Abstract
Background
It is well known that exposure to severe stress increases the risk for developing mood disorders. However, most chronic stress models in rodents involve at least some form of physically experiencing traumatic events.
Methods
This study assessed the effects of a novel social stress paradigm that is insulated from the effects of physical stress. Specifically, adult male C57BL/6J mice were exposed to either emotional (ES) or physical stress (PS) for ten minutes per day for ten days. ES mice were exposed to the social defeat of a PS mouse by a larger more aggressive CD-1 mouse from the safety of an adjacent compartment.
Results
Like PS mice, ES mice exhibited a range of depression- and anxiety-like behaviors both 24 hr and 1 month after the stress. Increased levels of serum corticosterone, part of the stress response, accompanied these behavioral deficits. Based on prior work which implicated gene expression changes in the ventral tegmental area (a key brain reward region) in the PS phenotype, we compared genome-wide mRNA expression patterns in this brain region of ES and PS mice using RNA-seq. We found significant overlap between these conditions, which suggests several potential gene targets for mediating the behavioral abnormalities observed.
Conclusions
Together, these findings demonstrate that witnessing traumatic events is a potent stress in adult male mice capable of inducing long-lasting neurobiological perturbations.
Keywords: emotional stress, social defeat, ventral tegmental area, RNA-seq, post-traumatic stress disorder, depression
Introduction
Recent estimates suggest that 20–30% of people in the United State will develop an anxiety or mood disorder sometime in their lifetime (1). Nearly 7% of the population will develop post-traumatic stress disorder (PTSD), a severe anxiety disorder characterized by a persisting fear of trauma-related stimuli, which may emerge after exposure to severe stress (2, 3). Surprisingly, the stress does not have to be directly experienced for an individual to develop PTSD. Instead, PTSD can occur vicariously in individuals who only witness a traumatic event (4–7). Such severe stress can precipitate a more depression-like syndrome in other individuals (8).
While animal models of stress have been successful in delineating much of the biological basis of stress responses, all current models of traumatic stress focus on physical stressors (i.e., chronic unpredictable or “mild” stress, social defeat, learned helplessness, etc.) and neglect the ability of psychological stress alone to cause PTSD or other stress-related disorders. This is troubling because recent studies indicate that traumatic events can be detrimental to mental health, even when experienced vicariously (9, 10). Unfortunately, animal models of such purely emotional stress are scarce.
The social defeat paradigm is one of the most robust models of PTSD, depression, and other stress-related illnesses. Socially defeated mice reliably demonstrate social avoidance for weeks after the last social defeat session along with a range of other depression- and anxiety-like behavioral abnormalities (11, 12). The social avoidance phenotype has proven particularly useful because this effect is robust, reliable, possesses ethological relevance, has strong face validity for the avoidance cluster of PTSD symptoms and social withdrawal seen in subsets of depressed patients, and is easily testable. However, the social defeat model is unable to tease apart the different effects of emotional vs. physical stress, since, as currently performed, socially defeated mice are exposed to both. Thus, the following set of experiments was designed to examine the enduring neurobiological effects of emotional stress alone on behavioral measures of mood and anxiety in adult male mice. We then used RNA-seq to measure the effects of emotional stress on gene expression within the ventral tegmental area, a dopaminergic brain reward region increasingly implicated in mood and anxiety disorders (13–17).
Materials and Methods
Animals
Detailed methods and experimental design are provided in Supplement 1.
Chronic Emotional and Physical Stress
Social defeat was performed as described previously (11, 12), with the addition of an ‘emotional stress’ component. Briefly, CD-1 mice with consistent attack latencies (≤30 sec on three consecutive screening tests) were housed in cages fitted with perforated Plexiglas dividers (FSU-Psychology, Engineering Group), allowing sensory, but not physical, contact. Naïve C57BL/6J mice were assigned to either emotional stress (ES) or physical stress (PS) conditions. ES mice were placed into the empty compartment adjacent to the CD-1 aggressor, while PS mice were placed into the compartment containing the aggressor, as previously described (11, 12, 14). During this time, the PS mouse was attacked by the CD-1 aggressor and adopted a defensive posture. After 10 min, the ES exposed mouse was transferred to a novel cage, in the compartment adjacent to a novel CD-1, to minimize exposure to any latent stimuli potentially produced by the PS-exposed mouse. The PS exposed mouse was left overnight in the compartment adjacent to the CD-1 that socially defeated it. This process was repeated for ten consecutive days, such that each day the ES exposed mouse ‘witnessed’ the defeat of a novel mouse by a novel CD-1. The term ‘witness’ in this model refers to all sensory stimuli associated with the ES experience and not visual stimuli alone. In parallel, separate groups of PS mice were generated by ten days of repeated daily defeats and continuous exposure to their aggressors as published (11, 12). Control mice were housed in pairs, one on each side of a perforated Plexiglas partition, and handled daily (12). Social interaction was assessed at 24 hr after the last stress exposure, with the subsequent behavioral experiments conducted either 48 hr or 1 month after stress exposure. As a control, we also generated one group of ES, PS, and control mice using an opaque divider without holes to blunt sensory cues related to the resident-intruder interactions. We then assessed social interaction 24 hr later (Fig. S1A in the Supplement).
Corticosterone Enzyme Immunoassay
One group of ES, PS, and control mice was used 40 min after a single stress or control session. A second group was used 24 hr after 10 days of stress. A third group was exposed to the various stress conditions and then used 40 min after the forced swim test. A corticosterone enzyme immunoassay was performed per manufacturer’s instructions (Assay Designs). See Supplement for details.
Behavioral Assays
All behavioral assays were conducted as described previously (see Supplement for details).
