Abstract
BACKGROUND
Methamphetamine (MA) abuse is a worldwide problem. Little is known about the co-morbidity of substance use disorders (SUD) and other psychiatric disorders of mothers who use MA prenatally. The Infant Development, Environment and Lifestyle (IDEAL) Study is a prospective, investigation of prenatal MA use and child outcome in the United States (US) and New Zealand (NZ). This study examined prenatal MA use and the co-morbidity of SUD and psychiatric disorders at 1-month postpartum.
METHOD
Mothers who used MA (US = 127, NZ = 97) were compared to a matched comparison group (US = 193, NZ = 110). The Substance Abuse Subtle Screening Inventory-3 was used to measure the probability of a SUD. The Brief Symptom Inventory was used to measure the likelihood of a positive diagnosis of a psychiatric disorder.
RESULTS
In US and NZ, the MA groups had lower SES, increased single parenting, delayed prenatal care, increased polydrug use. In the US only, MA mothers had lower income than the comparison group. MA users were 10 times more likely to have a SUD and twice as likely to meet Brief Symptom Inventory criteria for a diagnosable psychiatric disorder. In NZ, but not the US, MA users were five times more likely have co-morbidity of both. This disparity may be due to higher quantities of prenatal alcohol use associated with increased psychiatric symptoms.
CONCLUSION
These findings suggest that addressing both substance abuse and psychiatric disorders in mothers who use MA may be required to effectively treat maternal MA use.
Keywords: Methamphetamine, Maternal Drug Use, Comorbidity, Substance Use Disorder, Psychiatric Disorder
1. INTRODUCTION
Methamphetamine (MA) abuse continues to be the fastest growing illicit drug problem worldwide (United Nations Office on Drugs and Crime, 2004, 2007, 2010, 2011). The most recent UNODC World Drug Report (United Nations Office on Drugs and Crime, 2011) emphasized the substantial increases in MA use across East Asia and an increase in MA initiation in the United States (US). Oceania is the region with the highest prevalence of amphetamine-type substance (ATS) use, with Australia and New Zealand (NZ) having the highest annual estimates of 2.7% and 2.1%, respectively. One major indication of the health risks of ATS is the increased proportion of women requiring comprehensive health and mental health services in both the US and internationally (Jones et al., 2011; Wechsberg et al., 2010; Wright et al., 2012).
MA is one of the most potent of the ATS. Repeated MA use, higher doses, the onset of MA use at an earlier age and being female have all been associated with increased risk for psychosis and persistent psychiatric symptoms (Chen et al., 2003; Cohen et al., 2007; Hall et al., 1996; McKetin et al., 2006; Nelson-Zlupko et al., 1995; Sekine et al., 2001). In addition, MA use in combination with alcohol and marijuana has been associated with significantly higher symptom scores on depression, obsessiveness, paranoia, and psychoticism than the use of MA alone (Christian et al., 2007).
Evidence that MA is being abused during pregnancy comes from the Treatment Episode Data set that captures admissions to US federally funded treatment centers (Terplan et al., 2009). Admissions for pregnant women using MA increased from 8% in 1994 to 23.7% in 2006. During this period, an increase in co-occurring psychiatric disorders was noted among MA-using women admitted for treatment. Increases were also noted in the proportion in dependent-living situations and those with criminal justice involvement. The Substance Abuse and Mental Health Services (SAMSHA) report (Substance Abuse and Mental Health Services Administration, 2008) found 5.1% of pregnant women aged 15–44 years used illicit drugs during their pregnancy and a further study of a non-clinical population found approximately 5.2% of women reported using MA at least once during their pregnancy (Arria et al., 2006).
While little is known about the co-morbidity of substance use disorders (SUDs) and psychopathology among women who use MA during pregnancy, increased psychosocial problems and polydrug use are common in the often, chaotic lifestyles of women who are drug dependent (Benningfield et al., 2010; Della Grotta et al., 2009; Good et al., 2010; Oei et al., 2010; Wouldes and Woodward, 2010). Of particular concern is the evidence that links maternal psychopathology, drug dependence and other psychosocial problems with negative parenting behaviours and poor developmental outcomes for children (Hans et al., 1999; Wan and Green, 2009). Thus, understanding co-morbidity between SUD and psychiatric disorders among women who use MA during pregnancy is a clinically important issue. From a research perspective, one must disentangle the direction of these associations and the extent to which they are reflective of confounding by other drug use or lifestyle factors.
In 2007, Derauf et al. reported a number of adverse psychosocial circumstances surrounding women who used MA during pregnancy from early results of the US Infant Development, Environment and Lifestyle (IDEAL) Study. Most notable was the finding that the odds of a mother who used MA during pregnancy developing a SUD was 12 times that of mothers in the comparison group. However, no association between prenatal MA use and comorbid psychiatric disorders was observed.
The present investigation will extend the earlier findings from the US IDEAL Study in a larger combined sample of US and NZ biological mothers. In addition, it will provide an opportunity to determine whether these findings generalize to another culture where there is no legal mandate to report mothers to child protective services (CPS) for illicit drug use during pregnancy, where perinatal care is available at no cost and economic assistance provides a variety of monetary benefits for substance dependent mothers. Exploring these differences will improve our understanding of maternal substance use, the psychosocial issues surrounding mothers who continue to use MA during their pregnancy, and the factors that are associated with co-morbid psychopathology and SUDs.
The specific aims of this investigation were: 1) to describe and compare the psychosocial characteristics of women who use MA during pregnancy in the US and NZ; 2) to examine the associations between prenatal MA use and the probabilities of a SUD and a psychiatric disorder in the US and NZ, holding constant other drug use and lower socioeconomic status (SES); 3) to evaluate the risk of co-morbid SUD and psychiatric disorders in US and NZ mothers.
