Abstract
Parkinson’s disease (PD) patients often exhibit impaired regulation of heart rate by the autonomic nervous system (ANS) that may precede motor symptoms in many cases. Results of autopsy studies suggest that brainstem pathology, including the accumulation of α-synuclein, precedes damage to dopaminergic neurons in the substantia nigra in PD. However, the molecular and cellular mechanisms responsible for the early dysfunction of brainstem autonomic neurons are unknown. Here we report that mice expressing a mutant form of α-synuclein that causes familial PD exhibit aberrant autonomic control of the heart characterized by elevated resting heart rate and an impaired cardiovascular stress response, associated with reduced parasympathetic activity and accumulation of α-synuclein in the brainstem. These ANS abnormalities occur early in the disease process. Adverse effects of α-synuclein on the control of heart rate are exacerbated by a high energy diet and ameliorated by intermittent energy restriction. Our findings establish a mouse model of early dysregulation of brainstem control of the cardiovascular system in PD, and further suggest the potential for energy restriction to attenuate ANS dysfunction, particularly in overweight individuals.
Keywords: α-synuclein, acetylcholine, ANS, BDNF, brainstem, parasympathetic, Parkinson’s disease
1. Introduction
Parkinson’s disease (PD) Clinical findings and neuropathological staging of PD suggest that autonomic nervous system (ANS) dysfunction and brainstem pathology, including the accumulation of α-synuclein, precedes midbrain dopaminergic pathology and motor symptoms (Buob et al., 2010; Müller et al., 2005). Symptoms of ANS dysfunction in prodromal PD include constipation associated with reduce parasympathetic activation of gut motility (Savica et al., 2009), and orthostatic hypotension mediated by degeneration of sympathetic neurons (Jain and Goldstein, 2012). PD patients also often exhibit impaired autonomic regulation of sweating, urination, pupillary responses to light, and hypothalamic regulation of endocrine organs (Jain, 2011). Heart rate (HR) is modulated by cholinergic parasympathetic neurons of the brainstem dorsal motor nucleus of the vagus (DMNV) and nucleus ambiguous, and by noradrenergic sympathetic neurons. Self-aggregation and accumulation of α-synuclein is implicated in the dysfunction and degeneration of midbrain dopaminergic neurons in PD, but the role of α-synuclein in ANS dysfunction in PD is unknown. However, two recent studies have documented impaired intestinal motility associated with α-synuclein accumulation in enteric ANS neurons, and these abnormalities preceded motor dysfunction (Kuo et al., 2010; Hallett et al., 2012). In the present study we describe another ANS abnormality in α-synuclein mutant mice involving impaired brainstem cholinergic regulation of HR.
Epidemiological data suggest that being overweight in midlife is a risk factor for PD (Hu et al., 2006). In addition, dietary energy restriction protects midbrain dopaminergic neurons and improves functional outcome in neurotoxin-based animal models of PD (Duan and Mattson, 1999; Maswood et al., 2004), whereas a high energy diet is detrimental (Morris et al., 2010). Intermittent energy restriction is known to enhance brainstem parasympathetic activity, while high energy diets have the opposite effect (Wan et al., 2003; Thayer et al, 2010). Here we describe ANS dysfunction in α-synuclein mutant mice that is exacerbated by a high calorie diet (HCD) and ameliorated by intermittent energy restriction (intermittent fasting; IF). These findings provide a model for future studies of the molecular and cellular mechanisms responsible for ANS dysfunction in PD, and their potential modification by therapeutic interventions.
2. Methods
2.1 Animals and Diets
Male B6.Cg-Tg(THY1-SNCA*A53T)M53Sud/J (SNCA) mice (Chandra et al., 2005) (Jackson Laboratories; Bar Harbor, ME) and wild-type littermates (WT) were housed under a 12-hour light/dark cycle. SNCA mice express A53T mutant human α-synuclein under the control of the murine Thy-1 promoter and exhibit α-synuclein accumulation in the midbrain, and age-dependent motor impairment (Chandra et al., 2005). No previous studies have described autonomic deficits in this mouse model of PD. Mice were maintained on either standard chow or a high calorie diet (Dyets #101842; Dyets Inc.; with water containing 11% of a fructose/glucose mix) (Stranahan et al., 2008), or were maintained on an alternate day fasting diet (Arumugam et al., 2010). Weight and food intake were recorded throughout the study. All procedures were approved by the Institutional Animal Care and Use Committee of the National Institute on Aging.