Transcriptome-Wide Analysis of VTA
RNA was isolated from VTA punch dissections (1.0 mm diameter) taken 24 hr after the last stress session. Sequencing was performed at Mount Sinai’s core facility using an Illumina HiSeq2000 machine (see Supplement for details).
Results
Short-Term Effects of Emotional Stress
Behavioral and neuroendocrine abnormalities
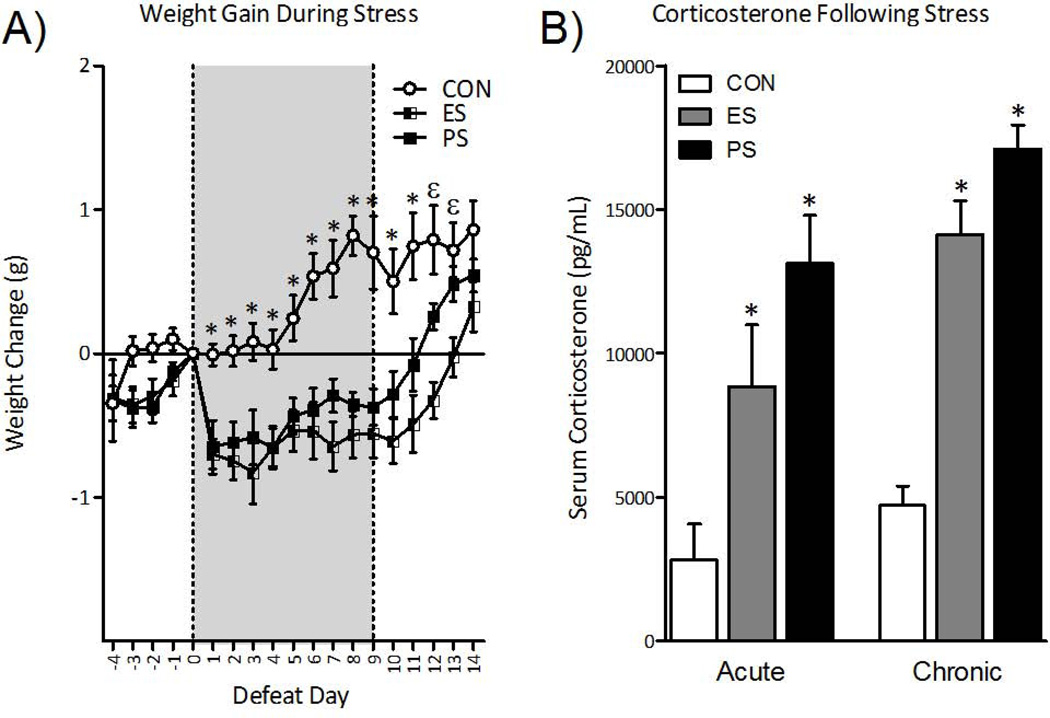
The effect of ES or PS on body-weight is shown in Fig. 1A. Repeated-measures ANOVA revealed that stress exposure significantly influenced body weight across days (within-subject main effect: F(18,558)= 14.4, p< 0.0001) and stress exposure (between-subject main effect: F(2, 558)= 20.8, p< 0.001). As previously reported (12), exposure to PS significantly reduced body-weight gain compared to control mice, and ES exposure induced a similar magnitude of weight loss. Both ES- and PS-exposed mice returned to control levels within 5 days of the last stress session.
Fig. 1.
Emotional (ES) and physical (PS) stress alters physiological and neuroendocrine reactivity 24-hr after the last stress exposure. (A) Exposure to ES and PS reduced weight gain across days of stress exposure, returning to control (CON) levels within 5 days of the last stress session (n=11–12). (B) Serum corticosterone concentrations varied 40 min after a single session of stress (left panel; n=9–10; Acute). Specifically, exposure to ES and PS significantly increased serum corticosterone levels when compared to the CON mice (p< 0.05, respectively). Separate groups of mice were exposed to 10 daily stress sessions and serum corticosterone levels were assessed 24-hr after the last exposure (right panel; n=10; Chronic). ES and PS exposure significantly increased serum corticosterone levels when compared to CON mice (p< 0.001, respectively). Data are presented as weight change in grams, and serum corticosterone concentrations as pg/mL (mean ± SEM).
To determine the acute effects of stress on serum corticosterone (CORT) levels, a group of mice was exposed to a single session of stress and CORT levels were assessed 40 min later (Fig. 1B, left panel). Serum CORT concentrations varied as a function of stress exposure (F(2,26)= 8.8, p< 0.01) across control, ES, and PS conditions. Both PS and ES exposure significantly raised CORT levels when compared to control mice (p<0.05, respectively). We next assessed the influence of chronic exposure to stress on CORT in a separate group of mice 24 hr after 10 days of stress (Fig. 1B, right panel). Serum CORT concentrations varied as a function of chronic stress exposure (F(2, 27)= 49.7, p< 0.001). PS and ES exposure induced similar increases in CORT levels when compared to control mice (p<0.05, respectively), demonstrating that ES alone can activate the glucocorticoid stress response.
