Abstract
The suprachiasmatic nucleus (SCN), site of the primary clock in the circadian rhythm system, has three major afferent connections. The most important consists of a retinohypothalamic projection through which photic information, received by classical rod/cone photoreceptors and intrinsically photoreceptive retinal ganglion cells, gains access to the clock. This information influences phase and period of circadian rhythms. The two other robust afferent projections are the median raphe serotonergic pathway and the geniculohypothalamic (GHT), NPY-containing pathway from the thalamic intergeniculate leaflet (IGL). Beyond this simple framework, the number of anatomical routes that could theoretically be involved in rhythm regulation is enormous, with the SCN projecting to 15 regions and being directly innervated by about 35. If multisynaptic afferents to the SCN are included, the number expands to approximately brain 85 areas providing input to the SCN. The IGL, a known contributor to circadian rhythm regulation, has a still greater level of complexity. This nucleus connects abundantly throughout the brain (to approximately 100 regions) by pathways that are largely bilateral and reciprocal. Few of these sites have been evaluated for their contributions to circadian rhythm regulation, although most have a theoretical possibility of doing so via the GHT. The anatomy of IGL connections suggests that one of its functions may be regulation of eye movements during sleep. Together, neural circuits of the SCN and IGL are complex and interconnected. As yet, few have been tested with respect to their involvement in rhythm regulation.
Keywords: circadian, neuroanatomy, suprachiasmatic, intergeniculate leaflet, efferent, afferent, retrograde, anterograde, serotonin, connectivity, transneuronal
The suprachiasmatic nucleus (SCN), master clock of the circadian system (Stephan and Zucker, 1972; Inouye and Kawamura, 1979; Green and Gillette, 1982; Ralph, et al., 1990), sits astride the supraoptic commissures, and well-positioned to receive photic input. This possibility became evident with the 1972 discovery of the retinohypothalamic tract (RHT) (Hendrickson, et al., 1972; Moore and Lenn, 1972). Since that time, there has been progressive elaboration of the circadian visual system. Exploration of the intergeniculate leaflet (IGL) began in 1974 with the demonstration that it projects to the SCN (Swanson, et al., 1974). Subsequent studies showed that the IGL is retinorecipient (Hickey and Spear, 1976), contains neuropeptide Y-IR cells (Card and Moore, 1982; Moore, et al., 1984) and provides NPY terminals to the SCN (Card and Moore, 1982). The IGL became the first site distal to the SCN acknowledged as contributing to circadian system regulation with the 1984 demonstration that NPY infused into the SCN would elicit circadian rhythm phase shifts (Albers and Ferris, 1984; Albers, et al., 1984).
With each experiment devoted to analysis of SCN or IGL efferent and afferent anatomy, the breadth of connections possibly contributing to the circadian rhythm system has grown. Presently, there are more than 100 brain regions that are potential contributors to circadian rhythm regulation. Eventually, each of these must be individually tested to determine whether it is a true member of the extended circadian rhythm system. The present reality is that very few brain regions are known to make such contributions because the rhythm-related functions of rather few regions have thus far been explored. Areas having or possibly having such functions include the SCN itself (Moore and Eichler, 1972), the IGL (Harrington and Rusak, 1986; Pickard, et al., 1987; Janik and Mrosovsky, 1994), paraventricular thalamus (Moga and Moore, 2000), the subparaventricular (sPVz) zone (Schwartz, et al., 2009), dorsomedial hypothalamus (DM) (Chou, et al., 2003), the habenula (Paul, et al., 2011), a poorly determined part of the pretectum and tectum (Marchant and Morin, 1999), and the dorsal (DR) and median (MnR) raphe nuclei (Meyer-Bernstein and Morin, 1999).
One intention of the present manuscript is to demonstrate the massive knowledge increase, both in breadth and depth, about the neuroanatomical substrate of the circadian system. Another is to demonstrate deficiencies in that knowledge. It is certainly true that not all brain regions identified here as projecting directly or indirectly to the SCN are necessarily part of an extended circadian rhythm system. But if such connections do exist, they may have a rhythm-related function. It is reasonable to expect that any route afferent to the circadian clock might alter its function in some fashion or other. The present anatomically-oriented discussion is necessarily open-ended precisely because, despite the large expansion in factual knowledge, there are few guiding principles that facilitate understanding of the routes by which the master SCN circadian clock is modified by various stimuli or the efferent routes through which it alters phase and function of various outputs.
The relationship between the sleep regulatory system and the circadian system exemplifies some of the difficulties. The sleep system functions on an oscillating foundation provided by the SCN, while also providing feedback to SCN function (Schaap and Meijer, 2001; Deboer, et al., 2003; Deboer, et al., 2007; Deboer, et al., 2007; Houben, et al., 2009). There are multiple candidate routes by which the SCN can influence the sleep system or, in turn, be influenced by it. These include connections with the ventrolateral preoptic area (VLPO) (Novak and Nunez, 2000; Chou, et al., 2002; Deurveilher and Semba, 2003), possibly with lateral hypothalamic (LH) neurons of the orexin (OX) system (Abrahamson, et al., 2001; Schwartz, et al., 2011) or through IGL connections with brainstem sleep regulatory nuclei (Morin and Blanchard, 2005). Neither system can be fully understood without knowing the contribution of each.
A clearly incomplete aspect of the SCN afferent anatomy concerns which stimuli modify rhythmicity and what anatomical routes convey the stimulus information to the circadian clock. The route by which light alters rhythm function is well established (Hendrickson, et al., 1972; Moore and Lenn, 1972) and is being refined regularly (Chen, et al., 2011). In contrast, the exact nature of the stimulus by which locomotion modifies circadian clock function is not known, nor is the route by which the stimulus reaches the IGL through which it exerts its effect on the SCN (Mistlberger, et al., 2003). In a few instances, as with the gonadotrophin releasing hormone (GnRH) pathway to the SCN (Jennes and Stumpf, 1980; Merchenthaler, et al., 1984), a general function can be inferred from the neuropeptide involved, but there are no data bearing on if, how or why a GnRH pathway alters rhythmicity although it is well known that a circadian clock controls GnRH release (Williams, et al., 2011).
There is also a large amount of information available concerning SCN efferents and their targets. It is likely that all efferent projections carry circadian rhythm phase information to distal targets in other systems. The truth of this statement has not been demonstrated, nor has it been shown that different projections carry similarly phased timing information. Those sorts of studies await the research efforts of neurophysiologists. The neuroanatomists have provided a list of many brain regions and their related systems which receive SCN efferents. Those systems must be examined to determine the extent to which each receives and utilizes information about rhythm phase. And, the hope is that each such system will be studied to determine the extent to which it feeds back onto the circadian clock, influencing the very system that provides the baseline timing information.
Suprachiasmatic Nucleus Intrinsic Anatomy
Distributions of Cell Phenotypes
Critical to the understanding of SCN intrinsic anatomy is the overall morphology of the nucleus and its species dependence. The SCN of hamster and mouse is an upright, roughly tear-drop shaped nucleus (somewhat more elliptical in the hamster), but in the rat, the shape is oblate. As a result, the robust retinorecipient region in the rat SCN, compared to that of mouse and hamster, has a larger medial-lateral axis than a dorsal-ventral one. This modification is associated with a change in the distribution of VP-IR neurons from a predominantly dorsomedial position to a position capping the more ventral, densely retinorecipient region (Morin, et al., 2006).
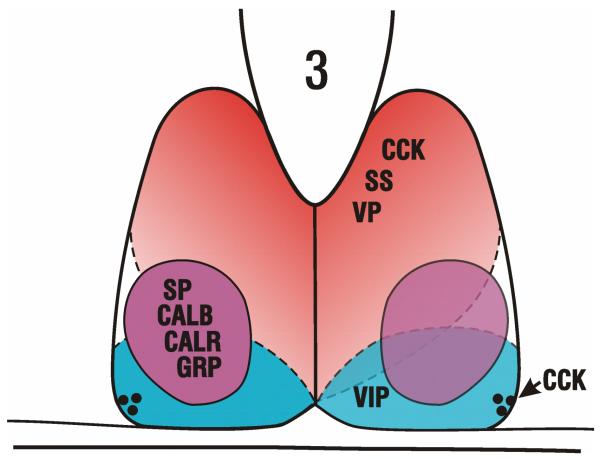
Several different neuron classes that are positive for one or more neuropeptides have been identified in the SCN (Fig. 1), including vasopressin (VP), vasoactive intestinal polypeptide (VIP), gastrin releasing peptide (GRP), substance P (SP), neurotensin (NT), enkephalin (ENK), somatostatin (SS) and cholecystokinin (CCK). Presence of VP- and VIP-immunoreactive (IR) cells is a constant across most mammals (but possibly not in the mink or musk shrew (Tokunaga, et al., 1992; Martinet, et al., 1995)). In addition, all SCN neurons appear to contain GABA (Moore and Speh, 1993; Abrahamson and Moore, 2001; Morin and Blanchard, 2001). Presence and location of the other neuromodulator phenotypes varies across species or have not been studied sufficiently to allow generalizations (see (Smale, et al., 1991; Goel, et al., 1999). A critical feature that appears common to rat, mouse and hamster is the presence of a central SCN subnucleus (SCNce) identifiable by a fairly circumscribed collection of GRP-IR neurons. This feature also appears in the SCN of a diurnal species, the Nile grass rat (Smale and Boverhof, 1999). The SCN of this species is very similar to those of the mouse, rat and hamster.
Figure 1.
Schematic showing the approximate locations of cell phenotypes in the hamster SCN. The regional boundaries are “fuzzy” and should not be rigidly applied. Also, the schematic applies only to the mid-caudal part of the SCN where the SCNce is evident. For example, both VP and CCK cells occupy most of the rostral third of the nucleus. The black dots ventrolaterally signify the presence of a few CCK neurons at that location. VP – vasopressin; SS – somatostatin; CCK – cholecystokinin; SP – substance P; CALB – calbindin; CALR – calretinin; GRP – gastrin releasing peptide; VIP – vasoactive intestinal polypeptide. The figure is modified after Fig. 1 in Morin and Allen (2006).
The hamster SCNce has cells identified as containing VIP, SP and GRP, and is easily identified by cells immunoreactive to calbindin (CALB) (LeSauter, et al., 2002). The bulk of the VIP-IR cells lie ventral to the SCNce. This raises a functional question, that is, which is more important, the neuropeptide content or the location of the cell(s)? Likewise, VIP-IR and GRP-IR are co-localized in some hamster SCN neurons (Aioun, et al., 1998), but obviously this can only occur where the distributions of the two cell types overlap. Again, whether the location of the cells or their neuromodulator content is of primary functional importance is not known.
The region containing the GRP-IR cells is fairly discrete and centrally located in the rat, but substantially overlaps with and extends more caudally than the region containing VIP-IR cells (Moore, et al., 2002). The evolving neuroanatomical work has been joined by more recent physiological and genetic studies of specific cell functions, especially as related to VIP (Cutler, et al., 2002; Harmar, et al., 2002; Aton, et al., 2005). This neuropeptide and its receptor, VPAC2, appear to be central to maintaining the cell-cell communication necessary for persistent SCN rhythmicity. The focus on neuropeptides capable of sustaining rhythmicity in an arrhythmic SCN has recently been expanded from VIP as the primary intrinsic neuropeptide synchronizer of SCN circadian rhythmicity to hierarchically include VP and GRP neurons as second and third order neuromodulators also capable of sustaining rhythmicity (Maywood, et al., 2011).
The topography of intrinsic SCN neuron phenotypes (Fig. 1) has been reviewed in detail (Morin and Allen, 2006; Morin, et al., 2006), and the appropriateness of various proposed organizational schemes has been questioned (e.g., core/shell; dorsomedial/ventrolateral). Contributing to the sense that such schemes are inadequate have been recent descriptions of cell distributions not consistent with the plan. In particular, the SCN of hamster, mouse and rat has a unique distribution of cells identified containing Fox-3 (formerly, NeuN) protein. These cells occupy a region that includes most of the lateral half of the SCN, including the SCNce (as seen in hamster and mouse) and the area lateral and dorsal to it (Geoghegan and Carter, 2008; Morin, et al., 2011). Similarly, the distributions of cells expressing the Magel2 gene or containing neuroglobin-IR include both portions of both nominal “core” and “shell” portions of the SCN (Kozlov, et al., 2007; Hundahl, et al., 2010).
A unique distribution of calcitonin-gene-related peptide-(CGRP) IR neurons has been described in the ventrolateral part of the caudal two thirds of the mouse SCN (Park, et al., 1993). The distribution of the CGRP-IR cells would probably overlap medially and rostrally with the VIP cell distribution. Sparse cells are found near the midline, with more evident laterally in the SCN or on its border. The distribution appears continuous with a decreasing concentration of cells extending dorsally into anterior hypothalamus. These cells are not present in rat, guinea pig or rabbit (Park, et al., 1993).
The complexity of the SCN with respect to the anatomy of its fields of innervation and distributions of its constituent cell groups has been discussed previously (Morin, et al., 2006). The presence of novel cell distributions that transcend widely referenced SCN “divisions” emphasizes SCN complexity as a characteristic consistent with molecular, cellular and physiological analyses of intercellular communication across the whole nucleus. Recent studies emphasize the view that SCN rhythmic function is an emergent property of a cellular network capable of harnessing and organizing rhythmic activities of individual and groups of cells (Buhr, et al., 2010; Welsh, et al., 2010; Mohawk and Takahashi, 2011).
SCN Intrinsic Connections
Cell-cell communication by neuropeptides may be the primary contributor to normal rhythmicity, but the network properties of the whole SCN appear to provide rhythm stability in the absence of genes contributing to the molecular circadian clockworks (Liu, et al., 2007; Welsh, et al., 2010). This fact demands more attention to gathering fundamental neuroanatomical information concerning cell-cell connectivity within the SCN.
In his 1980 paper, van den Pol (van den Pol, 1980) described the existence of extensive gap junctions between glial cells. Subsequently, the presence of presumptive gap junctions between neurons has been observed and confirmed (Colwell, 2000; Shinohara, et al., 2000; Rash, et al., 2001; Jobst, et al., 2004; Long, et al., 2005).
The dendritic arbor of neurons of the hamster SCNce, whether or not they contain CALB, orient a few degrees medial from the vertical (Jobst, et al., 2004). A small percentage of these cells apparently have gap junction contacts with adjacent neurons. The CALB cells in the hamster SCNce contact neurons identifiably VIP-, GRP- or CCK-IR and each of those cell types project back onto the CALB-IR cells. In contrast, VP-IR neurons appear to have no connections with CALB-IR cells (LeSauter, et al., 2002).
