | 95% –100% | 89% –93% |
| CAM-ICU (Mitasova2011)27 | 71.2 ± 11.5 | Stroke unit | 129 | 55 (47) | 88 (68) | 41 (31.8) | 21 (38) | NA | 76% | 98% |
| EEG (Thomas 2007)28 | 84.1 ± 3.9 | Geriatrics | 35 | 23 (65) | 0 (0) | 35 (100) | 12 (34) | NA | 67% | 91% |
| CAM (Hestermann2009)25 | 82.6 ± 6.7 | Geriatrics | 39 | 13 (33) | 26 (67) | 33 (85) | 11 (28) | NA | 77% | 96–100% |
Abbreviations: CAM, Confusion Assessment Method; DRS, Delirium Rating Scale; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; CTD, Cognitive Test for Delirium; EEG, electroencephalography; NA, non available; SPMSQ, Short-Portable Mental Status Questionnaire.
Patient Characteristics
The age range of the study populations was 34 to 84 (mean 75.8, standard deviation (SD) 11.5). Patients were tested mainly in three clinical settings: inpatient geriatric/medical units, stroke units, and the intensive care unit (ICU). Six studies were conducted in the USA, one study in Germany, one in Finland, and one in the Czech Republic.20–27The overall sample sizes ranged from 35 to 791 (mean 174, SD 243). Of the included studies only one28specifically evaluated delirium in patients with dementia, whereas the others included patients with the dementia as a subgroup of patients in the validation. The prevalence of dementia in individual studies ranged from 12% to 100%. The number of patients with identified DSD in individual studies ranged from 7 to 12; the total number of patients with DSD from the whole population studied was 50. None reported severity or subtype of dementia.
Delirium Screening Instruments
Six different tools (Confusion Assessment Method (CAM), Confusion Assessment Method for the Intensive Care Unit (CAM-ICU), Cognitive Test for Delirium (CTD), Delirium Rating Scale (DRS), electroencephalography (EEG), and the Short-Portable Mental Status Questionnaire (SPMSQ)) were used to assess DSD.20–27
Reporting Quality
89% of the studies reviewed achieved a “high” quality methodology rating (QUADAS score ≥ 10) and 67% of the studies had a “high” quality data reporting rating (STARD score ≥ 20) (Table 2). Importantly, 44% of the studies did not report the time interval between the delirium assessment of the DSM rater and the index tool rater (Table 2). Two studies20,21 allowed a maximum time of ≤ 4 hours, one study of ≤ 3 hours,25one study of ≤ 2 hours,27and one up to a maximum of 6 hours.24 The DSM raters for delirium were neurologists, geriatricians, geriatric psychiatrists or experienced neuropsychologists, providing a high standard of evaluation. Similarly, diagnosis of probable dementia or dementia was performed by expert clinicians (i.e., neurologists, psychiatrists or geriatric psychiatrists) (Table 1).
Table 2.
Characteristics of included studies: delirium raters, and quality of methodology and reporting.