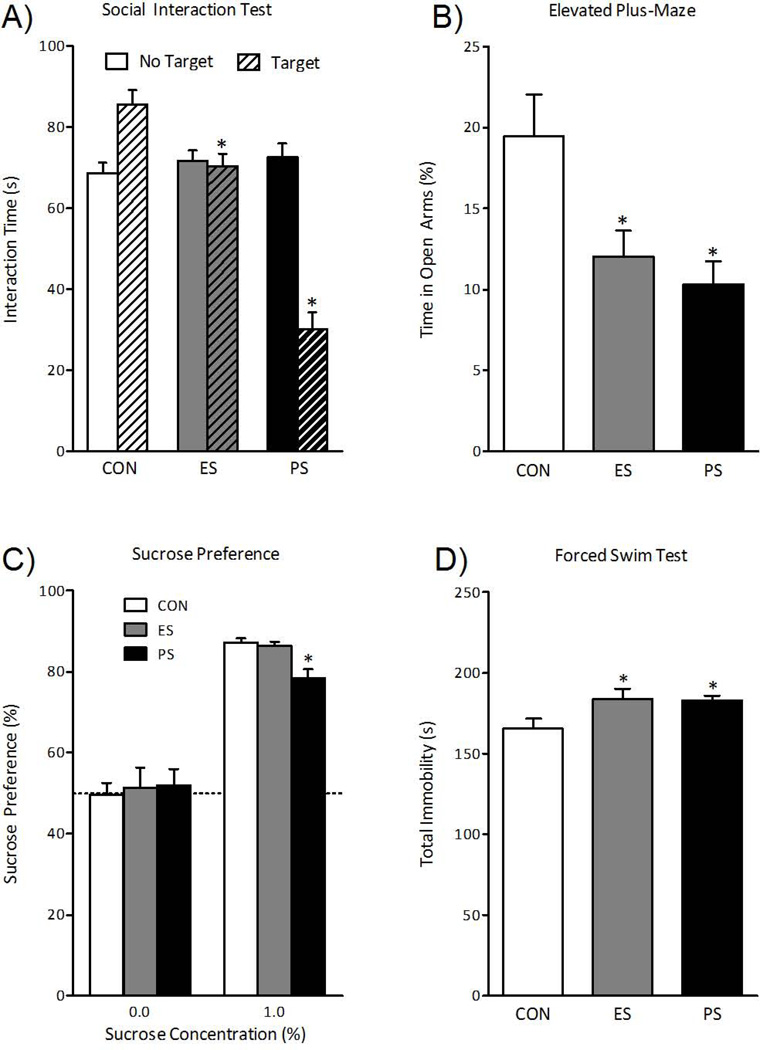
We then assessed the consequences of 10 days of ES or PS on social interaction 24 hr after the last stress session. Repeated-measures ANOVA (for presence of CD-1 mouse) revealed that social interaction time varied across target presence (within-subject main effect: F(1, 111)= 12.5, p< 0.001) and by stressor (between-subject main effect: F(2, 111)= 31.6, p < 0.001) across control, ES, and PS exposure. As expected, PS exposure reduced social interaction compared to control mice (Fig. 2A). ES-exposed mice also exhibited social avoidance, although to a much lesser degree that PS-exposed mice. To determine whether sensory contact during stress sessions was responsible for these effects, we used opaque non-perforated dividers to block or blunt sensory cues in separate groups of mice. While visual stimuli were completely blocked, it should be noted that transmission of auditory and chemosensory stimuli might have been blunted as well. We found that this manipulation completely blocked the acquisition of social avoidance in ES-exposed mice, supporting the view that the effects of ES are mediated by sensory stimuli from the aggressor’s compartment (Fig. S1A in the Supplement). In contrast, exposure of mice to soiled bedding from CD-1/PS mouse interactions had no effect on social interaction scores (Fig. S1F in the Supplement).
Fig. 2.
Emotional (ES) and physical (PS) stress alter mood and anxiety-related behavioral measures 24-hr after the last stress exposure. (A) ES and PS exposure reduced the time spent interacting with the CD-1 mouse when compared to control (CON) mice (n=38; p< 0.05). (B) A group of mice was exposed to the elevated plus-maze 48 hr after the last stress session (n=10). ES- and PS-exposed mice showed reduced time spent in the open arms of the EPM when compared to CON (p< 0.05, respectively). (C) Exposure to PS (p< 0.05), but not ES (p> 0.05), significantly reduced sucrose preference, a measure of natural reward, when compared to CON mice 24-hr after the last stress session (n=8). (D) To assess effects of CON, ES, or PS conditions on acute stress responses, a group of mice was exposed to the forced swim test (FST) 48 hr after the last stress session. Mice in the ES and PS conditions (n=10) spent more time immobile when compared to CON mice (p< 0.05, respectively). Data are presented as interaction times (in seconds), % time spent in the open arms, % preference for sucrose, and total immobility in seconds (Mean ± SEM).
To determine the effect of stress exposure on anxiety-like behavior, separate groups of mice were exposed to the elevated plus-maze (EPM) 48 hr after the last stress session. Time spent in the open arms of the EPM varied as a function of stress exposure (F(2,27)= 6.3; p< 0.01). Exposure to ES induced a robust decrease in the time spent in the open arms of the EPM (p<0.05), which provides a measure of anxiety-like behavior (Fig. 2B). ES- and PS-exposed mice avoided the open arms to a similar degree.
Stress exposure also influenced sucrose preference (F(2, 21)= 10.5, p< 0.05) 48 hr after the last stress session. As expected, PS-exposed mice displayed a decrease in sucrose preference, interpreted as a decrease in sensitivity to natural reward—or anhedonia—compared to control mice (Fig. 2C). In contrast, exposure to ES did not influence sucrose preference at this time point. Importantly, we did not observe changes in total liquid intake in ES- or PS-exposed mice (Fig. S1B in the Supplement).
To assess responses to acute stress, ES- and PS-exposed mice were analyzed in the forced swim test 48 hr after the last stress session. Figure 2D shows that total time spent immobile varied as a function of stress exposure (F(2,27)= 3.6, p< 0.05). ES exposure, like PS exposure, increased total time spent immobile, a depression-like behavior, when compared to control mice (p<0.05).
