Abstract
The current clinical mainstays for cancer treatment, namely, surgical resection, chemotherapy and radiotherapy, can cause significant trauma, systemic toxicity, and functional/cosmetic debilitation of tissue, especially if repetitive treatment becomes necessary due to tumor recurrence. Hence there is significant clinical interest in alternate treatment strategies like photodynamic therapy (PDT) which can effectively and selectively eradicate tumors and can be safely repeated if needed. We have previously demonstrated that the second-generation photosensitizer Pc 4 can be formulated within polymeric micelles, and these micelles can be specifically targeted to EGFR-overexpressing cancer cells using GE11 peptide ligands, to enhance cell-specific Pc 4 delivery and internalization. In the current study, we report on the in vitro optimization of the EGFR-targeting, Pc 4 loading of the micellar nanoformulation, along with optimization of the corresponding photoirradiation conditions to maximize Pc 4 delivery, internalization and subsequent PDT-induced cytotoxicity in EGFR-overexpressing cells in vitro. In our studies, absorption and fluorescence spectroscopy were used to monitor the cell-specific uptake of the GE11-decorated Pc 4-loaded micelles and the cytotoxic singlet oxygen production from the micelle-encapsulated Pc 4, to determine the optimum ligand density and Pc 4 loading. It was found that the micelle formulations bearing 10 mole% of GE11-modified polymer component resulted in the highest cellular uptake in EGFR-overexpressing A431 cells within the shortest incubation periods. Also, the loading of ~50 μg Pc 4 per mg of polymer in these micellar formulations resulted in the highest levels of singlet oxygen production. When formulations bearing these optimized parameters were tested in vitro on A431 cells for PDT effect, a formulation dose containing 400 nM Pc 4 and photoirradiation duration of 400 seconds at a fluence of 200 mJ/cm2 yielded close to 100% cell death.
Keywords: micelles, photodynamic therapy, photosensitizer, epidermal growth factor receptor
Introduction
Photodynamic therapy (PDT) is a novel treatment strategy with significant promise in several diseases including cancer, age-related macular degeneration and psoriasis. PDT involves the activation of a photosensitizer (PS) drug by appropriate wavelength of light which ultimately leads to generation of cytotoxic reactive oxygen species (ROS, e.g., singlet oxygen) from molecular oxygen via various energy transfer pathways 1,2. In the absence of photoirradiation, the PS itself is nontoxic, which is a significant advantage when compared to chemotherapy agents. Additionally, the activating light (often visible or near infra-red) is non-ionizing and therefore produces minimal tissue damage by itself, which is a significant advantage compared to radiotherapy. It is only through the drug-light-oxygen combination that PDT becomes cytotoxic. Hence, harnessing this effect through targeted delivery of the PS to the cancer tissue, followed by tumor region-specific light irradiation provides a way of dual selectivity for effective tumor eradication without harmful side-effects on neighboring healthy tissues 3–5.
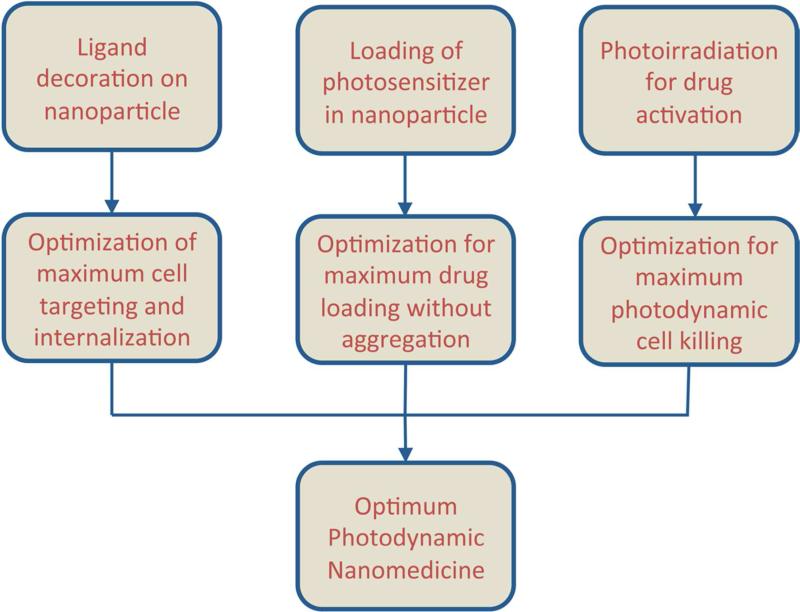
Based on the above rationale, for efficient PDT of cancers, it is necessary to ensure delivery of the PS selectively to the cancer cells, and also ensure that the delivered drug can produce sufficient ROS upon photoactivation to render significant killing of the cancer cells. In the context of cancer cell-specific delivery of the PS, the ‘targeted nanomedicine’ approach provides immense advantage. In this approach the drug is packaged within bioengineered nanoparticulate vehicles that can accumulate within the tumor tissue through the passive mechanisms of enhanced permeation and retention (EPR) and can then be further internalized within the cancer cells through the active mechanisms of receptor-mediated endocytosis 6. During the past decade this approach has revolutionized the delivery of chemotherapeutic agents to cancer, as evident from the clinical approval and application of nanoformulations like Doxil®, DuanoXome®, Onco-TCS®, Myocet®, Transdrug® and Abraxane®, and ongoing advanced phase clinical studies with several others 7. Driven by the success of the nanomedicine approaches in cancer chemotherapeutics, similar approaches are being explored in cancer PDT, as evident from recent research efforts in (a) formulation of various PS in different nanovehicles, and (b) investigation of cell-selective delivery of the PS-loaded vehicles using receptor-targeted antibodies, saccharides, aptamers, peptides, etc. decorated on the nanovehicle surface 8–20. In our research, we are studying PDT for cancer with a second-generation PS, the silicon phthalocyanine Pc 4, which is photoactivated at the tissue-penetrating wavelength of ~675 nm 21. Recently we have demonstrated the feasibility of formulating Pc 4 in polyethylene glycol-co-polycaprolactone (PEG-PCL)-based polymeric micelles (<100 nm in diameter) and actively targeting the Pc 4-loaded micelles using surface-conjugated peptide ligands to Epidermal Growth Factor Receptor (EGFR)-overexpressing model cancer cells (A431 epidermoid carcinoma cells) to render EGFR-mediated rapid internalization for enhanced Pc 4 delivery 22,23. Although these studies have established the viability of a nanomedicine strategy for Pc 4-based PDT of EGFR-overexpressing cancers, it is further necessary to optimize the density of EGFR-targeting ligands on the micelle surface, the extent of Pc 4 loaded within the polymeric micelles, and the appropriate photoirradiation parameters (fluence, fluence rate, and timing of photoirradiation) that maximize the targeted Pc 4-PDT induced killing of the cells in vitro (Figure 1). These optimized parameters can then be used for future investigations in appropriate in vivo models. To this end, here we report on the in vitro optimization of our micelle-based EGFR-targeted Pc 4-PDT strategy.