| Tools (Author, Year) | Definition of dementia and dementia rater | Delirium rater DSM criteria |
Delirium rater delirium tool |
Time between assessment for delirium and DSM rater |
Quality of methods (1–14)a |
Quality of data report (1–25)b |
|---|---|---|---|---|---|---|
| SPMSQ (Erkinjuntti1987)22 | DSM (Neurologist) | Neurologist | Psychologist | Not reported | 10 | 11 |
| CAM (Inouye 1990)24 | DSM-III-R (Psychiatrist) | Geriatric psychiatrists | Geriatrician | Maximum 6 hours | 13 | 25 |
| DRS (Rosen 1994)26 | DSM-III-R (Geriatric psychiatrists) | Geriatric psychiatrists | Research clinician | Not reported | 11 | 20 |
| CTD (Hart 1996)23 | DSM-III-R, MDRS (Neuropsychologist) | Psychiatrist | Research nurses | Not reported | 9 | 19 |
| CAM-ICU (Ely 2001)20 | DSM-IV;BDRS (geriatrician, geriatric psychiatrist ; nurses) | Geriatrician, geriatric psychiatrist neuropsychologist | Research nurses | ≤ 4 hours | 13 | 24 |
| CAM-ICU (Ely 2001)21 | DSM-IV;BDRS (geriatrician, geriatric psychiatrist ; nurses) | Geriatrician, geriatric psychiatrist | Research nurses, intensivists | ≤ 4 hours | 13 | 24 |
| CAM-ICU (Mitasova2011)27 | BDRS (neuropsychologist) | Neurologist, neuropsychologist, psychiatrist, speech therapist | Neurology resident | <2 hours | 13 | 23 |
| EEG (Thomas 2007)28 | IQCODE, DSM-IV, neuropsychological tests (geriatrician, geriatric psychiatrist, gerontologist, neurologist) | Geriatrician, geriatric psychiatrist, gerontologist, neurologist | Neurophysiologist | Not reported | 10 | 21 |
| CAM (Hestermann2009)25 | IQCODE, DSM-IV (psychologist, gerontologist;geriatric psychiatrist) | Geriatric psychiatrist, geriatrician | Psychologist, gerontologist | ≤ 3 hours | 13 | 19 |
Abbreviations: BDRS, Blessed dementia rating scale score; CAM, Confusion Assessment Method; DSM, Diagnostic and Statistical Manual of Mental Disorders; DRS, Delirium Rating Scale; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; CTD, Cognitive Test for Delirium; EEG, electroencephalography; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; Mattis Dementia Rating Scale, MDRS; NA, non available; SPMSQ, Short-Portable Mental Status Questionnaire.
Quality of study methodology was assessed with the Assessment of Methodological Quality (QUADAS tool): score range 1–14 (higher=better).19
Quality of data reporting was evaluated with the Assessment of Quality of Reporting (STARD criteria): score range 1–25 (higher=better).18
Screening for Delirium Superimposed on Dementia Test Characteristics
The CAM was assessed in two studies including patients with dementia.24,25 It was originally24 developed and validated in a population of elderly patients admitted to a medical/geriatric ward (N=56). Of these 12 (21%) had dementia and 9 of whom had DSD. Presence of dementia was defined according to DSM-III-R criteria following an evaluation by a geriatric psychiatrist. The sensitivity and specificity of the delirium tool in the entire sample ranged from 94% (95% Confidence Interval (CI):68–100) to 100% (CI: 54–100) and from 90% (CI: 54–100) to 95% (CI: 73–100), respectively, but specific measures for those with dementia were not reported. A subsequent validation study of a German translation of the CAM25 included a high percentage (85%) of patients with dementia (N=33). In the entire group (N=39) of elderly patients admitted to an acute geriatric unit, the CAM had high specificity (96% to 100%) and moderate sensitivity (77%)in delirium detection, with a likelihood ratio for a positive test (LHR) of 19.25. The number of patients with DSD was 11 (28% of the total sample; 33% of those with dementia), however, the test characteristics in this subgroup were not reported. Diagnosis of dementia was obtained by a consensus between a geriatric neuropsychiatrist and a geriatrician, blinded to the diagnosis of delirium, obtained through the CAM evaluation. A structured interview of the family was performed by the psychiatrist following the DSM-IV criteria. The final diagnosis of dementia was obtained combining this information with the IQCODE, a surrogate interview administered to a close relative of each patient by a psychologist. Additionally, no information was provided whether or not the raters of the SPMSQ and reference standard were blind to the results of the other test.