A confocal laser scanning microscopic study (Romijn, et al., 1997) analyzed connections between cells representing the major phenotypes in rat SCN, including VP-, VIP-, GRP- and SS-IR neurons. The study showed abundant and reciprocal synaptic connections between VP and VIP cells, VP and GRP cells, VP and SS cells, VIP and SS cells, and between SS and GRP cells. Neurons immunoreactive to VIP, GRP or both VIP/GRP also reciprocally connect with each other. The widespread interconnectivity of cells in groups differentially located within the SCN may facilitate the rapid distribution of both intrinsic pacemaker and afferent information across the entire SCN. In addition, the complex features of the SCN cellular network have been suggested to be characteristic of an “oscillatory/reverberative activity generated by a non-linear self-organizing system” (Romijn, et al., 1997).
The term, “dynamic anatomy,” has been used in reference to the large apparent changes in the spatial distribution of certain neuropeptidergic cell groups according to circadian time (Morin, 2007). Thus, the SCN volume occupied by cells expressing VP mRNA appears to vary by a factor of 5-10 across the day. The number of actual intrinsic SCN connections with cells seemingly devoid of VP may be a function of circadian time rather than absence of VP. The same logic applies to the connections of all other classes of neuropeptidergic cells not yet tested for their dynamic anatomy.
A related phenomenon may be reflected in the ability of certain groups of SCN neurons to functionally reorganize according to past experience. Thus, an SCN “division” may emerge under the influence of photoperiod or gonadal hormone history (Watanabe, et al., 2006; Yan, et al., 2010; Karatsoreos, et al., 2011) (see above statement by Romijn et al. (1997). Collectively, the information concerning functional reorganization within the SCN reinforces the notion that the entire SCN achieves and maintains its status as the master circadian clock precisely because of its complexity (Buhr, et al., 2010; Welsh, et al., 2010; Maywood, et al., 2011; Mohawk and Takahashi, 2011).
In mice, GRP neurons identify an SCN region thought to be homologous to the hamster SCNce. Because of this, and because GRP is known to be a stabilizer and modulator of the circadian clock (Antle, et al., 2005; Brown, et al., 2005; Maywood, et al., 2011), mouse GRP neurons have been used as the basis for a thorough, systematic and very necessary anatomical analysis of intrinsic SCN connectivity in mouse (Drouyer, et al., 2010). These studies show that GRP cells are structurally different than similarly located non-GRP cells, having a dendritic tree with more branches, greater overall dendritic length and longer axons. Additionally, the non-GRP cells have a preferred axonal orientation about 10 degrees lateral to the vertical. With respect to cell-cell appositions, there are more from non-GRP cells on VP somas or fibers than from GRP cells. There are also smaller differences between the two cell types. Further, dye-coupled cells were more commonly seen adjacent to GRP cells (3.8/cell vs 1.3/non-GRP cell) with the vast majority found in the region containing the GRP cells and only 15% in the area of VP-IR neurons (Drouyer, et al., 2010).
An important result obtained by Drouyer and colleagues (2010), consistent with the Romijn et al (1997) studies, was the demonstration reciprocal connections between centrally located GRP or non-GRP neurons and VP or non-VP cells in the dorsomedial regions SCN. This result overturns a previous conclusion (Leak, et al., 1999), based on virus tract tracing methods, that SCN connectivity is one-way, with centrally or ventrally located cells generally projecting dorsomedially. This conclusion has also been disputed by Luo and Aston-Jones (2009) who consistently observed a pattern of retrograde virus labeling opposite to that described by Leak et al. (1999) who used the same method. An unresolved issue related to the retrograde virus tracing studies concerns the extent to which the virus fills cells in the SCN. Studies from several labs (e.g., (Luo and Aston-Jones, 2009)) suggest that the Bartha strain of pseudorabies virus, whether injected into the brain or peripheral organs, may eventually fill all or nearly all SCN neurons. Accurate documentation of SCN cell filling would provide useful information regarding the extent to which SCN neurons are interconnected.
SCN Afferent Pathways
Retinohypothalamic Tract
Initial studies of the RHT demonstrated a simple projection from retina to the SCN (Hendrickson, et al., 1972; Moore and Lenn, 1972). With increasing investigation and newer methods, the RHT has been shown to be increasingly complex (Johnson, et al., 1988; Muscat, et al., 2003). The cells of origin are primarily melanopsin-containing, intrinsically photoreceptive retinal ganglion cells (ipRGCs) (Provencio, et al., 2000; Gooley, et al., 2001; Berson, et al., 2002; Hattar, et al., 2002; Provencio, et al., 2002; Morin, et al., 2003; Sollars, et al., 2003). These cells not only function as photoreceptors that pass phase-setting photic information to the SCN, they also function as conduits of photic information arriving from classical rod and cone photoreceptors (Goz, et al., 2008; Guler, et al., 2008; Hatori, et al., 2008).
Different types of ipRGCs have been observed in the mouse retina. Documentation exists for as many as five, currently designated M1-M5 (Ecker, et al., 2010; Chen, et al., 2011; Schmidt, et al., 2011). The dendritic stratification patterns allow division of the five types into three distinguishable classes with type M1 dendrites found exclusively in the OFF sublamina of the inner plexiform layer. The M3 type shows bilaminar dendritic stratification in the OFF and the ON sublamina of the inner plexiform layer. The M2, M4 and M5 types have dendrites exclusively in the ON sublamina. The M1 type is further divisible according to whether the cells express the transcription factor, Brn3b. The M2, M4 and M5 type are distinguishable according to quantitative morphological characteristics. Melanopsin has not been positively identified in the M4 or M5 types, but these cells show weak responses to light.
For the purposes of this review, the most important point is that functional differences related to the ipRGC axonal projection anatomy are emerging. The Brn3b-negative ipRGCs comprise about 10% of the melanopsin cell population, but the number is sufficient to allow normal rhythm entrainment (Badea, et al., 2009; Schmidt, et al., 2011; Schmidt, et al., 2011). In the absence of these cells, innervation of the SCN by the remaining ipRGCs remains robust and apparently normal. In contrast, ipRGC projections to the IGL are greatly reduced and those to the OPT are completely absent (Chen, et al., 2011). These anatomical alterations appear to account for the greatly reduced pupillary light reflex in mice lacking Brn3b-negative ipRGCs (absence of projection to the OPT), attenuation of the period lengthening effect of constant light (diminished projection to the IGL) and the maintenance of apparently normal entrainment (SCN innervation is apparently normal). This research represents the beginning of a process by which the RHT is being parsed into functional anatomical divisions (Badea, et al., 2009). Presumably, such efforts will eventually merge with existing reports that individual ipRGCs project to two or more retinorecipient nuclei (Pickard, 1985; Gooley and Saper, 2003; Muscat, et al., 2003). Bifurcating projections of ipRGCs are completely ipsilateral except those to the SCN which is innervated bilaterally.
It should be noted that the RHT projects to numerous hypothalamic regions in addition to the SCN (Johnson, et al., 1988; Morin and Blanchard, 1999; Major, et al., 2003). Some of these are sufficiently close to the SCN that most lesions directed at it are likely to destroy fibers of passage to neighboring or more caudal hypothalamic sites. Table 1 lists the brain nuclei, largely hypothalamic, receiving projections specifically from the RHT. For comparison purposes, Table 1 also indicates which of these nuclei have projections to or from the SCN and IGL.
Table 1.
Brain nuclei with retinal terminals received via the retinohypothalamic tract and connections of those nuclei with the IGL and SCN.a,b
| Retinorecipient Nuclei |
From IGL |
To IGL |
From Retina |
From SCN |
To SCN |
|---|---|---|---|---|---|
| AH | ++ | +/− | +++ | + | + |
| DA | + | − − | + | + | + |
| DM | + | − − | + | + | +++ |
| LH | − − | − − | ++ | ||
| LPO, ventral | + | − − | + | − − | − − |
| Pa | − − | +/− | + | + | |
| Re | +++ | − − | +/− | + | − − |
| sPVz | + | + | ++ | +++ | |
| SCN | ++++ | c | +++++ | +++d | d |
| MTu | + | +/− | + | + | ** |
| VLPO | ++ | − − | + | ++ | |
| VMH | ++ | − − | + | − − | +++ |
based on data from (Morin, et al., 1994; Moga and Moore, 1997; Morin and Blanchard, 1999; Moore, et al., 2000; Krout, et al., 2002)
−− no evident connection; +/− very sparse; +sparse; ++ modest; +++ moderate; ++++ dense; +++++ very dense
in the rat, SCN cells may project to the IGL as determined by retrograde tracer(Card and Moore, 1989), but in the hamster, the such labeled cells lie on the border or just outside the SCN as do cells projecting to other parts of the subcortical visual shell(Morin, et al., 1992; Morin and Blanchard, 1998; Morin and Blanchard, 1999)
dcells project to the contralateral SCN
The retina projects to approximately 30 brain regions, with ipRGCs projecting to most of them (Fig. 2). In addition to the large number retinorecipient nuclei, there are abundant connections between them, especially with respect to their relationships to the IGL. As indicated, the retinorecipient IGL projects to most of the subcortical visual nuclei which, in turn, project back to the IGL, creating a variety of theoretical loops through which photic information might indirectly influence function of the SCN.
Figure 2.
Diagrammatic representation of brain regions receiving retinal input from melanopsin-containing ipRGCs (blue lines; red fill) in the mouse (data from (Hattar, et al., 2006; Ecker, et al., 2010). The figure also identifies retinorecipient regions not innervated by ipRGCs (unfilled). Connections of the IGL with nuclei of the subcortical visual shell are also shown (green lines). The reciprocal connections between the DR and MnR, and the MnR to SCN projections are identified by yellow fill and yellow lines. The figure is modified after Fig. 21.3 in (Morin, 2012).
Retinorecipient nuclei of the hypothalamus are also heavily interconnected. For example, the medial preoptic area (MPOA), subparaventricular zone (sPVz), DM and posterior hypothalamus (PH) each receive projections from, and provide reciprocal innervation to, the SCN, median preoptic nucleus (MnPO) and ventrolateral preoptic nucleus (VLPO) (Chou, et al., 2002; Deurveilher, et al., 2002; Deurveilher and Semba, 2003; Kriegsfeld, et al., 2004).
It should also be noted, that among the convoluted pathways of the broader circadian visual system there is a direct visual projection to the dorsal raphe nucleus (DR) in several species, including the gerbil, degus, cat and rat (Foote, et al., 1978; Shen and Semba, 1994; Fite, et al., 1999; Fite and Janusonis, 2001), but has not been seen in the hamster (Morin and Blanchard, unpub. data).
SCN Terminal Fields
There are three major terminal fields in the SCN, that of the RHT, of the median raphe projection and of the GHT from the IGL (Fig. 3). Each field has its most robust density in a different location, but upon close examination terminals from the three input pathways can be found in essentially all parts of the nucleus.
Figure 3.
SCN schematic showing the relative positions of (A) retinal terminal fields, and (B) geniculohypothalamic tract terminal field (left side) containing terminals from four IGL neuron classes (NT – neurotensin; NPY – neuropeptide Y; ENK – enkephalin; GABA – gamma amino butyric acid) and (right side) the variably dense serotonergic (5-HT – serotonin) terminal field. In (A), the red area indicates the region of densest retinal input which arrives predominately from the contralateral retina. The green region indicates the area receiving input predominately from the ipsilateral retina. Note that the density is not uniform for any terminal distribution. As for Fig. 1, the boundaries should not be rigidly applied. The figure is modified after Fig. 1 in Morin and Allen (2006).
From the Retinohypothalamic Tract
There are species differences in the topography of RHT terminals in the SCN. Most obvious is the difference in ipsi- vs contralateral retinal contribution to SCN innervation which varies substantially. The predominant retinal input arrives from the contralateral retina. In the golden mantled ground squirrel, 100% of the terminals appear to be from the contralateral retina (Smale, et al., 1991), whereas in the mouse, each retina contributes 50% of SCN terminals (Morin, et al., 2006). The terminal field in the hamster SCN has been described, based on cholera toxin-HRP methods, to be approximately equal bilaterally (Johnson, et al., 1988), but more recently, a subtle ipsi-/contralateral difference in SCN innervation was noted (Fig. 3A). Cholera Toxin beta subunit (CTB) tract tracing revealed the ventral and medial SCN to be innervated predominately by the ipsilateral retina with the more centrally located region of densest retinal input arriving predominately from the contralateral eye (Muscat, et al., 2003). The pattern of hamster SCN innervation by the RHT places it between species (such as the ground squirrel mentioned above) receiving exclusively contralateral input and some primates with predominately ipsilateral input (Magnin, et al., 1989).
In the rat, there is virtually no retinal innervation of the dorsomedial SCN containing most of the VP-IR cells, although the retina provides terminals to the ventral 20-25% of the VP-IR neuron distribution (Morin, et al., 2006). Both hamster and mouse SCN have substantially greater dorsomedial retinal terminals than the rat (Muscat, et al., 2003; Morin, et al., 2006).
The RHT terminal field in the SCN substantially fills the nucleus, at least for hamster and mouse (Muscat, et al., 2003; Hattar, et al., 2006; Morin, et al., 2006).The rat, with its oblate SCN, presents a somewhat different situation and may represent a true species difference. It is commonly stated that the RHT terminal plexus in the rat occupies the ventrolateral SCN with no dorsomedial SCN innervation (but see Fig. 4 in Moore et al. (2002) for an example of terminal “patches” within the dorsomedial rat SCN). In terms of generalities across species, the important points are, first, that there are regions of dense retinal innervation in all species which grade into regions of less dense or even sparse innervation and, second, there is a far from perfect match between the retinal terminal field (whether tightly or loosely defined) and a particular group of cells. For example, most of the VP cells are found dorsomedially where retinal innervation is sparse, but such cells are also plentiful, especially in mouse, more centrally where retinal innervation is dense. The actual relationship between the retinal terminal field and cell phenotype is known only for CALB-IR cells on which electron microscopy has demonstrated RHT terminals (Bryant, et al., 2000).
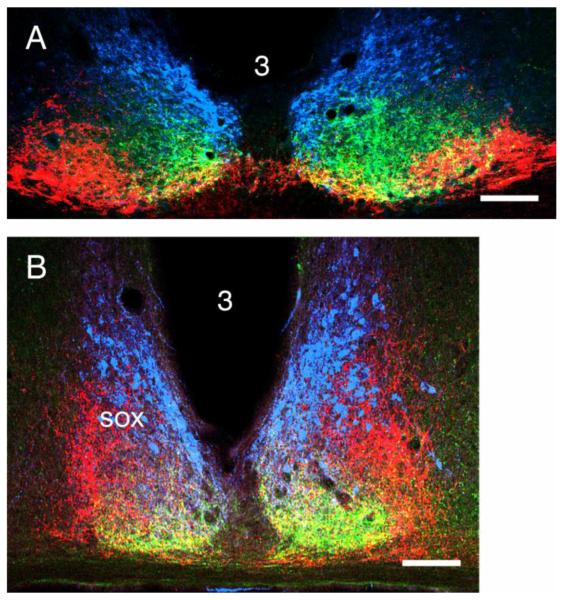
Figure 4.