Figure 1.
Optimization strategies to enhance the efficacy of nanomedicine-based cell-targeted PDT strategy.
Experimental Section
Materials
The EGFR-targeting GE11 peptide, YHWYGYTPQNVI, was custom synthesized by Abgent Inc. (San Diego, CA), with the addition of a cysteine residue on the N-terminus to yield a final sequence of CYHWYGYTPQNVI. The cysteine allows for a thioether-mediated conjugation to the maleimide-terminated PEG block of the PEG-PCL copolymer that forms the micellar nanoformulation. The polymer synthesis, GE11 conjugation and subsequent micelle fabrication have been described previously 23. The structures and molecular weights of mPEG-PCL, Mal-PEG-PCL and peptide functionalized PEG-PCL have been confirmed by 1H NMR and MALDI-TOF mass spectroscopy and have been reported previously 22,23. For analysis of the level of cytotoxic singlet oxygen production by the micelle-encapsulated Pc 4 upon photoirradiation, Singlet Oxygen Sensor Green (SOSG) was purchased from Invitrogen (Carlsbad, CA). A light-emitting diode array (EFOS, Mississauga, ONT, Canada) was used for photoirradiation at a fluence of 200 mJ/cm2 (λmax= 675 nm; width of output peak at half maximum 24 nm). For post-PDT cell viability analysis, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Aldrich (St. Louis, MO). Pc 4 was donated by the laboratory of Dr. Malcolm Kenney in the CWRU Department of Chemistry.
Cell Culture
Human epidermoid carcinoma A431 cells (American Type Culture Collection) were grown in Dulbecco's modified Eagle's medium (Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum, penicillin (50 units/mL) and streptomycin (50 μg/mL). All cultures were maintained in a humidified atmosphere of 5% CO2 in a 37 °C incubator. In all experiments, 70-85% confluent cultures were used. These cells are reported to have EGFR expression of 2-2.5 × 106 receptors/cell 24.
Optimization of ligand density on micelle surface to maximize cell targeting and extent of internalization of the targeted micelles
In an in vitro set up, where nanovehicles are incubated with cells in the culture medium, the nanoparticles can be internalized within cells even in the absence of ligand-based active receptor targeting mechanisms, through non-specific membrane processes (e.g., membrane fusion, fluid-phase pinocytosis, etc.) over long periods of incubation 25. However, when specific receptor-targeting ligands are present on the nanoparticle surface, receptor-mediated endocytotic mechanisms can accelerate the internalization of the nanoparticles25,26. In such scenarios, the cell-targeting efficacy and subsequent receptor-mediated internalization are dependent on nanoparticle size and shape, surface-density of ligands and availability of target receptors 27. In our previous work, we have demonstrated that surface-decoration of Pc 4-loaded polymeric micelles with EGFR-targeting GE11 peptides is a feasible way to achieve rapid internalization and targeted uptake of Pc 4 in EGFR-overexpressing A431 cells 23. Building on this work, in the current studies we aimed to determine the optimum GE11 modification density that allows maximum uptake of the Pc 4-loaded micelles in A431 cells within minimum incubation periods. For this, micelles were prepared bearing ligand-modified polymer component (GE11-PEG-PCL) at 5, 10 and 20 mole %, and the Pc 4-loading in the micelles was maintained at 70 μg per mg of polymer to allow for Pc 4 fluorescence (λem = 675 nm) to be used to assess extent of micelle uptake in cells. The micelles were incubated in aqueous suspension with 85% confluent A431 cells for 30 min, 2 hrs, 6 hrs and 24 hrs. At the end of each incubation period the medium was removed, the cells were gently washed to removed loosely-bound micelles, and the cellular internalization of micelles was assessed by monitoring Pc 4 fluorescence within the cells using a Carl Zeiss Axio Observer D1 Inverted Epifluorescence Microscope (Carl Zeiss, Oberkochen, Germany) fitted with a dichromatic filter cube (bandpass λex = 630-649 nm; bandpass λem = 650-690 nm).
Optimization of Pc 4 loading in micelles to maximize singlet oxygen production upon photoirradiation
Conventional nanovehicle-based delivery of chemotherapeutic agents (e.g., doxorubicin or paclitaxel) usually aims for maximizing the drug loading within nanoparticles which in turn can maximize the intratumoral/intracellular drug dose within tolerated ranges to render maximal cell-killing 28–31. However, in the case of PS loading in nanovehicles for targeted delivery and PDT, excessive PS encapsulation within the nanoparticles can lead to PS aggregation, which in turn reduces their photodynamic efficacy due to mutual quenching of the photo-activating energy among the PS molecules instead of being used for ROS formation. Hence, it is necessary to determine the optimum loading of PS in the nanovehicles that allows for efficient delivery without compromising the photodynamic efficacy of the formulation. Based on this rationale, in the current studies we aimed to determine the optimum Pc 4:polymer ratio in our micelle-encapsulated Pc 4 formulation that renders the maximum level of singlet oxygen (the major cytotoxic ROS component in PDT) production without affecting micelle stability. For these studies we used the fluorescent probe Singlet Oxygen Sensor Green (SOSG), which is weakly fluorescent until it binds irreversibly to singlet oxygen to yield a strong green fluorescence (λmax = 525 nm) signal 32,33. The intensity of the green fluorescence can be correlated to the level of singlet oxygen production. A working solution of SOSG was prepared as per the company's instructions. Micelle formulations of Pc 4 were prepared by keeping the Pc 4 concentration fixed at 1 μM and varying the PEG-PCL concentration from 0-100 mg/ml in 8 mg increments. This resulted in corresponding drug:polymer ratios of 0-180 μg Pc 4 per mg polymer. Samples were then exposed to Pc 4-activating photoirradiation using a light-emitting diode array (EFOS, Mississauga, ONT, Canada) at a fluence of 200 mJ/cm2 (λ=675 nm) for 200 seconds at room temperature. Pre- and post-photoirradiation, the fluorescence emission spectra of SOSG (λexcitation = 470 nm) were recorded to correlate and compare levels of singlet oxygen production for each micelle formulation. Because SOSG can be a very weak PS by itself, appropriate controls were used to determine baseline values and ensure accurate results. After assessing the singlet oxygen production upon photoirradiation of the formulations themselves, the SOSG assay was further used to confirm singlet oxygen production within A431 cells upon photoirradiation. For this, A431 cells were plated in 6-well plates at 2×105 cells/well. This study was done using the optimum drug:polymer ratio that yielded the highest singlet oxygen fluorescence as found through the previous experiments. After growing to 85% confluence, cells were incubated overnight with 1 μM micelle encapsulated Pc 4. The next day, the medium was removed from all wells and cells were washed twice with PBS to remove any residual micelles that were not taken up by the cells. It has been shown previously that SOSG binds to serum proteins, preventing cellular uptake 33. Therefore, we diluted the SOSG solution in Hank's Balanced Salt Solution (HBSS) and exposed the cells to 1 μM SOSG for 2 hours. Controls included cells incubated with only SOSG, cells incubated with only Pc 4 and cells incubated with neither. Two hours following SOSG uptake, the cells were photoirradiated as previously. ‘Dark’ controls were shielded from ambient light. Brightfield images of cells, as well as images of SOSG emission were taken using a Zeiss Axio Observer D.1 inverted fluorescence microscope, and overlaid to assess SOSG fluorescence in cells.
