Abstract
Purpose
To establish human retinal pigment epithelial (RPE) cell culture as a source for cell replacement therapy in ocular diseases.
Methods
Human cadaver globes were used to isolate RPE cells. Each globe was cut into several pieces of a few millimeters in size. After removing the sclera and choroid, remaining tissues were washed in phosphate buffer saline and RPE cells were isolated using dispase enzyme solution and cultured in Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12 supplemented with 10% fetal calf serum.
Results
Primary cultures of RPE cells were established and spheroid colonies related to progenitor/stem cells developed in a number of cultures. The colonies included purely pigmented or mixed pigmented and non-pigmented cells. After multiple cellular passages, several types of photoreceptors and neural-like cells were detected morphologically.
Conclusion
Cellular plasticity in RPE cell cultures revealed promising results in terms of generation of stem/progenitor cells from human RPE cells. Whether the spheroids and neural-like retinal cells were directly derived from retinal stem cells or offspring of trans-differentiating or de-differentiating RPE cells remains to be answered.
Keywords: Retinal Pigment Epithelium, Stem Cells
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of blindness in elderly subjects in developed countries; its prevalence has also increased in developing countries. Pathologic changes affect retinal pigment epithelial (RPE) cells, Bruch ’s membrane and the choriocapillaris.1 RPE cells play an important protective role for the neurosensory retina; photoreceptors in particular degenerate and die due to RPE dysfunction in patients with AMD.2
Despite the large volume of studies on AMD, the exact pathogenesis of the disease is not clearly known and therapeutic options remain limited. Many attempts have been made to halt the destructive process in the neurosensory retina and to reestablish normal function in the outer retina including RPE cells and Bruch ’s membrane. Currently available therapies include photodynamic therapy (PDT), laser therapy and anti-vascular endothelial growth factor (anti-VEGF) therapy.3 PDT en- tails adverse effects on normal tissues and laser therapy inevitably destroys the outer retina which may result in loss of central vision. Main drawbacks to anti-VEGF therapy are short half- life, intraocular route of administration and limited efficacy.4,5 These features have forced re- searchers to investigate other therapeutic modalities such as retinal cell transplantation for the treatment of end-stage retinal diseases.6 RPE cells are a main source for replacing destroyed cells.7,8 Many attempts have also been made to generate RPE cells from various sources of stem cells.9–11 The purpose of this study was to establish the role of primary culture of human RPE cells as a source of retinal cell replacement therapy.
METHODS
Isolation and Cultivation of RPE Cells
Human globes were obtained from cadavers earlier than 24 hours after death at the Eye Bank of I.R. Iran and sent to the biochemistry laboratory at the National Institute of Genetic Engineering and Biochemistry (NIGEB) for isolation of RPE cells. To prepare the globe for this purpose, the optic nerve was cut and peribulbar tissues were removed using scissors. The globe was emptied from its contents and the eurosensory retina was removed using a forceps. The inner surface of the globe was irrigated with phosphate buffered saline (PBS) and the RPE layer was separated by a forceps and cut into small sections. Thereafter, segments of the RPE layer were incubated for 90 minutes in 2 μ/ml dispase at 37°C. The mixture was then centrifuged for 5 min at 300 g and 4°C. The resulting cellular preparation from each globe was cultured in 25 cm2 flasks previously coated with fetal calf serum (FCS).
The culture media included Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12 (DMEM: F12), FCS (20% at the beginning of culture), penicillin (120 μg/ml), streptomycin (220 μg/ml), gentamicin (50 μg/ml) and amphotericin B (2.5 μg/ml). The medium was changed twice a week (DMEM: F12 supplemented with 10% FCS) until the cultures reached confluence which usually occurred by 2-3 weeks. Then the cultures were passaged using 3×105 cells per 25 cm2 culture flask. Confluent cultures from passages 3 to 7 were used for all experiments.
Immunocytochemistry
Immunocytochemistry (ICC) was performed on slides prepared from harvested RPE cells. Cultured RPE cells were first fixed by paraformaldehyde 4% in PBS (V/V) for 20 minutes at room temperature. The cells were made permeable using Teriton x–100 (0.25%) and cultures were blocked in 1% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature. Rabbit anti-human RPE 65 polyclonal antibody (1:1000; Santa Cruz, USA) against the specific protein of RPE microsomal membranes was used to confirm the RPE characteristics of isolated cells. Cytokeratines are intermediate keratin filaments present in the cytoskeleton of pithelial cells, therefore it was essential to check PRE cells for existence of these filaments. We utilized specific murine anti-human cytokeratine 8/18 monoclonal antibody (1:1000;Santa Cruz, USA) for reconfirmation of RPE characteristics of isolated cells. To identify the origin of the cells generated within the spheroid colonies, we examined the cultures with rabbit polyclonal anti-human Nestin, goat polyclonal anti-human Pax-6 and goat polyclonal anti-human Chx-10 (1:100; Santa Cruz, USA) antibodies.
