Abstract
Toxoplasma gondii is a protozoan parasite of medical and veterinary significance that is able to infect any warm-blooded vertebrate host. In addition to its importance to public health, several inherent features of the biology of T. gondii have made it an important model organism to study host-pathogen interactions. One factor is the genetic tractability of the parasite, which allows studies on the microbial factors that affect virulence and allows the development of tools that facilitate immune studies. Additionally, mice are natural hosts for T. gondii, and the availability of numerous reagents to study the murine immune system makes this an ideal experimental system to understand the functions of cytokines and effector mechanisms involved in immunity to intracellular microorganisms. In this article, we will review current knowledge of the innate and adaptive immune responses required for resistance to toxoplasmosis, the events that lead to the development of immunopathology, and the natural regulatory mechanisms that limit excessive inflammation during this infection.
Keywords: Toxoplasma gondii, T. gondii, immune response, immunopathology, pathology, infection, parasite
Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite that can infect any warm-blooded vertebrate, and is a pathogen of medical and veterinary significance [1]. Infection with T. gondii can be acquired through congenital infection [2], or through carnivory, if tissue cysts present in the chronically infected host are ingested [3,4]. Additionally, it can be acquired through the ingestion of food and water contaminated with parasites in the form of oocysts, which are shed in the feces of infected cats [5]. Following ingestion, the parasite converts to a fast replicating form known as the tachyzoite, which results in systemic dissemination of the parasite to all tissues. Under normal circumstances this systemic infection is effectively controlled by the host immune response [6,7]. The parasite then converts to a slow replicating form known as the bradyzoite, which persist in tissue cysts in the host neural and muscle tissues for the lifetime of the host [8].
The course of infection in humans can range from asymptomatic to severe, depending on the parasite strain and the immune status of the host. The majority of cases of human infection are regarded as asymptomatic and infection rates in some areas are as high as 70% [9]. In contrast, congenital infection can result in a number of birth defects, including hydrocephalus, chorioretinitis, intracerebral calcifications, or spontaneous abortion [10]. Toxoplasmosis can also cause severe disease in patients with primary or acquired deficiencies in T cell function, such as those present in patients with AIDS, Hyper IgM Syndrome, those receiving treatment for cancer, and transplant patients being treated with immunosuppressive drugs [11–16]. Although such instances are relatively rare, symptomatic disease in immunocompetent individuals can result from infection with highly virulent strains of T. gondii and can cause severe ocular disease or death [17,18]. In addition to its direct significance to public health, the genetic malleability of the parasite and its natural ability to infect laboratory animals, have made it an ideal model to study parasite genetics and host-pathogen interactions [19].
Invasion process and intracellular niche
The mechanisms by which T. gondii invades host cells and forms an intracellular niche have been extensively reviewed elsewhere [20], but several aspects of this process are directly relevant to immunity and pathogenesis. During invasion, three successive waves of proteins are secreted from parasite organelles, called the micronemes, dense granules, and rhoptries, into the host cell. These proteins can alter host cell function and inhibit the immune response directed towards the parasite [21]. They also serve to modify the lipid membrane surrounding the parasite, forming a specialized intracellular organelle called the parasitophorous vacuole (PV). The PV allows for the transport of essential nutrients from the host cell to the parasite, while preventing lysosomal fusion, which would lead to the killing of the parasite [22]. The sequestered nature of the parasite within the PV raises several fundamental questions regarding the mechanisms by which the parasite interacts with the immune system. For example, can host cells sense the invading parasite, and how would infected cells access parasite antigens for presentation to T cells as is required for the effective control of the parasite.
Parasite virulence
As is the case for many pathogens, the outcome of infection with T. gondii is highly dependent on the interplay of host and microbial factors. Genotypic studies have identified three lineages of T. gondii into which most strains found in North America and Western Europe can be broadly classified [23]. In mouse models, parasites belonging to the Type I lineage are highly virulent whereas the Type II and Type III lineages are considered avirulent [23,24]. These differences are also reflected in human disease, as ocular toxoplasmosis in humans is associated with Type I, but not Type II or Type III strains [17]. Given the lethality of Type I strains during murine infection, the vast majority of insights into the mechanisms by which the host immune response controls infection have been gained through studies using avirulent isolates. However, the use of reverse genetics to compare parasite strains that differ in virulence has allowed the identification of secreted T. gondii kinases that modify host cell function. Understanding how these parasite enzymes impact host anti-microbial mechanisms provides one approach to define the events that determine the outcome of infection [25].
Innate immune responses to T. gondii
Following challenge with T. gondii, monocytes, neutrophils and dendritic cells (DCs) are recruited to the site of infection, and all of these cell types have been implicated in resistance to this organism [26–32]. However, questions remain about their specific roles in controlling infection. One of the most critical functions of the innate immune response to T. gondii is the ability to sense the pathogen and produce the cytokine IL-12, which stimulates natural killer (NK) cells and T cells to produce the cytokine Interferon-gamma (IFN-γ) [33–35]. IFN-γ is the major mediator of resistance to T. gondii and promotes multiple intracellular mechanisms to kill the parasite and inhibit its replication. This Th1 immune response, defined by the production of IL-12 and IFN-γ, is characteristic of infection with many intracellular pathogens, and as is the case for infection with other intracellular pathogens, mice deficient in either IL-12 or IFN-γ that are infected with T. gondii succumb to acute disease and demonstrate an inability to control parasite burden [34,36].
The innate production of IL-12 during toxoplasmosis requires that the parasite first be sensed by the host. Innate immune receptors called Toll Like Receptors (TLRs) appear to have a role in this process. Thus, mice deficient in the adapter molecule MyD88, which is required for downstream signaling from most TLRs, are acutely susceptible to toxoplasmosis [37]. Specific TLRs implicated in the immune response to T. gondii include TLRs 2, 4, 9 and 11. TLR11 responds to a profilin-like molecule conserved among protozoan parasites [38,39] whereas TLRs 2 and 4 appear to recognize glycosylphosphatidylinositols on the surface of the parasite [40]. Additionally, following oral infection with T. gondii, bacterial antigens translocate from the gut, and TLRs 2, 4, and 9 respond to these microbial insults, thus contributing to the development of the Th1 immune response [41]. Although deficiency in any single TLR (of those tested to date) does not result in acute susceptibility to T. gondii, the relative contribution of each of these TLRs is illustrated by the increased cyst burden present in infected mice deficient in one or more of these receptors [38,40]. Despite the critical importance of MyD88, other mechanisms of sensing the parasite clearly exist, as protective immunity can be induced in MyD88-deficient mice using a vaccine strain of the parasite, and IL-12 responses are not completely abolished in the absence of MyD88 [37,42].
Numerous studies have aimed to define the primary cell types responsible for production of IL-12 in vivo and have identified neutrophils, inflammatory monocytes, macrophages and DCs as relevant sources [27,28,37,43–45,]. The relative contribution of DCs to the production of IL-12 during toxoplasmosis has been examined using two mouse models: one in which DCs can be selectively depleted, and another in which DCs specifically lack expression of MyD88 [32,46]. In both cases, altered function or numbers of DCs resulted in lower systemic levels of IL-12 and increased susceptibility to T. gondii. In these models, resistance can be restored by treatment with IL-12, suggesting that DCs are an essential source of IL-12 during toxoplasmosis. Other studies have aimed to define which subsets of DCs are the most relevant sources of IL-12. Following the in vivo administration of soluble T. gondii antigens, the CD8α+ subset of DCs produces IL-12 [47]. More recently, mice lacking the transcription factor Batf3, which have a deficiency in CD8α+ DCs, have been shown to succumb to T. gondii associated with a severe IL-12 defect, reduced CD8+ T cell responses, and high parasite burdens [48]. The finding that exogenous IL-12 restores survival of Batf3 KO mice is consistent with a model in which CD8α+ DCs are an essential source of IL-12.