Optimization of total Pc 4 dose and photoirradiation parameters to maximize cell killing in vitro
In vitro PDT efficacy of the optimized nanoparticle formulation with and without EGFR-targeting was evaluated using a standard MTT assay. A431 cells were plated in 96-well flat-bottomed plates at a density of 2 ×104 cells per well. Cells were incubated with nontargeted or targeted (bearing optimum ligand decoration component as determined by previous studies) Pc 4-loaded micelles (bearing optimum Pc 4 loading as determined by previous studies), with final effective Pc 4 concentrations of 200, 400 or 800 nM. Separate plates were prepared for testing ‘dark toxicity’ and different irradiation times (200-600 seconds). We selected 200 seconds as the shortest irradiation period because it has been demonstrated previously to induce maximum cell death in vitro with 250 nM free Pc 4 34. In our Pc 4-PDT studies, we have used 200 nM Pc 4 as the minimum dose and hence, we rationalize that 200 seconds of photoirradiation would be appropriate for the initial photoirradiation duration. PDT plates were photoirradiated as above while dark controls were shielded from ambient light. Plates were then incubated at 37 °C to for 24 hours followed by incubation with MTT for four hours at 37 °C to allow for conversion of MTT to purple formazan by live cells. Formazan was quantified by absorption at 540 nm on a microplate reader (BioTek, Winooski, Vermont) and the measurements for treated cells were normalized to that of controls to report cell viability.
Data Analysis
Where applicable, statistical analyses were performed using Minitab (Minitab, State College, Pennsylvania). Analyses of variance (ANOVA) were used to analyze the data from MTT assays. Significance was reported for p<0.05.
Results
Optimization of ligand density
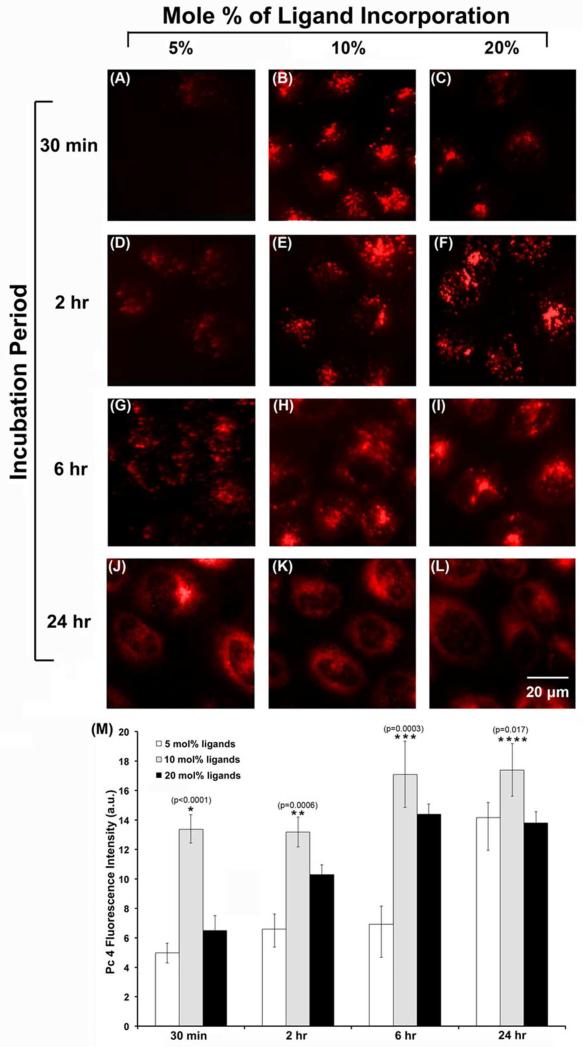
The inherent fluorescence of Pc 4 (λem = 675 nm) within the A431 cells was used to assess and compare the cellular internalization of the Pc 4-loaded micelles bearing varying surface densities of EGFR-targeted GE11 peptides. Figure 2A-L shows representative fluorescence images of the A431 cells incubated with the various micelle formulations for 30 minutes, 2 hours, 6 hours or 24 hours. At the shortest incubation time of 30 minutes, it is apparent that the micelle formulation bearing 10 mole% peptide component resulted in much enhanced intracellular delivery of Pc 4, compared to the other two formulations. This trend in cellular uptake appears to continue up to the 24 hour incubation time-point that we tested, as shown in Figure 2J-L. The results are further confirmed by the quantitative analysis of Pc 4 uptake shown in Figure 2M. These data suggest that, with a high level of target receptor expression on the cell surface, the optimum ligand density on the delivery vehicle is the primary factor influencing the cellular uptake while uptake due to non-specific processes such as membrane fusion and pinocytosis may contribute more with increasing incubation time.
Figure 2.
Representative fluorescence images (A-L) comparing the Pc 4 uptake in A431 cells at various incubation time periods when delivered via EGFR-targeted micelles bearing different mole percentages of GE11 peptide incorporation; (M) shows quantitative data for the Pc 4 fluorescence in these cells for the various targeted nanoformulations at the different incubation periods; all levels of significance are for p < 0.05 for the different formulations at each timepoint.