Fluorescein isothiocyanate (FITC) conjugated antibody (goat anti-mouse IgG, goat antirabbit IgG and donkey anti-goat IgG, Santa Cruz, USA) against the above-mentioned antibodies were diluted (1:400 to 1:1000) and used to detect immunoreactivity of cultures for primary antibodies. After final washing, the slides were incubated in 4,6-diamidino-2-phenyindole dihydrochloride (DAPI, 1 mg/ml, Santa Cruz, USA) for 10 minutes to counter-stain nuclear DNA. The slides were examined by Axiophot Zeiss fluorescence microscope (Zeiss, Germany) equipped with a 460 nm filter for DAPI dye and a 520 nm filter for fluorescein isothiocyanate (FITC) conjugated antibodies.
RNA Extraction
RNA extraction from RPE cells was performed using Tripure RNA isolation reagent (Roche, Germany) according to the manufacturer’s protocol. cDNA pools were made using a reverse primer and superscript III reverse transcriptase (Invitrogen, Belgium). Resulting cDNAs were amplified using iQ SYBER Green supermix kit (BioRad, Italy).
Quantitative Real-Time RT-PCR
Real-time PCR was performed to assess the expression of VEGF and pigment epithelium derived factor (PEDF) genes in harvested RPE cells. Since RPE cells are the only source of production and secretion of VEGF and PEDF in the human eye; detection of their expression in isolated cells would be a valuable marker for confirming the presence of RPE in cultures. The length of the amplicon was 141 base-pairs for VEGF and 342 base-pairs for PEDF. The primer sequences were as follows:
VEGF forward:
5´ACTTCTCTGGGCTGTTCTCG3´
VEGF reverse:
5´TCCTCTTCCTTCTCTCTTCC3´
PEDF forward:
5´TGCAGGCCCAGATGAAAGGG3´
PEDF reverse:
5´TGAACTCAGAGGTGAGGCTC3´
Specific primers for glyceraldehyde phosphate dehydrogenase (GAPDH) were used as intracellular controls beside the above mentioned genes. The length of this amplicon was 219 base-pairs and the primer sequences were:
GAPDH forward:
5´GCAGGGGGGAGCCAAAAGGGT3´
GAPDH reverse:
5´TGGGTGGCAGTGATGGCATGG3´
The PCR thermal profile was as follows:
Cycle 1:(1X)
Step 1:95.0°C for 5 minutes
Cycle 2:(35X)
Step 1:95.0°C for 1 minute
Step 2:61.5°C for 1 minute
Step 3:72.0°C for 1 minute
Step 4:80.0°C for 10 seconds
To draw the melting curve and confirm the precision of the reactions, the following steps were performed:
Cycle 3:(1X)
Step 1:95.0°C for 1 minute
Cycle 4:(1X)
Step 1:65.0°C for 1 minute
Cycle 5:(60X)
Step 1:65.0°C for 10 seconds
At this step, the temperature of the system was increased 0.5°C every 10 seconds until a final temperature of 95°C was reached.
For amplification of VEGF cDNA the above-mentioned protocol was performed at annealing temperature of 61.5°C. Amplification of PEDF cDNA and GAPDH cDNA was performed in a similar fashion with annealing temperature of 53.8°C and 62.8°C, respectively.
RESULTS
Cultivation of RPE Cells
Early stages of RPE cell culture were successful (fig. 1) and spheroid colonies pertaining to stem cells/progenitor cells were observed in the cultures (fig. 1D). When RPE cells were obtained from a fetus or newborn, as compared to those obtained from adult globes, the spheroids appeared earlier and in greater number.