Triple label images of SCN anatomy in (A) the rat and (B) mouse stained for vasopressin (blue), serotonin (green) and retinal projections (red). The yellow color does not indicate co-localization. Rather, it results from merging information from adjacent red and green pixels or because green and red items are superimposed in the original tissue. The oblate rat SCN enables the triple stain procedure to give the tissue a laminar appearance not visible in the more upright SCN of the mouse. The figure is modified after Figs. 5 and 8 in Morin et al. (2006).
Indirect evidence for abundant innervation of the entire SCN comes from studies of light-induced FOS protein. Here also, initial reports from the hamster focused on FOS in nuclei of SCNce neurons (Rusak, et al., 1990), but subsequent studies have demonstrated light-induced FOS throughout the SCN, specifically including VP-IR neurons (Castel, et al., 1997), as well as in adjacent hypothalamus (Guido, et al., 1999; Mahoney, et al., 2001; Muscat and Morin, 2006; Vidal and Morin, 2007). The little appreciated paper by Guido et al. (1999) is noteworthy for several reasons. The authors show (a) that light-induced FOS is present throughout the entire SCN, but is denser in the SCNce region; (b) there is abundant FOS induced in peri-SCN hypothalamus; (c) the intensity of FOS staining in the nucleus of any particular SCN cell varies from pale gray to fully black (i.e., there is cell-specific differential induction, rather than an all-or-none response); (d) the level of FOS induction by light varies according to circadian time; and (e) the spatial distribution pattern of light-induced FOS in the SCN also varies according to time (see also (Romijn, et al., 1996)). These results clearly demonstrate consistency with the view that the SCN is broadly innervated by the retina, but that events at the cellular levelmust be hindering or facilitating FOS expression according to time of day and locus within the SCN. The incomplete induction of FOS (e.g., pale gray vs black staining) also appears to vary according to region and time of day (Guido, et al., 1999).
From the Geniculohypothalamic Tract
NPY-IR has been the most widely used marker of the GHT terminal field in the SCN. The rat GHT terminal field demarcated by NPY-IR reveals a fairly uniform distribution within the ventral two thirds of the nucleus (Morin, et al., 2006). The region with densest retinal terminals in the rat overlaps the ventral and lateral distribution of NPY terminals (Fig. 3B). In the mouse, NPY-IR terminals appear to be sparser than in either rat or hamster. As with RHT terminals, NPY-IR terminals are very scarce in periventricular SCN, in the region closely correspondent to that containing most of the VP-IR neurons (Card and Moore, 1989; Morin, et al., 2006). The hamster SCN also has few GHT terminals in the dorsomedial SCN region. In fact, there is a relative paucity of NPY-IR terminals around the entire terminal field in the SCN (Morin and Blanchard, 2001). In the mouse, NPY-IR terminals appear to more thoroughly occupy the SCN (especially the perimeter), although the distribution is otherwise similar to that of the hamster (Morin and Blanchard, 2001; Morin, et al., 2006). In all three species, NPY-IR terminals are densest ventro-centrally, grading to a sparse distribution laterally, dorsally and dorsomedially.
In the hamster SCN, three additional terminal fields have been identified as arising from the IGL. Double label retrograde tracing studies identify IGL cells containing ENK-, NT- and GABA-IR cells as projecting to the SCN (Morin and Blanchard, 2001). The distributions of ENK-IR terminals in the SCN are very similar to the NPY terminal distribution. Lesions of the IGL abolish immunoreactivity of terminals in the SCN to NPY, ENK and NT. GABA in the SCN after IGL lesions is confounded with the GABA remaining in all SCN neurons and processes (Moore and Speh, 1993; Morin and Blanchard, 2001).
The calcitonin-gene-related peptide (CGRP) terminals in the mouse SCN (Park, et al., 1993) tend to be ventrolaterally located, with most found on or adjacent to the lateral SCN boundary, extending dorsally and laterally into anterior hypothalamus. CGRP-IR cells are found in the SCN proper, in adjacent hypothalamus, and in the IGL. At the present time, it is not known whether the IGL neurons containing CGRP project to the SCN through the GHT, but the vastly different distribution of CGRP terminals suggests that they are not from the IGL.
From the Median Raphe Nucleus
The serotonergic terminal field in the SCN arises from median raphe neurons and has features common to mouse, hamster and rat. In all three, the serotonergic terminals are found throughout the SCN, but are densest ventrally and medially; density is greatest ventromedially (Fig. 3B). Innervation density gradually becomes sparse dorsally and dorsolaterally; centrally, the innervation is modest (Meyer-Bernstein and Morin, 1996). In the rat, the plexus appears as a medio-laterally oriented ellipse contained within the ventral two thirds of the nucleus and shifted to the medial side, substantially sharing the location with VIP-IR neurons (Morin, et al., 2006). The serotonergic terminals also overlap with the ventral part of the dorsomedial SCN region containing most of the VP-IR neurons. Further dorsally among the VP-IR cells, the serotonergic terminals are sparse. The distributions of the serotonin terminals, retinal terminals and VP-IR neurons, although overlapping, differ in density and distribution to such an extent that under certain evaluation conditions, the rat SCN can be imaged as a three-part layered structure (Fig. 4).
In mouse and hamster, the serotonergic terminal distributions in the SCN are quite similar. The most obvious similarities are the nearly complete innervation of the nucleus, albeit with substantial region-dependent density differences, and the crescent shape of the terminal field pattern (Figs. 3,4). In both hamster and mouse, terminals are quite dense in an arc extending from a dorsomedial point and curving ventrally, laterally and then slightly dorsolaterally. The greatest terminal density is along the medial and ventral SCN border, grading to sparse innervation centrally and very sparse dorsolaterally. There is substantial overlap between the retinal and serotonergic terminals and the dorsomedial region containing most VP-IR cells, but it is not 100%. Because the mouse SCN is upright (unlike the oblate rat SCN), it does not appear layered as in the rat, but does allow clear spatial distinctions between the same two terminal fields and the region of VP-IR neurons, at least with respect to their regions of greatest density.
The rhythm related functions of the serotonergic system have been thoroughly reviewed (Morin, 1999; Mistlberger, et al., 2000). The serotonergic system modulates both photic and non-photic regulation of the circadian system.
From GnRH neurons
Release of GnRH is under the temporal control of the SCN and results in synthesis and release of luteinizing hormone from the anterior pituitary followed soon thereafter by ovulation (de la Iglesia and Schwartz, 2006; Tonsfeldt and Chappell, 2012). Robust projections of the gonadotrophin releasing hormone (GnRH) cell system are present in rat and hamster, but descriptions are limited, especially as they relate to the SCN (Jennes and Stumpf, 1980; Merchenthaler, et al., 1984). The hamster GnRH projections are visible as modest innervation of the rostral half of the SCN (Fig. 5A, B). Further caudally, the region of the SCNce is fairly devoid of GnRH fibers and terminals which remain evident dorsomedially and in adjacent hypothalamus ventrally, laterally and dorsally (Fig. 5C,D).
Figure 5.

(A) Parasagittal section through the hamster SCN showing GnRH fibers and terminals in rostral part of the nucleus and surrounding hypothalamus, but absent from the caudal SCN (arrow). (B-D) Coronal images of the SCN showing GnRH fibers and terminals in the SCN and adjacent hypothalamus, but also showing their absence from the SCNce (arrow). ox – optic chiasm; sox – supraoptic commissure. Bar = 100 μm.
In addition to the SCN afferent GnRH projections, there are SCN efferents that synapse on GnRH cells (De la Iglesia, et al., 1995; van der Beek, et al., 1997; Schwartz, et al., 2011). Thus, there is clear reciprocity between the gonadotropin regulatory and circadian rhythm systems, although it is not known whether the SCN cells contacting GnRH neurons also receive input from GnRH neurons.
From VIP neurons
The events in the foregoing paragraph occur prior to ovulation. If vaginocervical stimulation occurs in the female rat around the time of ovulation, one result is the appearance of two, accurately timed, daily peaks in prolactin secretion (Smith, et al., 1975). These events serve to transform a non-pregnant hypothalamo-hypophyseal-ovarian condition into one that can maintain pseudopregancy or pregnancy until the corpus luteum becomes functional. The daily prolactin peaks require the presence of a functional SCN and which synchronizes their phase to the prevailing photoperiod (Poletini, et al., 2010).
Prolactin is under inhibitory control by tuberoinfundibular dopamine neurons (Freeman, et al., 2000; Gerhold, et al., 2002), but its release can be stimulated by the posterior pituitary hormone, oxytocin (Arey and Freeman, 1992). Both the infundibular dopamine and paraventricular oxytocin cells receive direct input from VIP neurons located in the SCN with the VIP acting on via VPAC2R receptors (Horvath, 1997; TeclemariamMesbah, et al., 1997; Gerhold, et al., 2001; Gerhold, et al., 2002; Mahoney, et al., 2007). Rhythmic output from the VIP cells is presumed to inhibit both the critical dopamine neurons and the oxytocin neurons with an unknown excitatory factor facilitating oxytocin release from the VIP-inhibited neurons (Egli, et al., 2004; Egli, et al., 2006). Readers are encouraged to explore the large body of work by M.E. Freeman that addresses multiple facets of the neuroendocrine system and its temporal coordination by the SCN.
From other brain regions
The SCN sends projections directly to about 15 regions (depending on the definition of “region”), primarily in the hypothalamus (Watts, et al., 1987; Kalsbeek, et al., 1993; Morin, et al., 1994). In contrast, the SCN receives input from approximately 35 areas revealed by standard retrograde tracing (Table 2). Injection of virus into the SCN to obtain transneuronal retrograde cell labeling reveals approximately 48 additional sites that appear to connect to the SCN via two or more synapses. See Table 1 of Krout et al. (2002) for a large list that identifies regions in which CTB and retrograde transneuronal virus tracer identify cells projecting to the SCN by mono- or multisynaptic pathways. Krout et al. summarize their abundant anatomical observations by categorizing the SCN afferent regions as relating to olfaction, corticoid-sensitive temporal lobe function, chemosensitive circumventricular organ function and autonomically-regulated visceral organ function. Note that many of the brain regions evaluated by Krout et al. (2002) show substantial between-case variability in virus labeling. Definitive judgments regarding whether or not such regions actually project to the SCN are not always possible.
Table 2.
Brain regions projecting directly to the rat SCNa
| Amydalohippocampal zone | Lateral septum | Parastriatal N |
| Anterior Hth N | Laterodorsal tegmental N | Paraventricular thalamic N |
| Anterodorsal preoptic N | Locus coeruleus | Piriform cortex |
| Anteroventral periventricular Hth N | Medial amygdala | Posterior Hth N |
| Anteroventral preoptic N | Medial preoptic area | Precommissural N |
| Arcuate N | Medial preoptic N | Subfornical organ |
| BNST, principal N | Medial pretectal N | Subparaventricular zone |
| Claustrum | Median preoptic N | Tuberomammillary N |
| Dorsal Hth area | Median raphe N | Ventral subiculum |
| Dorsomedial Hth N | N incertus | Ventromedial Hth N |
| Infralimbic cortex | Olivary pretectal N | Zona incerta |
| Intergeniculate leaflet | Parabrachial N |
based on data from (Moga and Moore, 1997;Krout, et al., 2002), using the nomenclature of Krout et al.
The lateral habenula has recently been proposed as an extra-SCN rhythm regulatory site that primarily modulates the distribution of activity within a “window oscillator” controlled by the SCN (Paul, et al., 2011). Severance of the fasciculus retroflexus, the primary output pathway of the habenula greatly disrupts the temporal locomotor structure without damaging the overt circadian rhythmicity of hamsters. The habenula is one of the distal structures that projects multisynaptically to the SCN (Krout, et al., 2002). It also receives direct input from the SCN (Kalsbeek, et al., 1993; Morin, et al., 1994). It is likely that the SCN is the target of information arriving directly or indirectly from more than 80 brain areas. Except for the obvious locations (IGL, retina, raphe nuclei) there is very little known about the individual or collective impact of these inputs on circadian rhythm function.
SCN Efferent Pathways
Kalsbeek et al. (1993) used a somewhat unusual method to study SCN efferent anatomy by making lesions in the SCN and determining which neuropeptidergic pathways were lost. The hamster SCN has dense efferent projections immunoreactive to VP, VIP and GRP (Table 3). When the SCN is completely lesioned, these projections are absent, supporting the view that the cells of origin are in the SCN. Projections to the VMH, identified by anterograde tracing with Phaseolus, do not contain any of the three neuropeptides evaluated. Both VP- and VIP-IR projections extend rostrally to contact GnRH cells directly (van der Beek, et al., 1993; Mahoney and Smale, 2005).
Table 3.
Neuropeptide projections in the hamster brain that originate from cells in the SCN (after Kalsbeek et al., 1993).
| Structure & SCN inputa |
VP | VIP | GRP | ||||
|---|---|---|---|---|---|---|---|
| present | from SCN | present | from SCN | present | from SCN | ||
| BST | + | yes | no | yes | no | yes | no |
| LSv | + | sparse b | no | yes | no | ||
| POA | + | yes | partly | sparse | yes | ||
| MPN | + | yes | partly | sparse | yes | ||
| PVA | + | yes | yes | yes | yes | yes | yes |
| PVP | + | no b | − − | yes | no | ||
| APV | + | yes | yes | yes | no | ||
| PVN | + | yes | yes | yes | yes | yes | yes |
| subPVN c |
+ | yes | no | yes | yes | yes | yes |
| DMH | + | yes | yes | yes | yes | yes | yes |
| VMH | + | no | − − | no | − − | no | − − |
| SON | + | yes | no | no | − − | yes | yes |
| Ce | − | yes | no | yes | no | yes | no |
| LHb | + | no b | − − | ||||
| IGL | − | no | − − | no | − − | yes | no |
SCN input to the various nuclei is from PHAL tracing in hamster (Morin et al., 1994) except for the updated IGL information (see text);
yes, in mouse and rat;
equivalent to the upper retrochiasmatic region in Morin et al. (l994)
There is substantial agreement between the primary publications (Watts and Swanson, 1987; Watts, et al., 1987; Kalsbeek, et al., 1993; Morin, et al., 1994; Canteras, et al., 2011) providing information about rat and hamster SCN efferent projection patterns. Results from a more recent study of the California ground squirrel are consistent with prior results, but the authors have elected to emphasize innervation of a greater number of sub-regions, rather than a more limited number of primary regions (Canteras, et al., 2011). As initially described, there are three major projection patterns (Watts and Swanson, 1987; Watts, et al., 1987) (Fig. 6). Pattern 1, and the most robust, consists of input to the periventricular hypothalamic nuclei, including the several divisions of the preoptic region rostrally, the anterior parvocellular paraventricular nucleus, the subparaventricular zone and retrochiasmatic area, the dorsomedial and ventromedial nuclei and the premammillary area (Watts and Swanson, 1987). The hypothalamic innervation pattern in the rat suggests the presence of three projection zones, the rostral periventricular, the retrochiasmatic and a subparaventricular area that includes more caudal periventricular nuclei. Although the pattern is very similar in the hamster (Kalsbeek, et al., 1993; Morin, et al., 1994), equivalent zonal distinctions were not made because the hypothalamic radiation of efferent projections appears to be broad and non-specific with respect to proximal targets (Morin, et al., 1994). The point deserving emphasis is that hypothalamic nuclei caudal to the SCN are densely innervated by SCN projections in both rat and hamster.