Optimization of Singlet Oxygen Formation
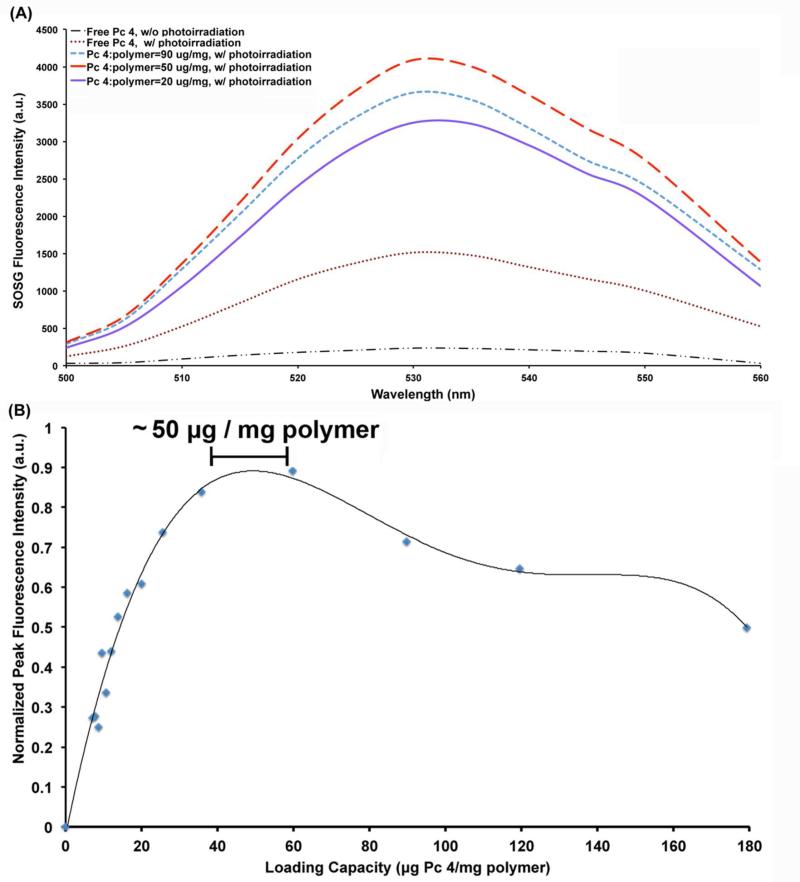
With Pc 4 amount fixed at 1 μM while varying the polymer amount for micelle formulation (hence varying the drug:polymer ratio), it was found that SOSG emission increases with polymer concentration until the ratio reaches approximately 50 μg per mg of polymer and then it decreases at higher ratios. This is evident from the representative SOSG fluorescence intensity spectra shown in Figure 3A and the Pc 4-loading versus SOSG fluorescence intensity correlation data shown in Figure 3B. The enhancement in SOSG fluorescence intensity upon appropriate photoirradiation of the micelle formulation is reflective of enhanced production of singlet oxygen. Hence 1 μM Pc 4 encapsulated in ~14 mg/mL PEG-PCL polymer (i.e., ~50 μg of Pc 4 per mg of polymer) seems to be the optimum loading threshold at which the Pc 4 exists in sufficiently un-aggregated form within the micelles so as to allow maximum photodynamic reactivity with molecular oxygen to produce singlet oxygen. If the polymer concentration is further increased beyond 14 mg/mL, the photodynamic reactivity of the loaded Pc 4 seems to decrease possibly due to dynamic perturbations in the micellar system at excessive polymer concentration (e.g., aggregation of micelles themselves, chain exchange between micelles, morphological transformations in micelle shapes, etc.).
Figure 3.
(A) Representative fluorescence emission spectra of SOSG in solution with either free Pc 4 or Pc 4-nanoformulations of different loading extents, with or without photoirradiation; only three representative loading values are shown for convenience; the loading capacity of around 50 μg of Pc 4 per mg of polymer showed maximum SOSG fluorescence upon photoirradiation. (B) Plot of peak SOSG fluorescence intensity versus loading capacity (μg of Pc 4 per mg of polymer) showing maximum intensity (hence maximum singlet oxygen production) for formulations having about 50 μg Pc 4 per mg of polymer.
Confirmation of Singlet Oxygen Production within cells in vitro
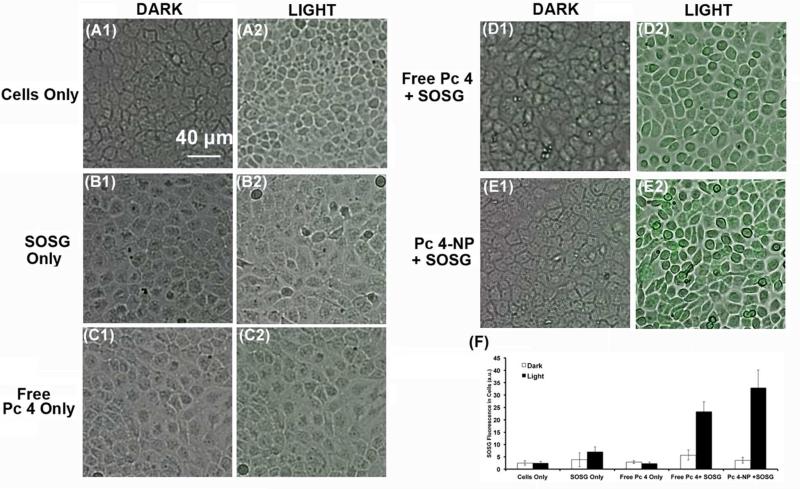
Figure 4 shows representative microscopy images of SOSG fluorescence (singlet oxygen production) in A431 cells incubated with free Pc 4 (Figure 4D1-D2) or the optimized micellar formulation of Pc 4 (Figure 4E1-E2), and in absence or presence of photoirradiation. Little or no green fluorescence was observed without photoirradiation, possibly due to the inherent weak fluorescence of the native SOSG, confirming that the Pc 4 formulation in micelles does not produce singlet oxygen unless activated by light (minimum dark toxicity). In contrast, upon appropriate photoirradiation, the SOSG-relevant green fluorescence in the A431 cells is significantly enhanced, suggesting a high level of production of singlet oxygen production. These results establish that the optimum Pc 4 loading in micelles of 50 μg per mg of polymer and internalization of these micelles within target cells allow for enhanced photodynamic reactivity of Pc 4 with intracellular molecular oxygen upon photoirradiation to produce cytotoxic singlet oxygen.
Figure 4.