2. Method
2.1 Study Design
Recruitment for the US study took place post-partum in seven hospitals at four sites in the US (Los Angeles, CA; Honolulu, HI; Tulsa, OK; and Des Moines, IA). Meconium specimens were collected from all infants for analysis of drug metabolites. Institutional Review Boards at all participating sites in the US approved the study. A NIDA Certificate of Confidentiality was obtained to ensure confidentiality was maintained for information regarding participants’ drug use, superseding mandatory reporting of substance use, but not evidence of child abuse or neglect. Mothers were approached postpartum to explain the study, and if interested and eligible, written informed consent was obtained, followed by detailed interviews of maternal drug use with the Substance Use Inventory, an interview that elicits retrospective reports of frequency and quantity of drug and alcohol use (Della Grotta et al., 2009). Socio-demographic information was obtained with the Lifestyle Interview (Della Grotta et al., 2009; Lester et al., 2002; Shankaran et al., 2004). Complete details of recruitment are reported elsewhere (Arria et al., 2006; Della Grotta et al., 2009).
In NZ, recruitment was conducted through referrals from maternity services at participating hospitals and through independent midwife practices. These referrals were screened prior to birth to determine if the mother met the study criteria. If the mother agreed, the study staff met with her to explain the study in detail and obtain written consent to participate. NZ study staff met with the mother again post-partum prior to discharge to review the study protocol, affirm consent, collect meconium from all infants and obtain substance use and lifestyle data consistent with the US protocol.
Approval to carry out this study in NZ was obtained from the following: Auckland and Waitemata District Health Boards (DHBs) and their Maori ethics committees, and the Ministry of Health’s Northern Regional Ethics Committee. There are no mandatory reporting laws for substance use during pregnancy in NZ, but confidentiality around maternal substance use was established according to the NZ Ministry of Health Ethics Committee guidelines. Consistent with the Ministry’s and US policies, any evidence of child abuse or neglect would require referral.
Mothers were excluded from participating in the study for the following: prenatal use of hallucinogens; history of intellectual disability; overt psychotic behavior or a documented history of psychosis; non-English speaking (except Maori in NZ); multiple gestation; newborn critically ill at birth or born with chromosomal disorder; previous child already enrolled in the study. Mothers in the US were excluded if they were < 18 at the infant’s birth. As the age of majority is 16 in NZ the exclusion age was reduced to < 17.5. MA-using mothers who also used cocaine in the US (n=17) or opiates in NZ (n=15) were accepted in the study as there was concern that these patterns of polydrug use were common among MA users in the respective countries.
Group designation (MA vs. comparison) was assigned using the following criteria: 1) MA group, either the mother self-reported any MA use during the current pregnancy or MA was detected in her infant’s meconium by gas chromatography-mass spectrometry (GCMS) or both; 2) comparison group assignment required both maternal denial of MA use during pregnancy and a negative meconium result for MA or other stimulants. Comparison mothers were also excluded if their infant’s meconium screened positive for cocaine or opiates or if they reported use of cocaine or opiates. US and NZ meconium samples were shipped to the United States Drug Testing Laboratory in Des Plaines, Illinois for analysis of the amphetamines, cocaine, cannabinoids, opiates and nicotine metabolites. Details of toxicology assays are reported elsewhere (Arria et al., 2006; Della Grotta et al., 2009; Gray et al., 2009).
Eligible MA-using and comparison mothers were matched on their infant’s birth weight (<1500 g; 1500–2500 g; >2500 g), self-identified ethnicity, adequate level of education (US: high school completed vs. not, NZ: 5th form certificate achieved or not or the equivalent according to the National Certificate of Educational Achievement [NCEA] in NZ); private vs. public health insurance (US only). Due to the referral process required in NZ, matching was geared to achieving equivalent ethnicity between the MA and comparison groups. The total number of mothers enrolled in the study was 633. Of this total, 412 were US mothers (MA = 204; comparison = 208) and 221 were NZ mothers (MA=111; comparison = 110).
2.2 Participants
All participants were biological mothers of infants enrolled in US and NZ IDEAL and available for follow-up at 1-month post-partum. The 1-month visit was missed by 27 US mothers and 11 NZ mothers. Further, the primary caregiver of the infant at 1-month was not the biological mother in 65 US families (MA=62) and 3 NZ families (MA=3). Newborn custody changes in the US were due in large part to mandatory reporting of prenatal drug and alcohol use by hospital staff or prior CPS referral. Thus, the final study sample in the US was 320 (MA=127; comparison=193) from the four US sites and in NZ was 207 (MA=97; comparison=110).
2.3 Study Procedures
The extent of problems related to maternal substance use and psychopathology was obtained at 1-month postpartum by trained interviewers. The Substance Abuse Subtle Screening Inventory—3 (SASSI-3) was used to measure problems related to substance abuse and determine the probability of a mother having a SUD (Lazowski et al., 1998). The SASSI-3 has 83 items and is divided into 2 parts. One part uses a true/false scale that produces 7 subscales. Six of those scales are used to describe characteristics associated with substance dependency including Obvious Attributes (OAT); Subtle Attributes (SAT); Defensiveness (DEF); Supplemental Addiction Measure (SAM); Family versus Control Subjects (FAM); Correctional (COR) and one scale identifies serious substance misuse and being part of a family system affected by addictions (Symptoms of Substance Misuse (SYM)). Part two uses a 4-point Likert Scale to answer questions on 2 face valid scales related to how an individual’s alcohol (Face Valid Alcohol (FVA), 12 questions) and other drug use (Face Valid Other Drug (FVOD), 14 questions) impacts their quality of life. Meeting criteria on one or more of these scales identifies mothers with a high probability of a SUD (Miller and Lazowski, 1999).
