Abstract
Background
Recent research has demonstrated that clinical depression can emerge as early as the preschool period. Here we examine brain function in children with a history of preschool onset depression (PO-MDD) in comparison to healthy children.
Methods
Participants were medication naïve school aged children (ages 7–11) with PO-MDD (N=22) or no psychiatric history (N=16) followed longitudinally as part of the Preschool Depression Study. We used fMRI measures of BOLD to examine functional brain activity in response to emotionally valenced faces (sad, fearful, angry, happy, neutral) following a negative mood induction provided to all children.
Results
In categorical group comparisons, children with PO-MDD demonstrated increased activity in parietal cortex in response to sad faces, but no differences in brain activity in a priori regions of interest (e.g., amygdala). However, in dimensional analyses, the severity of depression symptoms at the baseline preschool assessment predicted increased responses to sad faces in amygdala, hippocampal, parietal and orbital frontal regions.
Conclusions
School aged children with a history of PO-MDD show patterns of functional brain responses to emotionally evocative stimuli similar to patterns found in adults and adolescents with major depression. These patterns were most strongly related to the severity of depression during the preschool period, suggesting that the magnitude of early symptoms may be particularly important for understanding altered brain function. These findings suggest that an early episode of depression prior to age 6 may be associated with enduring brain change or may represent a biomarker that was present even prior to the preschool episode.
Keywords: Major Depression, Limbic System, Amygdala, FMRI, Emotional Processing, childhood
Major Depressive Disorder (MDD) is a major public health concern (1, 2). Recent research has shown that clinically significant depressive symptoms can also emerge in children as young as 3 (3–7), which is thought be an early onset form of childhood MDD referred to as preschool onset depression (PO-MDD) (4–7). Adults and adolescents with MDD show altered functional brain activation to emotionally evocative stimuli in limbic regions thought to be relevant for emotion processing, and frontal regions thought to be relevant for emotion regulation (8, 9). However, little is known as to whether children with a history of very early occurring depression also show altered functional brain responses to negative affective stimuli. It is important to understand whether the pathophysiology of PO-MDD is similar to childhood, adolescent or adult onset forms of MDD, as this may elucidate the developmental trajectory of the more commonly recognized later life forms of MDD. Thus, the goal of the current study was to examine functional brain responses to negative face stimuli in school-aged children with a history of PO-MDD as compared to healthy children.
Drevets and colleagues (10) have hypothesized that altered prefrontal-limbic interactions compromise the capacity for adaptive, regulatory responses to emotional challenge in MDD. In their model, dorsal prefrontal systems (DLPFC and anterior cingulate) provide top down regulatory control over ventral limbic systems via inputs into pre and subgenual cingulate and orbital frontal regions with more direct connections to amygdala, hippocampal and thalamic regions. Support for disruptions within this circuit comes from many studies of depressed adults, though not all (see (11, 12)). Numerous studies of MDD have shown increased amygdala responses to negative emotional cues as well as less deactivation in subgenual PFC (BA 25) in response to processing emotional faces (13–25). This limbic over activity is sometimes accompanied by altered activity in cognitive control areas in response to emotional distracters (16, 23, 26) or the need to regulate emotional responses (15, 27, 28).
Additional work in adolescents, while mixed, has also revealed alterations in ventral prefrontal-limbic regions. Adolescents with MDD have shown both enhanced and reduced amygdala activity while viewing facial expressions of emotion (29–31) (30, 32) and reduced activation in dorsal prefrontal and cingulate cortex during cognitive control tasks (33). Research in school aged children, adolescents and young adults at high risk for depression has also reported altered pregenual cingulate activity during an emotional stroop task (34) and enhanced amygdala activity to fearful faces (35). The normative developmental literature has demonstrated that prefrontal regions are still undergoing changes during the school-age period, with evidence that prefrontal emotion regulation systems are not yet mature (36). As such, it is not clear whether school-age children with a history of PO-MDD will show alterations in prefrontal regions that may be involved in emotion regulation, or whether they will primarily show alternations in limbic activity related to emotion processing.
Epidemiological studies have detected depression in children as young as 3 at multiple sites (3, 37). Validation of PO-MDD has been supported by the finding of a specific and stable symptom constellation, greater family history of related disorders, alterations in stress cortisol reactivity similar to those known in depressed adults (5, 38, 39) and homotypic continuity between PO-MDD and later childhood episodes (40). As such, investigating PO-MDD is of interest both for early intervention efforts that might capture a period of greater neuroplasticity and for investigations of the developmental etiologies of depressive disorders.