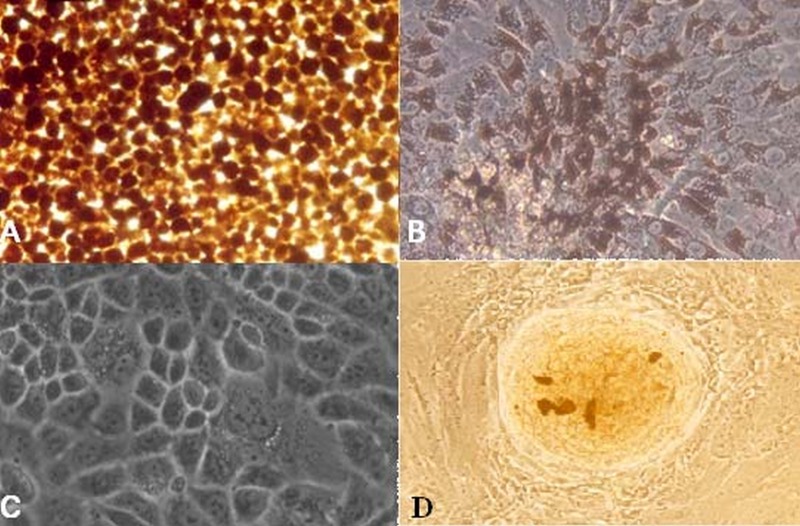
Figure 1.
(A) Retinal pigment epithelial (RPE) cells immediately after separation from a globe. (B) RPE cell culture after one week shows pigment dispersed on the cell surface. (C) Culture after 3 weeks revealed that RPE cells completely covered the floor of the container, retained the appearance of epithelial tissue and lost their pigment to some extent. (D) The appearance of a spheroid colony. The cells were visualized using Zeiss Axiovert 405M inverted microscope (magnification: 10×20 for A, B and D and 10×32 for C).
Serum Starvation in Culture
When confluent cultures were deprived from FCS, the cells that had grown as a monolayer began to establish floating spheroids. It appeared that serum starvation per se, without addition of growth factor supplements, increased the number of spheroids and accelerated the process (fig. 2).
Figure 2.
Culture of retinal pigment epithelial cells after deprivation from fetal calf serum. Cells which had grown in a monolayer began to establish spheroid colonies floating in the supernatant in a few days. A through D shows a typical process of spheroid formation (magnification: 10×20).
Reculturing Spheroids
To demonstrate the self renewal potential of colony forming cells, the spheroids were collected from the supernatant of the cultures and recultured in complete media supplemented with 10% FCS after dissociation using trypsin or without any treatment. New secondary spheroids were generated from reculturing the dissociated primary colonies (fig. 3). When the primary spheres were not treated with trypsin, they reattached to the surface of the culture vessels and began to grow in a mono-layer leading to new free floating colonies (figure. 3B,3C and 3D).
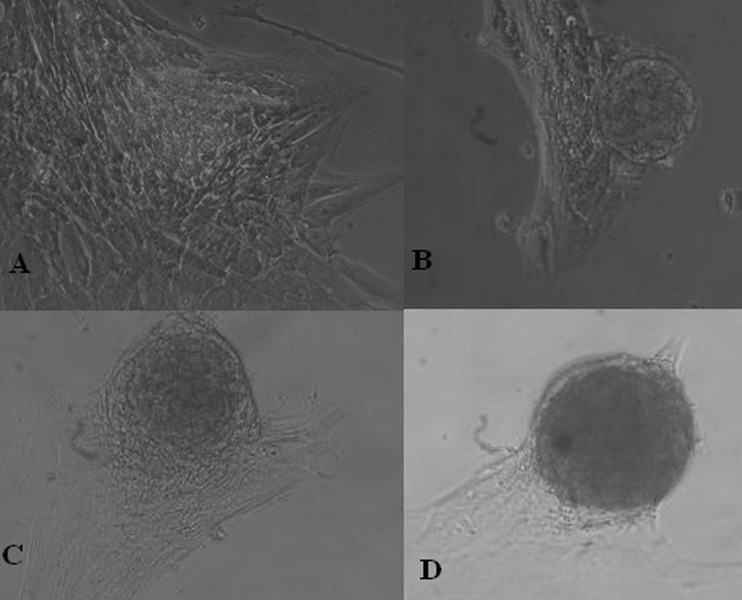
Figure 3.