The Beck Depression Inventory-II (BDI-II), a 21-item self-report questionnaire, was used to obtain the extent of current depressive symptoms. Mothers were asked to rate the intensity of their symptoms on a 4-point Likert scale, from which a summary score of depression was computed (Beck and Steer, 1987).
The Brief Symptom Inventory (BSI), a 53-item questionnaire, is a screening tool used to measure the extent of current psychiatric symptoms. Mothers were asked to rate their psychological distress using a 5-point Likert scale ranging from “not at all” [0] to “extremely” (Derogatis, 1992; Derogatis and Melisaratos, 1983). In this article we report on the 9 primary symptom dimensions of the BSI and two composite scales. Primary symptom dimensions include: Somatization, Obsessive-Compulsive, Interpersonal Sensitivity, Depression, Anxiety, Hostility, Phobic Anxiety, Paranoid Ideation and Psychoticism. The two composite scales include: 1) the Global Severity Index (GSI) which is based on the number and severity of symptoms, and 2) the presumptive positive diagnosis of psychiatric disorders. The following two criteria have been established as a procedure for determining a positive diagnosis for a psychiatric disorder based on adult, non-patient norms: 1) a T-score of 63 or greater on the GSI, or 2) T-scores of 63 or greater on any two primary symptom dimensions (Derogatis, 1992). For brevity, we will refer to this composite measure as a positive psychiatric diagnosis. No clinical diagnostic interview was conducted.
2.4 Statistical Analysis
One-way analysis of variance (ANOVA) and Chi-square statistics were used to compare MA using and comparison mothers in each country on demographic characteristics, obstetric history and prenatal drug use. The summary scores for problems related to substance abuse, symptoms of depression and psychopathology were tested for group effects (MA vs. comparison group) in each country using general linear models (GLM). These analyses were adjusted for the following a priori covariates: continuous measures of SES and prenatal drug use quantity per day: alcohol in ounces; tobacco in cigarettes; and marijuana in joints. For alcohol, standard drinks were converted to absolute alcohol ounces based on each country’s conventions. SES was measured by the Hollingshead four-factor index adapted to single parent and non-nuclear families. The continuous measure which was used in analysis was calculated as a weighted sum of the mother’s level of education and occupation averaged with similar measures of a contributing adult (if any; Hollingshead, 1975; LaGasse et al., 1999). For presentation purposes, the categorical low SES (Hollingshead V) was reported in Table 1. Tables 2 and 3 report adjusted means (SD) and N (%).
Table 1.
Comparison of maternal characteristics and other drug use between MA-using mothers and comparison mothers in the US and NZ.
| N (%) or Mean (SD) | N (%) or Mean (SD) | |||||
|---|---|---|---|---|---|---|
| US Mothers | NZ Mothers | |||||
|
| ||||||
| MA Group (N = 127) | Comparison (N = 193) | P - value | MA Group (N = 97) | Comparison (N = 110) | P - value | |
| Maternal Characteristics | ||||||
| Race/Ethnicity | .878 | .334 | ||||
| US or NZ European | 52 (41%) | 77 (40%) | 57 (59%) | 51 (46%) | ||
| Maori | 30 (31%) | 41 (37%) | ||||
| Hawaiian/Pacific Islands | 23 (18%) | 33 (17%) | 7 (7%) | 14 (13%) | ||
| Hispanic | 31 (24%) | 41 (21%) | ||||
| Asian | 12 (9%) | 26 (14%) | 3 (3%) | 3 (3%) | ||
| Black | 6 (5%) | 12 (6%) | ||||
| American Indian | 3 (2%) | 4 (2%) | ||||
| Indian-Pakistani | 0 | 1 (0.9%) | ||||
| Low SES (Hollingshead V) | 37 (29%) | 22 (12%) | <.001 | 46 (48%) | 20 (18%) | <.001 |
| Monthly Income | US$585 (753) | US$925 (906) | .001 | NZ$1736 (2250) | NZ$1681 (1394) | .833 |
| Partner Status – No Partner | 65 (51%) | 66 (34%) | .003 | 51 (53%) | 29 (26%) | <.001 |
| Education < high school (US)/< 5th form certificate (NZ) or equivalent | 49 (39%) | 72 (38%) | .803 | 59 (62%) | 55 (50%) | .082 |
| Maternal age, yr | 25.73 (5.76) | 24.55 (5.54) | .068 | 26.68 (6.17) | 25.44 (6.85) | .174 |
| Gestation at 1st prenatal visit, wk | 13.30 (7.61) | 9.44 (5.70) | <.001 | 15.65 (6.55) | 13.25 (5.51) | .006 |
| Number of prenatal visits | 12.91 (7.28) | 14.16 (5.50) | .085 | 16.03 (6.79) | 16.99 (5.59) | .271 |
| Other Drug Use During Pregnancy | ||||||
| Tobacco | 100 (79%) | 51 (26%) | .000 | 84 (87%) | 58 (53%) | .000 |
| Number cigarettes smoked/day | 6.86 (8.76) | 1.58 (4.52) | .000 | 8.52 (7.25) | 3.25 (5.46) | .000 |
| Alcohol | 56 (44%) | 25 (13%) | .000 | 61 (63%) | 61 (56%) | .322 |
| Ounces absolute alcohol/day | .128 (.55) | .003 (.02) | .000 | .328 (.83) | 118 (.28) | .165 |
| Marijuana | 43 (34%) | 7 (4%) | .000 | 61 (63%) | 23 (21%) | .000 |
| Number joints smoked/day | .10 (.28) | .01 (.09) | .000 | .43 (.96) | .17 (.64) | .000 |
Table 2.