Two validation studies of the CAM-ICU included a small number of patients with dementia, among the 134 total patients20,21 admitted to an Intensive Care Unit. The sensitivity and specificity in the entire sample were 98%–100% and 93%, respectively and a sensitivity of 100% (CI: 63–100) and specificity of 100% (CI: 3–100) for the diagnosis of DSD. The presence of dementia was defined combining a geriatrician/geriatric psychiatrist evaluation with the DSM-IV criteria or the BDRS performed by study nurses. According to these methods 23 patients were classed as having dementia. The age range was 55 to 66 years. Patients with suspected severe dementia were excluded. Though these studies specifically report the sensitivity and specificity of the CAM-ICU in the subgroup of patients with dementia, we could not retrieve the prevalence of DSD in one of the two validation studies. The CAM-ICU was also applied27 in 129 patients admitted to a stroke unit (mean age 71.2 ± 11.5 years), of whom 31.8% had probable dementia defined according to the BDRS performed by a neuropsychologist and 21 (38%) had DSD. As in the other two CAM-ICU studies patients with severe dementia were excluded. In the entire sample the sensitivity (76%) was moderate and specificity (98%) was high.
The quantitative EEG.28 showed high specificity (96–100%) and moderate sensitivity (77%) for the diagnosis of DSD in 35 patients with dementia admitted to an acute geriatric ward. Dementia was diagnosed according to the DSM-IV criteria by an expert panel (geriatric specialist, neurologist, geriatric psychiatrist, psychologist and gerontologist), using the complete medical history, chart information, caregiver questionnaires, and neuropsychological testing. It is unclear though how neuropsychological testing was used in the context of delirium. Patients with severe dementia were excluded. The EEG was performed by an expert neurophysiologist who was blinded to the clinical diagnosis of delirium. The time between the clinical delirium assessment and the EEG evaluation was not reported. The authors used two EEG techniques: resting EEG (rEEG) and quantitative EEG (qEEG) with eyes open. With the rEEG, pathological results were frequent but were not different between patients with DSD and patients with dementia alone. The rEEG provided sensitivity of only 42% and specificity of 86% in detecting DSD. The quantitative EEG (qEEG), with the presence of increased delta and reduced alpha2 activity during activation, provided an increased sensitivity (67%) and specificity (91%) in detection of DSD.
The SPMSQ was evaluated22 in 282 elderly patients with a mean age of 75 (SD 7.2). Of these, 34 (12%) had dementia previously documented and 7 (2%) experienced DSD. The sensitivity and specificity of the tool in the entire population ranged from 7.3% to 98% and from 82% to 100% respectively. The wide variation in the sensitivity is related to the numbers of errors detected with the SPMSQ, which could be used as different cut-offs for the diagnosis of delirium. The diagnosis of dementia was ascertained through a neurologist interview according to DSM criteria. The quality of the data reporting was lower compared to the other studies (STARD score, 11). In particular, the time between the SPMSQ evaluation and the reference standard rater was not reported, creating potential bias in the diagnosis of delirium given the fluctuation of this syndrome. Additionally, no information was provided whether or not the readers of the index tests and reference standard were blind to the results of the other test.
The CTD was23 tested in a group of 103 ICU patients (mean age 34.4–64.9), with a 25% prevalence of dementia. The overall tool sensitivity (100%) and specificity (95%) were high. The presence of dementia was assessed by a neuropsychologist with the DSM-III criteria and with the MDRS. The CTD was found to have lower quality methodology compared to the other tools (QUADAS score, 9). In particular, two features were found to be unclear: 1) if the reference standard for delirium was independent of the index test and 2) if the patients received the same reference standard regardless of the index result. As in the previous study the time interval between the CTD evaluation and the DSM-rater evaluation was not reported.
The DRS was evaluated26 in 791 geriatric patients admitted to an acute care psychogeriatric unit (mean age 72.6), with a prevalence of dementia of 27% (N=197). The number of patients with DSD was not reported, while the overall prevalence of delirium in the entire population was 9% (N=70). The sensitivity of the tool (DRS score ≥10) in the entire population was 94% and specificity 82%. As in the studies conducted by Erkinjuntti et al.22 and Hart23 time between the DRS evaluation and the DSM-rater evaluation was not reported. The diagnosis of dementia was obtained through a consensus conference attended by three to six geriatric psychiatrists according to the DSM-III criteria.