Recently, we reported preliminary evidence that the severity of depressive symptoms in preschool aged children with current PO-MDD predicts amygdala responses to sad faces (41). However, little is known about whether such heightened responses in ventral limbic regions are still present in school-aged children with a history of PO-MDD. The goal of the current study was to test specific hypotheses by examining functional brain responses to affective faces in school-aged children (7–11 years) with known PO-MDD and healthy children. The Drevet’s model postulates that altered prefrontal-limbic function and interactions compromise the capacity for adaptive, regulatory responses to emotional challenge. If PO-MDD shares biological substrates with adolescent and adult onset MDD that are consistent with this model, then we would predict: 1) children previously diagnosed with PO-MDD would show increased activation in ventral limbic regions such as the amygdala, the hippocampus, and the subgenual cingulate in response to negative faces; 2) these heightened responses would be most apparent for sad faces, given our prior work in preschoolers with MDD (41), and the prior work in adult depression suggesting some evidence for altered responses specifically to sad faces (24, 42), as well as evidence that responses to sad faces in MDD predict treatment response (18, 19); 3) increased ventral limbic activity would vary as a function of the severity of PO-MDD, given prior research suggesting that illness severity may predict the magnitude of functional and structural impairments in adult depression (43) and because early illness severity may reflect the magnitude of genetic or environmental contributions to illness onset; and 4) children with a history of PO-MDD may also show altered activity in lateral prefrontal and cingulate regions thought to be involved in emotion regulation (though this may be less apparent given that such regions may be less active in children normatively).
Methods
Participants
The participants were a subsample of the children in the Preschool Depression Study (PDS), a prospective longitudinal investigation of preschoolers and their families conducted in the Early Emotional Development Program (EEDP) at the Washington University School of Medicine (WUSM). See Supplemental Materials for details on recruitment for the PDS study (44). The current study reports on 38 children from the PDS psychotropically naïve at the time of this first scan. A history of head trauma, prematurity, neurological disease, developmental delay, or the use of psychoactive medications were exclusions for the current analyses. All PDS children with a history of either depression or no psychiatric were asked to participate in the imaging portion of the study. Participants were between the ages of 7 and 11 at the time of scan. One group had been diagnosed with PO-MDD between the ages of 3.0–5.11 (n=22) while the other group (healthy controls) did not exhibit any psychiatric diagnoses at any time point across the diagnostic assessment waves over a 4–6 year period (CON; n=16). Parental written consent and child assent were obtained prior to participation and the Institutional Review Board at WUSM approved all experimental procedures.
Diagnostic Assessment
Trained staff from the WUSM EEDP conducted up to four in-person assessment sessions with participants and their primary caregivers over the course of 4–6 years. The first three interviews used the Preschool-Age Psychiatric Assessment (PAPA) (45, 46) and the fourth used the Childhood and Adolescent Psychiatric Assessment (CAPA). Please see Supplement for details. Children were classified as having a history of PO-MDD if the child met symptom criteria for MDD on the PAPA prior to age 6.0 (5). For each in-person diagnostic time point, we computed depression severity sum scores (39) and summed severity scores for non-depression internalizing disorders and externalizing disorders (see Supplement). We used these scores to examine whether results were specific to depression or more generally to internalizing or externalizing psychopathology. Child and parent versions of the Children’s Depression Inventory (CDI-C and CDI-P) were completed at the time of scan (47) to assess current MDD symptom severity.
Functional Task and Stimuli
The fMRI task was an event-related facial emotion-processing task, chosen to provide continuity with research using similar tasks in adults and adolescents with depression (24, 29–32, 42). Children were shown faces that varied in affective content and were asked to decide whether the face was male or female. We chose to use a task that did not require explicit attention to the emotional content because of evidence that heightened amygdala responses associated with MDD may be more apparent with a less constrained response (16, 35). The face stimuli were from the MacArthur Network Face Stimuli Set (48). Children were shown sad, fearful, angry, happy and neutral expressions from 10 sets of individuals. We used several different negative face types in order to be able to examine the specificity of any alterations in brain responses to sad faces versus negative faces in general. In addition, we included neutral faces as another specificity control for general face processing alterations, and happy faces in order to examine whether children with a history of depression might show reduced responsivity to positive stimuli. In addition, we created intermediate sad, fearful, angry, and happy expressions by morphing the neutral expression for each individual with their emotional expression so that the resulting face was ½ way between neutral and the target emotion (MorphAge software). We included these stimuli because we thought it possible that behavioral and brain activation biases in depression may be more apparent at less strong emotional expressions where there may be a greater likelihood of detecting bias. Thus, each “actor” in the stimulus set provided a total of 9 expressions (neutral; 50% and 100% Sad, Fearful, Angry, and Happy).
We focused on the average functional activations to the full and ½ intensity faces, as we felt that explicit comparison of full to ½ intensity faces required a larger sample size for sufficient power. The results were not substantively different if we focused only on the full intensity faces. Each run consisted of 45 stimuli, 5 from each of the 9 conditions. Each stimulus was presented for 2500 ms, followed by an ITI ranging between 500 and 6500 ms. Each child was shown two runs, with no stimuli repetition. In addition, prior to performing the face task, all children went through a mood induction technique in the scanner based on the work of Gotlib and colleagues (24). We did so because not all of the children with a history of PO-MDD were depressed at the time of scanning (18% met MDD criteria), and there is evidence that affective processing biases (49) and hyperactivity of ventral prefrontal limbic regions (50) can be reactivated in individuals with a past history of MDD following a mood induction and can be elicited using mood induction in non-depressed children who were at risk due to maternal MDD (51–53). Thus, we felt it important to maximize the likelihood of seeing an influence of PO-MDD on functional brain responses in this first study in this population. We used a film clip from My Girl (see Joormann et al. (52)) that focuses on a child’s loss of a close friend to induce a negative mood state, coupled with directions to imagine the situation applying to one’s self (54). This mood induction occurred immediately before children began the face processing task. A positive mood repair clip was shown at the end of the session.