Long-Term Effects of Emotional Stress
Behavioral and neuroendocrine abnormalities
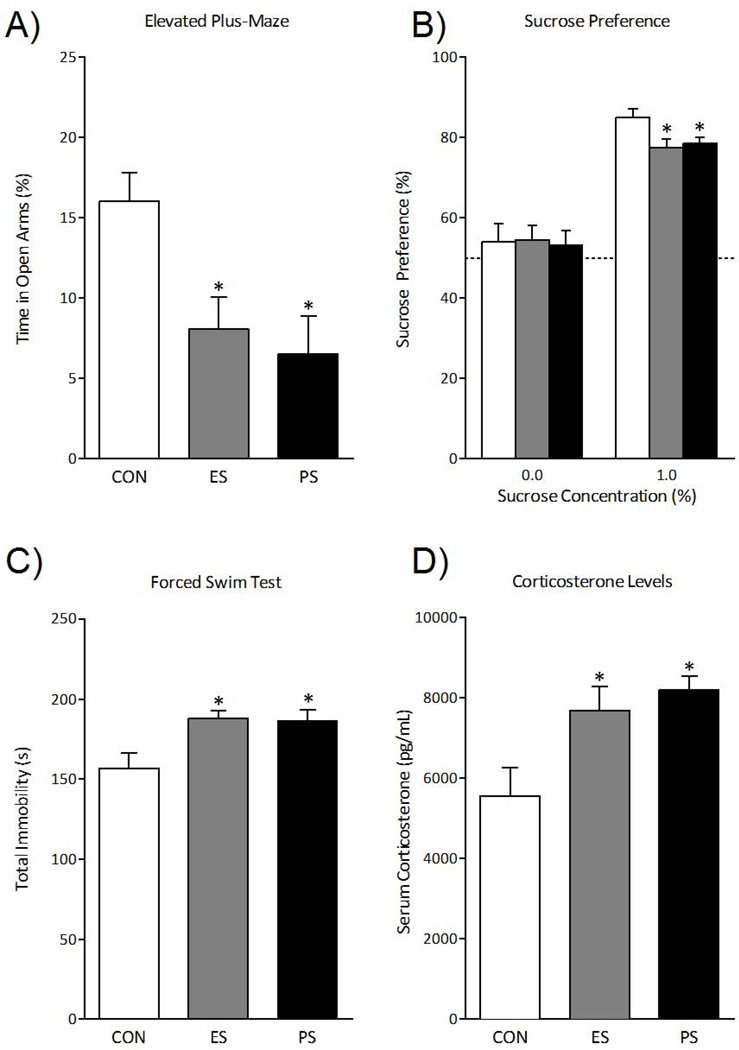
To determine the long-lasting effects of exposure to stress on anxiety-like behaviors, separate groups of control, ES- and PS-exposed mice were tested in the EPM 1 month after the last stress session. Time spent in the open arms of the maze varied as a function of stress exposure (F(2,21)= 6.1; p< 0.01). Figure 3A shows that ES exposure, like PS exposure, reduced time spent in the open arms of the maze as compared to control mice (p<0.05, respectively).
Fig. 3.
Emotional stress (ES) alters neuroendocrine and behavioral measures 1 month after the last stress exposure. (A) A separate group of mice was exposed to control (CON), ES, or physical stress (PS), and anxiety-like behavior was assessed 1 month after the last stress session using the elevated plus-maze (n=8). Exposure to ES and PS significantly reduced time spent in the open arms of the EPM when compared to the CON-exposed mice (p< 0.05, respectively). (B) Furthermore, ES or PS exposure influenced sucrose preference, a measure of natural reward, 1 month after the last stress session (n=8). Exposure to ES and PS significantly reduced preference for sucrose when compared to CON mice (p< 0.05, respectively). (C) To assess for the long-lasting effects of ES or PS on acute stress responses, a separate group of mice was exposed to the forced swim test (FST) 1 month after the last stress session (n=8). Exposure to either ES or PS increased total time spent immobile when compared to the CON-exposed mice (p< 0.05, respectively). (E) To determine the long-lasting effects of prior stress exposure on subsequent neuroendocrine stress responses, these mice were used 40 min after the FST exposure and serum corticosterone was assessed (n=8). ES and PS exposed mice had significantly increased serum corticosterone levels when compared to CON mice (p< 0.05, respectively). Data are presented as % time spent in the open arms, % preference for sucrose, total immobility (in seconds), and serum corticosterone concentrations as pg/mL (mean ± SEM).
We next assessed the long-term effect of prior stress on sucrose preference 1 month after the last stress session. Sucrose preference varied by stress exposure (F(2, 21)= 4.1, p< 0.05). Unlike the effects observed shortly after stress (Fig. 2C), mice tested a month after ES exposure showed a significant reduction in preference for sucrose, similarly to PS exposure, when compared to control mice (Fig. 3B; p<0.05, respectively). No effect was seen on total liquid intake in ES or PS mice (Fig. S1C in the Supplement). This is evidence that ES exposure uniquely induces behavioral abnormalities that only become apparent after a substantial incubation period.
Separate groups of ES, PS, and control mice were exposed to the forced swim test 1 month after the last stress session (Fig. 3C). Total immobility varied as a function of stress exposure (F(2, 21)= 5.6, p< 0.05). Exposure to PS increased total immobility, an effect also seen after exposure to ES, when compared to control mice (p<0.05, respectively). To characterize the lasting effects of the various stress conditions on neuroendocrine measures, CORT levels were assessed in these mice 40 min after forced swimming (Fig. 3D). Corticosterone concentrations varied as a function of stress exposure (F(2, 21)= 6.0, p< 0.01). Both ES and PS exposure significantly elevated CORT levels when compared to control mice (p<0.05, respectively), suggesting that witnessing stressful events causes long-lasting sensitivity of the neuroendocrine system.