Comparison of maternal risk for substance dependence (SASSI—3) between MA-using and comparison mothers in the US and NZ.
| N (%) or Mean (SD) | N (%) or Mean (SD) | |||||
|---|---|---|---|---|---|---|
| US Mothers | NZ Mothers | |||||
|
| ||||||
| SASSI—3 Scales | MA Group N = 126 |
Comparison N = 193 |
* Adjusted P Value | MA Group N = 91 |
Comparison N = 107 |
* Adjusted P Value |
| Face valid – Alcohol (FAV) | 3.13 (5.86) | .33 (1.02) | .000 | 3.37 (6.40) | 2.07 (4.66) | .898 |
| Face valid - Other drug (FVOD) | 14.85 (11.45) | .27 (1.49) | .000 | 11.44 (9.73) | 1.26 (3.33) | .000 |
| Symptoms of substance misuse (SYM) | 6.09 (2.47) | 2.53 (2.38) | .000 | 6.44 (2.36) | 3.54 (2.60) | .000 |
| Obvious attributes (OAT) | 5.91 (2.33) | 3.30 (2.12) | .000 | 6.51 (2.25) | 3.80 (2.34) | .000 |
| Subtle attributes (SAT) | 3.49 (1.48) | 2.39 (1.10) | .000 | 3.90 (1.37) | 2.50 (1.27) | .000 |
| Defensiveness (DEF) | 4.48 (2.01) | 5.74 (1.85) | .000 | 4.01 (1.90) | 5.29 (2.18) | .025 |
| Supplemental addiction measure (SAM) | 8.10 (2.01) | 5.39 (1.96) | .000 | 8.04 (1.69) | 5.45 (2.08) | .000 |
| Family vs. controls subjects (FAM) | 5.91 (1.79) | 7.66 (1.61) | .000 | 5.36 (1.72) | 6.89 (1.78) | .000 |
| Correctional (COR) | 6.76 (2.64) | 3.59 (2.39) | .000 | 8.27 (2.97) | 4.81 (2.65) | .000 |
| High probability of SUD | 89 (70.6%) | 18 (9.3%) | .000 | 65 (71.4%) | 22 (20.6%) | .000 |
GLM adjusted for continuous measures of SES, tobacco, alcohol and marijuana.
N varies due to missing data.
Table 3.
Comparison of psychopathology (BDI and BSI) between MA-using and comparison mothers in the US and NZ.
| N (%) or Mean (SD) | N (%) or Mean (SD) | |||||
|---|---|---|---|---|---|---|
| US Mothers | NZ Mothers | |||||
|
| ||||||
| MA Group N = 126 |
Comparison N = 193 |
* Adjusted P Value | MA Group N = 93 |
Comparison N = 107 |
* Adjusted P Value | |
| BDI Depression Total | 13.29 (9.51) | 9.96 (6.57) | .017 | 12.35 (9.25) | 9.68 (9.25) | .736 |
| BSI Scale | ||||||
| Somatization | .43 (.46) | .34 (.47) | .296 | .46 (.58) | .30 (.58) | .598 |
| Obsessive Compulsive | .93 (.85) | .78 (.74) | .382 | .94 (.80) | .69 (.80) | .208 |
| Interpersonal Sensitivity | .63 (.72) | .54 (.67) | .945 | .71 (.90) | .34 (.90) | .029 |
| Depression | .56 (.67) | .37 (.53) | .067 | .57 (.77) | .25 (.77) | .023 |
| Anxiety | .43 (.54) | .35 (.48) | .760 | .41 (.62) | .22 (.62) | .179 |
| Hostility | .62 (.62) | .48 (.60) | .393 | .65 (.75) | .44 (.75) | .639 |
| Phobic Anxiety | .36 (.63) | .27 (.48) | .989 | .43 (.72) | .21 (.46) | .210 |
| Paranoid Ideation | .83 (.73) | .52 (.70) | .023 | .82 (.90) | .36 (.54) | .015 |
| Psychoticism | .51 (.64) | .30 (.48) | .086 | .48 (.74) | .18 (.36) | .089 |
| Global Severity Index | .59 (.50) | .44 (.46) | .210 | .61 (.64) | .34 (.64) | .066 |
| Positive Psychiatric Diagnosis | 60 (48%) | 51 (26%) | .002 | 40 (43%) | 23 (22%) | .026 |
GLM adjusted for continuous measures of SES, tobacco, alcohol and marijuana.
N varies due to missing data.
Logistic regression analysis was used to test the following across the US and NZ cohorts: 1) the relationship between maternal MA use and a high probability of a SUD; 2) the relationship between MA use during pregnancy and a positive psychiatric diagnosis; 3) the relationship between MA use and a positive psychiatric diagnosis and the heightened risk of a SUD. Significant interactions involving country were examined by separate analyses of each country. Significance was accepted at p < .05.
3. Results
3.1 Maternal demographics and characteristics
As a result of the matching protocol there were no significant differences between groups in self-identified ethnicity or adequate level of education in either NZ or the US. The predominant ethnicity reported in both the US (40%) and NZ (52%) sample was white (Table 1). Across the US and NZ, mothers in the MA group were more likely to have low SES, live in a single parent household and more likely to present for their first prenatal visit later in pregnancy than comparison mothers. In the US, mothers in the MA-group had less monthly income than comparison mothers, but in NZ, there were no differences in monthly income between groups.
3.2 Maternal drug use and extent of substance abuse problems
A substantial proportion of NZ women in the MA group reported using a range of ATS in addition to MA including, amphetamine (14.6%) and Ecstasy (18.8%,) whereas only a small proportion of US women reported using other ATS (4.8% and 2.4%, respectively). In addition, over 35% of NZ mothers reported using MA in all three trimesters compared to only 15% in the US. In the US, 11 mothers who used MA also used cocaine. In NZ, 13 mothers who used MA also used one or more opiates including heroin (n = 1), methadone (n = 6), MISTI (n = 3), other opiates [e.g., codeine, Vicodin] (N = 8).