Functional Data Acquisition and Processing
Structural and functional scanning were performed on a 3.0 Tesla TIM TRIO Siemens whole body system. See Supplemental Materials for additional details on pulse sequences. The fMRI data were preprocessed using standard preprocessing steps as outlined in Supplemental Materials.
Analytical Approach
The present study used both a region of interest (ROI) analysis and a whole brain analysis. The ROI analysis focused on regions previously thought relevant to emotion processing in major depression (14–16, 55–60), and included the amygdala/hippocampus, the striatum, dorsolateral prefrontal cortex, dorsal anterior cingulate, pregenual anterior cingulate, and subgenual cingulate.
We conducted two types of analyses. The categorical analyses used a repeated measures ANOVA with diagnostic group as a between subject factor, and emotion type as a within subject factor. This analysis allowed us to examine any differences as a function of group across any of the face types. Significant interactions between group and condition were followed up by planned contrasts that allowed us to test the hypothesis that differences would be greatest to sad faces versus other face types. The dimensional analyses examined the relationship between functional brain activation and a) severity of PO-MDD at the baseline assessment when preschoolers were initially entered into the PDS study; and b) average severity of MDD across the four longitudinal assessment waves as a cumulative measure of depression severity. These correlations were conducted in the entire sample and focused on functional brain responses to sad versus neutral faces, based on the hypothesis that the processing of sad facial expressions may be particularly relevant (61). We followed up these analyses with planned analyses to further address specificity by asking whether any obtained results were also present for other faces type. All of these analyses were corrected for multiple comparisons using combined p-value/cluster size thresholds determined using Monte Carlo simulations (62, 63). These thresholds were p<.005 and 10 voxels for ROI analyses correcting for all ROIs simultaneously (corresponding to a false positive rate of p<.05 for the whole ROI mask), and p <.001 and 16 voxels for whole-brain analyses (corresponding to a whole-brain false positive rate of p<.05).
Results
Demographic and Clinical Characteristics
Table 1 summarizes demographic and clinical information for the groups. The PO-MDD group experienced a higher number of MDD symptoms at baseline (Time 1) and across all waves. The PO-MDD group experienced more externalizing and non-MDD internalizing symptoms. Six of the PO-MDD children had a comorbid diagnosis of ADHD, 3 of GAD, and 5 of either Separation Anxiety Disorder or Social Phobia. However, as described below, all results held when controlling for either externalizing or internalizing symptoms.
Table 1.
Demographic and Clinical Characteristics of Participants
| Healthy Control (N = 16) |
Pre School Onset Major Depression (PO-MDD) (N = 22) |
Group Comparison | |||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | |
| Age (in years) | 9.0 | 0.89 | 9.3 | 1.03 | T(36) = 0.85, p=.40 |
| Gender (% Males) | 44% | 41% | Χ2(2) = 0.56 | ||
| Ethnicity (% Caucasian) | 69% | 46% | Χ2(2) = 0.32 | ||
| Parental Education | 2.71 | 1.14 | 2.64 | 1.00 | T(36) = 0.22, p=.80 |
| Parental Income | 2.75 | 1.24 | 2.76 | 1.22 | T(36) = 0.03, p=.97 |
| # of MDD symptoms endorsed at Time 1 | 1.24 | 1.24 | 7.13 | 3.1 | T(36) = 6.98, p<.001 |
| Average # of MDD symptoms endorsed across all four assessments | 1.27 | 1.33 | 5.97 | 3.30 | T(36) = 5.38, p<.001 |
| Average # of Internalizing symptoms endorsed across all four assessments | 1.59 | 0.68 | 4.44 | 2.09 | T(36) = 5.24, p<.001 |
| Average # of externalizing symptoms endorsed across all four assessments | 1.47 | 1.50 | 7.87 | 5.23 | T(36) = 4.73, p<.001 |
| Parent CDI at time of scan | 6.86 | 5.52 | 10.10 | 5.70 | T(36) = 1.67, p=.11 |
| Child CDI at time of scan | 3.79 | 3.33 | 5.81 | 6.62 | T(36) = 1.06, p=.30 |
| Mood Rating Prior To Mood Induction | 4.6 | 0.7 | 4.4 | 0.7 | T(36) = 1.05, p=.30 |
| Mood After Mood Induction | 2.7 | 1.4 | 2.7 | 1.3 | T(36) = 0.09, p=.9 |
The healthy control and PO-MDD groups did not differ significantly in CDI scores at the time of scan, though the means were in the expected direction. The negative mood induction was effective in terms of self-reported mood pre versus post mood induction for both the healthy children (p<.001) and the PO-MDD children (p<.001) with no significant group differences (see Table 1). Based on the CAPA, 4 of the PO-MDD children met criteria for current MDD at the time of the fMRI scan (18%). All of the results reported below remained significant if those four children were excluded.