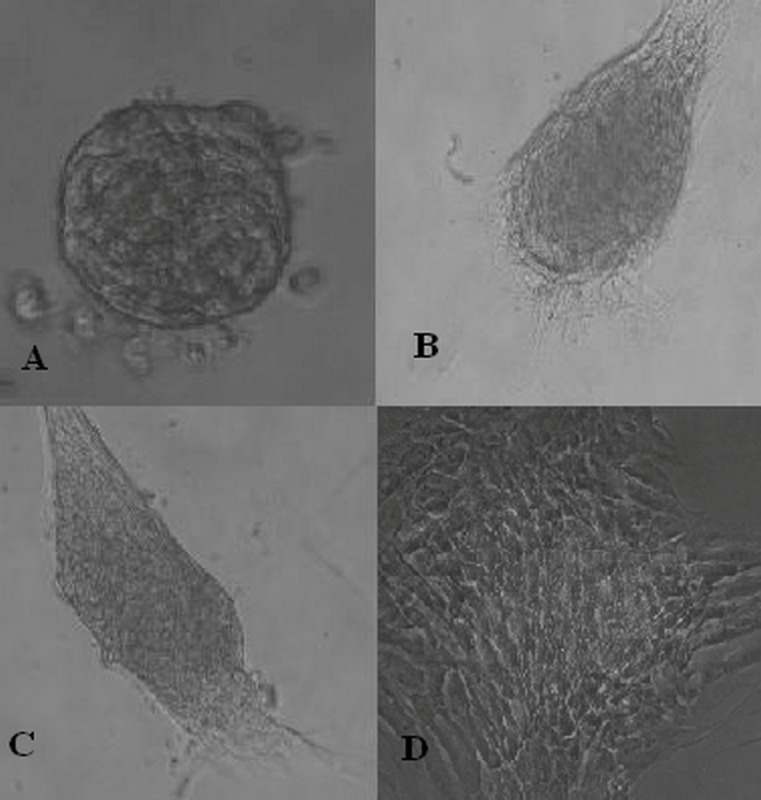
(A) A non-pigmented colony derived from dissociated spheroid colonies.(B and C) Recultured colonies derived from retinal pigment epithelial cell cultures. (D) They mostly reattached to the culture vessel surface and grew in a monolayer (magnification: 10×20).
Appearance of Photoreceptors and Neurallike Cells in High Passage Cell Cultures
Photoreceptors and neural-like cells were morphologically detected very late, usually after 8 passages in cultures (fig. 4).
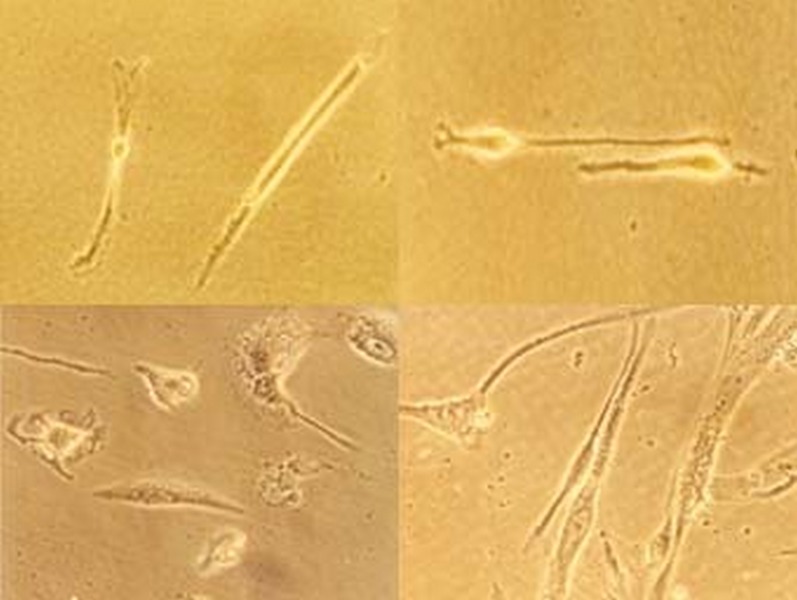
Figure 4.
Photoreceptors and neural-like cells observed within the cultures (magnification: 10×32).
Characterization of Cultures
RPE-Specific Markers
Results of evaluation for RPE-specific markers including RPE 65 and cytokeratine 8/18 are presented in figure 5 demonstrating RPE cells in green. All cells expressed RPE 65 and 30% reacted with antibody against cytokeratine 8/18.