2.2 Telemetry
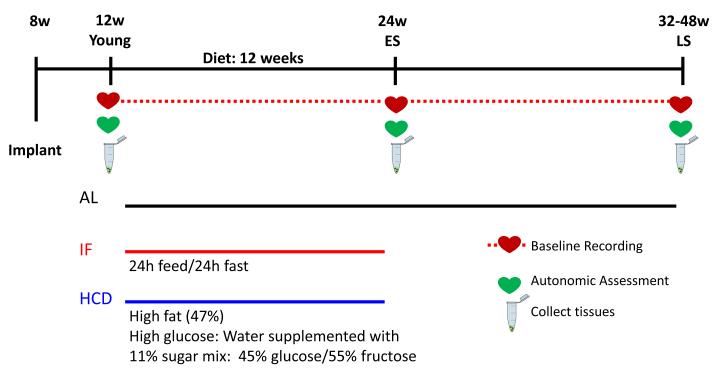
Telemetry transmitters were surgically implanted as described previously (Griffioen et al., 2008) at 8 weeks to continuously monitor biological parameters in the mice. Briefly, a transmitter, TA10ETA-F20 (Data Sciences International, St Paul, MN), which monitors electrocardiogram (ECG), core body temperature and general activity, was surgically implanted in each of the mice. Two biopotential leads were routed subcutaneously lateral to midline of the chest and secured to chest muscles with silk sutures (Ethicon). Telemetry data were continuously recorded in 2.5 minute bins, every 10 minutes. Mice were implanted with transmitters at 2 months of age and allowed to recover for a month before beginning recording. Baseline values for HR, activity and temperature were recorded in a preliminary group of WT controls (n = 8) and SNCA (n = 8) mice to determine the changes in these parameters throughout the lifespan, and to determine the period in which to apply the diets. In a second group of mice, after a 4-week recovery period, the mice were randomly assigned to one of three diet groups: standard chow ad libitum (AL; n=12), high calorie diet ad libitum (HCD; n=12), or alternate day fasting with standard chow (IF; n=12). 48-hour baseline recordings were made weekly to monitor changes in HR, activity and temperature throughout the study (See Fig. 1 for experimental design).
Figure 1. Experimental design.
Mice were implanted with telemetry transmitters at 8 weeks of age. After 4 weeks recovery, they were divided into AL, HCD and IF diet groups. At 12 weeks, 24 weeks and at 32-48 weeks (at the expression of late symptomatic motor impairment), autonomic assessment was made, and tissues were collected. 48-hour baseline parameters were recorded weekly throughout the study.
At 12 weeks and 24 weeks of age, all diet groups underwent restraint stress (n=8 per group). Baseline HR was recorded for 30 minutes. Mice were then individually restrained in their home cage using a modified plastic DecapiCone Restrainer (Braintree Scientific Inc.Braintree, MA) for one hour; HR was recorded continuously.
2.3 Drug Treatments
Autonomic blockade was performed by i.p. injection of the mAchR blocker atropine methyl nitrate (parasympathetic blockade; 2mg/kg), the beta-1 adrenergic receptor blocker atenolol (sympathetic blockade; 2mg/kg) or the nicotinic acetylcholine receptor blocker hexamethonium bromide (ganglionic blockade; 30 mg/kg). Preliminary tests with i.p. injection of PBS determined that HR returned to baseline levels within 30 minutes after injection. Therefore, change in HR was assessed as the difference between the averaged HR for 30 minutes before injection and the averaged HR between 45-60 minutes after injection.