Figure 6.
Schematic showing diencephalic and basal forebrain areas receiving projections from the SCN (black pathways) and IGL (red pathways). Blue fill identifies regions receiving input from both SCN and IGL; those receiving only IGL input have red fill. One region (PM) has yellow fill and is the only target of SCN projections that does not also receive input from the IGL. Line thickness indicates relative size of the projection. See the list of Abbreviations for anatomical names.
SCN projection patterns 2 and 3 are to the septum and to the anterior paraventricular thalamus plus several divisions of the bed nucleus of the stria terminalis, respectively. In general, the septal innervation pattern is similar for rat and hamster, although the majority of the innervation is seen in the intermediate division of the rat lateral septum, but in the ventral division in the hamster (Watts, et al., 1987; Morin, et al., 1994). In both species, the entire anterior paraventricular thalamic nucleus receives SCN innervation. Innervation from the SCN has also been described for the adjacent parataenial nucleus (Watts and Swanson, 1987; Watts, et al., 1987; Morin, et al., 1994; Leak and Moore, 2001).
A projection to the amygdala has been described (Morin, et al., 1994), but it is more likely that amygdaloid innervation arrives from cells in the retrochiasmatic area. It should be noted that cells in the retrochiasmatic area project to many of the same targets as the SCN (Morin, et al., 1994).
A projection to the intergeniculate leaflet (IGL) has been reported in the rat (Card and Moore, 1989), the cells for which are located in the dorsomedial SCN with larger numbers found in adjacent anterior hypothalamus. The initial hamster report concurred, but was based on anterograde tracing of SCN efferents (Morin, et al., 1994) (see also (Kriegsfeld, et al., 2004)). It appears more likely that hamster SCN neurons do not project to the IGL. Retrograde tracer injected into the IGL labels sparse cells on or adjacent to the dorsal and lateral borders of the caudal SCN, with more in the retrochiasmatic area (Morin, et al., 1992; Morin and Blanchard, 1999), but not in the SCN proper. Retrogradely labeled neurons are seen in the same areas after tracer injection into the hamster IGL, ventrolateral geniculate (VLG), posterior limitans nucleus (PLi), olivary pretectal nucleus (OPT), medial pretectal nucleus (MPT) and commissural pretectal area (CPT) (Morin and Blanchard, 1998). Visualization of such a SCN-IGL projection by anterograde tracing methods may be a false positive resulting because of tracer spillage from the injection site into adjacent hypothalamus or, just as likely, from uptake by peri-SCN neurons which have dendrites extending into the nucleus. Some of these dendritic connections with the SCN are as long as 250 μm (Morin, et al., 1994). The technical difference relating to the exact location of SCN/peri-SCN cells projecting to the IGL may very well be moot with respect to whether or not they acquire information from the SCN convey it to the IGL.
Particular effort has been made to identify multisynaptic pathways from the SCN to the locus coeruleus (LC) and from the SCN to the ventral tegmental area (VTA) (Aston-Jones, et al., 2001; Luo and Aston-Jones, 2009). In each study, Bartha strain pseudorabies virus was injected into the nucleus of interest and the tracer was followed retrogradely to the SCN as neurons became sequentially infected over a 60 hr interval. Simultaneous injection of the tracer, cholera toxin B subunit, revealed the circuit to have a synapse in the DM. This circuit appears to rhythmically modulate LC neuronal activity according to circadian time (Aston-Jones, et al., 2001), possibly regulating rhythmic arousal (Berridge, 2008).
In the case of the VTA, it too receives indirect input from the SCN, but in a more round-about fashion via the medial preoptic nucleus (MPN). Lesions of this area eliminated most cell labeling in the SCN after virus injection in the VTA. As in the LC, neuronal activity is rhythmically regulated, with maximal activity during the activity phase. Thus, a multisynaptic pathway appears able to rhythmically modulate the VTA, a region known to be involved in the regulation of sleep/arousal and reward (Tsujino and Sakurai, 2009).
Organization of SCN cells with efferent projections
The locations of SCN neurons projecting to specific brain regions is the subject of some controversy in part because there have been insufficient studies of the issue. Watts and Swanson (1987) examined SCN innervation of 9 different brain regions and Leak and Moore (2001) explored innervation of 11 brain regions. Detailed comparisons of the two sets of are difficult for several reasons. The Watts and Swanson (1998) study was the first of its kind and, as such, simply documented the positions of cells retrogradely labeled from the various injections. Leak and Moore (2001) provide a more schematic perspective consistent with the basic core and shell model set forth earlier (Moore, 1996). The contrast between the perspectives of the two publications is stark, with Watts and Swanson stating, “At the present time, there is no conclusive evidence for a functional dichotomy between dorsal and ventral parts of the [SCN].” The essential word in that view is “dichotomy.” It remains the focus of substantial research efforts directed toward demonstrating different “divisions” of the SCN and that they behave differently with respect to rhythm regulation, as suggested by Moore and colleagues (1996, 2001, 2002).
One technical point is worth emphasizing: no two tracer injections are ever made in the identical location, nor should there any expectation that the pattern of label uptake will be the same after two different injections. Given the small number of cases typically examined in tract tracing studies, the between-injection variability may overwhelm any consistency in the details of the results.
A second point concerns the locations to which groups of SCN neuropeptidergic cells project in the brain. The SCN lesion procedure used by Kalsbeek et al. (1993) specifically addressed this issue in the hamster. They showed that the DM and anterior paraventricular thalamus (PVA) receive moderately robust projections from both VP and VIP cells in the SCN. These cell types are found, in general, in the dorsomedial and ventral SCN, respectively, but retrograde tracer predominately labels cells in the SCN “shell” regardless of whether the injection was made in the DM or PVA (Leak and Moore, 2001). For a more thorough case by case comparison of the Watts and Swanson (1998) injection results with those of Leak and Moore (2001), see (Morin and Allen, 2006). The most important point seems to be that the exact distribution of SCN cells projecting to any target cannot be known at the present time because there are too few cases available for analysis.
Retrograde tracer injections have also been placed in targets of the hamster SCN (Kriegsfeld, et al., 2004). Microscopy images show numerous labeled cells scattered fairly homogeneously across the SCN following an injection in the DMH or ventral LS; and after a VLPO injection, small numbers of labeled cells are seen both in the SCNce and in a dorsal area containing VP-IR neurons. However, insufficient detail is available to support any conclusion about the relationship between tracer injection site and location of retrogradely labeled cells in the SCN of this species.
The above studies agree that the distributions of labeled cells following retrograde label injection are not uniform across the SCN. This result should not be surprising given the clustering of specific neuropeptide-containing cell types in the SCN and their moderately target-specific efferent, neuropeptidergic projections (Kalsbeek, et al., 1993). For example, anterograde Phaseolus tracing of SCN projections reveals a pattern of parataenial thalamic innervation that is closely mimicked by the distributions of VP- and VIP-IR fibers which are eliminated by SCN lesions. Immunohistochemical analysis indicates that VP and VIP neurons have virtually non-overlapping distributions in the SCN. This means that spatially distinct sets of SCN cells project to the same target region.
In their pioneering studies of rat IGL and SCN connections, Card and Moore (1989) injected each IGL with a different retrograde tracer. The results not only demonstrated retrogradely labeled cells in the SCN and peri-SCN region, they provided a unique demonstration that individual SCN neurons project bilaterally to each IGL. This result may be important because it speaks directly to the possibility that individual SCN neurons provide identical phase information to many targets. There has not been a similar study since the Card and Moore effort. Thus, there is presently no information concerning the extent to which individual SCN cells project to multiple targets.
Transneuronal retrograde viral tracer has been injected into the vitreous of the eye (Pickard, et al., 2002; Smeraski, et al., 2004), DM and sPVz (Leak, et al., 1999) and a variety of sympathetic or parasympathetic targets (reviewed in (Bartness, et al., 2001)). Each of these procedures yields cell labeling in the SCN. In the case of retinal injections, the virus appears to infect the SCN via a peripheral route from the eye to the nucleus of Edinger-Westphal, to the olivary pretectal nucleus and IGL, and ultimately to the SCN (Pickard, et al., 2002). An important technical note concerns the fact that the virus does not provide anterograde infection of the SCN via the RHT after an intravitreous eye injection.
To the extent that it is possible to discern from published photomicrographs, virtually all SCN cells eventually appear to be infected with virus (e.g., 60-67 hr after a VTA or paraventricular hypothalamus (Pa) injection (Aston-Jones, et al., 2001; Luo and Aston-Jones, 2009)). There has not been a systematic investigation of the percentage of SCN neurons ultimately infected by transneuronal virus or if there are cell types or regions in the SCN that do not become infected. Such studies would provide useful information regarding the extent to which SCN neurons are interconnected.
Intergeniculate Leaflet Intrinsic Anatomy
The IGL is homologous with the medial division of the cat VLG (Pu and Pickard, 1996; Van der Gucht, et al., 2003; Nakamura and Itoh, 2004) and with the primate pregeniculate nucleus (Moore, 1989; Van der Gucht, et al., 2003). The IGL is a nucleus of relatively large volume despite its typical presentation as a small laminar structure intercalated between the DLG and VLG. The overall length of the IGL is evident by embryonic day 14, well before the final adult shape of the lateral geniculate complex is completed (Botchkina and Morin, 1995).
The hamster IGL contains NPY-, ENK-, NT- and GABA-IR cells that project to the SCN. There does not appear to be any topographical order to the cell types, but there is co-localization of neuromodulators (Morin and Blanchard, 2001). The extent of co-localization varies substantially. About 100% of NT-IR cells contain NPY-IR and 50% of NPY-IR cells also contain NT-IR. About 50% of cells positive for NPY are also positive for ENK-IR, and the opposite is also true. In the rat, the anatomical details for ENK differ from those in the hamster with ENK-IR cells of the IGL projecting to the contralateral IGL and not to the SCN (Card and Moore, 1989; Takatsuji and Tohyama, 1989). All IGL neurons of the rat may contain GABA-IR (Moore and Speh, 1993), but this is not readily apparent in the hamster (Morin and Blanchard, 2001). Abundant CGRP-IR neurons have been demonstrated in the rat IGL, but not reported in other species. (Park, et al., 1993). At least some of these cells project to the contralateral IGL.
The IGL of the mouse, Siberian hamster, golden hamster and rat each contain a robust terminal plexus of SP-IR fibers (Morin, et al., 1992; Moore and Card, 1994; Piggins, et al., 2001) The origin of the SP projection is not known, although it may be from the laterodorsal tegmentum (LDTg) or the pedunculopontine tegmentum (PPTg) both of which have numerous SP-IR neurons (Kohlmeier, et al., 2002) and cells that project to the IGL (Horowitz, et al., 2004).
There is ample literature describing rhythm-related functions of the IGL and the innervation of the SCN by NPY projections through the GHT. Most of the functional studies have been with hamsters. In the rat, one study indicates a role of the IGL in entrainment (Edelstein and Amir, 1999). In the mouse, IGL lesions do not yield responses to constant light that are equivalent to what has been reported after similar lesions in hamsters (Pickard, 1994; Morin and Pace, 2002). However, other mouse studies have indicated that an intact IGL is necessary for rhythm regulation by certain non-photic stimuli (Marchant and Mistlberger, 1996; Marchant, et al., 1997).
Caution should be observed with respect to attributing all IGL functions exclusively to rhythm regulation or to NPY. It is apparent on anatomical grounds that the IGL has multiple functions (in particular, those related to eye movements during sleep; see below). In addition, NPY cells in the IGL project to pretectal nuclei (Morin and Blanchard, 2001) and could contribute to rhythm regulation via an indirect influence on the SCN. Note, for example, that there do not appear to be direct retinal connections to NPY cells in the IGL( (Thankachan and Rusak, 2005), but see (Takatsuji, et al., 1991)). Although light induces FOS protein in IGL neurons, including some that contain NPY (Janik, et al., 1995; Muscat and Morin, 2006), light-induced FOS is seldom found in cells projecting to the SCN (Peters, et al., 1996; Muscat and Morin, 2006). An explanation for the observation of light-induced FOS in NPY-IR IGL cells may lie with the fact that such cells also project to the pretectum. Retrograde tracer injected into the pretectum co-localizes with light-induced FOS-IR in IGL cells (Muscat and Morin, 2006) and there are abundant NPY- and ENK-IR projections from the IGL to the pretectum (Morin and Blanchard, 1997). The two results (Thankachan and Rusak, 2005; Muscat and Morin, 2006), suggest the presence of interneurons or other intervening cells between the retinal terminals and cells of origin of the GHT.
It should also be noted that novel wheel access induces FOS in both NPY and non-NPY neurons in the IGL (Janik and Mrosovsky, 1992; Janik, et al., 1995), but not in cells projecting directly to the SCN (Muscat and Morin, 2006). While the behavioral data strongly support the view that NPY released by GHT terminals in the SCN regulates circadian phase in response to certain non-photic stimuli (Johnson, et al., 1988; Biello, et al., 1994), the pathway by which those stimuli gain access to and influence the activity of IGL neurons is obscure.
The pretectum and/or deep superior colliculus are implicated in the regulation of triazolam-, but not novel wheel-induced, circadian rhythm phase control (Marchant and Morin, 1998). Electrical stimulation of the same region greatly attenuates light-induced phase shifts. These subcortical visual areas have substantial interconnections with the IGL (Morin and Blanchard, 1998), possibly accounting for the ability of pretectal/tectal and IGL lesions to block the response to triazolam. In the rat, the phenomenon of dark-induced REM sleep is also prevented by lesions of the pretectum/superior colliculus (Miller, et al., 1998; Miller, et al., 1999), but there is no information as to whether the IGL is a part of the sleep-regulatory circuitry.
IGL Efferent and Afferent Connections
Tracing studies have elaborated on the degree of IGL connectivity to the point of demonstrating projections to over 100 brain regions (Mikkelsen, 1990; Morin and Blanchard, 1998; Morin and Blanchard, 1999; Moore, et al., 2000; Morin and Blanchard, 2001; Vrang, et al., 2003). Three principles of IGL connectivity have emerged: 1) Abundance - the projections include many targets; 2) Bilaterality - most IGL projections are bilateral, a feature that is particularly evident in the subcortical visual shell; and 3) Reciprocity - about half the regions receiving IGL projections send efferents back to the IGL (Fig. 2; see (Morin and Blanchard, 2005) and (Morin and Blanchard, 1998)for a discussion). These attributes are not exclusive to the IGL, but are also characteristic of adjacent ventral thalamic nuclei, including the VLG, subthalamic nucleus and zona incerta (Harrington, 1997; Morin and Blanchard, 1998; Morin and Blanchard, 1999; Power and Mitrofanis, 2001; Horowitz, et al., 2004; Morin and Blanchard, 2005). Notably, the IGL projects to most regions innervated by the SCN (Fig. 6).