Neutrophils are another source of IL-12 during toxoplasmosis, as they contain pre-stored IL-12 and can secrete this cytokine in vitro and in vivo in response to T. gondii [28,43,49]. Additionally, there are reports that neutrophil depletion results in decreased levels of IL-12 and increased parasite replication [50]. These findings are complicated by the realization that the strategy used to deplete neutrophils also affects other cell types, including inflammatory monocytes [30]. Regardless, mice deficient in the chemokine receptor CXCR2, which is essential for neutrophil recruitment to the site of infection, have higher parasite levels in the CNS, suggesting a role for neutrophils during toxoplasmosis [31]. Neutrophils are also implicated in other effector mechanisms that directly kill parasites, including phagocytosis, the release of reactive chemical species, and the formation of extracellular traps [51–54]. While phagocytosis of T. gondii by neutrophils has been observed in vitro [51,52], several groups have reported that p47phox, an enzyme component necessary for the oxidative burst generated by neutrophils following phagocytosis, is unnecessary for resistance to T. gondii [55,56]. Indeed, in vivo imaging studies have observed swarms of neutrophils that congregate around infected cells, but the parasites present in the neutrophils appear to be largely intact [53]. However, infection with T. gondii does induce increased extracellular DNA at the site of infection, which is dependent upon the presence of neutrophils, and this may be explained by the release of DNA from neutrophils to form extracellular traps [54]. In vitro studies suggest that the formation of these traps results in decreased parasite vitality and may contribute to the control of T. gondii in vivo.
Monocytes are also required for resistance during toxoplasmosis, as mice deficient in the chemokine receptor CCR2, which in necessary for monocyte recruitment to the site of infection, exhibit increased susceptibility when challenged [30,57,58]. Inflammatory monocytes produce IL-12 in vitro and in vivo when stimulated with T. gondii, however it is not clear whether they are a critical source of this cytokine [27, 30,45,57–59]. It has also been proposed that these populations contribute to the direct control of T. gondii through the generation of nitric oxide (NO), which inhibits parasite replication [60]. In support of this model, inflammatory monocytes express inducible nitric oxide synthase (iNOS), the enzyme responsible for catalyzing the production of NO, and inflammatory monocytes are able to kill and inhibit the replication of T. gondii in vitro [61,62,27]. Additionally, CCR2 KO mice given a low dose oral challenge of T. gondii succumb approximately 3–4 weeks after infection, and this is associated with decreased expression of iNOS and increased parasite burdens in the CNS [58]. Although monocytes are clearly critical for survival during toxoplasmosis, their role is not limited to production of nitric oxide, as iNOS- deficient mice survive acute challenge, while deficiencies in monocyte recruitment can lead to acute susceptibility [30,57,63]. Monocytes also produce IL-1 in response to soluble toxoplasma antigens [64], and this factor can enhance anti-toxoplasmic effector mechanisms in macrophages and astrocytes in vitro [65,66]. Moreover, IL-1 can synergize with IL-12 to promote production of IFN-γ from innate and adaptive sources [67,68]. It is also possible that monocytes proceed to develop into DCs that are capable of inducing adaptive immune responses [69], or macrophages that can control infection through immune GTPase-mediated mechanisms, as will be discussed later in this article.
Natural killer (NK) cells are another innate population involved in immunity to T. gondii, and in mice that lack T cells they provide a limited mechanism of resistance through their ability to produce IFN-γ [35,70–72]. NK cell activity peaks early during infection, and although their activity is elevated during chronic toxoplasmosis, they do not appear to be significant contributors to immunity during the chronic stage of infection [35,70–74]. Consequently, most studies have focused on the early events that control NK cell activity, leading to a model in which IL-12 produced by other innate cells (e.g. neutrophils, monocytes and DCs) promotes NK cell-mediated production of IFN-γ [33,35]. In addition to IFN-γ, NK cells produce the cytokine IL-10, the significance of which will be discussed later in this review [75]. Human and murine NK cells can also be cytotoxic for cells infected with T. gondii [76,77], but it has been proposed that NK cells become infected with the parasite following the lysis of infected cells, which may promote the dissemination of the parasite [78].
NK cells can also act to promote adaptive immune responses. Thus, in the absence of CD4+ T cells, they can provide help to the CD8+ T cell response [79]. One mechanism by which this help is accomplished is by increasing IL-12 production from DCs through interactions dependent on the molecule NKG2D [80]. Additionally, production of IFN-γ by NK cells has been implicated in the development of optimal CD4+ T cell responses [81].
Adaptive Immune Responses to T. gondii
The importance of adaptive immune responses for resistance to T. gondii during human infection is demonstrated by the increased susceptibility of patients with primary or acquired defects in T cell function, and mice with deficiencies in B cells, CD4+ T cells or CD8+ T cells survive the acute stage of infection, but ultimately show increased susceptibility to T. gondii [82–84]. Understanding how these different cell populations are integrated to provide long-term resistance has been challenging, but several advances in technology have improved our ability to study adaptive immune responses to T. gondii. For example, the identification of the molecular epitopes of T. gondii recognized by CD8+ T cells has allowed the measurement of antigen-specific CD8+ T cell responses during infection, and provided insight into the mechanisms by which antigen is presented [85,86]. This has been complimented by the development of parasites that express model antigens such as ovalbumin, β-galactosidase, and EαRFP, as well as the use of two-photon imaging to allow visualization of immune cells following infection [19,87]. These advances are currently allowing a higher resolution analysis of the events that mediate the control of T. gondii, and may also provide insight into the strategies used by this parasite to persist despite the presence of an array of anti-microbial effector mechanisms.
CD4+ T cell responses: Initiation and mechanisms of controlling infection
As mentioned earlier, CD4+ T cells are critical for resistance during toxoplasmosis, as the emergence of severe toxoplasmosis is concomitant with the decline in T cell numbers in patients infected with HIV [88,11], and in mouse models, the lack of CD4+ T cells is associated with increased susceptibility during the chronic stage of infection [83]. CD4+ T cells provide several critical regulatory functions in mediating resistance to toxoplasmosis. During the early stages of infection they contribute to optimal B and CD8+ T cell responses (discussed in subsequent sections of this review) [83,89], and the ability of these cells to control chronic infection may be attributed to their production of cytokines such as IFN-γ, or their expression of CD40L (also referred to as CD154), which can activate effector mechanisms in macrophages and other innate cells expressing CD40 on their surface [90–95].
The initiation of T cell responses requires that naïve CD4+ or CD8+ T cells encounter antigen presenting cells bearing their cognate antigen in the context of MHCII or MHCI molecules respectively, in conjunction with co-stimulatory and cytokine signals needed for T cell activation [96–99]. During toxoplasmosis, ligation of the molecules CD28 and ICOS, expressed on the surface of T cells, contributes to the co-stimulatory signals, while IL-12 provides the cytokine signal required to promote proliferation and differentiation into populations that produce IFN-γ [34,100,101].
B cells, macrophages, and DCs are all capable of presenting antigen to CD4+ T cells, though DCs are generally considered the most crucial antigen presenting cell population in vivo [102]. Following infection with T. gondii, multiple populations of DCs undergo expansion and acquire an activated phenotype. Additionally, challenge of mice with parasites engineered to express the model antigen EαRFP revealed that CD8α+ and plasmacytoid DCs (pDCs) express complexes of MHC class II and Eα, a peptide derived from EαRFP, on their surfaces. While these studies implicate pDCs and CD8α+ DCs as responsible for presenting antigen to CD4+ T cells during toxoplasmosis, the use of mice with deficiencies in specific DC compartments, as well as mouse models that allow for the selective depletion of DCs or DC subsets, may be useful to further define the roles of these populations in antigen presentation [48,103,104].