2.4 Immunoblot analysis
The brain was rapidly removed on ice, and the medulla was dissected and flash frozen. To prepare the tissue for immunoblot analysis it was homogenized in RIPA buffer with protease inhibitors on ice. Protein concentrations were determined using a Bradford assay. Immunoblots were performed using 40 μg of total protein extract separated on 4-12% bis-tris gels and then transferred electrophoretically to nitrocellulose membranes. Membranes were blocked with 5% milk in TBS-t, washed in TBS-t and incubated overnight at 4° in an antibody to α-synuclein (LB509;Abcam) (1:500), or actin (Sigma-Aldrich; St. Louis, MO). Membranes were then incubated in a secondary antibody solution for 30 minutes (1:5000, then in the presence of horseradish peroxidase-conjugated anti-mouse IgG) for 30 minutes. The optical density of immunoreactive bands was detected and quantified by chemiluminescence using the ECL system and results were normalized to actin, averaged and compared for each age-matched group of SNCA mice and WT littermates.
2.5 Quantification of DMNV Cells
Brain slices (40 μm) were stained with cresyl violet after being mounted sequentially at 120 μm intervals traversing the entire brainstem to ensure that the entire length of the DMNV was counted. All neurons in the DMNV were counted unilaterally in a blinded manner (n=4 per diet group).
2.6 Statistical Analyses
All data are expressed as mean +/− standard error (SE). Data were analyzed by two-way ANOVA with Bonferroni’s posthoc test and Student’s t test for individual comparisons between groups using GraphPad (GraphPad software, Inc., San Diego, CA); significance was set at p < 0.05.
3. Results
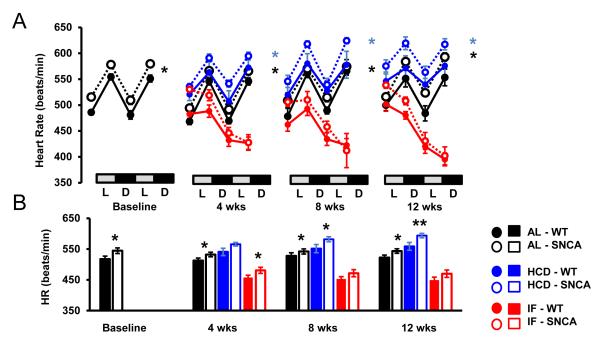
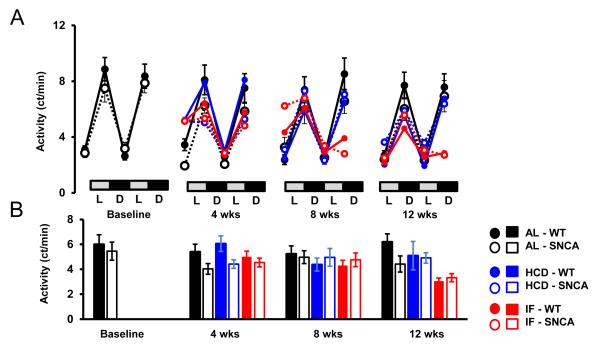
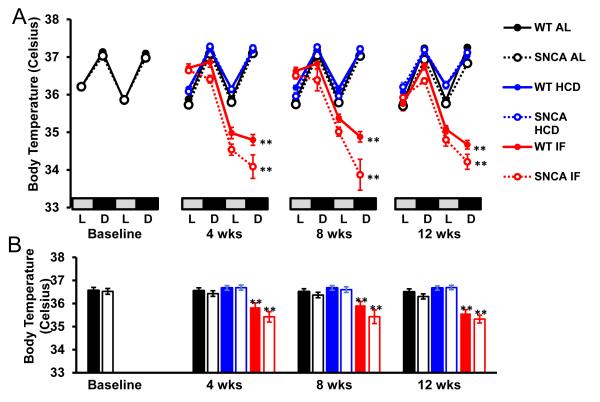
PD patients exhibit altered autonomic function and heart rate control, often preceding motor impairment (Jain, 2011). To test whether a transgenic mouse model of PD, which overexpresses human mutant A53T α-synculein, also exhibits altered autonomic control of HR, SNCA mice and WT littermates were implanted with telemetry transmitters which monitor HR, home cage activity and core body temperature. Consistent with a previous study (Hu et al., 2006), the lifespan of SNCA mice is between 6 and 10 months of age (Supplementary Fig. 1). The HR of young SNCA mice (n = 12; 3 months; Fig. 1), was significantly elevated compared to WT mice during the light and dark periods (Fig. 2A, B). The elevated HR in SNCA mice was not due to higher activity levels, as the activity of SNCA mice was similar to WT mice (Fig. 3 A, B). In addition, the body temperature of SNCA mice was similar to WT mice (Fig. 4 A, B). Therefore, this suggests that SNCA mice exhibit elevated HR, independent of their activity.