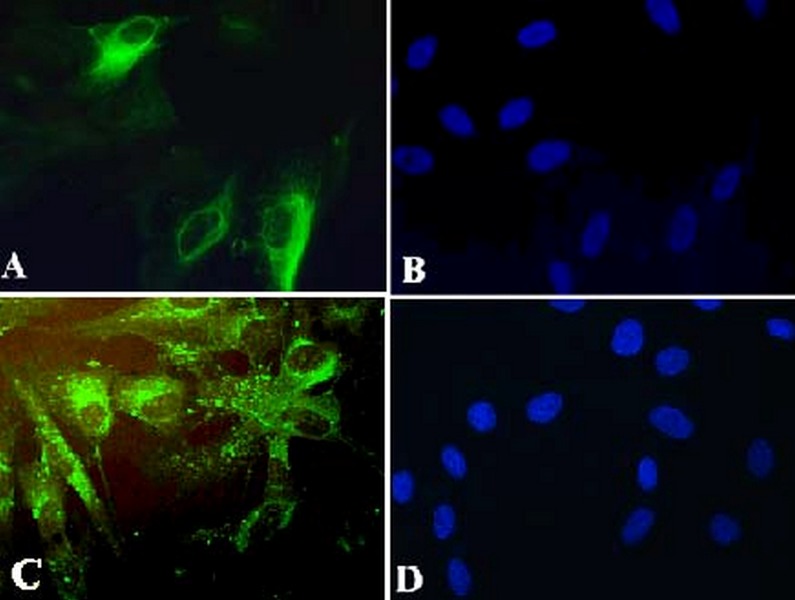
Figure 5.
(A) Retinal pigment epithelial (RPE) cells stained green with cytokeratine antibody.(B) Nuclei of RPE cells are stained blue using 4,6-diamidino-2-phenyindole dihydrocholoride (DAPI). (C) RPE cells are stained green using RPE 65 antibody. (D) Nuclei of the RPE cells stained blue with DAPI (magnification: 8×40).
Retinal Stem/Progenitor Cell Markers
Immunostaining for Pax-6, Nestin and Chx-10 revealed that cells and/or colonies within the cultures were positive for Chx-10 in 11.1%, for Pax-6 in 8.6% and for Nestin in almost all colonies (fig. 6). Cultures were simultaneously processed without primary and secondary antibodies served as controls which were negative for these markers.
Figure 6.
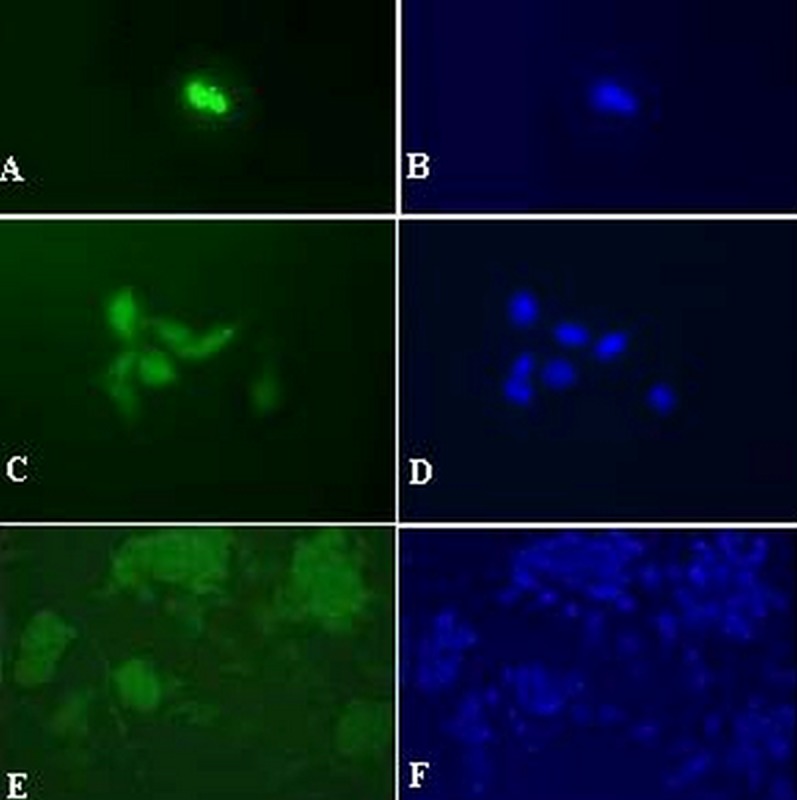
Immunostaining for retinal stem/progenitor cell markers including Chx-10 (A), Pax-6 (C) and Nestin (E). Slides (B), (D) and (F) show nuclei stained with 4,6-diamidino-2-phenyindole dihydrochloride and correspond to slides (A), (C) and (E), respectively (magnification: 8x40 for A-D and 8×20 for E-F).