There is very little known about the function of the vast majority of IGL efferent and afferent pathways. The GHT is the exception to that rule. Nevertheless, clues to function reside in the anatomy. One such is the fact that the IGL is innervated by orexin (hypocretin) neurons residing in the lateral hypothalamus and zona incerta (McGranaghan and Piggins, 2001; Mintz, et al., 2001; Nixon and Smale, 2004; Vidal, et al., 2005). A primary function of the OX system is sleep regulation (Sakurai, 2007; Burt, et al., 2011). The circadian basis of sleep can theoretically be influenced via direct SCN projections to OX-IR neurons (Abrahamson, et al., 2001; Schwartz, et al., 2011), but other studies suggest that there is little if any direct innervation of OX neurons by the SCN (Sakurai, et al., 2005; Yoshida, et al., 2006). The sleep-regulatory cells in the VLPO receive direct and indirect SCN input (Novak and Nunez, 2000; Chou, et al., 2002; Deurveilher and Semba, 2003) and sleep may be regulated through multisynaptic routes to brain stem sleep regulatory nuclei via OX projections to the IGL (Morin and Blanchard, 2005) or through the locus coeruleus or the ventral tegmental area (Aston-Jones, et al., 2001; Luo and Aston-Jones, 2009).
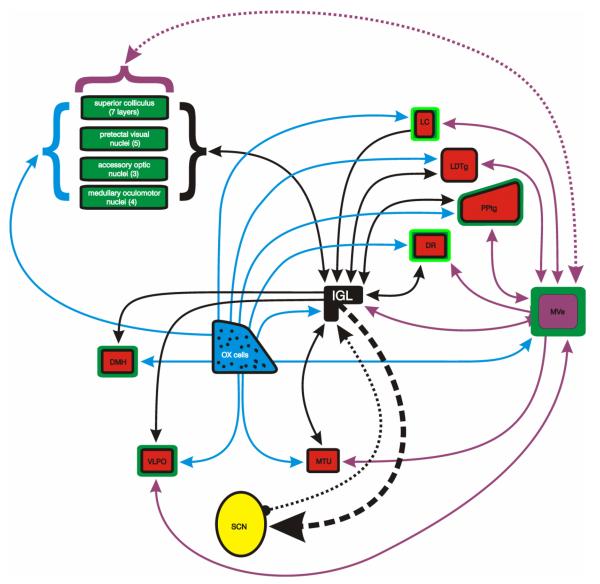
The IGL, because of its particular distribution of efferents, could conceivably act as a funnel through which rhythmically governed sleep regulatory information is distributed. Of potentially great, but unexplored importance, are the IGL projections to brain nuclei known to be involved with visuomotor, sleep functions, or the orexin system (Table 4). The IGL projects to 38 of 45 nuclei (84.4%) concerned with visuomotor function; to 14 of 16 nuclei (87.5%) concerned with sleep; and 57 of 65 areas (87.6%) bearing OX terminals. In addition, the IGL receives input from the medial vestibular nucleus (MVe), a region involved in maintaining equilibrium and coordination of head and eye movements (Leigh and Zee, 1999; Leigh and Zee, 1999) and possibly circadian rhythm regulation (Fuller, et al., 2002; Fuller and Fuller, 2006). The IGL also projects to many (36) regions innervated by MVe cells. A more thorough presented of the relationship between the several systems, the IGL connections and areas with MVe connections is provided elsewhere (see Table 1 in Morin and Blanchard, 2005). The MVe also projects to 12 out of 16 sleep-related nuclei and to 25 of 45 visuomotor nuclei, providing an anatomical rationale for the MVe being a regulator of eye movements during sleep as suggested earlier by lesion studies (Pompeiano and Morrison, 1965; Pompeiano and Morrison, 1966). The abundant connectivity of the IGL to sleep, equilibrium, and visuomotor control areas (Fig. 7) supports the view that, in addition to its role in circadian rhythm regulation, a second major function of the IGL concerns the control of eye movements during sleep.
Table 4.
Relationship between brain regions with direct IGL efferent or afferent connections and visuomotor, sleep, or orexin systems
| Functional Systems | Projections | ||||
|---|---|---|---|---|---|
| Visuomotor | Sleep | Orexin | From IGL (PHAL) | To IGL (CTB) | |
| Hypothalamus | |||||
| DM | + | + | + | + | − |
| MTu | + | + | + | + | + |
| VLPO | + | + | + | + | − |
| subPVz | + | + | + | + | |
| Thalamus | |||||
| IGL | + | + | + | + | |
| PP | + | + | + | + | |
| VLG | + | + | + | + | |
| ZI | + | + | + | + | |
| Pretectum | |||||
| APT | + | + | + | ||
| CPT | + | + | + | + | |
| NOT | + | +1 | + | − | |
| OPT | + | +1 | + | + | + |
| PLi | + | + | + | + | |
| Superior colliculus | |||||
| DpG | + | + | + | + | |
| DpWh | + | + | + | − | |
| SuG | + | + | + | + | |
| InG | + | + | + | + | |
| InWh | + | + | + | + | |
| Op | + | + | + | + | |
| Zo | + | + | + | − | |
| Accessory optic nuclei | |||||
| DT | + | + | + | + | |
| LT | + | + | + | + | |
| MT | + | + | + | + | |
| Periaqueductal gray | |||||
| DLPAG | + | + | + | + | |
| DMPAG | + | + | + | ||
| LPAG | + | + | + | + | |
| MPAG | + | + | + | + | |
| Oculomotor complex | |||||
| 3 | + | + | − | ||
| 4 | + | + | − | ||
| Dk | + | + | + | ||
| EW | + | + | + | − | |
| IMLF2 | + | + | − | ||
| MA3 | + | + | − | ||
| SU3 | + | + | + | + | |
| Additional midbrain nuclei | |||||
| CnF | + | + | + | ||
| DpMe | + | + | + | ||
| LDTg | + | + | + | + | |
| LDTgV | + | + | + | + | |
| PaR | + | − | + | + | |
| PPTg | + | + | + | + | + |
| PR | − | + | − | ||
| RRF | + | + | + | ||
| VLTg | + | + | − | ||
| Raphe nuclei | |||||
| CLi | + | + | + | − | |
| DR | + | + | + | + | |
| MnR | + | − | − | ||
| PMnR | − | + | − | ||
| RIP | + | − | + | − | |
| RLi | + | + | − | ||
| RMg | + | + | − | ||
| RPa | + | + | − | ||
| Pontine Region | |||||
| Bar | + | − | + | ||
| CGM | + | + | − | ||
| CGPn | + | + | − | ||
| KF | + | + | − | ||
| LC | + | + | + | − | + |
| LPBC | + | + | − | ||
| LPBE | + | + | − | ||
| LPBS | + | + | − | ||
| PnC | + | + | + | ||
| PnO | + | + | + | + | |
| PnV | + | + | − | ||
| RtTg | + | + | + | − | |
| SPTg | + | + | + | ||
| SubCA | + | + | − | − | |
| Medullary Region | |||||
| DPGi | + | + | + | − | − |
| GiA | + | + | − | ||
| GiV | + | + | − | ||
| IOM | + | + | − | ||
| lOPr | + | + | − | ||
| IRt | + | + | − | ||
| LPGi | + | + | + | − | |
| LVe | + | − | + | ||
| MVe | + | + | − | + | |
| PCRt | + | + | + | − | |
| Pr | + | + | − | B | |
| SpVe | + | + | − | + | |
| SuVe | + | − | − | + | |
| Cerebellum | |||||
| Int | + | + | + | − | |
| Med3 | + | + | − | − | |
OPT and/or NOT
IMLF, same as interstitial nucleus of Cajal
Med, same as fastigial nucleus
See Morin & Blanchard (2002) for references regarding the functional systems.
Figure 7.
Schematic presentation emphasizing the relationship between the IGL and sleep-regulatory nuclei (red fill), nuclei of the orexin system (blue fill and blue projections), nuclei of the visuomotor system (green fill or green outline) and the vestibular system (purple fill and purple projections). The dotted line between the MVe and superior colliculus indicates that only the deep gray later is connected reciprocally with the MVe, although there is also an intermediate gray projection to MVe. The GHT is indicated by the thick dashed line from IGL to SCN and the putative reciprocal connection by thinner dotted line.
Dozens of brain regions project to the IGL with the theoretical possibility that each could influence SCN function via the GHT. This is not likely, as demonstrated by studies involving injection of transneuronal virus tract tracer into the SCN. One clear result from this procedure is the absence of labeled neurons in the superior colliculus or lateral pretectal nuclei (Krout, et al., 2002; Horowitz, et al., 2004). This contrasts with the many retrogradely labeled cells present in those areas following CTB tracer injection in the IGL (Morin and Blanchard, 1998; Horowitz, et al., 2004). These observations demonstrate the existence of at least two functional sets of IGL cells, those that receive inputs from the visual midbrain, but do not project to the SCN, and those that project to the SCN, but do not receive input from visual midbrain. The former are likely to be concerned with visuomotor functions of the midbrain, and the latter with circadian rhythm regulation (Horowitz, et al., 2004).
The serotonergic system contributes to circadian rhythm regulation either through its direct projection from the MnR to the SCN or via an indirect DR projection to the IGL. There is evidence supporting function of both routes to the extent that electrical stimulation of DR or MR elicit similar behavioral and physiological responses-related pathways are involved in circadian rhythm function (Meyer-Bernstein and Morin, 1999; Glass, et al., 2003). In fact, under normal conditions, both DR and MR projections may participate in rhythm regulation because of their reciprocal connections (Tischler and Morin, 2003). See Fig. 5 in Morin and Allen (2006) for a diagrammatic representation of redundant routes for achieving serotonergic regulation of the circadian system by either the DR or the MnR.
Anatomy of the Human Circadian System
The literature concerning the intrinsic anatomy of the human SCN is sparse, as are studies of SCN connectivity. Readers are referred to the body of work by J.P. Dai, D.F. Swaab, R.M.Buijs and colleagues describing RHT in relationship to VP, VIP and neurotensin cell distributions (Dai, et al., 1998), co-localization of VIP and VP in SCN cells (Romijn, et al., 1999), SCN-efferent VP and VIP projections (Dai, et al., 1997), anterogradely labeled SCN efferent projections (Dai, et al., 1998), and afferent connections with the DMH (Dai, et al., 1998). There are several noteworthy differences reported between the human data and results from rodents such as unexpected co-localization of VIP and VP and the sparse, limited distribution of retinal fibers in the hypothalamus. Also of note is an earlier publication (Moore, 1989) describing the presence of an IGL in monkey and human, as indicated by NPY cell and fiber labeling. This study also noted a monkey-rodent difference with the human SCN to the extent that the human SCN contains NPY-IR neurons whereas the other species do not.
Summary
On the one hand, there is a large degree of complexity in the retina with respect to how the three photoreceptor classes provide visual information to the circadian system. On the other hand, the product of complex photoreception appears to be simplified in the process of being funneled through ipRGCs to the SCN where it acts on individual circadian clock cells (first order oscillators) to modulate overall SCN circadian rhythm phase and period. Although the cell groups in the SCN have been well described, there is still little information regarding how the cells connect together or what each phenotype contributes to circadian rhythm regulation. The generation and maintenance of stable SCN rhythmicity appears to be the interactive product of rhythmic gene expression in SCN neurons and the complex interaction of many rhythmic cells (second order oscillation). The SCN, as master circadian clock, provides phase-regulatory information to a relatively modest number of brain regions, but receives input from many additional locations. Thus, there is another layer of complexity, one by which the SCN efferent and afferent anatomy creates potential third order oscillatory circuits feeding back on the SCN to modify the baseline rhythmicity. It is even conceivable that expression of molecular clockworks in brain regions having input to the SCN may contribute to a greater circadian system than has been described here (see (Guilding and Piggins, 2007) for a review).
Single and multisynaptic connections provide communication between the SCN and other systems, especially the hypocretin and sleep systems. Such communication is two-way, there being clear anatomical routes by which oscillatory behavioral systems can modify circadian rhythmicity. One such pathway is the GHT through which the IGL and a great many brain areas have real or theoretical direct access to the SCN. In addition to the GHT, the IGL projects to many brain regions with both SCN efferents and afferents. The broad, reciprocal connections between the IGL and the SCN add a third layer of theoretical circuitry that may contribute to rhythm regulation. Further, the anatomical relationships between the sleep/vestibular/visuomotor systems create the strong possibility that the IGL regulates eye movements during sleep. The simple fact about the circadian system anatomy is that there are a great many possible circuits that may be involved in rhythm regulation, but only a small fraction have thus far been evaluated for possible inclusion as part an extended circadian rhythm system.