Figure 2. Elevated heart rate in SNCA is exacerbated by a high energy diet, and restored by intermittent fasting.
(A) 48-hour circadian HR recording in WT and SNCA mice. SNCA mice have significantly elevated HR compared to WT mice, during both the light (L) and dark (D) cycle at baseline, before diets started, and throughout the 12 week diet. HCD exacerbated tachycardia in SNCA mice, while IF significantly lowered HR in WT and SNCA mice. For IF mice, the second 24 hours represents a fasting day. (B) Average HR at each time point. *p < 0.05; n = 12 per group
Figure 3. Home cage activity is similar in WT and SNCA mice.
Elevated HR in SNCA mice was not due to increased activity, as SNCA mice become increasingly inactive as age increases. (A) 48-hour circadian home cage activity in WT and SNCA mice during the light (L) and dark (D) cycles. For IF mice, the second 24 hours represents a fasting day. (B) Average activity at each time point. n = 12 per group
Figure 4. Core body temperature is decreased on fasting days in both WT and SNCA mice.
Core body temperature was similar in WT and SNCA mice on AL or HCD diets, however IF significantly reduced body temperature in both WT and SNCA mice on fasting days. (A) 48-hour circadian core body temperature in WT and SNCA mice during the light (L) and dark (D) cycles. For IF mice, the second 24 hours represents a fasting day. (B) Average body temperature at each time point. n = 12 per group; **p<0.01 compared to AL and HCD groups.
High calorie diets and obesity are associated with cardiovascular dysfunction in humans, and can exacerbate dopaminergic degeneration in some animal models of PD (Morris et al., 2010; Bousquet et al., 2012). In contrast, caloric restriction is associated with improved cardiovascular health, and can protect dopaminergic neurons in mouse (Duan and Mattson, 1999) and monkey (Maswood et al., 2004) models of PD. Therefore, we tested whether intermittent fasting could improve, and high calorie diet could exacerbate the abnormal HR in SNCA mice. Mice were assigned to ad libitum control (AL; n = 12), intermittent (alternate day) fasting (IF; n = 12), or high calorie (high fat diet plus 11% fructose/glucose in the drinking water) diets (HCD; n = 12). WT mice on a HCD rapidly gained weight, while SNCA mice failed to gain weight on the HCD diet (Supplementary Fig. 2). In contrast, the weight of mice on the IF diet was reduced. Food intake for mice on the HCD was significantly reduced (Supplementary Fig. 3), consistent with consumption of a calorie-dense diet (Martin et al., 2007). Consistent with other reports, IF mice consumed less overall average food than those on the AL diet (Duan et al., 2003; Martin et al., 2007). At 4, 8 and 12 weeks after diet initiation (a time point designated as Early symptomatic (ES) with regards to HR changes) telemetry results revealed highly significant reductions in HR in WT and SNCA mice on IF compared to AL diets (Fig. 2A, B). Whereas IF ameliorated the abnormal elevation of HR in SNCA mice, HCD significantly exacerbated the elevated HR in SNCA mice (Fig. 2A, B). As expected from previous studies (Wan et al., 2003), WT mice in the IF group exhibited a significant reduction of body temperature on the fasting days (Fig. 4). The body temperature of the SNCA mice was also reduced during the fasting days, by an amounts similar to that of WT mice.