Cultured RPE Cells Express Transcripts for VEGF and PEDF
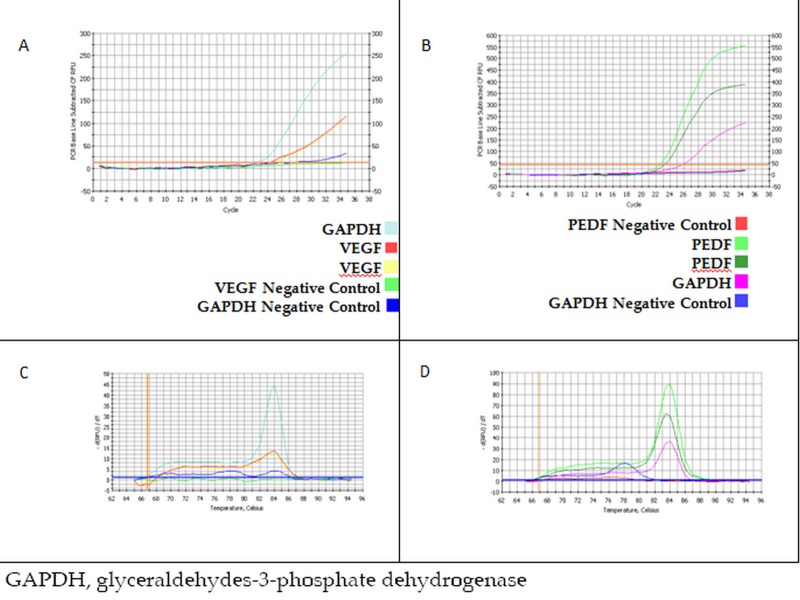
Results of real-time PCR for VEGF and PEDF are summarized in figure 7. Quantitative comparison of threshold cycles for PEDF and VEGF amplification in multiple independent tests revealed that the expression ratio for PEDF/VEGF was 1.37 in cultured RPE cells.
Figure 7.
(A) Quantitative amplification of cDNA for vascular endothelial growth factor (VEGF). (B) Quantitative amplification of cDNA for pigmented epithelial derived factor (PEDF). (C) Melting curve analysis of VEGF specific amplification. (D) Melting curve analysis of PEDF specific amplification.
DISCUSSION
It was long believed that mature stem cells were limited to the bone marrow, muscle, skin and gastrointestinal tract, however it is now recognized that stem cells may also be derived from brain, heart, bone, and adipose tissues as well as from various parts of the eye including the limbus, conjunctiva, ciliary epithelium and retina.12,13 Cell replacement therapy using human embryonic stem cells has had encouraging preliminary results in many conditions. These cells possess the unique ability to differentiate into all 3 embryonic germ cell layer derivatives. 14–16 Studies have revealed that in the absence of development inducing agents, embryonic cells pass through neural pathways by default.17 RPE cells obtained from human embryonic stem cells (hESC) can be used as an invaluable source for replacing destroyed cells in degenerative retinal disorders.18,19 In animal models of AMD and retinitis pigmentosa (RP), stem cell transplantation has been able to reduce the severity of the condition and transplantation in the fovea has led to visual improvement. 7
In recent years it has been shown that RPE cells can dedifferentiate into stem cells.20 RPE cell transplantation has been investigated in animal and human studies for treatment of disorders such as AMD and RP.21,22 Mannagh et al23 evaluated RPE cell behavior, and Fischer and Reh20 were able to isolate and culture human RPE cells using an enzymatic method. The method of separation of RPE cells from the globe is one of the most important factors in the establishment of successful RPE cell cultures. We were able to successfully separate RPE cells from globe using an enzymatic method, resulting in separation of intact and highly pure RPE cells leading to rapid induction of cell growth and proliferation in culture. When the ratio of PEDF/VEGF expression was utilized as a marker of functional and healthy RPE cells, our findings were comparable to in vivo studies.25 In addition to successful primary culture of RPE cells, spheroid colonies pertaining to stem/progenitor cells were observed. After several cellular passages, some cell types morphologically resembled photoreceptors and neural cells were detected on some cultures. Immunocytochemical analysis of cultured RPE cells showed co-expression of a considerable amount of stem cell markers including Chx-10, Nestin and Pax-6 involved in ocular development. Chen and colleagues26 compared serum containing media with serum free media for hESC proliferation. They reported that three hESC lines were established which grew at similar speed in both media but the serumcontaining medium yielded significantly higher percentages of morphologically qualified colonies and cells expressing stem cell markers. Mannello and Tonti27 aimed to define the optimal conditions for stem cell cultures in order to minimize the theoretical risk of using xenogenic compounds and to reduce immunological reactions once stem cells are transplanted but at the same time maintain characteristics such as multipotentiality, self-renewal and transplantability. There are published clinical trials on human stem cells using FCS for supplementing the culture medium without any significant side effects.28,29 FCS includes valuable bioactive factors with neuroprotective properties which play a crucial role in attenuating the cytotoxic consequences of necrotic and apoptotic factors. 