Documentation of depression
Information on the presence of depression was reported only in 3 studies.23,24,26 The proportion of patients with depression ranged from 16%to 29%.23,24 Rosen24 reported a prevalence of major depression of 28%. No specific subgroup analyses have been reported showing how the tools would perform differently in diagnosing delirium in the presence of depression.
Overall the evidence of marked heterogeneity of the studies as reflected by the differing populations and tests for delirium made a meta-analysis of this data not feasible.
DISCUSSION
This is the first systematic review of the literature on the performance of existing tools for delirium detection in patients with dementia. We found that the CAM and the derived CAM-ICU both had preliminary data supporting their use in the general ward and ICU settings, respectively. Nonetheless, the overall evidence base is small. Only 9 studies were of sufficient quality to meet our final inclusion criteria. Although 1569 patients were assessed in the included studies, only 50 patients had DSD as measured by validated methods. The prevalence of DSD ranged from 2% to 38%. Interestingly, none of the studies reported any effects of dementia severity or subtype. An expanded evidence base is required to draw firmer conclusions about the performance characteristics of delirium measurement tools among the growing and diverse populations of dementia patients.
On the basis of the available evidence, the CAM and the CAM-ICU have the most support for the use in the diagnosis of DSD. Both the CAM studies included patients with dementia, though neither specifically targeted a dementia subgroup. The Hestermann study25 provided a potential indication of the use of the CAM in demented patients given the high prevalence of dementia (85%). Here the CAM had high specificity for delirium but lower sensitivity. The probability of missing the diagnosis was almost 30%, a level that is somewhat low compared to delirium instruments that are used in a general population for routine clinical use. In the CAM-ICU validation study20,21 patients with severe dementia were excluded and no information was reported on the number of patients with probable mild and moderate dementia, though the overall sensitivity and specificity of the tools in demented patients were high. Serial EEGs have been proposed as a useful method in the diagnosis of delirium.29 The study conducted by Thomas and colleagues28 produced mixed results: the specificity of EEG was high, butsensitivity in the dementia population was only 67%. In addition, the studies generalizability is limited by the exclusion of patients with severe dementia. Thus, EEG might have a place in detecting DSD research studies, though further replication in larger groups that include a greater variation in degrees of cognitive impairments is needed.
This review has shown that the current evidence base is small and preliminary. We now discuss some issues that future work might address.
The differences between delirium and dementia provide an obvious focus for the development of scales with better ability to discriminate these conditions. One important such area is how to most effectively capture information from caregivers in making the diagnosis of DSD. Caregiver information is essential to ascertain if there has been an acute decline (characteristic of delirium) but also to establish if there has also been a much longer decline (characteristic of dementia). Caregivers can also clarify if fluctuations in the level of alertness or of cognitive functions are different from baseline fluctuations. It may be that a simple question asking about change is sufficient, but a more detailed dimension-based checklist might have additional value. Another valuable discriminating feature is level of consciousness. Alterations in level of consciousness are not always present in delirium, but when present they are highly specific to this diagnosis.30 It may that this feature is particularly valuable in situations where cognitive testing is hard to interpret due to the presence of significant underlying impairments, or where such testing or even interviews are impossible because of altered level of consciousness. Moreover, other non-cognitive domains might be exploited in this way. For example, the Trunk Control Test –a measure of the ability of a patient to control trunk position– was reported as a possible tool to distinguish DSD from dementia.31 Better descriptions of motor disturbance may also be useful, because compared to those with dementia, patients with may DSD have higher perturbation in motor agitation and retardation.32