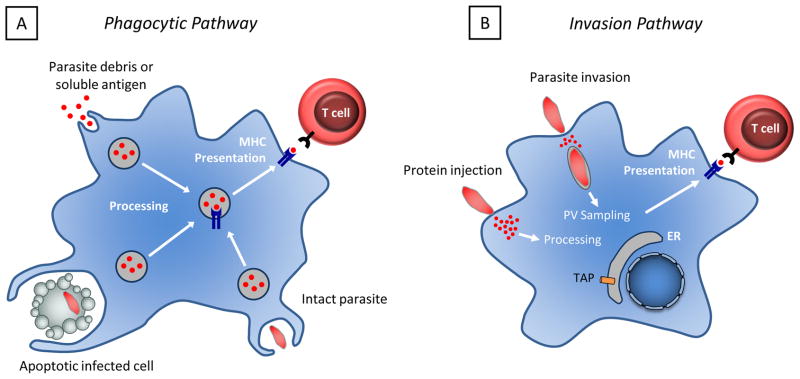
The mechanisms by which professional antigen presenting cells acquire parasite antigens for presentation in the context of MHCII are unclear, and there are several possible models to explain how this may be accomplished (Figure 1). Since there are multiple reports that murine DCs and monocytes infected with T. gondii express low levels of MHCII and co-stimulatory molecules, it has been suggested that infected cells would be poor presenters of antigen [105–107]. Thus, antigen acquisition might occur through the phagocytosis of parasites, infected cells, or parasitic debris, or through the endocytosis of antigens secreted by the parasite (Figure 1a). In vitro studies have demonstrated that murine DCs are able to present antigen derived from live and heat-killed parasites to CD4+ T cells [108]. Because heat-killed parasites cannot invade cells, these data are consistent with a model in which antigen is acquired via the phagocytosis of parasites.
Figure 1. Potential antigen presentation pathways.
A. Antigen may be acquired through the phagocytosis of infected cells, intact parasites, parasite antigens, or through the endocytosis of parasite debris. B. Antigen may also be acquired by infected cells through the release of soluble antigens from the parasite, or through the sampling of antigens from the parasitophorous vacuole, which may be mediated by fusion of the parasitopherous vacuole with the endoplasmic reticulum. Antigens may also be injected into the host cell through abortive invasion events.
Alternatively, antigen may be acquired by antigen presenting cells through active invasion mediated by the parasite, or through abortive invasion events in which antigens are injected into cells that are not subsequently infected (Figure 1b) [109]. This model is supported by experiments conducted using human monocytes and DCs, in which cells exposed to viable parasites upregulated MHCII and co-stimulatory molecules, whereas cells exposed to heat-killed parasites did not [110,111]. Additionally, these studies found that exposure to live parasites was necessary for antigen presentation ability. These findings are not entirely consistent with the reports described earlier, although this may be attributable to differences between human and murine cells. It is also relevant to note that results obtained from in vitro studies may not be representative of what occurs in vivo. Currently, in vivo studies examining the mechanisms by which antigen is presented to CD4+ T cells during toxoplasmosis are lacking. Other insights into the mechanisms by which antigen can be acquired during toxoplasmosis have been gained by studying antigen presentation to CD8+ T cells, and will be discussed later in this article.
Humoral immunity is essential for resistance to toxoplasmosis
It has long been recognized that infection with T. gondii promotes antibody responses, and that these antibodies can kill the parasite [112]. Indeed, parasite-specific IgM, IgA, IgE and IgG2 antibodies have been isolated from human patients, and detection of parasite specific antibodies is an effective diagnostic tool to distinguish newly infected individuals from those in the chronic stage of infection [112–116]. The critical role of antibody in immunity to T. gondii is demonstrated by the phenotype of μMT mice, which are deficient in B cells. These mice develop apparently normal IFN-γ responses, but succumb to infection within 3–4 weeks following challenge, associated with high parasite burdens in the CNS [82]. This increase in susceptibility is likely due to a lack of antibodies, as the passive transfer of antibodies confers protection to B cell-deficient mice [83]. Antibodies can mediate their protective effects through a variety of mechanisms. In vitro studies have found that they can opsonize parasites for phagocytosis, block invasion, and also activate the classical complement pathway [51,117–121]. The in vivo relevance of complement activation is illustrated by studies in which treatment of mice with an antibody that binds to the complement protein C3 results in acute susceptibility to toxoplasmosis [122]. Additional studies are required to define the contribution of other antibody-mediated functions.
As mentioned previously, CD4+ T cells are necessary to promote optimal B cell responses and mice deficient in or depleted of CD4+ T cells display lower parasite-specific antibody titers [83,123]. Furthermore, the increased susceptibility of CD4+ T cell-deficient mice can be ameliorated by the passive transfer of antibodies, indicating that the defect in antibody responses likely contributes to the failure to control parasite numbers [83]. Curiously, infection with T. gondii results in severe disruption of splenic architecture and the loss of distinct B cell zones [123,124]. Since B cell zones are considered the main location where CD4+ T cells provide help to B cells [125], this raises the question of whether there is a specialized microenvironment where T-B interactions occur when B cell zones are absent. Since disruption of secondary lymphoid structures is characteristic of many infections [126–131], murine models of toxoplasmosis may prove a useful system to interrogate the mechanisms by which CD4+ T cells help B cell responses, and the extent to which splenic architecture contributes to such interactions.
CD8+ T cell response: Initiation and control of parasite burden
Given that T. gondii is an intracellular pathogen, it is not surprising that CD8+ T cells, which are specialized to recognize and destroy cells infected with viral, bacterial and parasitic organisms, also have a critical role in mediating resistance to this infection. CD8+ T cells can control infection through the production of inflammatory cytokines such as IFN-γ, through CD40/CD40L interactions, and through the perforin-mediated cytolysis of infected host cells [84,90,91]. Indeed, mice deficient in CD8+ T cells show increased susceptibility to toxoplasmosis, succumbing approximately 50 days post-infection [84]. Furthermore, the adoptive transfer of CD8+ T cells from chronically infected mice, or mice vaccinated with an attenuated strain of T. gondii, is sufficient to confer resistance [132,133]. Additional evidence comes from in vivo depletion studies using chronically infected mice, in which depletion of CD8+ T cells and CD4+ T cells results in reactivation of the infection and severe disease, but depletion of CD4+ T cells alone had limited impact [90].
As previously described, CD8+ T cell responses are initiated when naïve CD8+ T cells encounter their cognate antigen in the context of MHCI on the surface of antigen presenting cells, accompanied by co-stimulatory and cytokine signals. Some of the earliest studies on the CD8+ T cell response identified the Surface Antigen 1 (SAG-1) protein as a target of CD8+ T cells, although the specific peptide sequence of SAG-1 that the CD8+ T cells recognize remains unknown [134]. More recently, technical advancements have accelerated the discovery of epitopes of T. gondii that are recognized by CD8+ T cells. Thus, in 2008, two studies identified peptides derived from T. gondii that are presented in the context of the H2-Ld allele of MHCI. These include peptides from the dense granule proteins GRA4 and GRA6, and the rhoptry protein ROP7 [86,135]. Of these, the GRA4 and ROP7 epitopes are conserved across multiple strains of T. gondii, whereas expression of the GRA6 epitope is limited to Type II strains. Another epitope, derived from the protein Tgd_057, is presented in the context of the MHCI allele H2-Kb, and is also conserved among multiple genotypic strains [136]. The function of Tgd_057 is unclear, but despite the presence of a secretory signal, it localizes primarily to the cytosol of the parasite. It is of interest that all of these proteins, with the possible exception of Tgd_057, are secreted from the parasite. While this observation likely reflects, in part, the methods used to screen for these epitopes, it is also in agreement with studies in which parasites are engineered to express model antigens, as these studies consistently demonstrate that antigens secreted from T. gondii induce more robust T cell responses than antigens expressed in the cytosol [137–139]. These findings may provide insight into the mechanisms by which antigen is presented to T cells, as will be discussed later in this article.