Categorical Group Comparison
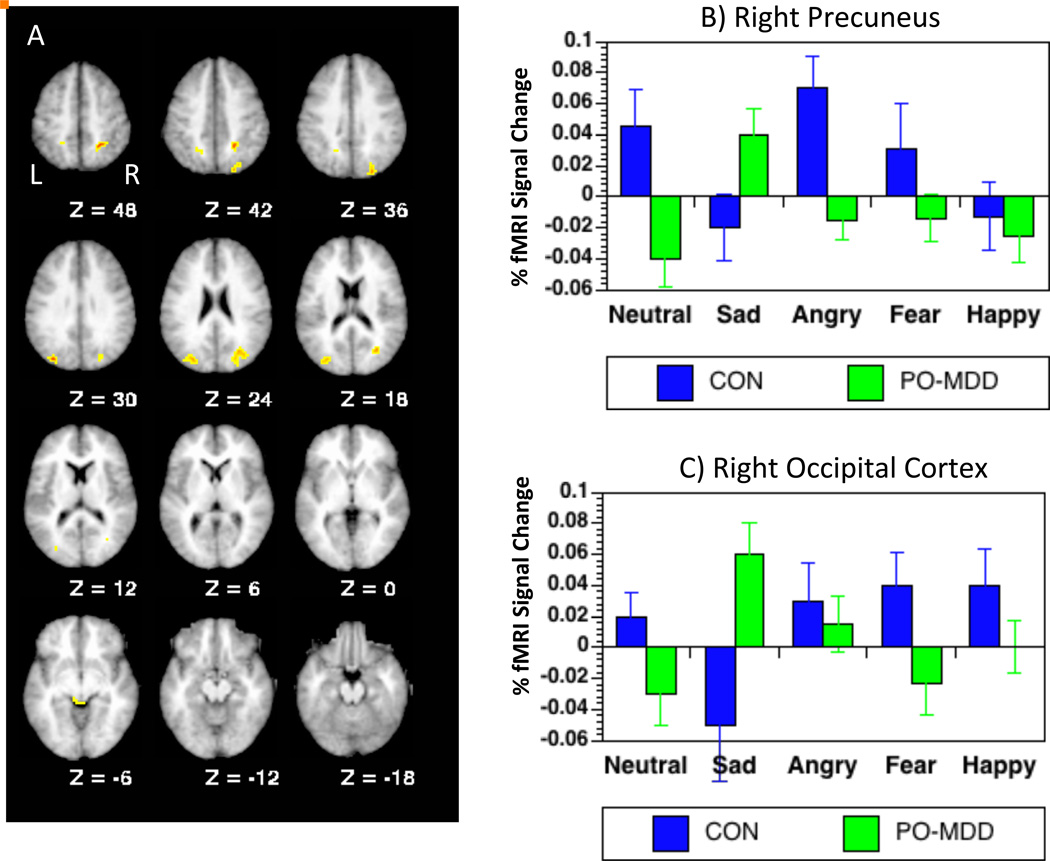
This analysis addressed the question of whether children with a history of PO-MDD would show enhanced ventral-limbic responsivity to negative faces (specifically sad faces),or altered activity in prefrontal and cingulate regions associated with emotion regulation. The voxel-wise ANOVAs did not reveal any regions showing significant main effects of group, emotion or group × emotion interactions. Similar ANOVAs at the whole brain level revealed a main effect of emotion in right parietal cortex (see Table 2), with greater activation to sad, fearful and happy faces than to neutral or angry faces (ps<.01). In addition, we found diagnostic group by face emotion type interactions in a number of regions, including left parietal cortex, bilateral precuneus, bilateral occipital cortex, brain stem and left temporal cortex (see Table 2). In all of these regions except for left temporal cortex, the interaction reflected the fact that the children with a history of PO-MDD showed greater activation to sad faces relative to control children and relative to the other face types (see Table 2). Comparisons on the other face types are shown in Table 2. In the left temporal cortex, control children, but not PO-MDD children, showed enhanced activation to fearful faces relative to the other face type. In all of these regions, the diagnostic group by emotion interactions remained significant when covarying for either externalizing and non-MDD internalizing symptoms and/or parent and child reported CDI scores at the time of the scan, suggesting that the results were specific to a history of PO-MDD rather than to psychopathology more broadly or current depressed mood. Thus, as a whole, these analyses did not support the hypothesis that as a group children with a history of PO-MDD would show increased ventral limbic responsivity or reduced frontal/cingulate responses to sad faces. However, they did show altered activity among PO-MDD children in a number of other regions, with the differences consistent for sad faces across the regions.
Table 2.