Cognitive differences between delirium and dementia could also be examined in more depth. Inattention is a core feature of delirium and thus differences in the severity and types of attentional deficits that occur in delirium versus dementia may be useful in future diagnostic tools. For example, sustained visual attention as assessed by an objective computerized instrument (the “Edinburgh Delirium Test Box”) was reported to be highly impaired in delirium but intact in Alzheimer’s dementia. Additionally, visual perception is impaired in delirium but relatively preserved in most types of dementia.33,34 By contrast, tests of memory are generally impaired in both delirium and Alzheimer’s dementia. Thus, objective evaluation of specific deficits in sustained attention may be useful in differentiating delirium-related inattention from typical dementia symptoms. The use of eye tracking technology, as described by Exton and Leonard,35 might represent a further novel approach to assess visual attention. Eye tracking might be used to specifically test visuospatial and perceptual attention, working memory, motor agitation or retardation.35 These tests should be then compared to existing delirium tools to identify which tools perform better in dementia and in different stages of dementia. These types of tasks appear to have good or excellent ability to discriminate between delirium and dementia. It is, however, unknown how these tasks perform in different stages and types of dementia. Another important knowledge gap is in understanding the impacts of the severity and subtype of dementia. This matters because severe dementia is associated with neuropsychological deficits, including of attentional functioning. Therefore some attentional tests might discriminate between mild and/or moderate dementia and delirium, but not severe dementia. Subtypes of dementia differ with respect to neuropsychological profile, fluctuations, and psychotic features. Therefore, research taking account of these parameters of the dementia patients would be informative. For instance, visual and visuospatial dysfunction, which has been studied in the context of delirium, is a prominent feature of Dementia with Lewy Bodies but it is relatively rare in AD,36 By contrast, patients with with fronto-temporal dementia have relatively preserved visuospatial abilities.37
The variation in dementia diagnostic procedures may add interpretational biases, and lead to populations with varying degrees of severity of dementia. A formal definition of dementia obtained via neuropsychological testing, neuroimaging and biomarkers would be ideal for improving comparisons across studies. As often happens in studies of acute hospitalized inpatients it is cumbersome and often not practical to obtain a complete pre-hospital evaluation. Although the included studies each used accepted methods to define the presence of dementia, future studies should strive to apply similar methods of diagnosis to enable better comparability of the research findings.
Finally, the overlap of delirium and depression might indeed interfere with the screening and diagnosis of delirium, and needs to be further elucidated in future work. Leonard and colleagues38 have previously highlighted how clinicians and researchers are limited in the diagnosis of delirium due to difficulties in differentiating delirium from emotional alterations such as depression. The current delirium tools do not include measures of mood/affect, though anxiety and depressed mood are features of delirium.39 This gap might lead to delirium and depression misclassification. Armstrong et al. found that 46% of patients referred to a psychiatric liaison consultation were misdiagnosed and in 31% of the patients depressive disorder was the most common incorrect diagnosis.40 An overlap of delirium, depression and dementia would create additional challenges to the diagnosis of delirium. Of the tools identified in this current systematic review as promising, only one study24 included a measure of depression but given the small number of patients with depression a formal analysis of the tool characteristics in patients with dementia and depression was not performed. Future studies are also warranted to provide further insights on the role of the overlap between delirium, depression and dementia for the diagnosis and screening of delirium.
Limitations of this systematic review include: (1) small number of identified studies; (2) small numbers of patients with dementia and delirium superimposed on dementia included in the studies; (3) lack of any studies done specifically to examine test performance in dementia patients or evaluate in different dementia severity subgroups; (4) only studies published in English were included; (5) studies evaluating solely alcohol-related delirium were excluded. It is important to note that the majority of these limitations are a result of the small number of studies of delirium diagnosis among dementia patients with a formal diagnosis in the literature to date. Although these limitations may decrease the generalizability and strength of the overall findings, they provide guidance on the future directions that research in this domain should move to improve our ability to diagnose DSD.
Recent underlined research priorities41 in patients with advanced dementia, include the need to develop “new advanced dementia-specific instruments for outcomes currently lacking valid measures.” This systematic review provides the most up-to-date and comprehensive information on test characteristics of existing tools for diagnosis of DSD, and highlights the gaps in the literature. There are currently three tools with preliminary evidence in support of their use in DSD: the CAM and the related CAM-ICU, and EEG, the latter of which lacks widespread clinical applicability. Further work is now needed to assess how these tools perform in the different stages and types of dementia, and studies with more DSD participants are required. Additional tests such as objective assessments of attention, and examination of the discriminatory value of level of consciousness are promising areas for future study.