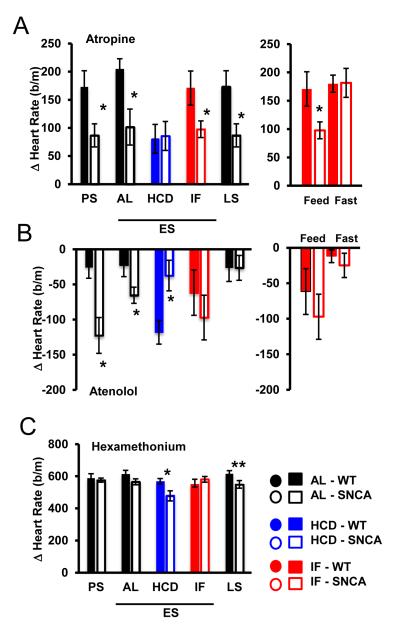
To determine the physiological basis of the elevated HR caused by mutant α-synuclein, we tested autonomic control of heart rate in AL, IF and HCD mice. We measured HR in WT and SNCA mice in the presence of antagonists of cholinergic (atropine) or β-adrenergic (atenolol) receptors. At young, ES and LS ages, AL SNCA mice exhibited a significantly reduced HR elevation in response to atropine compared to WT mice (Fig. 5A), suggesting impaired parasympathetic activity to the heart. The HR response to atropine was reduced in WT mice on HCD, but unaffected by IF. Interestingly, SNCA mice on the IF diet exhibited reduced parasympathetic input on feeding days, but normal parasympathetic activity was restored on fasting days (Fig. 5A). At young and ES ages, atenolol reduced HR to a greater degree in SNCA mice, and the HCD resulted in significantly reduced HR responses to atenolol in SNCA compared to WT mice (Fig. 5B), suggesting altered sympathetic activity to the heart. The intrinsic HR, determined by ganglionic blockade with hexamethonium bromide, was not different in young and ES SNCA mice fed AL, but was significantly reduced in LS SNCA mice and ES SNCA mice on the HCD (Fig. 5C). Therefore, SNCA mice exhibit impaired autonomic control of HR, and this is exacerbated by HCD and partially restored by IF.
Figure 5. Autonomic dysfunction in SNCA mice is exacerbated by a high energy diet, and restored by intermittent energy restriction.
(A) Change in heart rate after i.p. injection of atropine (2 mg/kg) indicates that parasympathetic activity to the heart is significantly reduced in SNCA mice before initiation of diets (PS; 3 mo). 12 weeks after diet initiation, HCD significantly reduced parasympathetic activity in WT mice, without changing parasympathetic input in SNCA mice. Whereas on feeding days SNCA mice on IF exhibit reduced parasympathetic input to the heart, this activity is restored on fasting days (right panel). Parasympathetic activity remains reduced at disease end stages (LS). (B) Sympathetic activity is significantly elevated in SNCA mice at presymptomatic stages, before diet initiation (3 mo), as assessed by i.p. administration of atenolol (2mg/kg). Sympathetic activity remains significantly elevated in SNCA mice at 24 weeks (ES). Whereas HCD increased sympathetic input in WT mice, it was significantly decreased in SNCA mice. Sympathetic activity in SNCA mice following IF is restored, and is similar to WT mice (right panel). (C) Intrinsic HR is not different between SNCA and WT mice at young (PS) or ES stages, as assessed by i.p. injection of hexamethonium bromide (30 mg/kg). However, at LS stages, intrinsic heart rate is significantly reduced in SNCA mice. The intrinisic heart rate of mice maintained on HCD was reduced compared to WT mice, similar to LS mice. * p>0.05; **p>0.01; n = 10 per group
PD patients may exhibit a reduced sympathetic cardiac response to stress (Nakamura et al., 2010). To test whether SNCA mice also exhibit dysregulation of HR control during stress, we exposed mice to restraint stress. Restraint stress is a well-characterized paradigm which elevates HR and is mediated by central cardiovascular control (Shapiro et al., 1993; Wan et al., 2003). During restraint stress, the change in HR in SNCA mice was significantly less than the HR response to stress in WT mice (Supplementary Fig. 4). The SNCA mice on the AL, IF and HCD each attained only a very small increase in HR during restraint stress. This suggests that similar to PD patients, SNCA mice also exhibit altered HR responses to stress.