Currently, there are several questions about the identity of the cell populations involved in antigen presentation to CD8+ T cells during toxoplasmosis. DCs are known to be efficient antigen presenting cells, and are crucial for the development of CD8+ T cell responses to T. gondii, however there is a lack of studies that clearly distinguish their role as producers of IL-12 from their role as presenters of antigen [29,32]. In vivo imaging studies have observed extensive interactions between DCs and antigen-specific CD8+ T cells, suggesting a role for DCs in antigen presentation [124,140]. In contrast, using a bone marrow chimera approach to generate mice in which MHCI expression was limited to non-hematopoietic cells, Dzierszinski et al. demonstrated that challenge of these mice with T. gondii resulted in an apparently normal CD8+ T cell response [141]. One interpretation of these data is that DCs are not necessary for antigen presentation during toxoplasmosis. Further experimentation is therefore necessary to determine which cell type presents antigen to CD8+ T cells.
As is the case for antigen presentation to CD4+ T cells, there are multiple pathways by which parasite antigens may be acquired for presentation to naïve CD8+ T cells (See figure). In vitro and ex vivo studies have found that infected DCs are able to present antigen, whereas cells exposed to parasites or parasite antigens that were not infected are unable to do so [108,141,142]. In contrast, the DCs observed to interact with CD8+ T cells in vivo appear to be largely uninfected [124,140], suggesting a possible role for uninfected cells in presenting antigen to naïve CD8+ T cells in vivo, which is consistent with numerous reports of cross-presentation in other models of infection [143].
While the cellular pathways by which phagocytosed antigens can come to be presented in the context of MHCI have been widely studied in a variety of systems [143], it is less clear how a cell infected with T. gondii can acquire antigen to be presented, given that the parasite resides in a specialized non-fusogenic vacuole. Several studies using reporter systems in which host cells respond to antigens derived from T. gondii have demonstrated that secreted antigens can enter the cytoplasm of infected cells [109,142]. These antigens would then be transported from the cytosol into the endoplasmic reticulum by the Transporter Associated with Antigen Processing (TAP) [142]. This model is consistent with studies demonstrating that secreted antigens from T. gondii are preferentially presented to T cells [86,135,137–139]. Alternatively, the PV can fuse with the endoplasmic reticulum, providing another mechanism by which antigens may escape sequestration and enter the protein transport pathway [108].
CD8+ T cell responses to T. gondii are influenced by help provided by CD4+ T cells [79,89]. Although depletion of CD4+ T cells does not affect the magnitude of the CD8+ T cell response during the early stage of CD8+ T cell expansion and activation, CD4+ T cells are necessary for the maintenance of CD8+ T cell effector functions during the chronic stage of infection, and this help must be provided during the acute stage of infection [89]. Further insights regarding the nature of CD4+ T cell help have been gained from studies using the attenuated vaccine strains of T. gondii ts-4 and cpsII, both of which require CD4+ T cell help for optimal protective CD8+ T cell responses [144,145]. In current models, ts-4 vaccination stimulates CD4+ T cells to produce the growth factor IL-2, which provides an essential signal for CD8+ T cells. Indeed, neutralization of IL-2 results in diminished CD8+ T cell responses and decreased protection [144]. Other potential mechanisms by which CD4+ T cells may provide help include the licensing of DCs, or direct interactions with CD8+ T cells through CD40/CD40L interactions [146].
The vast majority of studies examining the CD8+ T cell response have used avirulent Type II strains of T. gondii. Recently, it has become apparent that CD8+ T cell responses are dramatically decreased following infection with the highly virulent RH strain of the parasite and there are several possible explanations for this phenotype [29]. The defective CD8+ T cell response may be influenced by the activities of the parasite virulence factor ROP18 which (in addition to other functions) binds to the host protein Activating Transcription Factor 6 β (ATF6β), leading to its degradation [147]. In support of this model, ATF6β-deficient mice have a defective CD8+ T cell response when infected with T. gondii, and ROP18-deficient parasites from an RH background induce greater production of IFN-γ from CD8+ T cells, relative to WT RH parasites. Decreased CD8+ T cell responses may also result from an abbreviated DC response during RH infection, relative to infection with an avirulent Type II strain [148]. As the adoptive transfer of large numbers of antigen-specific CD8+ T cells is able to transiently reduce parasite burden during RH infection, it seems likely that the decreased CD8+ T cell response is a contributing factor to the virulence of the RH strain [29].
More subtle changes in CD8+ T cell responses may also help to explain differences in susceptibility among mouse strains. Whereas the C57B/6 inbred mouse strain succumbs to T. gondii during the chronic stage of infection, BALB/c mice are relatively resistant to toxoplasmic encephalitis. This difference in susceptibility has been genetically mapped to the MHC Class I H2-Ld allele, implicating CD8+ T cells as being responsible for this difference in susceptibility [149,150]. The recent identification of an immunodominant epitope from the protein GRA6, recognized by CD8+ cells, that binds to the H2-Ld Allele has led to the hypothesis that recognition of this peptide is crucial for controlling T. gondii infection in BALB/c mice, and may account for the differences in virulence among mouse strains [86]. Because expression of this epitope is restricted to Type II strains of T. gondii, its relative significance could be tested by replacing the peptide with the sequence present in Type I or Type III strains. Alternatively, it may be possible to tolerize mice to this epitope through vaccination, as has been reported in other systems [151]. Regardless, these studies highlight the importance of GRA6 as a target for protective CD8+ T cells.
Effector mechanisms controlling T. gondii infection
As discussed in the previous section, cellular immunity mediates protection through the production of inflammatory cytokines such as IFN-γ. Other molecular signals, such as the cytokine tumor necrosis factor alpha (TNF-α) and CD40 ligation are also required for resistance during chronic toxoplasmosis [91,152–154]. This section describes how these distinct pathways are integrated to engage specific effector mechanisms required to directly control infection with T. gondii.
Nitric oxide inhibits replication of T. gondii
Since the early 1980’s, it was recognized that IFN-γ can activate macrophages to kill a variety of intracellular organisms, including T. gondii [155], and during the late 1980’s it was reported that IFN-γ is also essential in vivo for resistance to T. gondii [36]. These findings raised the fundamental question of how this cytokine promotes control of T. gondii and other pathogens. It was proposed that the protective effects of IFN-γ may be mediated by inducing increased synthesis of Nitric Oxide (NO) [156]. Consistent with this hypothesis, expression of inducible nitric oxide synthase (iNOS), the enzyme responsible for catalyzing the reaction that results in production of NO, is increased in macrophages by stimulation with IFN-γ, and NO inhibits replication of T. gondii in macrophages and other cell types [157–161]. Importantly, IFN-γ alone is not typically sufficient to activate macrophages to kill T. gondii, and additional signals provided by factors like TNF-α or CD40L are required for optimal iNOS expression [158,162]. In vivo evidence for a role of NO in controlling toxoplasmosis came from a study in which administration of the iNOS inhibitor aminoguanidine to infected mice resulted in increased parasite burdens [163]. Subsequently, iNOS-deficient mice were developed and found to display increased susceptibility to toxoplasmosis, succumbing to disease in the chronic stage of infection [63]. Although the specific mechanism by which NO inhibits replication of T. gondii remains to be determined, studies using intracellular bacterial pathogens have shown that NO can inhibit bacterial enzymatic activity and directly damage DNA [164], which would preferentially affect pathogen replication and account for the static effects of NO.