ACKNOWLEDGEMENTS
Supported by NINDS grants NS061804 and NS22168 awarded to LPM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ABBREVIATIONS
| 3 | Oculomotor N |
| 4 | Trochlear N |
| AAA | Anterior amygdaloid area |
| AD | Anterodorsal thalamic N |
| AH | Anterior hypothalamic N |
| AHi | Amygdalohippocampal zone |
| APT | Anterior pretectal N |
| APTd | Anterior pretectal N, dorsal |
| APV | Anteroventral periventricular hypothalamic N |
| Bar | Barrington's N |
| BNST | Bed N. stria terminalis |
| BNSTpm | Bed N. stria terminalis, posteromedial |
| CGM | Central gray, medial part |
| CGPn | Central gray, pontine |
| Cl | Claustrum |
| CL | Centrolateral thalamic N |
| CLi | Caudal linear raphe N |
| CM | Centromedian thalamic N |
| CnF | Cuneiform N |
| CPT | Commissural pretectal N |
| DA | Dorsal hypothalamic area |
| Dk | N darkschewitsch |
| DLG | Dorsal lateral geniculate N |
| DLPAG | Dorsolateral periaqueductal gray |
| DM | Dorsomedial hypothalamic N |
| DMPAG | Dorsomedial periaqueductal gray |
| DpG | Superior colliculus, deep gray layer |
| DPGi | Paragigantocellular N, dorsal |
| DpMe | Deep mesencephalic, N |
| DpWh | Superior colliculus, deep white layer |
| DR | Dorsal raphe N |
| DT | Dorsal terminal N |
| EW | N Edinger-Westphal |
| GHT | Geniculohypothalamic tract |
| GiA | Gigantocellular reticular N, alpha part |
| GiV | Gigantocellular reticular N ventral part |
| IGL | Intergeniculate leaflet |
| IMLF2 | Interstitial N, medial longitudinal fasciculus |
| InG | Superior colliculus, intermediate gray layer |
| Int | Interposed cerebellar N |
| InWh | Superior colliculus, intermediate white layer |
| IOM | Inferior olive, medial N |
| IOPr | Inferior olive, principal N |
| IRt | Intermediate reticular N |
| KF | Kölliker-Fuse N |
| LC | Locus coeruleus |
| LD | Laterodorsal thalamic N |
| LDTg | Lateral dorsal tegmental N |
| LDTgV | Lateral dorsal tegmental N, ventral part |
| LH | Lateral hypothalamic N |
| LOT | N Lateral olfactory tract |
| LP | Lateral posterior N |
| LPAG | Lateral periaqueductal gray |
| LPBC | Lateral parabrachial N, central part |
| LPBE | Lateral parabrachial N, external part |
| LPBS | Lateral parabrachial N, superior part |
| LPGi | Lateral paragigantocellular N |
| LPO | Lateral preoptic N |
| LS | Lateral septum |
| LSV | Lateral septum, ventral |
| LT | Lateral terminal N |
| LVe | Lateral vestibular N |
| MA3 | Medial accessory oculomotor N |
| MeA | Medial amygdala |
| Med3 | Medial (fastigial) cerebellar N |
| MnR | Median raphe N |
| MPAG | Medial periaqueductal gray |
| MPN | Medial preoptic N |
| MPO | Medial preoptic area |
| MPT | Medial pretectal N |
| MT | Medial terminal N |
| MTu | Medial tuberal N |
| MVe | Medial vestibular N |
| NOT | N optic tract |
| Op | Superior colliculus, optic nerve layer |
| OPT | Olivary pretectal N |
| ot | Optic tract |
| ox | Optic chiasm |
| Pa | Paraventricular hypothalamic N |
| PAG | Periaqueductal gray |
| PaPA | Paraventricular hypothalamic N, anterior parvicellular |
| PaR | Pararubral N |
| PCRt | Parvicellular reticular N |
| PH | Posterior hypothalamus |
| Pir | Piriform cortex |
| PLi | Posterior limitans N |
| PM | Premammillary N |
| PMnR | Paramedian raphe N |
| PnC | Pontine reticular N, caudal part |
| PnO | Pontine reticular N, oral part |
| PnV | Pontine reticular N, ventral part |
| POA | Preoptic area |
| PP | Peripeduncular N |
| PPT | Posterior pretectal N |
| PPTg | Pedunculopontine tegmental N |
| Pr | Prepositus N |
| PrC | Precommissural N |
| PT | Parataenial thalamic N |
| PVA | Paraventricular thalamic N, anterior |
| PVP | Paraventricular thalamic N, posterior |
| RCh | Retrochiasmatic area |
| Re | N Reuniens, thalamus |
| RHT | Retinohypothalamic tract |
| RIP | Raphe interpositus N |
| RLi | Rostral linear raphe N |
| RMg | Raphe magnus N |
| RPa | Raphe pallidus N |
| RRF | Retrorubral field |
| RtTg | Reticulotegmental N, pons |
| S | Subiculum |
| SC | Superior colliculus |
| SCN | Suprachiasmatic N |
| SON | Supraoptic N |
| sox | Supraoptic commisures |
| SPTg | Subpedencular tegmental N |
| SpVe | Spinal vestibular N |
| sPVz | sub-Paraventricular hypothalamic zone |
| SU3 | Supraoculomotor periaqueductal gray |
| SubCA | Subcoeruleus N, alpha part |
| SubG | Subgeniculate N |
| SuM | Supramammillary N |
| SuVe | Superior vestibular N |
| Tu | Olfactory tubercle |
| VLG | Ventral lateral geniculate N |
| VLPO | Ventrolateral Preoptic N |
| VLTg | Ventrolateral tegmental area |
| VMH | Ventromedial hypothalamic N |
| VTA | Ventral tegmental area |
| ZI | Zona incerta |
| Zo | Superior colliculus, zonal layer |
REFERENCES
- 1.Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Research. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 4.Aioun J, Chambille I, Peytevin J, Martinet L. Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tissue Res. 1998;291:239–253. doi: 10.1007/s004410050994. [DOI] [PubMed] [Google Scholar]
- 5.Albers HE, Ferris CF. Neuropeptide Y: role in light-dark entrainment of hamster circadian rhythms. Neuroscience Letters. 1984;50:163–168. doi: 10.1016/0304-3940(84)90480-4. [DOI] [PubMed] [Google Scholar]
- 6.Albers HE, Ferris CF, Leeman SE, Goldman BD. Avian pancreatic polypeptide phase shifts hamster circadian rhythms when microinjected into the suprachiasmatic region. Science. 1984;223:833–835. doi: 10.1126/science.6546454. [DOI] [PubMed] [Google Scholar]
- 7.Antle MC, Kriegsfeld LJ, Silver R. Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J Neurosci. 2005;25:2447–2454. doi: 10.1523/JNEUROSCI.4696-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arey BJ, Freeman ME. Activity of oxytocinergic neurons in the paraventricular nucleus mirrors the periodicity of the endogenous stimulatory rhythm regulating prolactin secretion. Endocrinology. 1992;130:126–132. doi: 10.1210/endo.130.1.1727695. [DOI] [PubMed] [Google Scholar]
- 9.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nature Neuroscience. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 10.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat.Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol.Rhythms. 2001;16:196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- 13.Berridge CW. Noradrenergic modulation of arousal. Brain Res.Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 15.Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviorally induced phase shifts. Neuroscience. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 16.Botchkina GI, Morin LP. Specialized neuronal and glial contributions to development of the hamster lateral geniculate complex and circadian visual system. Journal of Neuroscience. 1995;15:190–201. doi: 10.1523/JNEUROSCI.15-01-00190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signalling. J Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryant DN, LeSauter J, Silver R, Romero MT. Retinal innervation of calbindin-D28K cells in the hamster suprachiasmatic nucleus: ultrastructural characterization. Journal of Biological Rhythms. 2000;15:103–111. doi: 10.1177/074873040001500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buhr ED, Yoo SH, Takahashi JS. Temperature as a Universal Resetting Cue for Mammalian Circadian Oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burt J, Alberto CO, Parsons MP, Hirasawa M. Local network regulation of orexin neurons in the lateral hypothalamus. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2011;301:R572–R580. doi: 10.1152/ajpregu.00674.2010. [DOI] [PubMed] [Google Scholar]
- 21.Canteras NS, Ribeiro-Barbosa ER, Goto M, Cipolla-Neto J, Swanson LW. The retinohypothalamic tract: Comparison of axonal projection patterns from four major targets. Brain Research Reviews. 2011;65:150–183. doi: 10.1016/j.brainresrev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Card JP, Moore RY. Ventral lateral geniculate nucleus efferents to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. Journal of Comparative Neurology. 1982;206:390–396. doi: 10.1002/cne.902060407. [DOI] [PubMed] [Google Scholar]
- 23.Card JP, Moore RY. Organization of lateral geniculate-hypothalamic connections in the rat. Journal of Comparative Neurology. 1989;284:135–147. doi: 10.1002/cne.902840110. [DOI] [PubMed] [Google Scholar]
- 24.Castel M, Belenky M, Cohen S, Wagner S, Schwartz WJ. Light-induced c-Fos expression in the mouse suprachiasmatic nucleus: Immunoelectron microscopy reveals co-localization in multiple cell types. European Journal of Neuroscience. 1997;9:1950–1960. doi: 10.1111/j.1460-9568.1997.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. Journal of Neuroscience. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colwell CS. Rhythmic coupling among cells in the suprachiasmatic nucleus. Journal of Neurobiology. 2000;43:379–388. doi: 10.1002/1097-4695(20000615)43:4<379::aid-neu6>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler DJ, Hardin H, Rees H, Maywood E, Hastings MH, Shena HM, Martens JM, Harrer C, Piggins HD. The mouse VPAC2 receptor confers SCN cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide (VIP) in vitro Society for Research on Biological Rhythms. 2002;8:37. [Google Scholar]
- 30.Dai JP, Swaab DF, Buijs RM. Distribution of vasopressin and vasoactive intestinal polypeptide (VIP) fibers in the human hypothalamus with special emphasis on suprachiasmatic nucleus efferent projections. Journal of Comparative Neurology. 1997;383:397–414. doi: 10.1002/(sici)1096-9861(19970714)383:4<397::aid-cne1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 31.Dai JP, Swaab DF, Van der Vliet J, Buijs RM. Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. Journal of Comparative Neurology. 1998;400:87–102. doi: 10.1002/(sici)1096-9861(19981012)400:1<87::aid-cne6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 32.Dai JP, Van der Vliet J, Swaab DF, Buijs RM. Human retinohypothalamic tract as revealed by in vitro postmortem tracing. Journal of Comparative Neurology. 1998;397:357–370. [PubMed] [Google Scholar]
- 33.Dai JP, Van der Vliet J, Swaab DF, Buijs RM. Postmortem anterograde tracing of intrahypothalamic projections of the human dorsomedial nucleus of the hypothalamus. Journal of Comparative Neurology. 1998;401:16–33. doi: 10.1002/(sici)1096-9861(19981109)401:1<16::aid-cne2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.De la Iglesia HO, Blaustein JD, Bittman EL. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport. 1995;6:1715–1722. doi: 10.1097/00001756-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 35.de la Iglesia HO, Schwartz WJ. Minireview: Timely ovulation: Circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- 36.Deboer T, Detari L, Meijer JH. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep. 2007;30:257–262. doi: 10.1093/sleep/30.3.257. [DOI] [PubMed] [Google Scholar]
- 37.Deboer T, Ruijgrok G, Meijer JH. Short light-dark cycles affect sleep in mice. European journal of neuroscience. 2007;26:3518–3523. doi: 10.1111/j.1460-9568.2007.05964.x. [DOI] [PubMed] [Google Scholar]
- 38.Deboer T, Vansteensel MJ, D,t ri L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat.Neurosci. 2003;6:1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 39.Deurveilher S, Burns J, Semba K. Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: a dual tract-tracing study in rat. European Journal of Neuroscience. 2002;16:1195–1213. doi: 10.1046/j.1460-9568.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 40.Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to the median preoptic nucleus in rat. Brain Research. 2003;987:100–106. doi: 10.1016/s0006-8993(03)03295-5. [DOI] [PubMed] [Google Scholar]
- 41.Drouyer E, LeSauter J, Hernandez AL, Silver R. Specializations of gastrin-releasing peptide cells of the mouse suprachiasmatic nucleus. The Journal of comparative neurology. 2010;518:1249–1263. doi: 10.1002/cne.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edelstein K, Amir S. The role of the intergeniculate leaflet in entrainment of circadian rhythms to a skeleton photoperiod. Journal of Neuroscience. 1999;19:372–380. doi: 10.1523/JNEUROSCI.19-01-00372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: Action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology. 2004;145:3386–3394. doi: 10.1210/en.2003-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME. Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. American Journal of Physiology-Endocrinology and Metabolism. 2006;290:E566–E572. doi: 10.1152/ajpendo.00427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fite KV, Janusonis S. Retinal projection to the dorsal raphe nucleus in the Chilean degus (Octodon degus) Brain Research. 2001;895:139–145. doi: 10.1016/s0006-8993(01)02061-3. [DOI] [PubMed] [Google Scholar]
- 47.Fite KV, Janusonis S, Foote W, Bengston L. Retinal afferents to the dorsal raphe nucleus in rats and Mongolian gerbils. Journal of Comparative Neurology. 1999;414:469–484. doi: 10.1002/(sici)1096-9861(19991129)414:4<469::aid-cne4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 48.Foote WE, Taber-Pierce E, Edwards L. Evidence for a retinal projection to the midbrain raphe of the cat. Brain Research. 1978;156:135–140. doi: 10.1016/0006-8993(78)90089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 50.Fuller PM, Fuller CA. Genetic evidence for a neurovestibular influence on the mammalian circadian pacemaker. Journal of Biological Rhythms. 2006;21:177–184. doi: 10.1177/0748730406288148. [DOI] [PubMed] [Google Scholar]
- 51.Fuller PM, Jones TA, Jones SM, Fuller CA. Neurovestibular modulation of circadian and homeostatic regulation: vestibulohypothalamic connection? Proceedings of the National Academy of Science of the United States of America. 2002;99:15723–15728. doi: 10.1073/pnas.242251499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geoghegan D, Carter DA. A novel site of adult doublecortin expression: neuropeptide neurons within the suprachiasmatic nucleus circadian clock. BMC.Neurosci. 2008;9:2. doi: 10.1186/1471-2202-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Research. 2001;919:48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- 54.Gerhold LM, Sellix MT, Freeman ME. Antagonism of vasoactive intestinal peptide mRNA in the suprachiasmatic nucleus disrupts the rhythm of FRAs expression in neuroendocrine dopaminergic neurons. Journal of Comparative Neurology. 2002;450:135–143. doi: 10.1002/cne.10307. [DOI] [PubMed] [Google Scholar]
- 55.Glass JD, Grossman GH, Farnbauch L, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J Neurosci. 2003;23:7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goel N, Lee TM, Smale L. Suprachiasmatic nucleus and intergeniculate leaflet in the diurnal rodent Octodon degus: Retinal projections and immunocytochemical characterization. Neuroscience. 1999;92:1491–1509. doi: 10.1016/s0306-4522(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 57.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neuroscience. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 58.Gooley JJ, Saper CB. A broad role for melanopsin in non-visual photoreception based on neuroanatomical evidence in rats. Journal of Neuroscience. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green DJ, Gillette R. Circadian rhythms of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Research. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- 61.Guido ME, Goguen D, De Guido L, Robertson HA, Rusak B. Circadian and photic regulation of immediate-early gene expression in the hamster suprachiasmatic nucleus. Neurosci. 1999;90:555–571. doi: 10.1016/s0306-4522(98)00467-9. [DOI] [PubMed] [Google Scholar]