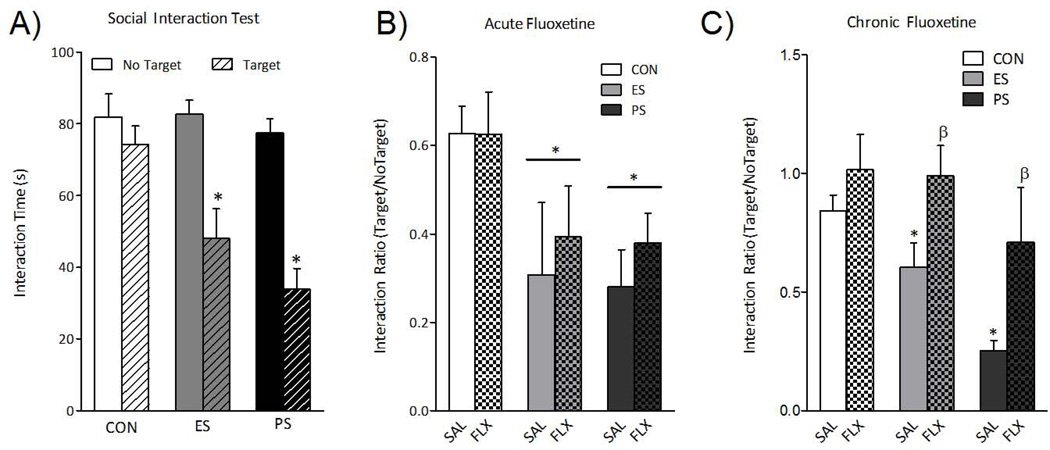
Given findings that social defeat induces an enduring social avoidance (11, 12), we assessed the lasting effects of ES and PS exposure on this behavioral abnormality. Repeated-measures ANOVA (for presence of CD-1 mouse) revealed that interaction time varied across testing condition (within-subject main effect: F(1,33)= 35, p< 0.001) and by stress exposure (between-subject main effect: F(2,33)= 7.9, p< 0.01). One month after the last stress session, mice were re-exposed to the social interaction test. As expected, PS mice displayed reduced time interacting with the social target. ES also resulted in robust social avoidance when compared to control mice (Fig. 4A), indicating that vicarious stress-induced social avoidance persists for up to 1 month.
Fig. 4.
(A) One month after the last exposure to the various stress conditions, a group of mice was re-exposed to the social interaction test (n=12). ES and PS exposure significantly reduced the time the mice spent interacting when compared to CON mice (p< 0.05, respectively). (B) A single day of fluoxetine injections was unable to reverse stress-induced social avoidance (p> 0.05, respectively). (C) Chronic (30 days) treatment with fluoxetine reversed the social interaction deficits previously observed in both ES- and PS-exposed mice (n=5–8). Data are presented as interaction times (in seconds) and as interaction ratios (Target/No Target) (mean ± SEM).
Fluoxetine reversal of ES effects
Given our results that witnessing stressful events induces behavioral and physiological dysregulation reminiscent of PTSD or depression, we tested whether antidepressant treatment could reverse the deficits in social interaction observed in ES-exposed mice. Groups of mice were first exposed to ES, PS, or control conditions for 10 days. Twenty-four hours after the last stress exposure, social interaction was assessed, mice were then divided into either acute or chronic fluoxetine treatment groups with equivalent mean social interaction scores, and were given either a single day or thirty days of fluoxetine injections (20 mg/kg/day). As previously demonstrated (11, 18, 19), a single injection of fluoxetine was not capable of reversing stress-induced social avoidance in PS-exposed mice, and we now expand this finding to ES-exposed mice (Fig. 4B). However, following chronic treatment with fluoxetine, a two-way ANOVA revealed main effects of drug (F(1,35)= 10; p< 0.01) and stress (F(2,35)= 6.4; p< 0.01), but no significant interaction (F(2,35)= .635; p> 0.05). Specifically, chronic fluoxetine treatment reversed stress-induced social avoidance in both ES- and PS-exposed mice (Fig. 4C), demonstrating that chronic, but not acute, fluoxetine is capable of reversing ES-induced social aversion.
Gene expression profiling after ES
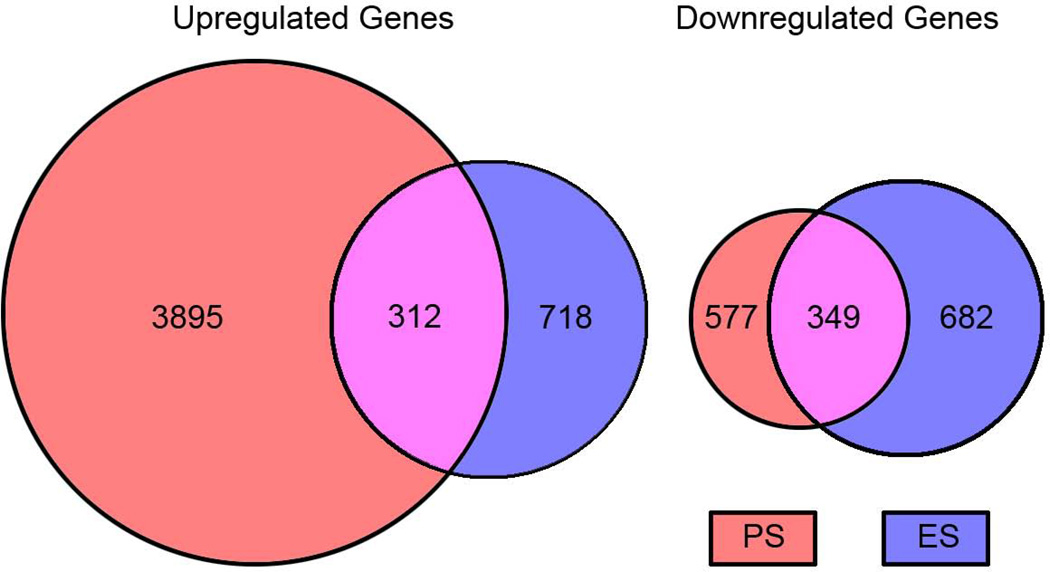
Previous work demonstrated robust changes in VTA gene expression after PS and directly linked several of these changes to the stable behavioral abnormalities seen under these conditions (12, 14). Therefore, as a first step to explore the neurobiological underpinnings of the ES-induced behavioral abnormalities, RNA was isolated from the VTA of mice 24-hr after the last exposure to 10 days of ES, PS, or control conditions and analyzed by RNA-seq using Illumina’s HiSeq 2000. Table S1 in the Supplement summarizes the read numbers from these analyses. Venn diagrams illustrate the considerable overlap in genes regulated similarly by ES and PS exposure compared to control conditions (Fig. 5). In ES-exposed mice, 718 transcripts were upregulated, and 682 were downregulated when compared to control mice. In PS-exposed mice, 3,895 transcripts were found to be upregulated and 577 transcripts were downregulated when compared to control mice. Interestingly, 312 transcripts were upregulated in both ES- and PS-exposed mice, while 349 transcripts were downregulated in both ES- and PS-exposed mice when compared to controls. See Table S3 in the Supplement for a complete list of similarly regulated transcripts.