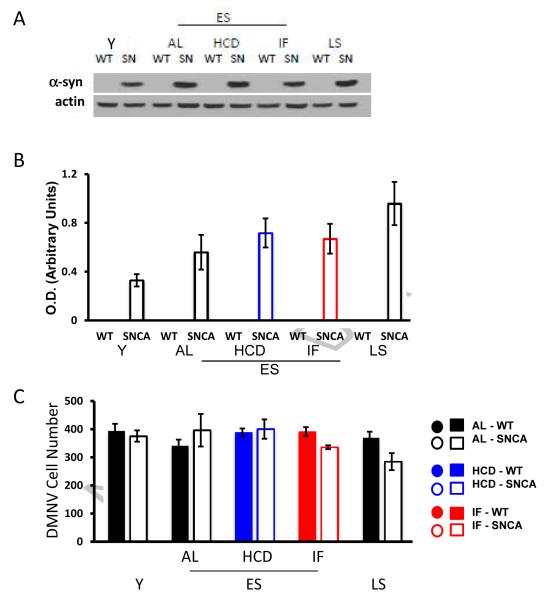
Finally, we evaluated α-synuclein protein levels in the brainstems of WT and SNCA mice in AL, IF and HCD groups. Immunoblot analysis demonstrated high amounts of α-synuclein in the brainstems of SNCA mice compared to WT mice. In SNCA mice the levels of α-synuclein increased with age, and were not significantly affected by HCD or IF (Fig. 6A, B). There were no significant differences in DMNV cell number in WT and SNCA mice, nor did age or diet affect the number of DMNV cells (Fig. 6C). Collectively, these findings suggest that IF preserves DMNV cell function despite the presence of mutant α-synuclein, a finding similar to a study in a mouse model of Alzheimer’s disease where IF did not reduce amyloid β-peptide accumulation, but nevertheless preserved cognitive function (Halagappa et al., 2007). On the other hand, the HCD exacerbated the impairment of autonomic control of HR caused by mutant α-synuclein, via a mechanism that apparently involves increased vulnerability of parasympathetic brainstem neurons to the α-synuclein (i.e., a mechanism downstream of α-synuclein accumulation).
Figure 6. Accumulation of α-synuclein in the brainstem increases with age, and is unaffected by energy intake.
(A) Immunoblot analysis revealed that α-synuclein accumulates in the medulla in and age-dependent manner in SNCA mice. (B) Neither the HCD or IF diets affected the expression of α-synuclein in ES mice (values are the mean and SEM of analysis of samples from 4 mice of each age and diet condition). (C) Results of counts of numbers of cells in the DMNV (see Methods). Values are the mean and SE of measurements made in 4 mice per group.
4. Discussion
The brainstem α-synuclein pathology and imbalance of autonomic regulation of HR early in the disease process in SNCA mice demonstrated here is comparable to the early brainstem pathology and ANS dysfunction that occurs in humans with PD (Braak et al., 2003; Ghebremedhin et al., 2009). Our findings suggest an adverse effect of mutant α-synuclein on parasympathetic (cholinergic) neurons in the brain stem. The latter finding is consistent with a recent report demonstrating α-synuclein pathology in the efferent DMNV axons that innervate the gut (Noorian et al., 2012). The brainstem pathology in PD is not limited to the DMNV. PD patients exhibit damage to noradrenergic neurons in the locus coeruleus (Chan-Palay and Asan, 1989; Remy et al., 2005) and serotonergic neurons in the raphe nucleus (Halliday et al., 1990; Basso and Evinger, 1996). The locus coeruleus is also adversely affected in mouse models of familial PD, including α-synuclein transgenic mice (Sotiriou et al., 2010) and Parkin-deficient mice (Von Coelln et al., 2004). Additional findings suggest that deficits of noradrenergic and serotonergic signaling contribute to psychiatric symptoms and motor deficits in PD patients and animal models (Rommelfanger et al., 2007; Rodriquez-Oroz et al., 2009; Huot et al., 2011). Collectively, the available data suggest that the accumulation of α-synuclein in brainstem neurons occurs early in PD and contributes to both peripheral (ANS) manifestations (constipation, aberrant HR regulation, and others) and central nervous system manifestations (anxiety, depression and motor dysfunction) of the disease.