Supplementary Material
ACKNOWLEDGMENTS
We thank Prof Melissa McPheeters for her advice on the study design.
Funding/support: Dr. Vasilevskis is supported by the National Institutes of Health (K23AG040157), the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr Bellelli has received honoraria from Novartis, Pfizer, Lilly, and Lundbeck. Dr. Ely is supported by the VA Clinical Science Research and Development Service (VA Merit Review Award), and the National Institutes of Health (AG027472). Dr Ely has received honoraria from GSK, Pfizer, Lilly, Hospira, and Aspect. Dr. Fick acknowledges partial support for this work by Award Number R01 NR011042 from the National Institute of Nursing Research (NINR). Prof MacLullich is supported by grants from the UK Medical Research Council. Prof MacLullich has received honoraria from Lundbeck, Novartis and Shire. Dr Shenkin and Prof MacLullich are members of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative: funding from the BBSRC, EPSRC, ESRC and MRC is gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINR or NIH.
Sponsor role: none. The authors’ funding sources did not participate in the planning, collection, analysis or interpretation of data or in the decision to submit for publication. The investigators had full access to the data and were responsible for the study protocol, progress of the study, analysis, reporting of the study and the decision to publish.
Footnotes
Author contribution: Study conception and design – All authors. Acquisition of data – Morandi, McCurley, Vasilevskis, Fick, Bellelli, Jackson, Lee. Interpretation of results – All authors. Drafted manuscript – Morandi. Critically revised the manuscript – All authors Final approval of manuscript – All authors.
Potential conflict of interest: Prof MacLullich holds patents on instruments for assessment of attentional deficits in delirium.The other authors report no financial conflict of interest.
REFERENCES
- 1.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: A systematic review. J Am Geriatr Soc. 2002;50:1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph JL, Boustani M, Kamholz B, et al. Delirium: A strategic plan to bring an ancient disease into the 21st century. J Am Geriatr Soc. 2011;59(Suppl 2):S237–S240. doi: 10.1111/j.1532-5415.2011.03670.x. [DOI] [PubMed] [Google Scholar]
- 4.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty JH, Rudolph J, Shay K, et al. Delirium is a serious and under-recognized problem: Why assessment of mental status should be the sixth vital sign. J Am Med Dir Assoc. 2007;8:273–275. doi: 10.1016/j.jamda.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Foreman MD, Mion LC, et al. Nurses' recognition of delirium and its symptoms: Comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 7.Morandi A, Solberg LM, Habermann R, et al. Documentation and management of words associated with delirium among elderly patients in postacute care: A pilot investigation. J Am Med Dir Assoc. 2009;10:330–334. doi: 10.1016/j.jamda.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Spronk PE, Riekerk B, Hofhuis J, et al. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35:1276–1280. doi: 10.1007/s00134-009-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: Value of bedside instruments. JAMA. 2010;304:779–786. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third Edition. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 12.American Psychiatric Association. Delirium, dementia and amnestic and other cognitive disorders. Diagnostic and Statistical Manual of Mental Disorders. (4th ed) 1994 [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 15.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 16.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003;138:40–44. doi: 10.7326/0003-4819-138-1-200301070-00010. [DOI] [PubMed] [Google Scholar]
- 19.Fontela PS, Pant PN, Schiller I, et al. Quality and reporting of diagnostic accuracy studies in TB, HIV and malaria: Evaluation using QUADAS and STARD standards. PLoS One. 2009;4:e7753. doi: 10.1371/journal.pone.0007753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 22.Erkinjuntti T, Sulkava R, Wikstrom J, et al. Short portable mental status questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987;35:412–416. doi: 10.1111/j.1532-5415.1987.tb04662.x. [DOI] [PubMed] [Google Scholar]