Fig. 5.
Venn diagrams showing overlap in significantly upregulated or downregulated mRNAs within the ventral tegmental area of mice exposed to emotional (ES) or physical (PS) stress conditions 24-hr after the last exposure to stress (p< 0.05).
Discussion
We demonstrate that witnessing stressful events (i.e., social defeat) serves as a potent stressor in adult mice capable of inducing long-lasting dysregulation in several functional outputs. Our data show that both acute and repeated exposure to vicarious stress altered weight gain, serum CORT levels, and responsiveness to both rewarding and aversive stimuli. Importantly, the effects of chronic ES are enduring and at least some of the abnormalities are normalized by chronic antidepressant treatment. Witnessing stress also potently dysregulated VTA gene expression, with considerable overlap between ES- and PS-exposed mice.
Our results indicate that experiencing ES elicits a strong stress response, based on elevated CORT levels both 24 hr and 1 month after the last stress, and these effects are similar to those observed in PS-exposed mice. Likewise, the reduced weight gain observed in mice exposed to ES was similar to that seen after PS, further indicating that ES is a potent stressor (12, 20–22). These findings are intriguing, since mice that witness, but do not physically experience, stress have nearly identical changes in these classic stress measures as those subjected to PS. Deficits in weight gain are not influenced by differences in pre-stress weight, because body weight did not differ prior to stress exposure (Fig. 1A). These findings agree with reports demonstrating that PS exposure increases CORT levels for up to 24 hr (12, 23) and reduces weight gain in adult mice (24–26).
Exposure to ES enhances sensitivity to aversive situations 24 hr after cessation of the stress regimen. ES exposure induced a small decrease in social interaction, a measure of social avoidance. A more robust decrease was seen in PS-exposed mice, as reported (11, 12, 18). Under normal conditions, a naïve mouse interacts more with a novel mouse. After repeated social defeat, however, most mice avoid these interactions (11, 12, 24, 27). The finding that ES vicariously induces a social avoidance is particularly striking, since these mice never had physical contact with a CD-1 aggressor. Exposure to ES also increased immobility in the forced swim test and decreased time spent in the open arms of the EPM, like PS-exposed mice. These data are in full agreement with reports showing that PS exposure increases social avoidance and anxiety-like measures 24 hr after the last stress session (12, 28), and these findings are now extended to mice witnessing stressful events.
Exposure to ES induced a more robust long-lasting sensitivity to aversive situations, comparable to that seen following PS exposure. ES exposure caused pronounced decreases in social interaction 1 month after cessation of stress exposure. This is important because it suggests that mice that witness aggression can vicariously develop a lasting sensitivity to trauma-related stimuli that is similar to that seen in mice that actually experience physical harm. In addition, ES exposure increased immobility in the forced swim test and reduced time spent in the open arms of the EPM in mice tested 1 month after cessation of stress exposure, indicating that mice that experience ES form a persistent increased sensitivity to stress- and anxiety-eliciting situations. It is unlikely that these differences are due to changes in general locomotor activity, since total locomotion did not differ during the social interaction test (target not present; Fig. S1D in the Supplement). Together, these observations strongly agree with reports demonstrating that exposure to PS induces a lasting sensitivity to anxiety- and stress-eliciting situations (11, 12, 14, 28), findings now extended to ES.
Reduced interaction with a social target is particularly interesting, since avoidance of trauma-related cues is a hallmark of PTSD and subsets of depression (29, 30). This behavioral abnormality is corrected by chronic fluoxetine treatment; fluoxetine is a selective serotonin reuptake inhibitor, which is a robust antidepressant and one of the few effective treatments for a subset of patients with PTSD. However, the efficacy of fluoxetine in PTSD patients is highly variable, in contrast to the ability of the drug to robustly reverse ES- and PS-induced social avoidance. One possibility is that social avoidance reflects more of a depression-like symptom as opposed to PTSD per se. However, we must emphasize that clinical diagnoses of depression, PTSD, and anxiety are based on behavioral abnormalities only, with no known biologically-based diagnostic distinctions. Further appreciation of our ES and PS model must thus await better characterization of the clinical syndromes. Meanwhile, it would be interesting to test the ability of fluoxetine to reverse other sequelae of ES exposure.