30 Human mesenchymal stem cells (hMSC) expanded in FCS-supplemented medium displayed omparable morphology, phenotype and differentiation capacities to hMSC expanded n medium supplemented with platelet lysate.27 Furthermore, it has been shown that FCS supplemented culture media is more convenient in terms of preventing alloreactivityrelated mmune complications.31 In summary, the generation of primitive retinal cells from cultured RPE cells in serum containing media was in line with recently published data.22,25 Our data showed that serum starvation caused a great increase in the number of formed spheroids without need for exogenous growth factors. Due to the observed plasticity in cell cultures, RPE cells seems to be an encouraging source of stem cells for retinal cell replacement therapy.
Acknowledgements
This study was supported by grant no. 253 provided by the National Institute of Genetic Engineering and Biotechnology. We would like to extend our gratitude to Tahereh Chamani, Zahra Ataei and Mahnaz Kavari for their contributions to this work.
REFERENCES
- 1.Lotery A, Trump D. Progress in defining the molecular biology of age related macular degeneration. Hum Genet. 2007;122:219–236. doi: 10.1007/s00439-007-0406-3. [DOI] [PubMed] [Google Scholar]
- 2.Wang AL, Lukas TJ, Yuan M, Neufeld AH. Increased mitochondrial DNA damage and downregulation of DNA repair enzymes in aged rodent retinal pigment epithelium and choroid. Mol Vis. 2008;14:644–651. [PMC free article] [PubMed] [Google Scholar]
- 3.Lanzetta New treatments for CNV secondary to AMD: what evidence exists to support a treatment recommendation? Graefe ’s Arch Clin Exp Ophthalmol. 2002;240:885–888. doi: 10.1007/s00417-002-0532-5. [DOI] [PubMed] [Google Scholar]
- 4.Singh CN, Saperstein DA. Combination treatment with reduced-fluence photodynamic therapy and intravitreal injection of triamcinolone for subfoveal choroidal neovascularization in macular degeneration. Retina. 2008;28:789–793. doi: 10.1097/IAE.0b013e31817082d7. [DOI] [PubMed] [Google Scholar]
- 5.Pieramici DJ, Rabena MD. Anti-VEGF therapy: comparison of current and future agents. Eye. 2008;22:1330–1336. doi: 10.1038/eye.2008.88. [DOI] [PubMed] [Google Scholar]
- 6.Spaide R. New treatments for AMD. Ophthalmology. 2006;113:160–161. doi: 10.1016/j.ophtha.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Binder S, Stanzel B, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26:516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Petrukhin K. New therapeutic targets in atrophic age-related macular degeneration. Expert Opin Ther Targets. 2007;11:625–639. doi: 10.1517/14728222.11.5.625. [DOI] [PubMed] [Google Scholar]
- 9.Carr AJ, Vugler A, Lawrence J, Chen LL, Ahmado A, Chen FK, et al. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283–295. [PMC free article] [PubMed] [Google Scholar]
- 10.Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009 doi: 10.1016/j.neulet.2009.04.035. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Sheridan CM, Mason S, Pattwell DM, Kent D, Grierson I, Williams R. Replacement of the RPE monolayer. Eye. 2009 doi: 10.1038/eye.2008.420. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Nadri S, Soleimani M, Kiani J, Atashi A, Izadpanah R. Multipotent mesenchymal stem cells from adult human eye conjunctiva stromal cells. Differentiation. 2008;76:223–231. doi: 10.1111/j.1432-0436.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 13.Tropepe V, Coles BLK, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 14.Maclaren RE, Uppal GS, Balaggan KS, Tufail A, Munro PMG, Milliken AB, et al. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular agerelated macular degeneration. Ophthalmology. 2007;114:561–570. doi: 10.1016/j.ophtha.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 15.Limb GA, Daniels JT. Ocular regeneration by stem cells: present status and future prospects. Br Med Bull. 2008;85:47–61. doi: 10.1093/bmb/ldn008. [DOI] [PubMed] [Google Scholar]