Results of Emotion Type × Diagnostic Group ANOVA
| Region | BA | X | Y | Z | ROI Z Value |
# of Voxels |
Pattern in Control Children |
Pattern in PO- MDD Children |
|---|---|---|---|---|---|---|---|---|
| ROI Based Analyses | ||||||||
| NONE | ||||||||
| Whole Brain Analyses | ||||||||
| Main Effect of Emotion | ||||||||
| Right Parietal Cortex | 40 | 63 | −27 | 19 | 3.99 | 15 | S, F, H > N, A | S, F, H > N, A |
| Emotion × Group Interaction | ||||||||
| Left Parietal Lobe | 7 | −22 | −49 | 51 | 3.42 | 13 | A, N > S, F, H | S > N, A, F, H |
| Right Precuneus | 7 | 22 | −51 | 45 | 4.34 | 51 | N, A, F, H > S | S > N, A, F, H |
| Left Precuneus | 7 | −19 | −56 | 39 | 3.79 | 16 | N, A, H > S, F | S > N, A, F, H |
| Left Occipital Cortex | 19 | −30 | −80 | 23 | 4.00 | 69 | N=A=S=F=H | S > N, A, F, H |
| Right Occipital Cortex | 31 | 26 | −75 | 27 | 4.16 | 108 | N, A, F, H > S | S > N, A, F, H |
| Brain Stem | 30 | −4 | −37 | −5 | 3.91 | 14 | H > N, S, A, F | S > N, A, F, H |
| Left Temporal Lobe | 20 | −52 | −31 | −28 | 4.21 | 14 | F > N, S, A, H | N, A, H > S, F |
Note: N = Neutral, S = Sad, A = Angry, F = Fear, and H = Happy; Bold indicates group differences at p<.05. Bold and underline indicates group differences at p<.01.
Dimensional Analyses
These analyses were designed to address the hypothesis that the magnitude of ventral limbic activity in response to sad faces would vary as a function of the severity of PO-MDD and were conducted using all participants.
A Priori ROIs
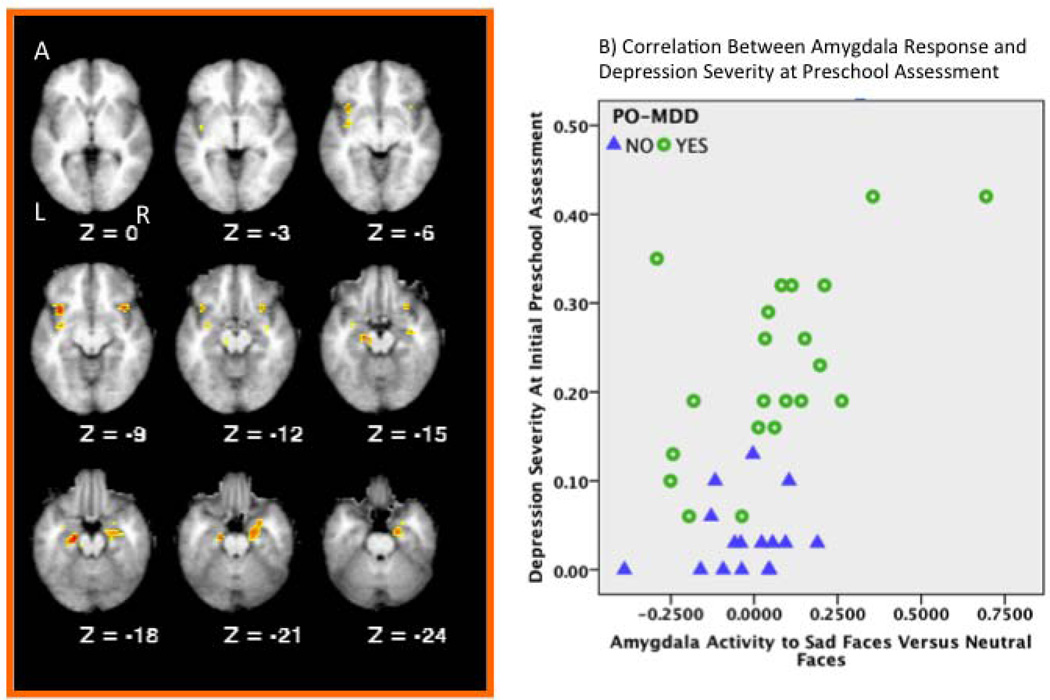
The voxel-wise correlations between baseline (Time 1) preschool MDD severity and brain activation in response to sad versus neutral faces in our a priori ROIs revealed positive correlations in several regions (see Table 3, and Figure 2), including bilateral orbital frontal cortex, right hippocampus, right amygdala, left claustrum and bilateral parahippocampal gyrus. In all of these regions, a greater number of MDD symptoms at baseline were associated with greater activation to sad faces relative to neutral faces. Further, all of these correlations remained significant when externalizing and non-MDD internalizing symptoms were included as covariates in the same model, as well as parent and child reported CDI scores at the time of scan. Since the contrast used in these analyses was the difference between sad and neutral faces, we also examined whether the correlations were due to increased activation to sad faces or decreased activation to neutral faces. All seven regions showed significant positive correlations with activation to sad faces (rs of .39 to .59, ps of .02 to .001). In contrast, only two of the regions (left and right parahippocampal gyrus) showed significant correlations with neutral faces, both of which were negative (rs of −.40 and −.37, ps .01 and .02 respectively). Further, we also confirmed that the correlations remained significant in all 7 regions when the analyses were conducted in only those children with a history of PO-MDD (rs of .47 to .75, ps of .03 to .0001).