IFN-γ mediates protection through the p47 GTPases
The increased susceptibility of iNOS-deficient mice to toxoplasmic encephalitis clearly implicated iNOS in immunity to T. gondii, but also pointed toward iNOS-independent mechanisms by which IFN-γ mediates protection during the acute phase of infection. Like iNOS, members of the p47 GTPase family (also referred to as the immune related GTPase family (IRGs)) are also upregulated in response to IFN-γ [165,166], but the importance of this family was first apparent when mice that lack the p47 GTPase IGTP (Irgm3) were infected with T. gondii. These mice have normal IFN-γ responses, but succumb to acute toxoplasmosis due to high parasite burdens [167]. Subsequent studies revealed other members of this family, including LRG-47 (Irgm1), IRG-47 (Irgd), IIGP1 (Irga6), and TGTP (Irgb6) to be involved in immunity to T. gondii as well [161,168–170]. The specific mechanisms by which individual members of the p47 GTPase family promote the clearance of T. gondii are the subject of ongoing studies in many laboratories [166]. There are reports that in IFN-γ activated cells p47 GTPases colocalize to the PV, which then develops a tight fitting morphology followed by a rough and disrupted appearance before being stripped away [161,170–177]. Once free in the cytosol, the parasite egresses the infected cell or becomes permeabilized and killed [161,178]. In the latter studies the host cell was observed to undergo necrosis after killing the parasite. Additionally, other studies have observed the exposed cytosolic parasite to be disposed of by xenophagy, the process by which foreign bodies within a cell are eliminated using the same cellular machinery involved in autophagy [172]. In further support of a role for autophagic machinery in immunity to T. gondii, the autophagy protein Atg5 has been found to be necessary for the disruption of the PV and resistance to this infection in vivo [174]. Additionally, CD40 ligation has been observed to induce xenophagic elimination of parasites independently of p47 GTPases, as will be discussed later in this review [93].
Given the important role of the p47 GTPases in immunity to T. gondii, it is not surprising that the parasite has evolved strategies to interfere with their function. At least three members of the p47 GTPase family, Irga6, Irgb6 and Irgb10, are phosphorylated by ROP18, resulting in changes in their functionality or cellular localization associated with increased virulence [170,177]. Additionally, the recruitment of GBP1, a member of the guanylate-binding protein family (GBPs), to the PV is also inhibited by the parasite-derived virulence factors GRA15, ROP16 and ROP18 [179]. As the GBP family has recently been implicated in immunity to intracellular bacteria [180], this finding may be indicative of a role for GBPs in immunity to T. gondii, although further research will be necessary to directly test this hypothesis.
The role of tryptophan degradation as a defense mechanism
IFN-γ can also mediate protective effects against T. gondii by promoting tryptophan degradation in a variety of infected cell types, including fibroblasts, macrophages, and brain cells [181–184]. Treatment of cells with IFN-γ results in the upregulation of the genes indolamine 2,3-dioxygenase 1 and 2 (IDO-1 and IDO-2), which catalyze the degradation of tryptophan [182,185]. Because T. gondii is a natural tryptophan auxotroph, the increased degradation of tryptophan by host cells inhibits parasite growth [186]. The in vivo relevance of this pathway is illustrated by the finding that long-term treatment of infected mice with inhibitors of IDO-1 and 2 results in increased susceptibility and increased parasite burdens during chronic infection [187]. Interpretation of this finding is complicated by the fact that IDO has other known immune functions such as suppression of DC and effector T cell functions, as well as promotion of regulatory T cell responses [188].
Members of the TNF family are necessary for immunity to T. gondii
In addition to IFN-γ, members of the TNF family such as CD40L, TNF-α and LT-α, are also required for protection during the chronic stage of infection [91,153,154]. The critical role of TNF-α is demonstrated by studies in which neutralization of this cytokine results in increased susceptibility and higher parasite burdens [189]. Additionally, mice deficient in TNF-α (TNF-α KO) or the components of its receptor (TNFR KO) succumb to infection approximately 3–4 weeks post-challenge despite having functional IFN-γ responses [152–154]. TNF-α is produced by a number of cell populations in response to T. gondii or T. gondii antigens, including neutrophils [43,190], DCs [190], macrophages [191], microglia [192], and T cells [193]. TNF-α synergizes with IFN-γ to promote anti-parasitic mechanisms in macrophages, as well as non-hematopoietic cells [194,195]. In vitro studies have demonstrated that this can be mediated through the production of nitric oxide [157,158]. Additionally, TNF-α KO mice, TNFR KO mice, and mice treated with a neutralizing antibody for TNF-α display decreased iNOS expression [152,154,189]. Collectively, these data support a model in which TNF-α mediates its protection by inducing expression of iNOS. However, there are also data that suggest that susceptible TNFR KO mice infected with T. gondii can have appropriate levels of iNOS, suggesting that TNF-α can mediate protection through iNOS-independent mechanisms [153]. Because TNF-α KO and TNFR KO mice are capable of surviving the acute stage of infection, it is clear that TNF-α is not required for the IGTP-mediated elimination of the parasite [152–154]. This notion is also supported by in vitro studies, in which macrophages show no defect in their ability to kill parasites in the absence of TNF-α signaling [196]. However, interpretation of these results is complicated by the finding that TNF-α plays a more prominent role in activating macrophages when concentrations of IFN-γ are limiting [153]. Thus, the chronic susceptibility of mice deficient in TNF-α signaling may result from changes in the expression of IFN-γ during the course of infection rather than a deficiency in any one specific effector mechanism that is absolutely dependent upon TNF-α.
Another component of the TNF family involved in immunity to T. gondii is CD40L, which is expressed on T cells and binds to CD40 expressed on macrophages and other cell populations [197]. The importance of CD40/CD40L interactions to promote immunity to T. gondii is evidenced by the increased susceptibility of patients with Hyper-IgM syndrome, a disease characterized by defective CD40L expression [12–14]. During human toxoplasmosis, CD40/CD40L interactions are necessary to promote optimal production of IFN-γ and class switched antibody [12,198]. In contrast, these interactions are not critical for production of IFN-γ in the murine model, yet mice deficient in CD40L display increased susceptibility during chronic infection [91]. While CD40L can act synergistically with IFN-γ to inhibit parasite replication, there is also evidence that CD40L can act independently of IFN-γ [91–93,95]. One IFN-γ independent mechanism by which CD40L controls infection is through the induction of xenophagic killing of the parasite, which has been shown to be independent of the p47 GTPase family, but dependent upon the autophagic molecule Beclin-1 [93–95]. Beclin-1-heterozygous mice also demonstrate increased susceptibility to T. gondii infection, indicating that CD40-mediated xenophagy may be a unique and critical mechanism for controlling chronic toxoplasmosis.
Lymphotoxin alpha (LT-α) is another member of the TNF family essential for immunity to T. gondii. Like TNF and TNFR KO mice, LT-α KO mice succumb to this infection within the first 4 weeks, associated with a high parasite burden [154]. These mice display functional but delayed IFN-γ responses and antibody titers, and decreased expression of iNOS. These defects may conceivably result from a critical role for LT-α in signaling to directly promote effector functions, or they may be a secondary consequence of the defective splenic architecture observed in LT-α KO mice [199].
Thus, cytokines and the effector mechanisms they induce are able to control toxoplasmosis, allowing the parasite and the host to co-exist. Parasite virulence factors or immunodeficiency can disrupt this equilibrium, leading to severe disease or the death of the host. However, the immune effector mechanisms that control parasite burden can also bear a fitness cost upon the host, as will de described in the following section.