Mothers in the US and NZ who used MA were significantly more likely to report the use of marijuana and cigarettes during pregnancy and at higher levels than comparison mothers (Table 1). A similar pattern of alcohol use was reported in the US. In NZ, there were no differences in alcohol consumption between the mothers who used MA during pregnancy and comparison mothers. Overall, a higher proportion of NZ mothers reported using tobacco (US 151 [47%] versus NZ 142 [69%], p<.001), alcohol (US 81 [25%] versus NZ 122 [59%], p<.001, and marijuana (US 50 [16%] versus NZ 84 [41%], p<.001, during pregnancy than US mothers. Prenatal use of hashish, benzodiazepines and barbiturates were rare (<5% in US and NZ).
Psychosocial problems related to MA use are reported in Table 2 (SASSI-3). In both the US and NZ, mothers who used MA had higher mean scores than comparison mothers on 5 scales; OAT; SAT; SAM (differentiates defensiveness related to substance dependence); COR (extensive histories of legal problems) and SYM (symptoms of substance misuse). Similarly, mothers in the MA group in the US and NZ had lower mean scores on two subscales, DEF (lower scores: unwillingness to acknowledge any personal issues and faults) and FAM (lower scores: more likely to have significant others who misuse substances). The mean scores on the FVOD scale that directly measures other drug use were also higher in the MA groups in both countries than in the comparison groups. A higher score on the FVA scale (alcohol use) was also found in the US MA group versus the US comparison group, however, there was no significant difference between the MA and comparison groups in the NZ cohort. Overall, a larger proportion of women who used MA during their pregnancy in both the US (71%) and NZ (71%) sample were determined to have a high probability of developing a SUD. Notable was the finding that 21% of NZ comparison mothers also met criteria for a SUD versus 10% of US mothers (p =.006).
Similar results were shown by final logistic regression analysis testing the association between maternal MA use and the probability of a SUD across the US and NZ sample. Mothers who used MA during pregnancy were 10 times more likely (OR 10.4, CI 6.5–16.7)) to meet criteria for a SUD than comparison mothers. There were no effects for quantities of alcohol, marijuana or country. However, tobacco quantity was associated with a heightened risk of having a SUD (OR 1.09, CI 1.05–1.13). There were no interactions with country.
3.3 Maternal Psychopathology
In the US, mothers in the MA group reported more symptoms of depression on the BDI-II than comparison mothers, but there were no differences between groups in NZ (Table 3). Further, in both countries, mothers in the MA group had higher scores for paranoid ideation. In NZ only, mothers in the MA group also reported increased scores on the BSI for interpersonal sensitivity and depression. Although the GSI was not different between the MA and comparison groups in either the US or NZ, a significantly higher proportion of MA using women in the US (47.6%) and NZ (43.0%) had a positive psychiatric diagnosis than comparison mothers in the US (26.4%) and NZ (21%), respectively.
Similar findings resulted from logistic regression analyses testing the association between prenatal MA use and increased likelihood of a positive psychiatric diagnosis. Mothers in the MA group were twice as likely to have a positive psychiatric diagnosis (Table 4). An effect of country and a country by alcohol quantity interaction independent of MA exposure was also associated with a positive psychiatric diagnosis. Separate logistic regression analyses for the US and NZ samples found mothers who used MA during their pregnancy were more likely to have a positive psychiatric diagnosis. The amount of alcohol used during pregnancy also increased the odds of having a positive psychiatric diagnosis in NZ mothers but not US mothers.
Table 4.
Summary of the logistic regression models for the association of MA use and a positive diagnosis for a psychiatric disorder
| Combined US and NZ Model | Odds Ratio (OR) | 95% CI |
|---|---|---|
| MA use during pregnancy (Yes/No) | 2.34 | 1.54 – 3.58 |
| Country | .62 | .41 – .95 |
| SES | .99 | .97 – 1.01 |
| Marijuana use (joints/day) | .82 | .53 – 1.26 |
| Alcohol (drinks/day) | .19 | .02 – 1.58 |
| Tobacco (cigarettes/day) | 1.02 | .98 – 1.05 |
| Country by alcohol interaction | 14.09 | 1.49 – 133.50 |
|
| ||
| US Model | Odds Ratio (OR) | 95% CI |
|
| ||
| MA use during pregnancy (Yes/No) | 2.37 | 1.37 – 4.07 |
| SES | .99 | .96 – 1.01 |
| Marijuana use (joints/day) | 1.44 | .37 – 5.67 |
| Alcohol (drinks/day) | .14 | .02 – 1.33 |
| Tobacco (cigarettes/day) | 1.01 | .978 – 1.05 |
|
| ||
| NZ Model | Odds Ratio (OR) | 95% CI |
|
| ||
| MA use during pregnancy (Yes/No) | 2.19 | 1.10 – 4.36 |
| SES | .99 | .96 – 1.02 |
| Marijuana use (joints/day) | .78 | .49 – 1.23 |
| Alcohol (drinks/day) | 2.75 | 1.24 – 6.13 |
| Tobacco (cigarettes/day) | 1.01 | .97 – 1.07 |
3.4 Co-morbid SUD and other psychiatric disorders
Having a positive psychiatric diagnosis significantly increased the odds (OR 2.67, CI 1.63–4.35) of having a high probability of a SUD. Increased odds were also found for MA use during pregnancy (OR 9.75, CI 6.04–15.75), but not for country, SES, alcohol or marijuana quantity. Tobacco quantity was associated with increased odds (OR 1.09, CI 1.05–1.14). Further analysis revealed a significant interaction of country by a positive psychiatric diagnosis (OR 3.37, CI 1.16–9.96). Separate logistic regression analyses for the US and NZ samples showed that having a positive psychiatric diagnosis co-morbid with a SUD was observed only in NZ (OR 5.55, CI 2.46–12.52), but not in the US (OR 1.73, CI .897–3.32). The effect of MA use and a SUD was maintained in both countries.