Table 3.
Correlations Between Baseline Depression Severity Scores and Functional Brain activation in A Priori ROIs
| Region | BA | X | Y | Z | ROI Z Value |
# of Voxels |
Correlation with Sad vs. Neutral Faces |
Correlation with Angry vs. Neutral Faces |
Correlation with Fear vs. Neutral Faces |
|---|---|---|---|---|---|---|---|---|---|
| Right Orbital Frontal Cortex | 13 | 32 | 13 | −12 | 4.13 | .62 | .19+ | .32+ | |
| Left Orbital Frontal Cortex | 13 | −35 | 13 | −10 | 3.80 | .59 | .06+ | .25+ | |
| Right Hippocampus | -- | 35 | −13 | −14 | 3.14 | .50 | .21+ | .33+ | |
| Right Amygdala | -- | 27 | −02 | −21 | 3.25 | .51 | .32+ | .23+ | |
| Left Claustrum | -- | −36 | −9 | −5 | 3.04 | .48 | .08+ | .17+ | |
| Right Parahippocampal Gyrus | 28 | 23 | −15 | −21 | 3.48 | .54 | .47* | .33+ | |
| Left Parahippocampal Gyrus | 35 | −16 | −22 | −16 | 3.92 | .60 | .37+ | .42*+ |
Note: Grey shading indicates regions that displayed significant correlations only with activation to sad versus neutral faces.
p<.05
Indicates that the correlation between baseline depression severity and activity to sad faces (sad – neutral) was significantly greater than the correlation between baseline depression severity and either activity to angry faces (angry - neutral) or fearful faces (fear - neutral) using the procedures developed by Meng, Rosenthal and Rubin (69).
Figure 2.
Results of a priori ROI analyses examining the correlations between depression severity at the baseline preschool assessment and functional brain response to sad faces. A) Brain slices illustrating regions showing regions displaying significant correlations between functional brain responses to sad versus neutral faces and the severity of depression at the initial preschool assessment. Z values represent millimeters above or below the line bisecting the anterior and posterior commissures. B) Scatter plot illustrating the correlation in the amygdala between functional brain responses to sad versus neutral faces and the dimensional assessment of depression severity at the initial preschool assessment. Blue triangles indicate healthy control children and green circles indicate children with a history of PO-MDD.
We then examined whether these associations were specific to sad faces, or also present for angry or fearful faces. Baseline MDD severity was only correlated with activation to sad faces (Table 3), and not angry or fearful faces, in bilateral orbital frontal cortex, hippocampus, amygdala and claustrum, and the correlations with activity to sad faces were significantly stronger than the correlations to angry or fearful faces. The right parahippocampal gyrus also showed a significant correlation between baseline preschool MDD severity and activation to angry faces, and the left parahippocampal gyrus also showed a correlation between baseline preschool onset MDD severity and activation to fearful faces, suggesting that the relationship between depression severity and functional activation is not specific to sad faces in these regions. However, the correlations with sad face activity for the left parahippocampal gyrus were still significantly stronger than the correlations with activity to either angry or fear faces. To further address the question of specificity of any effects to sad faces, we conducted voxel wise correlations between baseline depression severity and brain activity to either angry-neutral faces or fear-neutral faces, and did not find any significant clusters in our a priori ROIs.
We also examined correlations with average depression severity across the first 4 assessment, but did not identify any significant regions in the a priori ROI mask. We also examined whether baseline (Time 1) depression severity was correlated with activation to either angry or fearful faces (versus neutral) in voxel-wise correlational analyses, but did not identify any significant regions in the a priori ROI mask. Thus, within our a priori ROI mask, the associations between overall depression severity and functional brain activity appeared specific to depression severity at the initial pre-school assessment (compared to average severity) and to functional brain responses to sad faces (versus neutral faces) compared to angry or fearful faces. See Supplement for whole brain results.
Discussion
The dimensional analyses using MDD severity revealed evidence that more severe depression in the preschool period was associated with greater activity to sad faces in bilateral orbital frontal cortex, amygdala, claustrum hippocampal and parahippocampal gyrus. Importantly, this enhanced activity was specific to sad faces as compared to angry or fearful faces in all regions but the parahippocampal regions. These results are consistent with our recent work in depressed preschoolers, showing that greater depression severity was associated with greater amygdala responses to sad faces (41). These correlations remained even after controlling for comorbid internalizing and externalizing symptoms and depression at the time of scanning, suggesting they were specific to the severity of depression in the preschool period rather than the cumulative severity of depression across childhood or other comorbid symptomatology. This suggests that the occurrence of very early depression may be of unique importance for understanding the developmental trajectory of this illness.