Severe immunopathology and the mechanisms that prevent it
As is true for many infections, maintaining immune homeostasis during toxoplasmosis requires not only the ability to limit the replication of the pathogen, but also the ability to control the host immune response. In WT mice this is illustrated by severe infection-induced inflammation in the gut and central nervous system that is mediated by CD4+ T cells. This section will review the factors that contribute to these pathologic events and the mechanisms by which they are controlled.
Intestinal Ileitis following infection with T. gondii
Immunopathology can occur in the ileum following oral infection with T. gondii in mice and other species, and this ileitis has been proposed as a model to understand the basis for immune-mediated gastrointestinal disease in humans [200,201]. The infection-induced ileitis is characterized by the development of severe necrosis and inflammatory foci, and is dependent upon the host’s sex and genetic background [200,202]. That this process is immune mediated is demonstrated by studies in which C57B/6 mice lacking CD4+ T cells or mice depleted of CD4+ T cells fail to develop this phenotype [200]. Development of the ileitis is a complex process involving numerous cell types, including intraepithelial lymphocytes, natural killer T cells, and NK cells [203–206]. Factors that promote T cell responses, such as CD40/CD40L interactions and the cytokines IL-12 and IL-23 also contribute to ileitis development [207–209]. Other cytokines, such as IFN-γ, TNF-α, IL-18, IL-22, and the macrophage migration inhibitory factor (MIF) have also been implicated in mediating pathology [208–211]. The Th2 cytokines IL-4 and IL-5 have also been implicated in ileitis development, although another report found IL-4-deficient mice to be more susceptible to oral challenge [212–214].
As work on this model has progressed, it has become clear that the commensal bacteria present in the gut contribute to the development of this infection-induced ileitis [215]. Recent findings have led to a model in which oral challenge with T. gondii results in a dramatic increase in the quantity of Gram-negative bacteria in the gut flora and bacterial translocation to subepithelial tissues [215] where TLR4 senses these bacteria and amplifies the local inflammation [216]. Additionally, mice deficient in TLR11, which binds to profilin expressed by the parasite, do not develop ileitis, suggesting that the innate response to T. gondii also contributes to this process [41]. While these findings implicate both parasitic and bacterial antigens in stimulating the pathologic immune response, it remains to be determined for which antigens the CD4+ T cells that mediate ileitis are specific.
CD4+ T cell mediated immunopathology during Toxoplasmic encephalitis
Another example of severe pathology occurs in mouse models of chronic toxoplasmic encephalitis. Although CD4+ T cells are essential for long-term resistance to T. gondii, they also can induce severe pathology in the central nervous system. Thus, in susceptible mice large numbers of CD4+ T cells are present in the brain during the chronic encephalitis, and depletion of CD4+ T cells can ameliorate pathology without affecting parasite burden [217]. Similarly, CD28 KO mice infected with T. gondii have normal parasite burdens but exhibit enhanced resistance to TE that correlates with decreased numbers of CD4+ T cells in the brain [218]. This work contrasts with studies in which depletion of CD4+ T cells was sufficient to reactivate disease [219]. These seemingly conflicting results may be partially explained by differences in depletion efficiency, as complete depletion of CD4+ T cells in the central nervous system can be difficult to obtain, and is not necessarily reflected by the depletion efficiency in other tissue sites [220]. Thus, while CD4+ T cells are required for control of infection, partial inhibition of CD4+ T cell responses may be beneficial to the host in the context of chronic toxoplasmic encephalitis.
IL-10 inhibits CD4+ T cell mediated immunopathology
Since 1996, the use of various knockout mice has led to the identification of factors critical for limiting the development of immune pathology during toxoplasmosis. These studies have provided a novel insight into the nature of host-pathogen interactions, which is perhaps best illustrated by studies in which mice deficient in the cytokine IL-10 (IL-10 KO) were challenged with T. gondii [221]. IL-10 is produced by a number of cell types, including macrophages, NK cells, T cells and B cells, and functions by inhibiting the activation of accessory cells and adaptive immune responses [222]. The central role for IL-10 in limiting inflammation was confirmed by the finding that IL-10 KO mice develop spontaneous colitis [223]. However, IL-10 is also a potent antagonist of the ability of macrophages to kill intracellular bacteria and parasites, such as T. gondii, and infection with a number of pathogens, including T. gondii, increases expression of IL-10 [189,224–227]. These findings led to the idea that pathogens induce IL-10 production as a means to evade the immune response [227]. However, challenge of IL-10 KO mice with T. gondii revealed that these mice display normal parasite burdens, but develop severe liver damage, increased production of pro-inflammatory cytokines, and succumb to a CD4+ T cell mediated hyper-inflammatory response [221]. These results provide one of the first examples of an infection in which the host must control its own immune response and tolerate pathogen persistence in order to survive.
While IL-10 is clearly critical for survival during the acute stage of infection, studies analyzing the role of IL-10 during toxoplasmic encephalitis have yielded more ambiguous results. There is general agreement that IL-10 expression is upregulated in the brain during chronic toxoplasmosis, and macrophages and CD4+ T cells represent a local source of IL-10 in the central nervous system [220,228]. One study observed that neutralization of IL-10 during chronic toxoplasmosis was not lethal to infected mice, and resulted in decreased parasite burden [228]. In contrast, another study reported that blocking of the IL-10R resulted in decreased survival of chronically infected mice [229]. Similarly, in a system that allowed IL-10 KO mice to survive acute infection, the chronically infected IL-10 KO mice displayed normal parasite burden but also developed severe immunopathology mediated by CD4+ T cells associated with increased production of pro-inflammatory cytokines [220]. Together these results suggest that while IL-10 may partially inhibit effector mechanisms that could otherwise reduce parasite burden, it is crucial in the acute and chronic stage of infection to prevent severe immunopathology.
Attempts to identify the cellular sources of IL-10 during toxoplasmosis have revealed that there are multiple contributors, including macrophages [228], NK cells [75], CD4+ T cells [228,229] and CD8+ T cells [228]. Innate sources of IL-10 are significant during toxoplasmosis, as the loss of IL-10 expression from SCID mice can dramatically extend their survival [230], and NK cells are regarded as a major innate source of IL-10 during the acute stage of infection [75]. Nevertheless, the use of a cre-flox system to selectively eliminate IL-10 production from CD4+ and CD8+ T cells revealed that these mice still develop severe immunopathology upon infection [231], indicating that non-T cell sources of IL-10 were not sufficient to limit pathology. Subsequent work has demonstrated that depletion of CD4+ T cells dramatically decreases expression of IL-10, and that the CD4+ T cells that produce IL-10 express the transcription factor T-bet and low levels of CD25, suggesting that they are effector T cells as opposed to regulatory T cells [229].
IL-27 inhibits CD4+ T cell mediated immunopathology
IL-27 is another cytokine critical for regulating CD4+ T cell responses during toxoplasmosis. Initial reports concluded that the IL-27 receptor was important in promoting Th1 immune responses [232], however when mice deficient in WSX1, a component of the IL-27 receptor, were infected with T. gondii, these mice were found to exhibit enhanced Th1 responses and dramatically increased susceptibility to this challenge [233]. Similar to the IL-10 KO mice, infected WSX1 KO mice have normal parasite burdens but develop severe liver and lung pathology, and increased numbers of activated CD4+ and CD8+ T cells. Furthermore, depletion of CD4+ T cells prevents the infection-induced pathology. Although initial studies indicated that the suppressive effects of IL-27 were independent of IL-10 [233], subsequent work has demonstrated that IL-27 promotes IL-10 expression [234]. However, this property of IL-27 does not fully explain its suppressive activities. IL-27 can also act directly on CD4+ T cells to inhibit production of IL-2, a growth and survival factor for T cells, and neutralization of IL-2 results in enhanced survival of WSX1-KO mice [233]. Another recent study found that deficiency in EBI3, a second component of the IL-27 receptor, correlated with decreased expression of the inhibitory molecule PD-L1 on CD4+ T cells during toxoplasmosis, providing another potential mechanism by which IL-27 may mediate its protective effects [235].