Chronic exposure to ES results in long-lasting reductions in sensitivity to sucrose, a measure of anhedonia, similar to that observed after PS exposure. In contrast, at 24 hr after stress exposure, only PS mice displayed decreased sucrose preference. This suggests that the neurobiological adaptations that result in anhedonia must incubate before they emerge following exposure to ES, but not PS. Evidence for incubation is also seen with social avoidance, which is more extreme in ES mice at 1 month versus 24 hr. This is remarkable because it suggests that a more complicated and insidious mechanism may be involved in ES. Although both ES and PS exposure decreased body weight, this did not likely influence sucrose consumption because body weight rapidly returned to normal and remained at control levels 1 month after the last stress session (Fig. S1E in the Supplement), when preference for sucrose was altered. Further, no differences in total liquid intake were observed (Fig. S1B and S1C in the Supplement). Therefore, decreased sucrose preference is likely due to the influence of stress on the brain’s reward circuitry (12, 31). Dopaminergic output from the VTA to the nucleus accumbens (NAc) and other forebrain regions is critical for regulating responses to rewarding stimuli (17, 32). Dysregulation of the VTA-NAc circuitry, as occurs in depressive-like conditions, results in anhedonic-like responses to sucrose (17, 31, 33, 34).
To explore the role of the VTA-NAc circuitry in mediating aberrant behavior after exposure to ES and PS, we performed RNA-seq on the VTAs of ES, PS, and control mice 24 hr after the last stress session. We found considerable overlap in gene expression changes between ES and PS conditions. This is significant because it provides a unique list of transcripts that may be important in mediating the effects of both physically experienced and witnessed trauma, and may prove informative for understanding neurobiological mechanisms underlying behavioral pathology caused by witnessing stressful events. Several of these transcripts have been previously identified in microarray studies of the VTA in PS-exposed mice (12, 14), making those exciting targets for future study and excellent candidates for potential therapeutic intervention. Specifically, we found several molecules related to the ERK/MAP-kinase signaling pathway to be regulated. This is not surprising, since a growing literature indicates that this pathway is highly involved in mood disorders (13, 35–37). We also found the cell adhesion molecule, cadherin 1, to be upregulated after exposure to both ES and PS. This is particularly interesting because cadherin has been shown to interact with beta-catenin, a molecule essential to the wingless (WNT) signaling pathway (38), which has also been implicated in mood disorders (19, 39, 40). Shank3, a scaffold protein involved in spinogenesis, anchoring of membrane proteins including glutamate receptors, and intracellular signaling, was found to be downregulated in the VTA of both ES and PS exposed mice. Mutant forms of Shank3 have been found in a subset of individuals with autism spectrum disorders, which are characterized by compromised social behavior (41), and mice with mutations in Shank3 have deficits in social interaction (42, 43). These transcripts, found to be dysregulated 24 hr after the last stressor, may yield targets that could be useful in preventing PTSD symptoms from manifesting. However, it is currently unknown whether these alterations persist beyond 24 hr after stress exposure. It will be important in future studies to delineate the full time course of these differences in gene expression, as well as to interrogate other limbic brain regions, since PTSD can be a highly chronic condition (44, 45).
Overall, our present findings in PS-exposed mice agree with previous reports: exposure to PS blunts sensitivity to rewarding stimuli and enhances sensitivity to aversive stimuli (11–14, 28, 46). Interestingly, the present study shows that ES exposure results in similar behavioral phenotypes: increased social avoidance, increased anxiety-like behavior, anhedonia, and increased depression-like behavior. Additionally, deficits in social avoidance and sucrose preference are particularly salient 1 month after ES. This indicates that neuronal adaptations underlying these behavioral effects likely occur and build over time, in the absence of continued stress (47, 48).
Note that we did not find a consistent increase in social interaction—more time spent in the interaction zone in the presence versus absence of a social target—in control mice (Fig. 4). The reason for this is unknown, as we used highly replicable protocols that have been published widely (e.g., 11, 12). It is possible that the stress associated with 30 daily IP injections of fluoxetine or saline may have influenced behavior in the control mice. Regardless, our data clearly reveal robust deficits exhibited by PS and ES mice compared to controls.
In summary, the present study demonstrates that exposure to ES robustly influences stress responses in adult male mice. Further, it shows that ES induces a negative emotional state characterized by blunted sensitivity to reward and increased sensitivity to stress- and anxiety-eliciting situations. This study further demonstrates that these ES-induced effects are strikingly similar to those seen after PS exposure alone, suggesting that simply witnessing trauma is a potent stressor in male mice. This supports the view that ES exposure can be as critical as PS exposure for the long-lasting behavioral and neuroendocrine effects observed after social defeat. Within this context, these findings suggest that repeated ES exposure could be used as a relevant animal model for PTSD, depression, and other stress-related disorders. Moreover, gene discovery experiments such as those shown here have the potential of providing novel paths forward in the development of new treatments for these disorders.
Supplementary Material
Table 1.