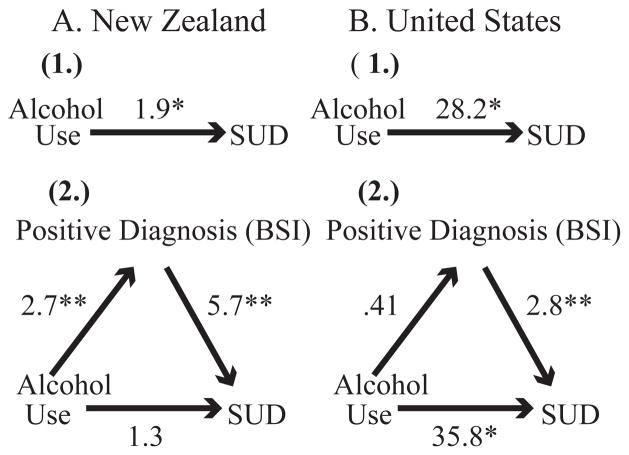
We conducted secondary analyses to explore why mothers in NZ were more likely than US mothers to have a positive psychiatric diagnosis co-morbid with a SUD. Previous findings showed that mothers in the US and NZ had similar rates of a positive psychiatric diagnosis and similar rates of SUDs in the MA group. However, mothers in the comparison group in NZ were twice as likely to have a SUD than US comparison mothers. We also noted that the amount of prenatal alcohol consumed was associated with a positive psychiatric diagnosis in NZ but not in the US. We tested the possibility that a positive psychiatric diagnosis could mediate the relationship between alcohol quantity and diagnosis of a SUD. We conducted mediation analysis using established procedures (Mackinnon and Dwyer, 1993; Sobel, 1982). In both countries, quantity of prenatal alcohol was significantly associated with a SUD (Figure 1). Having a positive psychiatric diagnosis was only a significant mediator between quantity of alcohol and diagnosis of a SUD in NZ (Sobel Z=2.51, p=.01) not in the US (Sobel Z=−0.95, p=.34) These findings suggest that prenatal alcohol quantity and a positive psychiatric diagnosis are strongly related in NZ and may in part support the observed comorbidity in NZ.
Figure 1.
Mediation effects of a positive diagnosis of a psychiatric disorder for the relationship between ounces of absolute alcohol and SUD in NZ. (1) shows the direct relationship between ounces of alcohol and SUD. (2) shows the relationship between ounces of alcohol and a positive diagnosis of a psychiatric disorder, as well as between ounces of alcohol and SUD, adjusting for a positive diagnosis. B. Similar mediation effects in the US. Numbers presented are odds ratios from logistic regression models. *P <.05, **P <.01. The Sobel test was used to examine the significance of the mediated pathway in each country. NZ (Sobel Z = 2.51, p = .01), US (Sobel Z = −0.95, p =.34)
4. DISCUSSION
Despite the distinct differences between the US and NZ health, legal and welfare systems in relation to maternal drug use, MA use during pregnancy was associated with the abuse of alcohol and other drugs and a complex array of family, social, relationship, legal, and psychiatric problems. These findings support and reinforce earlier findings from the US-IDEAL Study (Derauf et al., 2007) as well as other research that has shown that women who use drugs during pregnancy have lifestyles that are burdened by multiple adverse psychosocial circumstances that are likely to impair their ability to parent their new born child (Benningfield et al., 2010; Good et al., 2010; Oei et al., 2010; Terplan et al., 2009; Wouldes and Woodward, 2010). However, the current findings provide the first evidence that mothers recruited from community populations in two different cultures who reported using MA during pregnancy were also more likely to have multiple psychosocial problems.
An important question raised by these findings concerns the extent to which MA use during pregnancy heightens the risk of having a SUD, a positive diagnosis for a psychiatric disorder or the co-occurrence of both. In this study we were able to control the associations between MA use during pregnancy and these outcomes for a number of prospectively measured covariates that included other prenatal drug use and socioeconomic status. These analyses revealed the following conclusions about the extent of substance dependence and psychopathology at one-month post-partum.
First, consistent with the earlier findings of Derauf et al. (2007) that found MA use during pregnancy increased the risk of having a SUD by 12-fold, the combined findings from the US and NZ-IDEAL studies showed a 10-fold increase for a SUD. Second, mothers who used MA in both the US and NZ were over twice as likely have a positive diagnosis for a psychiatric disorder than comparison mothers. Derauf et al. (2007) failed to find an association between MA use during pregnancy and measures of psychopathology in their preliminary study. One explanation for this difference may be the increased sample size in the present study, which might have yielded the statistical power to detect a difference. However, the current findings do reinforce reports from studies of clinical populations where the risk for psychiatric problems was heightened in women MA users who were in prison or seeking treatment for MA dependence (Christian et al., 2007; Hser et al., 2004; Terplan et al., 2009; Vik, 2007), and in populations of women who reported continuing to use other psychoactive substances during pregnancy (Benningfield et al., 2010; Good et al., 2010; Oei et al., 2010; Wouldes and Woodward, 2010).
Finally, we found a significantly heightened risk (five-fold) for co-morbid SUD and a positive diagnosis for a psychiatric disorder in NZ mothers who used MA during pregnancy, but not US mothers. This disparity was likely the result of the quantity of alcohol consumed by NZ mothers leading to psychiatric distress, which in turn led to a higher probability of NZ mothers meeting criteria for a SUD. These findings are consistent with reports from investigators who have found a relationship between psychopathology and alcohol-use disorders (Swendsen et al., 2010), especially in women (Greenfield et al., 2010), in combination with MA (Chen et al., 2003) and with other stimulant dependence (Ford et al., 2009).