Although WSX1 KO mice normally do not survive to chronic infection, treatment with anti-toxoplasma drugs or immune blockade can prevent acute susceptibility, allowing the role of IL-27 during chronic infection to be examined [236]. Under these circumstances, WSX1 KO mice show no defect in their ability to control parasite burden but do develop severe immunopathology in the central nervous system, which correlated with decreased production of IL-10 and increased production of IL-17 [234,236]. These findings led to the recognition that IL-27 can directly inhibit production of IL-17 from Th17 cells [236]. These results collectively support a model in which IL-27 controls immunopathology during toxoplasmosis by inhibiting multiple facets of T cell activation, and the principles established using T. gondii have been shown to be relevant to the immunosuppressive effects of IL-27 in a variety of systems, including infections with other intracellular parasites [237,238], helminth infection [239], bacterial infection [240, 241], and numerous autoimmune models [242–247].
The role of Ahr and Lipoxin in controlling immunopathology
Another factor that contributes to the control of chronic toxoplasma infection is Lipoxin A4 (LXA4), a product of the reaction catalyzed by the enzyme 5-lipoxygenase (5LO). Mice deficient in 5LO succumb to toxoplasmosis approximately one month post infection, having reduced parasite burdens relative to WT controls [248]. 5LO- deficiency is associated with increased infiltration of inflammatory cells into the brain, and elevated production of the cytokines IL-12, IFN-γ, and TNF-α. Expression of LXA4 is induced by infection with T. gondii and administration of an LXA4 analogue can rescue 5LO-deficient mice, implicating this molecule as a negative regulator of inflammation. One known mechanism by which LXA4 can inhibit immune responses is by serving as a ligand for the aryl hydrocarbon receptor (Ahr) [249]. Ahr is an intracellular signaling molecule that translocates to the nucleus upon binding to its ligand [250]. Ligation of Ahr by LXA4 inhibits the production of IL-12 from DCs in a manner dependent upon the signaling molecule Suppresser of Cytokine Signaling 2 (SOCS2), which is also required for survival during toxoplasmosis [251,252]. Consistent with this mechanism as a means to control the immune response during toxoplasmic encephalitis, mice deficient in Ahr have reduced parasite burdens, but succumb to chronic infection with T. gondii [253].
Other factors involved in controlling immune pathology
TLR11 is another factor necessary to control the immune response during acute toxoplasmosis [254]. In the absence of TLR11, mice develop fat necrosis and pancreatic inflammation, which does not correlate with increased parasite burden in the pancreas. This inflammation is mediated by IL-12, IFN-γ and NK cells, but is independent of T lymphocytes. The cytokines IL-18 and IL-1β are also partially responsible for the development of pancreatic inflammation, as neutralization of either of these cytokines decreases inflammation. These results raise the question as to what anti-inflammatory mechanisms may be initiated by TLR11 ligation. Given the prominent role of IL-12 in eliciting production of IL-10 from NK cells [75], it may be that decreased IL-12 levels in the absence of TLR11 result in insufficient amounts of IL-10 to prevent pancreatic pathology, although additional experiments are required to test this model.
Another pathway involved in protection of mice against severe immunopathology during acute toxoplasmosis involves the cleavage of fibrinogen to produce fibrin, as part of the cascade responsible for blood clotting [255]. Fibrin levels are increased upon infection with T. gondii in a TNF-α dependent manner, while IFN-γ negatively regulates these events [256]. Mice lacking fibrin exhibit normal control of parasite burden and intact immune responses, but succumb to infection within the first 15 days, associated with an IFN-γ dependent liver pathology characterized by necrosis, hemorrhaging, and diffuse inflammatory infiltrates [257]. Thus, fibrin prevents hemorrhaging and severe pathology during toxoplasmosis.
Concluding remarks and future perspectives
Since the discovery that T. gondii infects humans 75 years ago, there have been many advancements in understanding the mechanisms by which this parasite establishes persistent infection and how the host’s immune system can control it. Additionally, the study of immunity to T. gondii has advanced our understanding of basic immunology, host-pathogen interactions, and provided a model system to study other inflammatory conditions. Despite these advances, there are many fundamental questions that remain unanswered, as highlighted throughout this review. The identification of parasite virulence factors shows great promise in providing insights into the pathogenesis of this infection. These studies have led to the identification of a role for ATF6β in immunity to T. gondii, and highlighted the potential role of GBPs. The mechanisms by which other parasite factors, such as ROP5, mediate virulence remain unknown [258], but determining their mechanisms of action may elicit the identification of novel components of the host immune response.
There are also many open questions about the mechanisms by which antigen is presented to T cells following infection with T. gondii. Recently, protective CD8+ T cell responses were found to be induced in mice vaccinated with a replication-deficient strain of T. gondii [133,145,259]. Understanding the fate of these live attenuated parasites, and the mechanisms by which host cells acquire and present antigens from them may provide insights for designing efficacious vaccines for humans that elicit T cell mediated memory– a long-standing goal for vaccine design that has remained elusive [260].
The advent of two-photon imaging technology has had a profound impact on the study of immunology by enabling researchers to visualize the interactions of immune populations as they occur in vivo or in situ. Most recently, this technology was utilized to model the migratory patterns of CD8+ T cells in the brains of mice chronically infected with T. gondii [261]. This study found that CD8+ T cells use a migration pattern that is conserved among other predatory animals such as sharks, which maximizes their searching efficiency. This approach provides a foundation for other studies examining the migration patterns of other cell types in other tissue locations. An improved understanding of the migration patterns of effector cells and their interactions with infected cells may provide insight into how these cells can efficiently eliminate parasites while minimizing damage to the host, or how parasites manage to evade destruction. A recurring theme throughout this review has been the ability of CD4+ T cells to mediate severe immunopathology through the production of inflammatory cytokines. However, relatively little is known about the downstream effector mechanisms involved in this process. Nitric oxide has been implicated in the pathology mediated in the gut following infection, and could potentially contribute to the pathology that occurs in the absence of IL-10 or IL-27. Further insight may by provided by imaging the CD4+ T cells in the context of immunopathology. Such studies have the potential to greatly advance our understanding of host-pathogen interactions and the development of immunopathology.
Table.
Cytokines necessary for survival during toxoplasmosis
Acknowledgments
This work was made possible by funding from the Commonwealth of Pennsylvania and the following grants from the National Institute of Health: R01-AI-41158 (C.A.H.), R01-AI-42334 (C.A.H.), and T32-AI007532 (CDD). We also thank Alan J. Dupont for his critical reading of our manuscript.