We found that energy intake modifies ANS dysfunction in SNCA mice, with energy restriction reversing the abnormalities and a high energy diet exacerbating the disease process. It is believed that energy intake might affect the vulnerability of neurons during aging by either increasing (energy restriction) or decreasing (energy excess) the production of neurotrophic factors including BDNF and GDNF (Maswood et al., 2004; Morris et al., 2010; Arumugam et al., 2010). Both BDNF and GDNF can enhance the cholinergic phenotype (Brodski et al., 2002; Yang et al., 2002), suggesting roles for one or both of these neurotrophic factors in the adverse effects of α-synuclein and a high energy diet, and the beneficial effects of energy restriction, on ANS brainstem neurons. Indeed, recent findings suggest that BDNF signaling in brainstem cholinergic neurons can enhance parasympathetic activity resulting in a reduction of heart rate and improved cardiovascular stress adaptation (Griffioen et al., 2012). If similar mechanisms occur in humans, then intermittent energy restriction could mitigate early brainstem ANS dysfunction in PD.
PD patients often exhibit abnormalities in sweating and reactivity of blood vessels to changes in body temperature (Goetz et al., 1986). We found no evidence for disturbed thermoregulation in the SNCA mice, suggesting that either α-synuclein does not accumulate in the (hypothalamic) neurons that control body temperature, or those neurons are not adversely affected by the mutant α-synuclein. In addition, the SNCA mice exhibited levels of daily activity that were similar to those of wild type mice. These findings suggest that ANS control of HR becomes perturbed at early stage in the disease process in SNCA mice, during which time there are no discernible abnormalities of locomotor activity or thermoregulation.
Our findings establish an animal model for early brainstem ANS dysfunction in PD. Measuring HR and HR variability as a ‘readout’ of ANS functional status (Wan et al., 2003; Mager et al., 2006) provides the opportunity to better understand the relationships between perturbed activity in brainstem neurons and a range of abnormalities in PD, including disturbed circadian rhythms, stress responses and energy metabolism. In addition, this model can be used to understand the molecular and cellular mechanisms by which α-synuclein impairs the function of ANS neurons. Moreover, the normalization of ANS function in SNCA mice by intermittent fasting provides proof-of-principle evidence that this model of ANS dysfunction in PD can be used to evaluate the therapeutic efficacy of interventions designed to prevent or treat PD.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Evinger C. An explanation for reflex blink hyperexcitability in Parkinson’s disease. II. Nucleus raphe magnus. J Neurosci. 1996;16:7318–7330. doi: 10.1523/JNEUROSCI.16-22-07318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet M, St-Amour I, Vandal M, Julien P, Cicchetti F, Calon F. High-fat diet exacerbates MPTP-induced dopaminergic degeneration in mice. Neurobiol Dis. 2012;45:529–538. doi: 10.1016/j.nbd.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brodski C, Schaubmar A, Dechant G. Opposing functions of GDNF and NGF in the development of cholinergic and noradrenergic sympathetic neurons. Mol. Cell. Neurosci. 2002;19:528–538. doi: 10.1006/mcne.2001.1093. [DOI] [PubMed] [Google Scholar]
- Buob A, Winter H, Kindermann M, Becker G, Möller JC, Oertel WH, Böhm M. Parasympathetic but not sympathetic cardiac dysfunction at early stages of Parkinson’s disease. Clin. Res. Cardiol. 2010;99:701–706. doi: 10.1007/s00392-010-0170-6. [DOI] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. Alphasynuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol. 1989;287:373–392. doi: 10.1002/cne.902870308. [DOI] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- Ghebremedhin E, Del Tredici K, Langston JW, Braak H. Diminished tyrosine hydroxylase immunoreactivity in the cardiac conduction system and myocardium in Parkinson’s disease: an anatomical study. Acta Neuropathol. 2009;118:777–784. doi: 10.1007/s00401-009-0596-y. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Lutge W, Tanner CM. Autonomic dysfunction in Parkinson’s disease. Neurology. 1986;36:73–75. doi: 10.1212/wnl.36.1.73. [DOI] [PubMed] [Google Scholar]