Representative images of A431 cells when incubated with free Pc 4 (D1-D2) versus Pc 4 nanoformulation (E1-E2) formulations with (light) or without (dark) photoirradiation; appropriate controls consisted of cells without any Pc 4 or SOSG (A1-A2), cells with SOSG only (B1-B2) and cells with free Pc 4 only (C1-C2); significantly enhanced singlet oxygen production was observed for cells incubated with Pc 4-NP and SOSG; (M) shows quantitative data of SOSG fluorescence intensity in the cells for the various test and control samples.
Optimization of Drug Dose and Photoirradiation Parameters
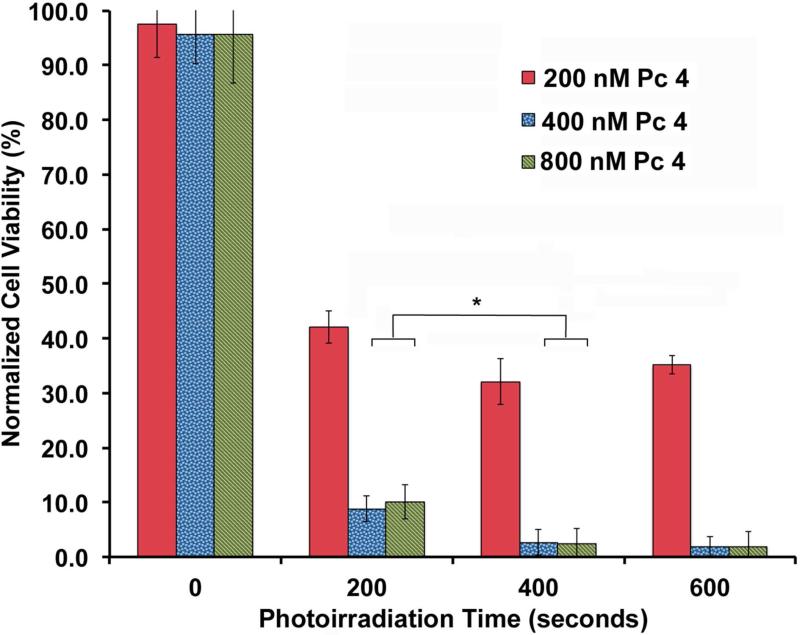
Figure 5 shows results of the cell viability assays when incubated with the optimized EGFR-targeted micelle-encapsulated Pc 4 formulation at total doses of 200, 400 and 800 nM Pc 4. As evident from the data, dark conditions (no photoirradiation) confer little cytotoxicity, while even 200 seconds of photoirradiation leads to significant cell death (p<0.001). The highest extent of cell death occurred following 400 seconds of photoirradiation and subsequent increase of light dose to 600 seconds did not enhance this effect. Similarly, it was found that increasing the Pc 4 concentration from 200 to 400 nM and maintaining 400 seconds of photo-irradiation resulted in significant increase of cell death, but further enhancement of cell death in vitro was not observed when the Pc 4 dose was increased to 800 nM or photoirradiation duration was increased beyond 400 seconds. This suggests that the optimum dose of 400 nM of Pc 4 delivered via EGFR-targeted polymeric micelles can achieve maximum possible cell death in vitro for our target A-431 cells, when activated with the optimum photoirradiation duration of 400 seconds.
Figure 5.
A431 cell response to PDT with various doses of Pc 4 delivered via micelles bearing 10 mole% EGFR-targeted ligands (the optimum targeting ligand parameter) and 50 μg Pc 4 per mg of polymer loading (the optimum loading parameter), with various irradiation times using 200 mJ/cm2 fluence.
Discussion
In cancer nanomedicine, it is critical to optimize the formulation parameters and the therapy conditions that can ensure maximum cell-killing. Often research reports are shown to simply demonstrate feasibility of targeted delivery and cytotoxic effects on various cancer cell lines in vitro and tumor models in vivo, without further determining the optimum targeting and therapy parameters that maximize the therapeutic effect. From a practical perspective, lack of such information is an important barrier towards translating such nanomedicine technologies into clinical application. These considerations are even more important in treatment modalities like PDT, in which multiple factors of targeting, drug loading and light dose need to be optimized in tandem for achieving dual selectivity of tumor-targeted delivery of a PS and tumor-focused photoirradiation for maximum therapeutic efficacy. We have previously demonstrated the feasibility of encapsulating Pc 4 in block-copolymer based micelles and targeting this formulation to EGFR-overexpressing cancer cells by decorating the micelle surface with EGFR-targeting peptide ligands. To further build on this work and establish Pc 4-PDT strategies for various EGFR-overexpressing cancers, it is necessary to optimize the targeting ligand decoration density and the Pc 4-loading extent of the micelles, as well as, the incubation dose and photoirradiation conditions that lead to maximum cell-killing in vitro. This would help identify the candidate formulation and corresponding PDT conditions that can be subsequently investigated in vivo in appropriate EGFR-overexpressing xenograft models.
As evident from our results in vitro, the EGFR-mediated cell-targeting and internalization efficacy of the micelles seem to be maximum at a micelle formulation bearing 10 mole% EGFR-targeting GE11 peptide-bearing polymer component in the micelles, as this formulation resulted in maximum internalization within a short (≤ 2 hrs) period of time. For our studies, at the end of the various incubation periods we thoroughly washed the cell cultures to remove possible loosely-bound micelles from the cell surface, added fresh media and then carried out direct imaging of cell-associated Pc 4 or carried out appropriate photo-irradiation for PDT. In these studies we did not differentiate between vehicles that may have been tightly bound to the cell membrane versus those that are already internalized, because achieving enhanced levels of either scenarios results in ultimate enhanced cell death upon photoirradiation, albeit through different molecular mechanisms 35,36. Research has shown that the reactive oxygen species can diffuse ~0.02 μm during its lifetime in a biological environment and hence we rationalize that photo-activated Pc 4 from both cell surface-bound micelles and internalized micelles can induce PDT effects 37. While the increase in cellular targeting and internalization in going from micelles bearing 5 mol% ligand components to that bearing 10 mol% ligand components can be rationalized by higher extent of EGFR-specific interactions of the micelles, it is interesting to find that increasing the ligand-bearing component further to 20 mole% does not result in a further increase of targeting and internalization. In fact, this resulted in an apparent decrease in targeting and internalization in our in vitro experimental conditions. This is in accordance with the theoretical findings of nanomedicine design optimization reported by Wang et al., which reports that increasing the number of ligand decorations on a nanoparticle surface does not necessarily guarantee a corresponding increase in receptor targeting (and internalization in the case of receptors which undergo endocytosis), due to possible entropic barriers associated with the conformational changes that a ligandte-thered random coil polymer (e.g., PEG in our case) and heterogeneously distributed receptors (e.g., EGFRs on a non-planar cell surface) may possess 38. It is also to be noted that for the cell-targeted assays with the GE11-modified micelles, the intracellular fluorescence at short time periods (up to 6 hrs) appears punctate while after longer time periods (e.g., 24 hrs) the fluorescence seems to be distributed more homogenously throughout the cytoplasm. This may be indicative of the fact that at the shorter incubation periods the internalized micelles (hence Pc 4 fluorescence) are within the endosomal spaces in the cells, while over time the endosomal/lysosomal acidic environment may degrade and disassemble the micelles, resulting in release and distribution/partitioning of the hydrophobic Pc 4 into intracellular organelle membranes (e.g., mitochondria, Golgi, ER, etc.).