- 62.Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? European Journal of Neuroscience. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- 63.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood E, Hastings MH. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 65.Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: Interrelated structures in the visual and circadian systems. Neuroscience and Biobehavioral Reviews. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- 66.Harrington ME, Rusak B. Lesions of the thalamic intergeniculate leaflet alter hamster circadian rhythms. Journal of Biological Rhythms. 1986;1:309–325. doi: 10.1177/074873048600100405. [DOI] [PubMed] [Google Scholar]
- 67.Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS.ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. Journal of Comparative Neurology. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hendrickson AE, Wagoner N, Cowan WM. An autoradiographic and electron microscopic study of retinohypothalamic connections. Zeitschrift fur Zellforschung. 1972;135:1–26. doi: 10.1007/BF00307084. [DOI] [PubMed] [Google Scholar]
- 71.Hickey TL, Spear PD. Retinogeniculate projections in hooded and albino rats: an autoradiographic study. Experimental Brain Research. 1976;24:523–529. doi: 10.1007/BF00234968. [DOI] [PubMed] [Google Scholar]
- 72.Horowitz SS, Blanchard JH, Morin LP. Intergeniculate leaflet and ventral lateral geniculate nucleus afferent connections: an anatomical substrate for functional input from the vestibulo-visuomotor system. Journal of Comparative Neurology. 2004;474:227–245. doi: 10.1002/cne.20125. [DOI] [PubMed] [Google Scholar]
- 73.Horvath TL. Suprachiasmatic efferents avoid phenestrated capillaries but innervate neuroendocrine cells, including those producing dopamine. Endocrinology. 1997;138:1312–1320. doi: 10.1210/endo.138.3.4976. [DOI] [PubMed] [Google Scholar]
- 74.Houben T, Deboer T, van Oosterhout F, Meijer JH. Correlation with behavioral activity and rest implies circadian regulation by SCN neuronal activity levels. Journal of Biological Rhythms. 2009;24:477–487. doi: 10.1177/0748730409349895. [DOI] [PubMed] [Google Scholar]
- 75.Hundahl CA, Hannibal J, Fahrenkrug J, Dewilde S, Hay-Schmidt A. Neuroglobin expression in the rat suprachiasmatic nucleus: colocalization, innervation, and response to light. J Comp Neurol. 2010;518:1556–1569. doi: 10.1002/cne.22290. [DOI] [PubMed] [Google Scholar]
- 76.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proceedings of the National Academy of Science of the United States of America. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janik D, Mikkelsen JD, Mrosovsky N. Cellular colocalization of Fos and neuropeptide Y in the intergeniculate leaflet after nonphotic phase-shifting events. Brain Research. 1995;698:137–145. doi: 10.1016/0006-8993(95)00878-t. [DOI] [PubMed] [Google Scholar]
- 78.Janik D, Mrosovsky N. Gene expression in the geniculate induced by a nonphotic circadian phase shifting stimulus. Neuroreport. 1992;3:575–578. doi: 10.1097/00001756-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 79.Janik D, Mrosovsky N. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Research. 1994;651:174–182. doi: 10.1016/0006-8993(94)90695-5. [DOI] [PubMed] [Google Scholar]
- 80.Jennes L, Stumpf WE. LHRH-SYSTEMS IN THE BRAIN OF THE GOLDEN-HAMSTER. Cell and Tissue Research. 1980;209:239–256. doi: 10.1007/BF00237629. [DOI] [PubMed] [Google Scholar]
- 81.Jobst EE, Robinson DW, Allen CN. Potential pathways for intercellular communication within the calbindin subnucleus of the hamster suprachiasmatic nucleus. Neuroscience. 2004;123:87–99. doi: 10.1016/j.neuroscience.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 82.Johnson RF, Morin LP, Moore RY. Retinohypothalamic projections in the hamster and rat demonstrated using cholera toxin. Brain Research. 1988;462:301–312. doi: 10.1016/0006-8993(88)90558-6. [DOI] [PubMed] [Google Scholar]
- 83.Johnson RF, Smale L, Moore RY, Morin LP. Lateral geniculate lesions block circadian phase shift responses to a benzodiazepine. Proceedings of the National Academy of Science of the United States of America. 1988;85:5301–5304. doi: 10.1073/pnas.85.14.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalsbeek A, Teclemariam-Mesbah R, Pevet P. Efferent projections of the suprachiasmatic nucleus in the golden hamster (Mesocricetus auratus) Journal of Comparative Neurology. 1993;332:293–314. doi: 10.1002/cne.903320304. [DOI] [PubMed] [Google Scholar]
- 85.Karatsoreos IN, Butler MP, LeSauter J, Silver R. Androgens Modulate Structure and Function of the Suprachiasmatic Nucleus Brain Clock. Endocrinology. 2011;152:1970–1978. doi: 10.1210/en.2010-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kohlmeier KA, Burns J, Reiner PB, Semba K. Substance P in the descending cholinergic projection to REM sleep-induction regions of the rat pontine reticular formation: anatomical and electrophysiological analyses. European Journal of Neuroscience. 2002;15:176–196. doi: 10.1046/j.0953-816x.2001.01829.x. [DOI] [PubMed] [Google Scholar]
- 87.Kozlov SV, Bogenpohl JW, Howell MP, Wevrick R, Panda S, Hogenesch JB, Muglia LJ, Van Gelder RN, Herzog ED, Stewart CL. The imprinted gene Magel2 regulates normal circadian output. Nat.Genet. 2007;39:1266–1272. doi: 10.1038/ng2114. [DOI] [PubMed] [Google Scholar]
- 88.Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J.Comp Neurol. 2004;468:361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience. 2002;110:73–92. doi: 10.1016/s0306-4522(01)00551-6. [DOI] [PubMed] [Google Scholar]
- 90.Leak RK, Card JP, Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Research. 1999;819:23–32. doi: 10.1016/s0006-8993(98)01317-1. [DOI] [PubMed] [Google Scholar]
- 91.Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. Journal of Comparative Neurology. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- 92.Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford University Press; New York: 1999. [Google Scholar]
- 93.Leigh RJ, Zee DS. The vestibular-optokinetic system, The Neurology of Eye Movements. ed.3 Oxford University; New York: 1999. pp. 19–89. [Google Scholar]
- 94.LeSauter J, Kriegsfeld LJ, Hon J, Silver R. Calbindin-D 28K cells selectively contact intra-SCN neurons. Neuroscience. 2002;111:575–585. doi: 10.1016/s0306-4522(01)00604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, III, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nature Neuroscience. 2005;8:61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- 97.Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. Eur.J Neurosci. 2009;29:748–760. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Magnin M, Cooper HM, Mick G. Retinohypothalamic pathway: a breach in the law of Newton-Muller-Gudden? Brain Research. 1989;488:390–397. doi: 10.1016/0006-8993(89)90737-3. [DOI] [PubMed] [Google Scholar]
- 99.Mahoney M, Bult A, Smale L. Phase response curve and light-induced Fos expression in the suprachiasmatic nucleus and adjacent hypothalamus of Arvicanthis niloticus. Journal of Biological Rhythms. 2001;16:149–162. doi: 10.1177/074873001129001854. [DOI] [PubMed] [Google Scholar]
- 100.Mahoney MM, Ramanathan C, Smale L. Tyrosine hydroxylase positive neurons and their contacts with vasoactive intestinal polypeptide-containing fibers in the hypothalamus of the diurnal murid rodent, Arvicanthis niloticus. Journal of Chemical Neuroanatomy. 2007;33:131–139. doi: 10.1016/j.jchemneu.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 101.Mahoney MM, Smale L. Arginine vasopressin and vasoactive intestinal polypeptide fibers make appositions with gonadotropin-releasing hormone and estrogen receptor cells in the diurnal rodent Arvicanthis niloticus. Brain Research. 2005;1049:156–164. doi: 10.1016/j.brainres.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 102.Major DE, Rodman HR, Libedinsky C, Karten HJ. Pattern of retinal projections in the California ground squirrel (Spermophilus beecheyi): anterograde tracing study using cholera toxin. J Comp Neurol. 2003;463:317–340. doi: 10.1002/cne.10764. [DOI] [PubMed] [Google Scholar]
- 103.Marchant EG, Mistlberger RE. Entrainment and phase-shifting of circadian rhythms in mice by forced treadmill running. Physiology and Behavior. 1996;60:657–663. doi: 10.1016/s0031-9384(96)80045-x. [DOI] [PubMed] [Google Scholar]
- 104.Marchant EG, Morin LP. Superior colliculus mediates triazolam-induced phase shifts of hamster circadian rhythms. Society for Neuroscience. 1998;24:1919. [Google Scholar]
- 105.Marchant EG, Morin LP. The hamster circadian rhythm system includes nuclei of the subcortical visual shell. Journal of Neuroscience. 1999;19:10482–10493. doi: 10.1523/JNEUROSCI.19-23-10482.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marchant EG, Watson NV, Mistlberger RE. Both neuropeptide Y and serotonin are necessary for entrainment of circadian rhythms in mice by daily treadmill running schedules. Journal of Neuroscience. 1997;17:7974–7987. doi: 10.1523/JNEUROSCI.17-20-07974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martinet L, Bonnefond C, Peytevin J, Monnerie R, Marcilloux JC. Vasoactive intestinal polypeptide in the suprachiasmatic nucleus of the mink (Mustela vison) could play a key role in photic induction. Journal of Neuroendocrinology. 1995;7:69–79. doi: 10.1111/j.1365-2826.1995.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 108.Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McGranaghan PA, Piggins HD. Orexin A-like immunoreactivity in the hypothalamus and thalamus of the Syrian hamster (Mesocricetus auratus) and Siberian hamster (Phodopus sungorus), with special reference to circadian structures. Brain Research. 2001;904:234–244. doi: 10.1016/s0006-8993(01)02463-5. [DOI] [PubMed] [Google Scholar]
- 110.Merchenthaler I, Gorcs T, Setalo G, Petrusz P, Flerko B. Gonadotropin-releasing Hormone (GnRH) Neurons and Pathways in the Rat-brain. Cell and Tissue Research. 1984;237:15–29. doi: 10.1007/BF00229195. [DOI] [PubMed] [Google Scholar]
- 111.Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. Journal of Neuroscience. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meyer-Bernstein EL, Morin LP. Electrical stimulation of the median or dorsal raphe nuclei reduces light-induced FOS protein in the suprachiasmatic nucleus and causes circadian activity rhythm phase shifts. Neurosci. 1999;92:267–279. doi: 10.1016/s0306-4522(98)00733-7. [DOI] [PubMed] [Google Scholar]
- 113.Mikkelsen JD. Projections from the lateral geniculate nucleus to the hypothalamus of the Mongolian gerbil (Meriones unguiculatus): An anterograde and retrograde tracing study. Journal of Comparative Neurology. 1990;299:493–508. doi: 10.1002/cne.902990409. [DOI] [PubMed] [Google Scholar]
- 114.Miller AM, Miller RB, Obermeyer WH, Behan M, Benca RM. The pretectum mediates rapid eye movement sleep regulation by light. Behav.Neurosci. 1999;113:755–765. doi: 10.1037//0735-7044.113.4.755. [DOI] [PubMed] [Google Scholar]
- 115.Miller AM, Obermeyer WH, Behan M, Benca RM. The superior colliculus-pretectum mediates the direct effects of light on sleep. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8957–8962. doi: 10.1073/pnas.95.15.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mintz EM, van den Pol AN, Casano AA, Albers HE. Distribution of hypocretin-(orexin) immunoreactivity in the central nervous system of Syrian hamsters (Mesocricetus auratus) Journal of Chemical Neuroanatomy. 2001;21:225–238. doi: 10.1016/s0891-0618(01)00111-9. [DOI] [PubMed] [Google Scholar]
- 117.Mistlberger RE, Antle MC, Glass JD, Miller JD. Behavioral and serotonergic regulation of circadian rhythms. Biological Rhythm Research. 2000;31:240–283. [Google Scholar]
- 118.Mistlberger RE, Antle MC, Webb IC, Jones M, Weinberg J, Pollock MS. Circadian clock resetting by arousal in Syrian hamsters: the role of stress and activity. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2003;285:R917–R925. doi: 10.1152/ajpregu.00222.2003. [DOI] [PubMed] [Google Scholar]
- 119.Moga MM, Moore RY. Paraventricular thalamus lesions alter circadian period and activity distribution in the blinded rat. Biological Rhythm Research. 2000;31:212–219. [Google Scholar]
- 120.Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends in Neurosciences. 2011;34:349–358. doi: 10.1016/j.tins.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moore RY. The geniculohypothalamic tract in monkey and man. Brain Research. 1989;486:190–194. doi: 10.1016/0006-8993(89)91294-8. [DOI] [PubMed] [Google Scholar]
- 122.Moore RY. Entrainment pathways and the functional organization of the circadian timing system. In: Buijs R, Kalsbeek A, Romijn HJ, Pennartz CMA, Mirmiran M, editors. Hypothalamic integration of circadian rhythms. Elsevier; Amsterdam: 1996. pp. 101–117. [Google Scholar]
- 123.Moore RY, Card JP. Intergeniculate leaflet: An anatomically and functionally distinct subdivision of the lateral geniculate complex. Journal of Comparative Neurology. 1994;344:403–430. doi: 10.1002/cne.903440306. [DOI] [PubMed] [Google Scholar]
- 124.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 125.Moore RY, Gustafson EL, Card JP. Identical immunoreactivity of afferents to the rat suprachiasmatic nucleus with antisera against avian pancreatic polypeptide, molluscan cardiaoexcitatory peptide and neuropeptide Y. Cell and Tissue Research. 1984;236:41–46. doi: 10.1007/BF00216511. [DOI] [PubMed] [Google Scholar]
- 126.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. Journal of Comparative Neurology. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 127.Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neuroscience Letters. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- 128.Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- 129.Moore RY, Weis R, Moga MM. Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. Journal of Comparative Neurology. 2000;420:398–418. doi: 10.1002/(sici)1096-9861(20000508)420:3<398::aid-cne9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 130.Morin LP. Serotonin and the regulation of mammalian circadian rhythmicity. Annals of Medicine. 1999;31:12–33. doi: 10.3109/07853899909019259. [DOI] [PubMed] [Google Scholar]
- 131.Morin LP. SCN organization reconsidered. Journal of Biological Rhythms. 2007;22:3–13. doi: 10.1177/0748730406296749. [DOI] [PubMed] [Google Scholar]
- 132.Morin LP. The Mammalian Circadian Visual System. In: Lazareva OF, Shimizu T, Wasserman EA, editors. How Animals See the World. Oxford University Press; Oxford: 2012. p. 548. [Google Scholar]
- 133.Morin LP, Allen CN. The circadian visual system, 2005. Brain Res.Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 134.Morin LP, Blanchard JH. Neuropeptide Y and enkephalin immunoreactivity in retinorecipient nuclei of the hamster pretectum and thalamus. Visual Neuroscience. 1997;14:765–777. doi: 10.1017/s0952523800012712. [DOI] [PubMed] [Google Scholar]
- 135.Morin LP, Blanchard JH. Interconnections among nuclei of the subcortical visual shell: The intergeniculate leaflet is a major constituent of the hamster subcortical visual system. Journal of Comparative Neurology. 1998;396:288–309. [PubMed] [Google Scholar]
- 136.Morin LP, Blanchard JH. Forebrain connections of the hamster intergeniculate leaflet: comparison with those of ventral lateral geniculate nucleus and retina. Visual Neuroscience. 1999;16:1037–1054. doi: 10.1017/s0952523899166069. [DOI] [PubMed] [Google Scholar]
- 137.Morin LP, Blanchard JH. Neuromodulator content of hamster intergeniculate leaflet neurons and their projection to the suprachiasmatic nucleus or visual midbrain. Journal of Comparative Neurology. 2001;437:79–90. doi: 10.1002/cne.1271. [DOI] [PubMed] [Google Scholar]
- 138.Morin LP, Blanchard JH. Descending projections of the hamster intergeniculate leaflet: relationship to the sleep/arousal and visuomotor systems. Journal of Comparative Neurology. 2005;487:204–216. doi: 10.1002/cne.20546. [DOI] [PubMed] [Google Scholar]