These findings should be interpreted in the context of potential methodological limitations. First, screening tools were used to ascertain the risk for psychiatric and substance use disorders rather than systematic, formal diagnoses. Therefore, the associations between MA use and the heightened risk of a SUD, a positive diagnosis for a psychiatric disorder and the comorbidity of these disorders should be taken as estimates. Furthermore, drug use was mostly obtained by self-report due in part to limitations in the meconium assay (Gray et al., 2009). Although education was part of SES as well as one of the group matching criteria, findings of lower SES in MA using mothers suggest that the overlap does not obscure evaluation of SES. Finally, this report did not include assessments of Axis II disorders which have been implicated in treatment failure, ongoing substance abuse and interfering with the developing relationship between the mother and child (Hans et al., 1999; Killeen et al., 1995; Newman et al., 2007).
5. CONCLUSION
Despite the above limitations, our study provides the first prospective evidence that MA use during pregnancy across two different cultures and in a diverse range of ethnicities is associated with a complex array of difficult social circumstances, psychiatric problems and multiple SUDs. Moreover, this is the first evidence that a significant proportion of mothers who used MA in combination with alcohol during pregnancy were at a heightened risk for co-morbidity of SUD and a positive diagnosis for a psychiatric disorder. In terms of clinical relevance, our findings underscore the importance of considering the following: (i) surveillance of MA, alcohol and other drug use during pregnancy; (ii) integration of psychiatric and social support services in prenatal and postnatal care where continued maternal drug use is identified. Healthcare organizations that do so may better identify the needs of mothers who are drug dependent and more effectively tailor early interventions.
Acknowledgments
Role of Funding Source
This work is part of the US and NZ Infant Development, Environment and Lifestyle Study funded by NIH grants: National Institutes on Drug Abuse, 2RO1DA014948 and RO1DA021757. NIH and/or NIDA had no further role in study design; in the collection, analysis and interpretation of date; in the writing of the manuscript; or in the decision to submit the paper for publication.
Footnotes
Contributors
Dr. Trecia Wouldes wrote the first draft of the manuscript. All authors are responsible for the reported research and have participated in the concept and design, analysis and interpretation of data, drafting or revising of the manuscript. In addition, all authors have approved the manuscript as submitted.
Conflict of Interest
None of the authors have a financial association with any product associated with this research or other conflict of interest. There were no commercial sponsors involved in the above matters. There was no honorarium, grant, or other form of payment given to anyone to produce this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arria AM, Derauf C, LaGasse LL, Grant P, Shah R, Smith L, Haning W, Huestis M, Strauss A, Della Grotta S, Liu J, Lester BM. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Maternal Child Health J. 2006;10:293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Benningfield MM, Arria AM, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Fischer G, Jones HE, Martin PR. Co-occurring psychiatric symptoms are associated with increased psychological, social and medical impairment in opioid dependent pregnant women. Am J Addict. 2010;19:416–421. doi: 10.1111/j.1521-0391.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC, Chian YL, Ree SC, Lee CH, Murray RM. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychol Med. 2003;33:1407–1414. doi: 10.1017/s0033291703008353. [DOI] [PubMed] [Google Scholar]
- Christian DR, Huber A, Brecht ML, McCann M, Marinelli-Casey P, Lord R, Reiber C, Lu TH The Methamphetamine Project Galloway G.P. Methamphetamine users entering treatment: characteristics of the methamphetamine treatment project sample. Subst Use Misuse. 2007;42:2207–2222. doi: 10.1080/10826080701209341. [DOI] [PubMed] [Google Scholar]
- Cohen JB, Greenberg R, Uri J, Halpin M, Zweben J. Women with methamphetamine dependence: research on etiology and treatment. J Psychoactive Drugs SARC Suppl. 2007;4:347–351. doi: 10.1080/02791072.2007.10399896. [DOI] [PubMed] [Google Scholar]
- Della Grotta S, LaGasse LL, Arria A, Derauf C, Grant P, Smith LM, Shah R, Huestis M, Liu J, Lester BM. Patterns of methamphetamine use during pregnancy: results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Maternal Child Health J. 2009;14:519–527. doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, LaGasse LL, Smith LM, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, Liu J, Lester BM. Demographic and psychosocial characteristics of mothers using methamphetamine during pregnancy: preliminary results of the Infant Development, Environment, and Lifestyle Study (IDEAL) Am J Drug Alcohol Abuse. 2007;33:281–289. doi: 10.1080/00952990601175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. BSI: Administration, Scoring, and Procedures Manual--II. Clinical Psychometric Research Towson; MD: 1992. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- Ford JD, Gelernter J, DeVoe JS, Zhang W, Weiss RD, Brady K, Farrer L, Kranzler HR. Association of psychiatric and substance use disorder comorbidity with cocaine dependence severity and treatment utilization in cocaine-dependent individuals. Drug Alcohol Depend. 2009;99:193–203. doi: 10.1016/j.drugalcdep.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good MM, Solt I, Acuna JG, Rotmensch S, Kim MJ. Methamphetamine use during pregnancy. Obstet Gynecol. 2010;116:330–334. doi: 10.1097/AOG.0b013e3181e67094. [DOI] [PubMed] [Google Scholar]