It has been reported that polymeric micelles without specific receptor targeting can still get internalized within cells through membrane-mediated endocytotic and fluid-phase pinocytic mechanisms depending on long incubation periods and micelle concentration, and in such cases micelle disassembly and subsequent drug dispersion throughout the cytoplasm can occur with equilibrium being reached between 16-24 hours 39–42. Hence at some longer incubation time point, micelles (and micellar payload) may be in the cell membrane, endosomal/lysosomal domain and distributed in cytoplasm. There was a significant difference in cell-associated Pc 4 fluorescence in our studies for the various GE11-decorated micelles incubated with the A431 cells at shorter time periods, and the differences seemed to decrease over longer incubation period. We have interpreted this observation as receptor-mediated uptake occurring predominantly at shorter incubation period (hence bigger difference in uptake based on ligand density), while both receptor-mediated and passive endocytotic uptake occurring at longer incubation period (hence the passive uptake process reducing the fluorescence difference to some extent). It is possible that the some of the micelles uptaken through receptor-mediated mechanisms in earlier time periods have already undergone endosomal/lysosomal degradation by the 24 hr time point resulting in heterogenous distribution of the Pc 4 within the cell, along with the Pc 4 internalized through the passive endocytotic uptake of micelles. These cumulative effects possibly result in comparable fluorescence intensity for all ligand concentration. However, our quantitative analysis still shows that for the cells incubated with the 10 mol% ligand decorated micelles, cell-associated Pc 4 fluorescence is still statistically higher than the other mol% decorations, even at 24 hr. We rationalize this by the possibility that the receptor-mediated uptake for the 10 mol% ligand-decorated ligands is higher in the cells through the earlier time periods and the passive endocytotic uptake is comparable for all mol% ligand decorations. So the cumulative Pc 4 uptake for the cells incubated with the 10 mol% ligand-decorated micelles is still higher than the other groups.
Our results also indicate that for loading of a PS within micelles, there is an optimum loading capacity (mass of drug per unit mass of polymer). It may be possible to physically increase the loading capacity beyond this value (i.e., more drug per unit mass of polymer), but then the drug may be too aggregated within the micelle core to allow maximum photodynamic interaction with molecular oxygen, due to mutual quenching of activating energy by the PS molecules. On the other hand, using a polymer amount higher than the optimal threshold does not necessarily lead to further enhancement of the dis-aggregated state of the photosensitizer (hence, further enhanced photodynamic reaction capability) but may actually destabilize the micellar assembly state of the polymers due to dynamic macromolecular morphological interactions. Stability of micelles involves (a) thermodynamic stability and (b) kinetic stability 43. Thermodynamic stability is rendered by using polymer concentrations at or above CMC. In such situations, the polymer will exist both as micelles and as single chains, with thermodynamic equilibrium between them. Kinetic stability, on the other hand, is dependent on the rate of unimer (single chain) exchange between the micellar assembly and the existent single chains 43. This rate will be influenced if the concentration of polymer is varied, and the macromolecular exchange perturbations will also potentially affect the drug distribution in the micelles, since the drug is associated with the hydrophobic block of the amphiphilic chain undergoing dynamic exchange and assembly. It has also been reported in the literature that at polymer concentration much higher than CMC, inter-micellar aggregation can occur, which can also in effect cause aggregation of the photosensitizer drug resulting in a reduction of photodynamic efficacy 30,44. Hence, once the optimum loading capacity is reached to ensure sufficiently disaggregated state of the photosensitizer within the micelles and allow for maximum singlet oxygen production upon photoirradiation, further addition of polymers may affect the kinetic stability, even if the concentrations are above the CMC (hence in thermodynamic stability range). In our studies, the optimum drug loading was found to be ~50 μg of Pc 4 per mg of PEG-PCL polymer. It is to be noted that this optimum loading is specific for this drug and this polymer system; changing the drug or changing the molecular weight or chemistry of the polymer would require investigation of other corresponding optimized loading conditions for maximum photodynamic efficacy.
Following the establishment of optimum ligand density and optimum Pc 4 loading parameters, it was necessary to determine if these optimum conditions resulted in maximum cell-killing upon photoirradiation in vitro, and thereby establish the corresponding PDT efficacy. Our Pc 4-PDT assays showed significant cell killing, even with the shortest (200 sec) photoirradiation. As the photoirradiation was increased to 400 sec, a significant increase in cell death was observed, but further increase to 600 sec did not further increase cell death. Regarding drug formulation dosage, a statistical difference in cell death was observed between cells incubated with 400 nM Pc 4 compared to the lower dosage of 200 nM Pc 4, for fixed photoirradiation time. However, the increase to 800 nM Pc 4 did not cause additional cell death. This may imply that in translation in vivo, a lower amount of PS may allow sufficient PDT effect, provided the drug is effectively targeted to the tumor (e.g., EGFR-targeting in our case) and the tumor is effectively photoirradiated for an appropriate extent of time. We would like to point out that the EGFR-targeted Pc 4 nanoformulation dose and the photoirradiation conditions that exhibited the highest level of cell-killing in vitro, are specific to the particular cell line and the particular nanovehicle platform used in our studies. For a different cell line and for a different targeted nanoformulation strategy, the optimum conditions can be similarly determined in vitro. Furthermore, the formulations parameters and photoirradiation conditions that show maximum cell-killing in vitro are to be additionally validated by appropriate clonogenic assays, to ensure tumor eradication potential in vivo. It is also to be noted that in vitro the ‘tissue penetration depth’ of the photoactivating light is not a limiting factor, whereas in vivo, proper delivery of light will be another critical factor to be optimized, in addition to using the optimum fluence and fluence rate. In this context, PS like Pc 4 that are photoactivated by red to near infra-red wavelengths provide significant advantage since these wavelengths have high tissue penetration compared to those photoactivated at shorter wavelengths. Also, in vivo the availability of sufficient molecular oxygen can be an additional limiting factor for tumors with regions of hypoxia. We are currently carrying out studies using 3D spheroids which will provide important insight regarding additional parameters relevant to in vivo PDT, namely, diffusion of the nanovehicles and light penetration 45,46. Nonetheless, optimizing the targeting, drug formulation and photoirradiation conditions in vitro provides important insight about the appropriate candidate formulation and PDT strategy that can be further tested in vivo.