- 23.Hart RP, Levenson JL, Sessler CN, et al. Validation of a cognitive test for delirium in medical ICU patients. Psychosomatics. 1996;37:533–546. doi: 10.1016/S0033-3182(96)71517-7. [DOI] [PubMed] [Google Scholar]
- 24.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 25.Hestermann U, Backenstrass M, Gekle I, et al. Validation of a German version of the Confusion Assessment Method for delirium detection in a sample of acute geriatric patients with a high prevalence of dementia. Psychopathology. 2009;42:270–276. doi: 10.1159/000224151. [DOI] [PubMed] [Google Scholar]
- 26.Rosen J, Sweet RA, Mulsant BH, et al. The Delirium Rating Scale in a psychogeriatric inpatient setting. J Neuropsychiatry Clin Neurosci. 1994;6:30–35. doi: 10.1176/jnp.6.1.30. [DOI] [PubMed] [Google Scholar]
- 27.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2012;40:484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 28.Thomas C, Hestermann U, Walther S, et al. Prolonged activation EEG differentiates dementia with and without delirium in frail elderly patients. J Neurol Neurosurg Psychiatry. 2008;79:119–125. doi: 10.1136/jnnp.2006.111732. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson S, Jerrier H. EEG in delirium. Semin Clin Neuropsychiatry. 2000;5:86–92. doi: 10.153/SCNP00500086. [DOI] [PubMed] [Google Scholar]
- 30.Chester JG, Grande LJ, Milberg WP, et al. Cognitive screening in community-dwelling elders: performance on the clock-in-the-box. Am J Med. 2011;124:662–669. doi: 10.1016/j.amjmed.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellelli G, Speciale S, Morghen S, et al. Are fluctuations in motor performance a diagnostic sign of delirium? J Am Med Dir Assoc. 2011;12:578–583. doi: 10.1016/j.jamda.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Meagher DJ, Leonard M, Donnelly S, et al. A comparison of neuropsychiatric and cognitive profiles in delirium, dementia, comorbid delirium-dementia and cognitively intact controls. J Neurol Neurosurg Psychiatry. 2010;81:876–881. doi: 10.1136/jnnp.2009.200956. [DOI] [PubMed] [Google Scholar]
- 33.Perry RJ, Watson P, Hodges JR. The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer's disease: Relationship to episodic and semantic memory impairment. Neuropsychologia. 2000;38:252–271. doi: 10.1016/s0028-3932(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 34.Brown LJ, Fordyce C, Zaghdani H, et al. Detecting deficits of sustained visual attention in delirium. J Neurol Neurosurg Psychiatry. 2011;82:1334–1340. doi: 10.1136/jnnp.2010.208827. [DOI] [PubMed] [Google Scholar]
- 35.Exton C, Leonard M. Eye tracking technology: A fresh approach in delirium assessment? Int Rev Psychiatry. 2009;21:8–14. doi: 10.1080/09540260802675106. [DOI] [PubMed] [Google Scholar]
- 36.Johnson DK, Morris JC, Galvin JE. Verbal and visuospatial deficits in dementia with Lewy bodies. Neurology. 2005;65:1232–1238. doi: 10.1212/01.wnl.0000180964.60708.c2. [DOI] [PubMed] [Google Scholar]
- 37.Blair M, Kertesz A, McMonagle P, et al. Quantitative and qualitative analyses of clock drawing in frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc. 2006;12:159–165. doi: 10.1017/S1355617706060255. [DOI] [PubMed] [Google Scholar]
- 38.Leonard M, Spiller J, Keen J, et al. Symptoms of depression and delirium assessed serially in palliative-care inpatients. Psychosomatics. 2009;50:506–514. doi: 10.1176/appi.psy.50.5.506. [DOI] [PubMed] [Google Scholar]
- 39.Matsushima E, Nakajima K, Moriya H, et al. A psychophysiological study of the development of delirium in coronary care units. Biol Psychiatry. 1997;41:1211–1217. doi: 10.1016/s0006-3223(96)00219-3. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong SC, Cozza KL, Watanabe KS. The misdiagnosis of delirium. Psychosomatics. 1997;38:433–439. doi: 10.1016/S0033-3182(97)71420-8. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.