Examples of Genes Regulated in the VTA by Physical or Emotional Stress
| Gene (Definition) | Function | PS | ES |
|---|---|---|---|
| Cdh1 (cadherin 1) | Cell adhesion | ↑ | ↑ |
| Fgfr1 (fibroblast growth factor receptor 1) | Stimulus Response | ↑ | ↑ |
| Gabrd (GABA A receptor, delta) | Inhibition | ↓ | ↓ |
| Grin2C (N- methyl-D-aspartate receptor subunit 2C) | Long- term potentiation | ↓ | ↓ |
| Hspd1 (heat shock 60kDa protein 1) | Chaperon | ↑ | ↔ |
| Kcnh3 ( potassium voltage- gated channel) | Potassium channel | ↔ | ↓ |
| Kif1b (kinesin family member 1B) | Motor protein | ↑ | ↔ |
| Mbp (myelin basic protein) | Myelination | ↑ | ↓ |
| Pcdh20 (protocadherin 20) | Cell adhesion | ↑ | ↔ |
| Pik3r2 (PI3K regulatory subunit beta) | Kinase adapter | ↑ | ↔ |
| Prkcd (protein kinase C delta type) | Protein kinase | ↓ | ↓ |
| Rasgrp1 (RAS guanyl releasing protein 1) | Activates Erk cascade | ↓ | ↓ |
| Shank3 (SH3 and multiple ankyrin repeat domains 3) | Scaffold Protein | ↓ | ↓ |
| Stat1 (signal transducer and activator of transcription 1) | Transcription activator | ↑ | ↔ |
| Tcf12 (transcription factor 12) | Transcription regulator | ↑ | ↔ |
| Wnt9a (wingless- type MMTV integration site 9A) | Activates frizzled | ↓ | ↓ |
Significantly up-regulated (↑), down-regulated (↓), or no change (↔) when compared to controls. Genes in bold have been verified by qPCR.
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse R21DA022351 and R01DA026854 to CABG, and R01MH51399 and P50MH66172 to EJN. BLW was supported by a Neuroscience Fellowship from Florida State University. SDI was supported by a McKnight Fellowship from the Florida Education Fund, a Neuroscience Fellowship from Florida State University, and a NRSA (F31DA027300) from NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: results from the National Comorbidity Survey Replication (NCSR) Psychol Med. 2009:1–13. doi: 10.1017/S0033291709990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newport DJ, Nemeroff CB. Neurobiology of posttraumatic stress disorder. Current opinion in neurobiology. 2000;10:211–218. doi: 10.1016/s0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard EB, Kuhn E, Rowell DL, Hickling EJ, Wittrock D, Rogers RL, et al. Studies of the vicarious traumatization of college students by the September 11th attacks: effects of proximity, exposure and connectedness. Behav Res Ther. 2004;42:191–205. doi: 10.1016/S0005-7967(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 5.Schlenger WE, Caddell JM, Ebert L, Jordan BK, Rourke KM, Wilson D, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans' Reactions to September 11. JAMA. 2002;288:581–588. doi: 10.1001/jama.288.5.581. [DOI] [PubMed] [Google Scholar]
- 6.Cougle JR, Resnick H, Kilpatrick DG. Does prior exposure to interpersonal violence increase risk of PTSD following subsequent exposure? Behav Res Ther. 2009 doi: 10.1016/j.brat.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. The New England journal of medicine. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 9.Beck CT. Secondary traumatic stress in nurses: a systematic review. Arch Psychiatr Nurs. 2011;25:1–10. doi: 10.1016/j.apnu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Motta RW. Secondary trauma. Int J Emerg Ment Health. 2008;10:291–298. [PubMed] [Google Scholar]
- 11.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, et al. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brainderived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- 21.Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 22.Zelena D, Haller J, Halasz J, Makara GB. Social stress of variable intensity: physiological and behavioral consequences. Brain Res Bull. 1999;48:297–302. doi: 10.1016/s0361-9230(98)00176-2. [DOI] [PubMed] [Google Scholar]
- 23.Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- 24.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 25.Reber SO, Obermeier F, Straub RH, Falk W, Neumann ID. Chronic intermittent psychosocial stress (social defeat/overcrowding) in mice increases the severity of an acute DSSinduced colitis and impairs regeneration. Endocrinology. 2006;147:4968–4976. doi: 10.1210/en.2006-0347. [DOI] [PubMed] [Google Scholar]
- 26.Chuang JC, Krishnan V, Yu HG, Mason B, Cui H, Wilkinson MB, et al. A beta3- adrenergic-leptin-melanocortin circuit regulates behavioral and metabolic changes induced by chronic stress. Biol Psychiatry. 2010;67:1075–1082. doi: 10.1016/j.biopsych.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, et al. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLoS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Foa EB. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67(Suppl 2):40–45. [PubMed] [Google Scholar]
- 31.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 32.Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- 33.Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 34.Bolaños CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- 35.Warren BL, Iniguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, et al. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci. 2011;31:10347–10358. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trainor BC, Crean KK, Fry WH, Sweeney C. Activation of extracellular signalregulated kinases in social behavior circuits during resident-intruder aggression tests. Neuroscience. 2010;165:325–336. doi: 10.1016/j.neuroscience.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eaton S, Cohen S. Wnt signal transduction: more than one wat to skin a (beta-)cat? Trends Cell Biol. 1996;6:287–290. doi: 10.1016/0962-8924(96)20026-1. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, et al. Wnt2 Expression and Signaling Is Increased by Different Classes of Antidepressant Treatments. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gould TD, Picchini AM, Einat H, Manji HK. Targeting glycogen synthase kinase-3 in the CNS: implications for the development of new treatments for mood disorders. Curr Drug Targets. 2006;7:1399–1409. doi: 10.2174/1389450110607011399. [DOI] [PubMed] [Google Scholar]
- 41.Herbert MR. SHANK3, the synapse, and autism. N Engl J Med. 2011;365:173–175. doi: 10.1056/NEJMcibr1104261. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golier JA, Harvey PD, Legge J, Yehuda R. Memory performance in older trauma survivors: implications for the longitudinal course of PTSD. Ann N Y Acad Sci. 2006;1071:54–66. doi: 10.1196/annals.1364.006. [DOI] [PubMed] [Google Scholar]
- 45.Yehuda R, Tischler L, Golier JA, Grossman R, Brand SR, Kaufman S, et al. Longitudinal assessment of cognitive performance in Holocaust survivors with and without PTSD. Biol Psychiatry. 2006;60:714–721. doi: 10.1016/j.biopsych.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 46.LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, et al. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.