Conclusion
We have established the optimal in vitro nanoformulation parameters and photoirradiation conditions for maximizing cell killing in targeted Pc 4-PDT of EGFR-overexpressing cancers. This study demonstrates that EGFR-targeted Pc 4-loaded polymeric micelles with a ligand-bearing polymer composition of 10 mole% percent and Pc 4-loading of ~50μg/mg of PEG-PCL polymer maximizes the killing of EGFR-overexpressing A431 cells at an effective formulation dose of 400 nM Pc 4 and a minimum effective photoirradiation duration of 400 sec.
Supplementary Material
Acknowledgements
The authors are grateful to Dr. Malcolm E. Kenney of CWRU Department of Chemistry for providing the Pc 4. A.M. Master is funded by an F31-DE019998 award from the NIDCR.
Abbreviations
- PDT
Photodynamic therapy
- PS
photosensitizer
- Pc 4
silicon phthalocyanine 4
- SOSG
singlet oxygen sensor green
Footnotes
Supporting Information: The focus of this manuscript was to determine the targeting, drug-loading and photo-irradiation parameters that maximize PDT effect on EGFR-overexpressing model A431 cell lines in culture exposed to a Pc 4 nanoformulation in EGFR-targeted micelles, in vitro, to be able to establish an initial metric of targeted PDT conditions for subsequent in vivo studies. In our previous report we have already established the efficacy of GE11-decorated Pc 4-loaded micelles to deliver Pc 4 to EGFR-overexpressing A431 cells, by comparing against two negative control conditions: GE11-decorated Pc 4-loaded micelles incubated with low-EGFR MCF-7 cells and undecorated Pc 4-loaded micelles incubated with high-EGFR A431 cells 23. In both negative control conditions, the uptake of the micelles happened at much longer incubation times due to passive processes in the in vitro set-up. Hence in the current manuscript, we have presented data focused more on optimizing the conditions for the targeted PDT scenario with GE11-decorated Pc 4-loaded micelles on A431 cells, and have provided the data of the ‘control conditions’ only in supporting information. Representative data from the control groups is available in the supporting information section, free of charge, via the Internet at http://pubs.acs.org/.
References
- 1.Allison RR, Bagnato VS, Sibata CH. Future of oncologic photodynamic therapy. Future Oncology. 2010;6:929–940. doi: 10.2217/fon.10.51. [DOI] [PubMed] [Google Scholar]
- 2.Robertson C. a, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B, Biol. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Solban N, Ortel B, Pogue B, Hasan T. Targeted optical imaging and photodynamic therapy. Ernst Schering Res. Found. Workshop. 2005:229–58. doi: 10.1007/3-540-26809-x_12. [DOI] [PubMed] [Google Scholar]
- 4.Bugaj AM. Targeted photodynamic therapy - a promising strategy of tumor treatment. Photochem. Photobiol. Sci. 2011;10:1097–109. doi: 10.1039/c0pp00147c. [DOI] [PubMed] [Google Scholar]
- 5.Solban N, Rizvi I, Hasan T. Targeted photodynamic therapy. Lasers Surg Med. 2006;38:522–31. doi: 10.1002/lsm.20345. [DOI] [PubMed] [Google Scholar]
- 6.Praetorius NP, Mandal TK. Engineered nanoparticles in cancer therapy. Recent Pat Drug Deliv Formul. 2007;1:37–51. doi: 10.2174/187221107779814104. [DOI] [PubMed] [Google Scholar]
- 7.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–46. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Chen B, Pogue B, Hasan T. Liposomal delivery of photosentising agents. Expert Opinion on Drug Delivery. 2005;2:477–487. doi: 10.1517/17425247.2.3.477. [DOI] [PubMed] [Google Scholar]
- 9.Derycke A, de Witte PA. Liposomes for photodynamic therapy. Advanced Drug Delivery Reviews. 2004;56:17–30. doi: 10.1016/j.addr.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Zhang G. Polyion complex micelles entrapping cationic dendrimer porphyrin: effective photosensitizer for photodynamic therapy of cancer. Journal of Controlled Release. 2003;93:141–150. doi: 10.1016/j.jconrel.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Van Nostrum CF. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Advanced Drug Delivery Reviews. 2004;56:9–16. doi: 10.1016/j.addr.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Tang W, Xu H, Kopelman R, Philbert MA. Photodynamic characterization and in vitro application of methylene blue-containing nanoparticle platforms. Photochemistry and Photobiology. 2005;81:242–249. doi: 10.1562/2004-05-24-RA-176.1. [DOI] [PubMed] [Google Scholar]
- 13.Pitsillides CM, Joe EK, Wei X, Anderson RR, Lin CP. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophysical Journal. 2003;84:4023–4032. doi: 10.1016/S0006-3495(03)75128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopelman R, Leekoo Y, Philbert M, Moffat B, Ramachandrareddy G, Mcconville P, Hall D, Chenevert T, Bhojani M, Buck S. Multifunctional nanoparticle platforms for in vivo MRI enhancement and photodynamic therapy of a rat brain cancer. Journal of Magnetism and Magnetic Materials. 2005;293:404–410. [Google Scholar]
- 15.Sharman WM, Van Lier JE, Allen CM. Targeted photodynamic therapy via receptor mediated delivery systems. Adv. Drug Deliv. Rev. 2004;56:53–76. doi: 10.1016/j.addr.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Wieder ME, Hone DC, Cook MJ, Handsley MM, Gavrilovic J, Russell D. a Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: cancer therapy using a “Trojan horse”. Photochem. Photobiol. Sci. 2006;5:727–34. doi: 10.1039/b602830f. [DOI] [PubMed] [Google Scholar]
- 17.Rosenkranz AA, Jans DA, Sobolev AS. Targeted intracellular delivery of photosensitizers to enhance photodynamic efficiency. Immunology and Cell Biology. 2000;78:452–464. doi: 10.1046/j.1440-1711.2000.00925.x. [DOI] [PubMed] [Google Scholar]