- Gray TR, LaGasse LL, Smith LM, Derauf C, Grant P, Shah R, Arria AM, DellaGrotta S, Strauss A, Haning W, Lester BM, Huestis M. Identification of prenatal amphetamines exposure by maternal interview and meconium toxicology on the Infant Development, Environment and Lifestyle (IDEAL) Study. Drug Monit. 2009;1231:769–775. doi: 10.1097/FTD.0b013e3181bb438e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Hando J, Darke S, Ross J. Psychological morbidity and route of administration among amphetamine users in Sydney, Australia. Addiction. 1996;91:81–87. doi: 10.1046/j.1360-0443.1996.9118110.x. [DOI] [PubMed] [Google Scholar]
- Hans SL, Bernstein VJ, Henson LG. The role of psychopathology in the parenting of drug-dependent women. Dev Psychopathol. 1999;11:957–977. doi: 10.1017/s0954579499002400. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. Yale University, Department of Sociology; New Haven: 1975. [Google Scholar]
- Hser YI, Evans E, Huang YC. Treatment outcomes among women and men methamphetamine abusers in California. J Subst Abuse Treat. 2004;28:77–85. doi: 10.1016/j.jsat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Jones HE, Browne FA, Myers BJ, Carney T, Ellerson RM, Kline TL, Poulton W, Zule WA, Wechsberg WM. Pregnant and nonpregnant women in Cape Town, South Africa: drug use, sexual behavior, and the need for comprehensive services. Int J Pediatr. 2011 doi: 10.1155/2011/353410. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen TK, Brady KT, Thevos A. Addiction severity, psychopathology and treatment compliance in cocaine-dependent mothers. J Addict Dis. 1995;14:75–84. doi: 10.1300/J069v14n01_08. [DOI] [PubMed] [Google Scholar]
- LaGasse LL, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, Shankaran S, Bada H, Smeriglio V. The Maternal Lifestyle Study (MLS): the caretaking environment of infants exposed to cocaine/opiates. Pediatr Res. 1999;45(4) Part 2. [Google Scholar]
- Lazowski LE, Miller FG, Boye MW, Miller GA. Efficacy of the Substance Abuse Subtle Screening Inventory--3 (SASSI-3) in identifying substance dependence disorders in clinical settings. J Person Assess. 1998;71:114–128. doi: 10.1207/s15327752jpa7101_8. [DOI] [PubMed] [Google Scholar]
- Lester BM, Lagasse L, Seifer R, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Liu J, Finnegan LP, Maza PL. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Pediatr. 2002;142:279–285. doi: 10.1067/mpd.2003.112. [DOI] [PubMed] [Google Scholar]
- Mackinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev. 1993;17:144–158. [Google Scholar]
- McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101:1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Miller FG, Lazowski LE. The adult SASSI-3 manual. The SASSI Institue; Springville, IN: 1999. [Google Scholar]
- Nelson-Zlupko L, Kauffman E, Dore MM. Gender differences in drug addiction and treatment: implications for social work intervention with substance-abusing women. Soc Work. 1995;40:45–53. [PubMed] [Google Scholar]
- Newman LK, Stevenson CS, Bergman LR, Boyce P. Borderline personality disorder, mother-infant interaction and parenting perceptions: preliminary findings. Aust NZ J Psychiatr. 2007;41:598–605. doi: 10.1080/00048670701392833. [DOI] [PubMed] [Google Scholar]
- Oei J, Abdel-Latif ME, Clark R, Craig F, Lui K. Short-term outcomes of mothers and infants exposed to antenatal amphetamines. Arch Dis Child Fetal Neonatal Ed. 2010;95:F36–F41. doi: 10.1136/adc.2008.157305. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Das A, Bauer CR, Bada H, Lester BM, Wright LL, Smeriglio V. Association between patterns of maternal substance use and infant birth weight, length, and head circumference. Pediatrics. 2004;114:e226–234. doi: 10.1542/peds.114.2.e226. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhart S, editor. Sociological Methodology. Jossey-Bass; San Francisco: 1982. pp. 290–312. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-34, DHHS Publication No SMA 08–4343. Office of Applied Studies; Rockville, MD: 2008. Results from the 2008 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR, Sampson N, Kessler RC. Mental disorders as risk factors for substance use, abuse and dependence: results from the 10-year follow-up of the National Comorbidity Survey. Addiction. 2010;105:1117–1128. doi: 10.1111/j.1360-0443.2010.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report. United Nations Publication; Vienna, Austria: 2004. [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report. United Nations Office on Drugs and Crime; Vienna, Austria: 2007. [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2010. 2010. [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2011. 2011. [Google Scholar]
- Vik PW. Methamphetamine use by incarcerated women: comorbid mood and anxiety problems. Womens Health Issues. 2007;17:256–263. doi: 10.1016/j.whi.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Wan MW, Green J. The impact of maternal psychpathology on child--mother attachment. Arch Women’s Ment Health. 2009;12:123–134. doi: 10.1007/s00737-009-0066-5. [DOI] [PubMed] [Google Scholar]
- Wechsberg WM, Jones HE, Zule WA, Myers BJ, Browne FA, Kaufman MR, Luseno W, Flisher AJ, Parry CDH. Methamphetamine (“tik”) use and its association with condom use among out-of-school females in Cape Town, South Africa. Am J Drug Abuse. 2010;36:208–213. doi: 10.3109/00952990.2010.493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouldes TA, Woodward LJ. Maternal methadone dose during pregnancy and infant clinical outcome. Neurotoxicol Teratol. 2010;32:406–413. doi: 10.1016/j.ntt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Wright TE, Schuetter R, Fombonne E, Stephenson J, Hanning WF., III Implementation and evaluation of a harm-reduction model for clinical care of substance using pregnant women. Harm Reduction Journal. 2012:9. doi: 10.1186/1477-7517-9-5. http://ww.harmreductionjournal.com/contents/9/1/5. [DOI] [PMC free article] [PubMed]