There are at least two explanations for these findings relating to the severity of PO-MDD. One is that more severe depression in the preschool period is an indicator of increased genetic or environment liability for depression, and thus reflects altered brain activity associated with trait factors that put children at risk for depression. Such an explanation would be consistent with work in at-risk children (based on parental mood disorders) showing enhanced activation to negative faces (35). An alternative explanation is that enhanced brain activity in response to sad faces reflects a “scar” caused by an episode of preschool depression. Prospective studies of at-risk children are needed to distinguish between functional brain changes that are associated with risk for depression versus those that are the consequences of the experience of depression. Further, it was surprising that the cumulative severity of depression was not as predictive of functional brain responses as depression severity specifically in the preschool period. This could reflect the fact that greater severity in the preschool period is associated with a stronger contribution of genetics to illness onset, though evidence suggesting that childhood onset depression in general has a stronger environmental contribution than adult onset depression argues against this explanation (64). It may also reflect the fact that the preschool period assessments capture particularly important variance in depression scores, as children were specifically recruited at a time of increased depressive symptoms, while the longitudinal follow-up captures children naturalistically at periods of both depression and non-depression.
Our categorical comparisons of children with and without a history of PO-MDD revealed enhanced activation in several parietal, occipital and lateral temporal regions, specifically to sad faces. The specificity to sad faces is consistent with our previous findings in currently depressed preschoolers, showing correlations between depression severity and amygdala and occipital activity to sad faces (41). This result is also consistent with findings in depressed adults which have shown some evidence for altered responses specifically to sad faces (24, 42), and evidence that responses to sad faces in MDD predict treatment response (18, 19).The parietal and precuneus regions showing enhanced activity in the PO-MDD children are thought to be involved in a variety of aspects of attention and cognitive control (65, 66). These activations, as well as those in occipital cortex, may reflect increased attentional modulation of face processing, potentially due to the increased salience of sad stimuli and a bias towards negative stimuli that is often associated with depression (67, 68). The increased brain stem activity could also be associated with increased periaqueductal gray (PAG) activation related to alerting or orienting to salient stimuli, as PAG activation is highly influenced by amygdala and related limbic inputs (9, 10). Finally, the lateral temporal activity may reflect activity associated with the semantic processing or object classification of the stimuli into relevant categories. Although the majority of the brain regions did show PO-MDD related effects specifically to sad faces, there were bilateral parahippocampal gyrus regions in the ROI analyses that also showed effects to angry or fearful faces, and a number of regions in the whole brain analysis (primarily in temporal cortex and cerebellum) that showed effects to angry or fearful faces as well. Thus, there may be some PO-MDD effects related to the processing of all negative emotions.
We did not find evidence for altered activity in the amygdala or VMPFC in categorical group analyses, though as discussed above, we did see enhanced amygdala activity associated with depression severity. The fact that the depression severity analyses revealed greater amygdala responsivity, while the group analysis did not, speaks to the importance of addressing the effect of clinical heterogeneity in early onset depression, a factor acknowledged to be important in the adult literature as well. Importantly, this increased amygdala activity as a function of PO-MDD severity occurred in the absence of depression related effects in brain regions typically associated with regulation (e.g., dorsolateral prefrontal cortex), suggesting that at least in early onset depression, limbic hyperactivity may not simply be the result of failures of top-down control.
There were several limitations. First, the sample sizes were relatively small. Additional children with PO-MDD were not included in the analyses because they were currently taking medications or had a history of medication use, two factors which could limit interpretability. Second, the majority of children with a history of PO-MDD did not meet criteria for current MDD, and CDI scores at the times of scanning did not differ between the groups. It is possible that we would have seen additional evidence for altered brain activity in the categorical analysis if all of the PO-MDD children were currently depressed. Third, all children underwent a negative mood induction prior to the facial affect task. As such, we do not know how specific our results are to the use of mood induction and future studies directly comparing results with and without mood induction will be needed to clarify this issue.
In summary, the current study provides the first evidence that we are aware of demonstrating that school aged children with a history of PO-MDD showed altered functional brain responses to negative stimuli. These patterns were generally consistent with findings in adults and adolescents and contribute to the growing evidence for the clinical and neurobiological validity of preschool onset depression, and provide preliminary support for common neural pathophysiology with later onset forms of depression.
Supplementary Material
Figure 1.
Results of whole brain analyses comparing PO-MDD children and healthy controls. A) Brain slices illustrating regions showing regions displaying significant diagnostic group (PO-MDD vs. Control) × emotion (neutral, sad, fear, angry, happy) interactions B) Graph illustrating the pattern of responses in right precuneus. C) Graph illustrating the pattern of responses in right occipital cortex.