- Griffioen KJ, Wan R, Brown TR, Okun E, Camandola S, Mughal MR, Phillips TM, Mattson MP. Aberrant heart rate and brainstem brain-derived neurotrophic factor (BDNF) signaling in a mouse model of Huntington’s disease. Neurbiol. Aging. 2012;7:1481.e1–1481.e5. doi: 10.1016/j.neurobiolaging.2011.11.030. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O. Alpha-synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis. 2012;47:258–267. doi: 10.1016/j.nbd.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, Blessing WW, Geffen LB. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol. 1990;27:373–385. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67:1955–1959. doi: 10.1212/01.wnl.0000247052.18422.e5. [DOI] [PubMed] [Google Scholar]
- Huot P, Fox SH, Brotchie JM. The serotonergic system in Parkinson’s disease. Prog Neurobiol. 2011;95:163–212. doi: 10.1016/j.pneurobio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Jain S. Multi-organ autonomic dysfunction in Parkinson disease. Parkinsonism Relat. Disord. 2011;17:77–83. doi: 10.1016/j.parkreldis.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis. 2012;46:572–580. doi: 10.1016/j.nbd.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM, Bruneau BG, Giasson BI, Smeyne RJ, Gershon MD, Nussbaum RL. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 2010;19:1633–1650. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Bomhoff GL, Stanford JA, Geiger PC. Neurodegeneration in an animal model of Parkinson’s disease is exacerbated by a high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1082–1090. doi: 10.1152/ajpregu.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CM, de Vos RA, Maurage CA, Thal DR, Tolnay M, Braak H. Staging of sporadic Parkinson disease-related alpha-synuclein pathology: inter- and intra-rater reliability. J. Neuropathol. Exp. Neurol. 2005;64:623–628. doi: 10.1097/01.jnen.0000171652.40083.15. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hirayama M, Yamashita F, Uchida K, Hama T, Watanabe H, Sobue G. Lowered cardiac sympathetic nerve performance in response to exercise in Parkinson’s disease. Mov. Disord. 2010;25:1183–1189. doi: 10.1002/mds.23127. [DOI] [PubMed] [Google Scholar]
- Noorian AR, Rha J, Annerino DM, Bernhard D, Taylor GM, Greene JG. Alphasynuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiol Dis. 2012 Jun 18; doi: 10.1016/j.nbd.2012.06.005. 2012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S A. 2007;104:13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica R, Carlin JM, Grossardt BR, Bower JH, Ahlskog JE, Maraganore DM, Bharucha AE, Rocca WA. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. 2009;73:1752–1758. doi: 10.1212/WNL.0b013e3181c34af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro PA, Sloan RP, Horn EM, Myers MM, Gorman JM. Effect of innervation on heart rate response to mental stress. Arch Gen Psychiatry. 1993;50:275–279. doi: 10.1001/archpsyc.1993.01820160045004. [DOI] [PubMed] [Google Scholar]
- Sotiriou E, Vassilatis DK, Vila M, Stefanis L. Selective noradrenergic vulnerability in α-synuclein transgenic mice. Neurobiol Aging. 2010;31:2103–2114. doi: 10.1016/j.neurobiolaging.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, Hess EJ, Dawson VL, Dawson TM. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci U S A. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J. Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Yang B, Slonimsky JD, Birren SJ. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat. Neurosci. 2002;5:539–545. doi: 10.1038/nn0602-853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.