- 139.Morin LP, Blanchard JH, Moore RY. Intergeniculate leaflet and suprachiasmatic nucleus organization and connections in the hamster. Visual Neuroscience. 1992;8:219–230. doi: 10.1017/s095252380000287x. [DOI] [PubMed] [Google Scholar]
- 140.Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet and visual midbrain: bifurcation and melanopsin immunoreactivity. Journal of Comparative Neurology. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- 141.Morin LP, Goodless-Sanchez N, Smale L, Moore RY. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience. 1994;61:391–410. doi: 10.1016/0306-4522(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 142.Morin LP, Hefton S, Studholme KM. Neurons identified by NeuN/Fox-3 immunoreactivity have a novel distribution the hamster and mouse suprachiasmatic nucleus. Brain Research. 2011;1421:44–51. doi: 10.1016/j.brainres.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morin LP, Pace L. The intergeniculate leaflet, but not the visual midbrain, mediates hamster circadian rhythm response to constant light. Journal of Biological Rhythms. 2002;17:217–226. doi: 10.1177/07430402017003005. [DOI] [PubMed] [Google Scholar]
- 144.Morin LP, Shivers KY, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience. 2006;137:1285–1297. doi: 10.1016/j.neuroscience.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 145.Muscat L, Huberman AD, Jordan CL, Morin LP. Crossed and uncrossed retinal projections to the hamster circadian system. J.Comp Neurol. 2003;466:513–524. doi: 10.1002/cne.10894. [DOI] [PubMed] [Google Scholar]
- 146.Muscat L, Morin LP. Intergeniculate leaflet: contributions to photic and non-photic responsiveness of the hamster circadian system. Neurosci. 2006;140:305–320. doi: 10.1016/j.neuroscience.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura H, Itoh K. Cytoarchitectonic and connectional organization of the ventral lateral geniculate nucleus in the cat. Journal of Comparative Neurology. 2004;473:439–462. doi: 10.1002/cne.20074. [DOI] [PubMed] [Google Scholar]
- 148.Nixon JP, Smale L. Individual differences in wheel-running rhythms are related to temporal and spatial patterns of activation of orexin A and B cells in a diurnal rodent (Arvicanthis niloticus) Neuroscience. 2004;127:25–34. doi: 10.1016/j.neuroscience.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 149.Novak CM, Nunez AA. A sparse projection from the suprachiasmatic nucleus to the sleep active ventrolateral preoptic area in the rat. Neuroreport. 2000;11:93–96. doi: 10.1097/00001756-200001170-00019. [DOI] [PubMed] [Google Scholar]
- 150.Park HT, Baek SY, Kim BS, Kim JB, Kim JJ. Calcitonin gene-related peptide-like immunoreactive (CGRPI) elements in the circadian system of the mouse: an immunohistochemistry combined with retrograde transport study. Brain Research. 1993;629:335–341. doi: 10.1016/0006-8993(93)91342-p. [DOI] [PubMed] [Google Scholar]
- 151.Paul MJ, Indic P, Schwartz WJ. A role for the habenula in the regulation of locomotor activity cycles. European Journal of Neuroscience. 2011;34:478–488. doi: 10.1111/j.1460-9568.2011.07762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peters RV, Aronin N, Schwartz WJ. c-Fos expression in the rat intergeniculate leaflet: Photic regulation, co-localization with Fos-B, and cellular identification. Brain Research. 1996;728:231–241. doi: 10.1016/0006-8993(96)00414-3. [DOI] [PubMed] [Google Scholar]
- 153.Pickard GE. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neuroscience Letters. 1985;55:211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- 154.Pickard GE. Intergeniculate leaflet ablation alters circadian rhythms in the mouse. Neuroreport. 1994;5:2186–2188. doi: 10.1097/00001756-199410270-00049. [DOI] [PubMed] [Google Scholar]
- 155.Pickard GE, Ralph MR, Menaker M. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. Journal of Biological Rhythms. 1987;2:35–56. doi: 10.1177/074873048700200104. [DOI] [PubMed] [Google Scholar]
- 156.Pickard GE, Smeraski CA, Tomlinson CC, Banfield BW, Kaufman J, Wilcox CL, Enquist LW, Sollars PJ. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. Journal of Neuroscience. 2002;22:2701–2710. doi: 10.1523/JNEUROSCI.22-07-02701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Piggins HD, Samuels RE, Coogan AN, Cutler DJ. Distribution of substance P and neurokinin-1 receptor immunoreactivity in the suprachiasmatic nuclei and intergeniculate leaflet of hamster, mouse, and rat. J.Comp Neurol. 2001;438:50–65. doi: 10.1002/cne.1301. [DOI] [PubMed] [Google Scholar]
- 158.Poletini MO, Kennett JE, McKee DT, Freeman ME. Central Clock Regulates the Cervically Stimulated Prolactin Surges by Modulation of Dopamine and Vasoactive Intestinal Polypeptide Release in Ovariectomized Rats. Neuroendocrinology. 2010;91:179–188. doi: 10.1159/000254379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pompeiano O, Morrison AR. Vestibular influences during sleep. I. Abolition of the rapid eye movements of desynchronized sleep following vestibular lesions. Arch.Ital.Biol. 1965;103:569–595. [PubMed] [Google Scholar]
- 160.Pompeiano O, Morrison AR. Vestibular origin of the rapid eye movements during desynchronized sleep. Experientia. 1966;22:60–61. doi: 10.1007/BF01897775. [DOI] [PubMed] [Google Scholar]
- 161.Power BD, Mitrofanis J. Zona incerta: Substrate for contralateral interconnectivity in the thalamus of rats. Journal of Comparative Neurology. 2001;436:52–63. [PubMed] [Google Scholar]
- 162.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. Journal of Neuroscience. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 164.Pu ML, Pickard GE. Ventral lateral geniculate nucleus afferents to the suprachiasmatic nucleus in the cat. Brain Research. 1996;725:247–251. doi: 10.1016/0006-8993(96)00340-x. [DOI] [PubMed] [Google Scholar]
- 165.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 166.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. Journal of Neuroscience. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Romijn HJ, Sluiter AA, Pool CW, Wortel J, Buijs RM. Differences in colocalization between fos and PHI, GRP, VIP and VP in neurons of the rat suprachiasmatic nucleus after a light stimulus during the phase delay versus the phase advance period of the night. Journal of Comparative Neurology. 1996;372:1–8. doi: 10.1002/(SICI)1096-9861(19960812)372:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 168.Romijn HJ, Sluiter AA, Pool CW, Wortel J, Buijs RM. Evidence from confocal fluorescence microscopy for a dense, reciprocal innervation between AVP-, somatostatin-, VIP/PHI-, GRP-, and VIP/PHI/GRP-immunoreactive neurons in the rat suprachiasmatic nucleus. Eur J Neurosci. 1997;9:2613–2623. doi: 10.1111/j.1460-9568.1997.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 169.Romijn HJ, van Uum JFM, Emmering J, Goncharuk V, Buijs RM. Colocalization of VIP with AVP in neurons of the human paraventricular, supraoptic and suprachiasmatic nucleus. Brain Research. 1999;832:47–53. doi: 10.1016/s0006-8993(99)01468-7. [DOI] [PubMed] [Google Scholar]
- 170.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 171.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nature Reviews Neuroscience. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 172.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin by a genetically encoded neurons revealed tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 173.Schaap J, Meijer JH. Opposing effects of behavioural activity and light on neurons of the suprachiasmatic nucleus. European Journal of Neuroscience. 2001;13:1955–1962. doi: 10.1046/j.0953-816x.2001.01561.x. [DOI] [PubMed] [Google Scholar]
- 174.Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends in Neurosciences. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schmidt TM, Do MTH, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-Positive Intrinsically Photosensitive Retinal Ganglion Cells: From Form to Function. Journal of Neuroscience. 2011;31:16094–16101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schwartz MD, Nunez AA, Smale L. Rhythmic cfos expression in the ventral subparaventricular zone influences general activity rhythms in the nile grass rat, arvicanthis niloticus. Chronobiology International. 2009;26:1290–1306. doi: 10.3109/07420520903415742. [DOI] [PubMed] [Google Scholar]
- 177.Schwartz MD, Urbanski HF, Nunez AA, Smale L. Projections of the suprachiasmatic nucleus and ventral subparaventricular zone in the Nile grass rat (Arvicanthis niloticus) Brain Research. 2011;1367:146–161. doi: 10.1016/j.brainres.2010.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shen H, Semba K. A direct retinal projection to the dorsal raphe nucleus in the rat. Brain Research. 1994;635:159–168. doi: 10.1016/0006-8993(94)91435-4. [DOI] [PubMed] [Google Scholar]
- 179.Shinohara K, Funabashi T, Mitushima D, Kimura F. Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neuroscience Research. 2000;38:43–47. doi: 10.1016/s0168-0102(00)00141-3. [DOI] [PubMed] [Google Scholar]
- 180.Smale L, Blanchard JH, Moore RY, Morin LP. Immunocytochemical characterization of the suprachiasmatic nucleus and the intergeniculate leaflet in the diurnal ground squirrel, Spermophilus lateralis. Brain Research. 1991;563:77–86. doi: 10.1016/0006-8993(91)91517-5. [DOI] [PubMed] [Google Scholar]
- 181.Smale L, Boverhof J. The suprachiasmatic nucleus and intergeniculate leaflet of Arvicanthis niloticus, a diurnal murid rodent from East Africa. Journal of Comparative Neurology. 1999;403:190–208. doi: 10.1002/(sici)1096-9861(19990111)403:2<190::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 182.Smeraski CA, Sollars PJ, Ogilvie MD, Enquist LW, Pickard GE. Suprachiasmatic nucleus input to autonomic circuits identified by retrograde transsynaptic transport of pseudorabies virus from the eye. J Comp Neurol. 2004;471:298–313. doi: 10.1002/cne.20030. [DOI] [PubMed] [Google Scholar]
- 183.Smith MS, Freeman ME, Neill JD. Control of progesterone secretion during estrous-cycle and early pseudopregnancy in rat – prolactin, gonadotropin and steroid levels associated with rescue of corpus-luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 184.Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Visual Neuroscience. 2003;20:601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]
- 185.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Science of the United States of America. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Swanson LW, Cowan WM, Jones EG. An autoradiographic study of the efferent projections of the ventral lateral geniculate nucleus in the albino rat and cat. Journal of Comparative Neurology. 1974;156:143–164. doi: 10.1002/cne.901560203. [DOI] [PubMed] [Google Scholar]
- 187.Takatsuji K, Miguel-Hidalgo JJ, Tohyama M. Retinal fibers make synaptic contact with neuropeptide Y and enkephalin immunoreactive neurons in the intergeniculate leaflet of the rat. Neuroscience Letters. 1991;125:73–76. doi: 10.1016/0304-3940(91)90134-f. [DOI] [PubMed] [Google Scholar]
- 188.Takatsuji K, Tohyama M. Geniculo-geniculate projection of enkephalin and neuropeptide-y containing neurons in the intergeniculate leaflet of the thalamus in the rat. Journal of Chemical Neuroanatomy. 1989;2:19–27. [PubMed] [Google Scholar]
- 189.TeclemariamMesbah R, Kalsbeek A, Pevet P, Buijs RM. Direct vasoactive intestinal polypeptide-containing projection from the suprachiasmatic nucleus to spinal projecting hypothalamic paraventricular neurons. Brain Research. 1997;748:71–76. doi: 10.1016/s0006-8993(96)01246-2. [DOI] [PubMed] [Google Scholar]
- 190.Thankachan S, Rusak B. Juxtacellular recording/labeling analysis of physiological and anatomical characteristics of rat intergeniculate leaflet (IGL) neurons. Journal of Neuroscience. 2005;25:99195–99204. doi: 10.1523/JNEUROSCI.2672-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tischler RC, Morin LP. Reciprocal serotonergic connections between the hamster median and dorsal raphe nuclei. Brain Research. 2003;981:126–132. doi: 10.1016/s0006-8993(03)02994-9. [DOI] [PubMed] [Google Scholar]
- 192.Tokunaga A, Ono K, Kondo S, Tanaka H, Kurose K, Nagai H. Retinal projections in the house musk shrew, Suncus murinus, as determined by anterograde transport of WGA-HRP. Brain, Behavior and Evolution. 1992;40:321–329. doi: 10.1159/000113922. [DOI] [PubMed] [Google Scholar]
- 193.Tonsfeldt KJ, Chappell PE. Clocks on top: The role of the circadian clock in the hypothalamic and pituitary regulation of endocrine physiology. Molecular and Cellular Endocrinology. 2012;349:3–12. doi: 10.1016/j.mce.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tsujino N, Sakurai T. Orexin/Hypocretin: A Neuropeptide at the Interface of Sleep, Energy Homeostasis, and Reward System. Pharmacological Reviews. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 195.van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. Journal of Comparative Neurology. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- 196.van der Beek EM, Horvath TL, Wiegant VM, Van den HR, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 197.van der Beek EM, Wiegant VM, van der Donk HA, Van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotropin-releasing-hormone neurons in the female rat. Journal of Neuroendocrinology. 1993;5:137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 198.Van der Gucht E, Hof PR, Arckens L. Neurochemical organization, architectonic subdivision and three-dimensional reconstruction of cat ventral lateral geniculate nucleus and monkey pregeniculate nucleus. Society for Neuroscience. 2003;33:68.67. [Google Scholar]
- 199.Vidal L, Blanchard J, Morin LP. Hypothalamic and zona incerta neurons expressing hypocretin, but not melanin concentrating hormone, project to the hamster intergeniculate leaflet. Neuroscience. 2005;134:1081–1090. doi: 10.1016/j.neuroscience.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 200.Vidal L, Morin LP. Absence of normal photic integration in the circadian visual system: response to millisecond light flashes. J Neurosci. 2007;27:3375–3382. doi: 10.1523/JNEUROSCI.5496-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vrang N, Mrosovsky N, Mikkelsen JD. Afferent projections to the hamster intergeniculate leaflet demonstrated by retrograde and anterograde tracing. Brain Research Bulletin. 2003;59:267–288. doi: 10.1016/s0361-9230(02)00875-4. [DOI] [PubMed] [Google Scholar]
- 202.Watanabe T, Naito E, Nakao N, Tei H, Yoshimura T, Ebihara S. Bimodal clock gene expression in mouse suprachiasmatic nucleus and peripheral tissues under a 7-hour light and 5-hour dark condition. Journal of Biological Rhythms. 2006;22:58–68. doi: 10.1177/0748730406295435. [DOI] [PubMed] [Google Scholar]
- 203.Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. Journal of Comparative Neurology. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 204.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. Journal of Comparative Neurology. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 205.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu.Rev.Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Williams WP, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian Control of Kisspeptin and a Gated GnRH Response Mediate the Preovulatory Luteinizing Hormone Surge. Endocrinology. 2011;152:595–606. doi: 10.1210/en.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yan L, Silver R, Gorman M. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J Biol.Rhythms. 2010;25:19–27. doi: 10.1177/0748730409352054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. Journal of Comparative Neurology. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]