- 16.Vugler A, Lawrence J, Walsh J, Carr A, Gias C, Semo M, et al. Embryonic stem cells and retinal repair. Mech Dev. 2007;124:807–829. doi: 10.1016/j.mod.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Smukler SR, Runciman SB, Xu S, van der Kooy D. Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J Cell Biol. 2006;172:79–80. doi: 10.1083/jcb.200508085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atmaca-Sonmez P, Li Y, Yamauchi Y, Schanie CL, Ildstad ST, Kaplan HJ, et al. Systemically transferred hematopoietic stem cells home to the subretinal space and express RPE-65 in a mouse model of retinal pigment epithelium damage. Exp Eye Res. 2006;83:1295–1302. doi: 10.1016/j.exer.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Lamba DA, Karl ME, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer A J, Reh TA. Trans-differentiation of pigmented epithelial cells: a source of retinal stem cells. Dev Neurosci. 2001;23:268–276. doi: 10.1159/000048710. [DOI] [PubMed] [Google Scholar]
- 21.Liang L, Yan RT, Ma W, Zhang H, Wang SZ. Exploring RPE as a source of photoreceptors: differentiation and integration of transdifferentiating cells grafted into embryonic chick eyes. Invest Ophthalmol Vis Sci. 2006;47:5066–5074. doi: 10.1167/iovs.06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kholodenko IV, Buzdin AA, Kholodenko RV, Baibikova JA. Mouse retinal progenitor cell (RPC) cocultivation with retinal pigment epithelial cell culture affects features of RPC differentiation. J Biochem. 2006;71:767–774. doi: 10.1134/s0006297906070091. [DOI] [PubMed] [Google Scholar]
- 23.Mannagh J, Arya DV, Irvine AR. Tissue culture of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1973;12:52–64. [PubMed] [Google Scholar]
- 24.Flood MT, Gouras P, Kjeldbye H. Growth characteristics and ultrastructure of human retinal pigment epithelium in vitro. Invest Ophtalmol Vis Sci. 1980;19:1309–1320. [PubMed] [Google Scholar]
- 25.Kim SY, Mocanu C, Mcleod DS, Bhutto I A, Merges C, Eid M, et al. Expression of pigment epitheliumderived factor (PEDF) and vascular endothelial growth factor (VEGF) in sickle cell retina and choroid. Exp Eye Res. 2003;77:433–445. doi: 10.1016/s0014-4835(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 26.Chen HF, Kuo HC, Chien CL, Shun CT, Yao YL, Ip PL, et al. Derivation, characterization and differentiation of human embryonic stem cells: comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum Reprod. 2007;22:567–577. doi: 10.1093/humrep/del412. [DOI] [PubMed] [Google Scholar]
- 27.Mannello F, Tonti G A. Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serumfree, serum replacement nonconditioned medium, or Ad hoc formula? All that glitters is not gold! Stem Cells. 2007;25:1603–1609. doi: 10.1634/stemcells.2007-0127. [DOI] [PubMed] [Google Scholar]
- 28.Berger MG, Veyrat-Masson R, Rapatel C, Descamps S, Chassagne J, Boiret-Dupre N. Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells. 2006;24:2888–2890. doi: 10.1634/stemcells.2006-0387. [DOI] [PubMed] [Google Scholar]
- 29.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Cell culture medium composition and translational adult bone marrowderived stem cell research. Stem Cells. 2006;24:1409–1410. doi: 10.1634/stemcells.2005-0654. [DOI] [PubMed] [Google Scholar]
- 30.Kume T, Taguchi R, Katsuki H, Akao M, Sugimoto H, Kaneko S, et al. Serofendic acid, a neuroprotective substance derived from fetal calf serum, inhibit mitochondrial depolarization and caspase-3 activation. Eur J Pharmacol. 2006;542:69–76. doi: 10.1016/j.ejphar.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 31.Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E, et al. Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell therapy approaches: further insight in the search for a fetal calf serum substitute. J Cell Physiol. 2007;211:121–130. doi: 10.1002/jcp.20911. [DOI] [PubMed] [Google Scholar]