Acknowledgements
Grant numbers MH64769 (JL) and MH090786 (JL, DB, KB) from the National Institute of Mental Health funded the current study. Dr. Belden’s work on this manuscript was supported by a grant from the National Institute of Mental Health (1K01MH090515-01). The NIMH had no further role in the design and conduct of the study, collection, management, analysis, and interpretation of data, or preparation, review, or approval of the manuscript. Author DMB had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. We would like to thank all participants and their families that provided time and effort to making this study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, et al. Development and natural history of mood disorders. Biological Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- 3.Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. Journal of child psychology and psychiatry, and allied disciplines. 2006;47:313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 4.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. Journal of Affective Disorders. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KM, Hildebrand T. Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry. 2002;41:928–937. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Luby JL, Heffelfinger AK, Mrakotsky C, Brown KM, Hessler MJ, Wallis JM, Spitznagel EL. The clinical picture of depression in preschool children. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:340–348. doi: 10.1097/00004583-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Gaffrey MS, Belden AC, Luby JL. The 2-week duration criterion and severity and course of early childhood depression: Implications for nosology. Journal of Affective Disorders. 2011 doi: 10.1016/j.jad.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neuroscience and Biobehavioral Reviews. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain structure & function. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. Journal of Psychiatric Research. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend JD, Eberhart NK, Bookheimer SY, Eisenberger NI, Foland-Ross LC, Cook IA, et al. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Research. 2010;183:209–217. doi: 10.1016/j.pscychresns.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 14.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant threatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 15.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. Journal of Psychiatric Research. 2007;41:511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 19.Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. 2008;64:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 20.van Wingen GA, van Eijndhoven P, Cremers HR, Tendolkar I, Verkes RJ, Buitelaar JK, Fernandez G. Neural state and trait bases of mood-incongruent memory formation and retrieval in first-episode major depression. Journal of Psychiatric Research. 2010;44:527–534. doi: 10.1016/j.jpsychires.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biological Psychiatry. 2010;67:155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Lee BT, Seong Whi C, Hyung Soo K, Lee BC, Choi IG, Lyoo IK, Ham BJ. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2007;31:1487–1492. doi: 10.1016/j.pnpbp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. Journal of Affective Disorders. 2009;114:131–142. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- 25.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biol Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau JY, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, et al. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry. 2009;65:349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 33.Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, et al. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naive adolescents with depression compared to controls. J Child Psychol Psychiatry. 2009;50:307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- 34.Mannie ZN, Norbury R, Murphy SE, Inkster B, Harmer CJ, Cowen PJ. Affective modulation of anterior cingulate cortex in young people at increased familial risk of depression. Br J Psychiatry. 2008;192:356–361. doi: 10.1192/bjp.bp.107.043398. [DOI] [PubMed] [Google Scholar]
- 35.Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 36.Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, et al. The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental psychobiology. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavigne JV, Hopkins J, Gouze KR, Bryant FB, LeBailly SA, Binns HJ, Lavigne PM. Is smoking during pregnancy a risk factor for psychopathology in young children? A methodological caveat and report on preschoolers. Journal of pediatric psychology. 2011;36:10–24. doi: 10.1093/jpepsy/jsq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Hessler M, Spitznagel E. Modification of DSM-IV criteria for depressed preschool children. The American journal of psychiatry. 2003;160:1169–1172. doi: 10.1176/appi.ajp.160.6.1169. [DOI] [PubMed] [Google Scholar]
- 39.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004;161:1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- 40.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Archives of General Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaffrey MS, Luby JL, Belden AC, Hirshberg JS, Volsch J, Barch DM. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. Journal of Affective Disorders. 2011;129:364–370. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. Journal of affective disorders. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Luby JL, Belden AC. Clinical characteristics of bipolar vs. unipolar depression in preschool children: an empirical investigation. J Clin Psychiatry. 2008;69:1960–1969. doi: 10.4088/jcp.v69n1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- 46.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs M. The Children's Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 48.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25 doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Taylor L, Ingram RE. Cognitive reactivity and depressotypic information processing in children of depressed mothers. Journal of Abnormal Psychology. 1999;108:202–1210. doi: 10.1037//0021-843x.108.2.202. [DOI] [PubMed] [Google Scholar]
- 52.Joormann J, Talbot L, Gotlib IH. Biased Processing of Emotional Information in Girls at Risk for Depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- 53.Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol. 2006;115:705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- 54.Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: A meta-analysis. European Journal of Social Psychology. 1996;26:557–580. [Google Scholar]
- 55.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 57.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 60.Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112:206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J Affect Disord. 2010;120:120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 62.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 63.McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. NeuroImage. 2001;13:S198. [Google Scholar]
- 64.Rice F. Genetics of childhood and adolescent depression: insights into etiological heterogeneity and challenges for future genomic research. Genome Med. 2010;2:68. doi: 10.1186/gm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 66.Corbetta M, Shulman GL, Miezin FM, Petersen S. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- 67.Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Current Opinion in Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- 68.Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: a review. The Australian and New Zealand journal of psychiatry. 2010;44:681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- 69.Meng X, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111:172–175. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.