- 18.Derycke ASL, Kamuhabwa A, Gijsens A, Roskams T, De Vos D, Kasran A, Huwyler J, Missiaen L, De Witte PAM. Transferrin-conjugated liposome targeting of photosensitizer AlPcS4 to rat bladder carcinoma cells. Journal Of The National Cancer Institute. 2004;96:1620–1630. doi: 10.1093/jnci/djh314. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv. Drug Deliv. Rev. 2008;60:1627–37. doi: 10.1016/j.addr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Allison RR, Mota HC, Bagnato VS, Sibata CH. Bio-nanotechnology and photodynamic therapy--state of the art review. Photodiagnosis Photodyn Ther. 2008;5:19–28. doi: 10.1016/j.pdpdt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Oleinick NL, Antunez AR, Clay ME, Rihter BD, Kenney ME. New phthalocyanine photosensitizers for photodynamic therapy. Photochemistry and Photobiology. 1993;57:242–247. doi: 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 22.Master AM, Rodriguez ME, Kenney ME, Oleinick NL, Gupta AS. Delivery of the photosensitizer Pc 4 in PEG–PCL micelles for in vitro PDT studies. J Pharm Sci. 2010;99:2386–2398. doi: 10.1002/jps.22007. [DOI] [PubMed] [Google Scholar]
- 23.Master AM, Qi Y, Oleinick NL, Gupta AS. EGFR-mediated Intracellular Delivery of Pc 4 Nanoformulation for Targeted Photodynamic Therapy of Cancer: In Vitro Studies. Nanomedicine. 2011;8:655–64. doi: 10.1016/j.nano.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes M, Shi C. Phosphorylation of extracellular signal-regulated kinase 1 and 2, protein kinase B, and signal transducer and activator of transcription 3 are differentialy inhibited by an epidermal growth factor receptor inhibitor, EKB-569, in tumor cells and normal hum. Molecular Cancer Therapeutics. 2004;3:21–27. [PubMed] [Google Scholar]
- 25.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007;59:748–58. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanai S, Sugiyama Y, Kim DC, Iga T, Fuwa T, Hanano M. Kinetic analysis of receptor-mediated endocytosis of epidermal growth factor by isolated rat hepatocytes. Am. J. Physiol. 1991;260:C457–67. doi: 10.1152/ajpcell.1991.260.3.C457. [DOI] [PubMed] [Google Scholar]
- 27.Yuan H, Zhang S. Effects of particle size and ligand density on the kinetics of receptor-mediated endocytosis of nanoparticles. Applied Physics Letters. 2010;96:033704. [Google Scholar]
- 28.Nyström AM, Xu Z, Xu J, Taylor S, Nittis T, Sheila A, Leonard J, Wooley KL. SCKs as nanoparticle carriers of doxubicin: investigation of core composition on the loading, release and cytotoxicity profiles. Chemical Communication. 2008:3579–3581. doi: 10.1039/b805428b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milane L, Duan Z-F, Amiji M. Pharmacokinetics and biodistribution of lonidamine/paclitaxel loaded, EGFR-targeted nanoparticles in an orthotopic animal model of multi-drug resistant breast cancer. Nanomedicine. 2011;7:435–44. doi: 10.1016/j.nano.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J Control Release. 2005;101:59–68. doi: 10.1016/j.jconrel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson a M., Bhar P, Hawkins MJ. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006;17:1263–8. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 32.Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green®. J. Exp. Bot. 2006;57:1725. doi: 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- 33.Gollmer A, Arnbjerg J, Blaikie FH, Pedersen BW, Breitenbach T, Daasbjerg K, Glasius M, Ogilby PR. Singlet Oxygen Sensor Green®: photochemical behavior in solution and in a mammalian cell. Photochem. Photobiol. 2011;87:671–9. doi: 10.1111/j.1751-1097.2011.00900.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang KK-H, Wilson JD, Kenney ME, Mitra S, Foster TH. Irradiation-induced enhancement of Pc 4 fluorescence and changes in light scattering are potential dosimeters for Pc 4-PDT. Photochemistry and Photobiology. 2007;83:1056–1062. doi: 10.1111/j.1751-1097.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 35.Piette J, Volanti C, Vantieghem A, Matroule J-Y, Habraken Y, Agostinis P. Cell death and growth arrest in response to photodynamic therapy with membrane-bound photosensitizers. Biochem. Pharmacol. 2003;66:1651–9. doi: 10.1016/s0006-2952(03)00539-2. [DOI] [PubMed] [Google Scholar]
- 36.Moor AC. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B, Biol. 2000;57:1–13. doi: 10.1016/s1011-1344(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 37.Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991;53:549–53. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Dormidontova EE. Monte carlo simulations of metallo-supramolecular micelles. Macromol Rapid Commun. 2010;31:897–903. doi: 10.1002/marc.200900900. [DOI] [PubMed] [Google Scholar]
- 39.Allen C, Yu Y, Eisenberg A, Maysinger D. Cellular internalization of PCL(20)-b-PEO(44) block copolymer micelles. Biochim. Biophys. Acta. 1999;1421:32–8. doi: 10.1016/s0005-2736(99)00108-x. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Shi Y, Kim JY, Park K, Cheng J-X. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Deliv. 2010;7:49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 41.Pratten M, Lloyd JB, Ringsdorf H. Micelle-forming block copolymers: Pinocytosis by macrophages and interaction with model membranes. Makromolekulare Chemie Macromolecular Chemistry And Physics. 1985;186:725–733. [Google Scholar]
- 42.Savic R, Luo L, Eisenberg A, Maysinger D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science. 2003;300:615–8. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]
- 43.Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids and Surfaces B: Biointerfaces. 1999;16:3–27. [Google Scholar]
- 44.Li W, Li J, Gao J, Li B, Xia Y, Meng Y, Yu Y, Chen H, Dai J, Wang H, Guo Y. The fine-tuning of thermosensitive and degradable polymer micelles for enhancing intracellular uptake and drug release in tumors. Biomaterials. 2011;32:3832–44. doi: 10.1016/j.biomaterials.2011.01.075. [DOI] [PubMed] [Google Scholar]
- 45.Foster TH, Hartley DF, Nichols MG, Hilf R. Fluence rate effects in photodynamic therapy of multicell tumor spheroids. Cancer Res. 1993;53:1249–54. [PubMed] [Google Scholar]
- 46.Madsen SJ, Sun C-H, Tromberg BJ, Cristini V, De Magalhães N, Hirschberg H. Multicell tumor spheroids in photodynamic therapy. Lasers Surg Med. 2006;38:555–64. doi: 10.1002/lsm.20350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.