Footnotes
This article is published as part of the Special Issue on Immunoparasitology [35:1]
References
- 1.Dubey JP. The history of Toxoplasma gondii--the first 100 years. J Eukaryot Microbiol. 2008;55(6):467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolf A, Cowen D, Paige B. Human Toxoplasmosis: Occurrence in Infants as an Encephalomyelitis Verification by Transmission to Animals. Science. 1939;89(2306):226–227. doi: 10.1126/science.89.2306.226. [DOI] [PubMed] [Google Scholar]
- 3.Desmonts G, Couvreur J, Alison F, Baudelot J, Gerbeaux J, Lelong M. Epidemiological study on toxoplasmosis: the influence of cooking slaughter-animal meat on the incidence of human infection. Rev Fr Etud Clin Biol. 1965;10(9):952–958. [PubMed] [Google Scholar]
- 4.Kean BH, Kimball AC, Christenson WN. An epidemic of acute toxoplasmosis. Jama. 1969;208(6):1002–1004. [PubMed] [Google Scholar]
- 5.Frenkel JK, Dubey JP, Miller NL. Toxoplasma gondii in cats: fecal stages identified as coccidian oocysts. Science. 1970;167(3919):893–896. doi: 10.1126/science.167.3919.893. [DOI] [PubMed] [Google Scholar]
- 6.Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. International journal for parasitology. 2009;39(8):895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson LL. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect Immun. 1992;60(9):3719–3724. doi: 10.1128/iai.60.9.3719-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frenkel JK. Toxoplasma in and around us. BioScience. 1973;23(6):343–352. [Google Scholar]
- 9.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. International journal for parasitology. 2009;39(12):1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Havelaar AH, Kemmeren JM, Kortbeek LM. Disease burden of congenital toxoplasmosis. Clin Infect Dis. 2007;44(11):1467–1474. doi: 10.1086/517511. [DOI] [PubMed] [Google Scholar]
- 11.Israelski DM, Remington JS. Toxoplasmic encephalitis in patients with AIDS. Infect Dis Clin North Am. 1988;2(2):429–445. [PubMed] [Google Scholar]
- 12.Leiva LE, Junprasert J, Hollenbaugh D, Sorensen RU. Central nervous system toxoplasmosis with an increased proportion of circulating gamma delta T cells in a patient with hyper-IgM syndrome. J Clin Immunol. 1998;18(4):283–290. doi: 10.1023/a:1027337923709. [DOI] [PubMed] [Google Scholar]
- 13.Tsuge I, Matsuoka H, Nakagawa A, Kamachi Y, Aso K, Negoro T, Ito M, Torii S, Watanabe K. Necrotizing toxoplasmic encephalitis in a child with the X-linked hyper-IgM syndrome. Eur J Pediatr. 1998;157(9):735–737. doi: 10.1007/s004310050925. [DOI] [PubMed] [Google Scholar]
- 14.Yong PF, Post FA, Gilmour KC, Grosse-Kreul D, King A, Easterbrook P, Ibrahim MA. Cerebral toxoplasmosis in a middle-aged man as first presentation of primary immunodeficiency due to a hypomorphic mutation in the CD40 ligand gene. J Clin Pathol. 2008;61(11):1220–1222. doi: 10.1136/jcp.2008.058362. [DOI] [PubMed] [Google Scholar]
- 15.Israelski DM, Remington JS. Toxoplasmosis in patients with cancer. Clin Infect Dis. 1993;17(Suppl 2):S423–435. doi: 10.1093/clinids/17.supplement_2.s423. [DOI] [PubMed] [Google Scholar]
- 16.Derouin F, Pelloux H. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect. 2008;14(12):1089–1101. doi: 10.1111/j.1469-0691.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 17.Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. The Journal of infectious diseases. 2001;184(5):633–639. doi: 10.1086/322800. [DOI] [PubMed] [Google Scholar]
- 18.Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, Valery N, Peneau C, Daigre JL, Aznar C, Cottrelle B, Terzan L, Darde ML, Carme B. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin Infect Dis. 2007;45(7):e88–95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- 19.Dzierszinski FS, Hunter CA. Advances in the use of genetically engineered parasites to study immunity to Toxoplasma gondii. Parasite immunology. 2008;30(4):235–244. doi: 10.1111/j.1365-3024.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 20.Sibley LD. Invasion and intracellular survival by protozoan parasites. Immunological reviews. 2011;240(1):72–91. doi: 10.1111/j.1600-065X.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim DC, Cooke BM, Doerig C, Saeij JP. Toxoplasma and Plasmodium protein kinases: roles in invasion and host cell remodelling. International journal for parasitology. 2012;42(1):21–32. doi: 10.1016/j.ijpara.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesbron-Delauw MF, Gendrin C, Travier L, Ruffiot P, Mercier C. Apicomplexa in mammalian cells: trafficking to the parasitophorous vacuole. Traffic. 2008;9(5):657–664. doi: 10.1111/j.1600-0854.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- 23.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. The Journal of infectious diseases. 1995;172(6):1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 24.Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359(6390):82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 25.Weilhammer DR, Rasley A. Genetic approaches for understanding virulence in Toxoplasma gondii. Brief Funct Genomics. 2011;10(6):365–373. doi: 10.1093/bfgp/elr028. [DOI] [PubMed] [Google Scholar]
- 26.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29(2):306–317. doi: 10.1016/j.immuni.2008.05.019. S1074-7613(08)00326-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mordue DG, Sibley LD. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J Leukoc Biol. 2003;74(6):1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- 28.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. Journal of immunology. 2000;165(8):4515–4521. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 29.Tait ED, Jordan KA, Dupont CD, Harris TH, Gregg B, Wilson EH, Pepper M, Dzierszinski F, Roos DS, Hunter CA. Virulence of Toxoplasma gondii is associated with distinct dendritic cell responses and reduced numbers of activated CD8+ T cells. J Immunol. 2010;185(3):1502–1512. doi: 10.4049/jimmunol.0903450. jimmunol.0903450 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun. 2010;78(4):1564–1570. doi: 10.1128/IAI.00472-09. IAI.00472-09 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J Immunol. 2001;167(11):6503–6509. doi: 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- 32.Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, Machado FS, Aliberti J. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177(1):31–35. doi: 10.4049/jimmunol.177.1.31. 177/1/31 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153(6):2533–2543. [PubMed] [Google Scholar]
- 35.Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62(7):2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 37.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. Journal of immunology. 2002;168(12):5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 38.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308(5728):1626–1629. doi: 10.1126/science.1109893. 1109893 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Jenkins MC, Tuo W, Feng X, Cao L, Murphy C, Fetterer R. Neospora caninum: cloning and expression of a gene coding for cytokine-inducing profilin. Exp Parasitol. 2010;125(4):357–362. doi: 10.1016/j.exppara.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, Gazzinelli RT, Schwarz RT. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179(2):1129–1137. doi: 10.4049/jimmunol.179.2.1129. 179/2/1129 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe. 2009;6(2):187–196. doi: 10.1016/j.chom.2009.06.005. S1931-3128(09)00217-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sukhumavasi W, Egan CE, Warren AL, Taylor GA, Fox BA, Bzik DJ, Denkers EY. TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. Journal of immunology. 2008;181(5):3464–3473. doi: 10.4049/jimmunol.181.5.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. Journal of immunology. 1999;162(12):7369–7375. [PubMed] [Google Scholar]
- 44.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. Journal of immunology. 1996;157(2):798–805. [PubMed] [Google Scholar]
- 45.Whitmarsh RJ, Gray CM, Gregg B, Christian DA, May MJ, Murray PJ, Hunter CA. A critical role for SOCS3 in innate resistance to Toxoplasma gondii. Cell Host Microbe. 2011;10(3):224–236. doi: 10.1016/j.chom.2011.07.009. S1931-3128(11)00230-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou B, Benson A, Kuzmich L, DeFranco AL, Yarovinsky F. Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their Toll-like receptors. Proc Natl Acad Sci U S A. 2011;108(1):278–283. doi: 10.1073/pnas.1011549108. 1011549108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. The Journal of experimental medicine. 1997;186(11):1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, Sher A, Ploegh HL, Murphy TL, Sibley LD, Murphy KM. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35(2):249–259. doi: 10.1016/j.immuni.2011.08.008. S1074-7613(11)00313-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bliss SK, Zhang Y, Denkers EY. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-gamma-independent IL-12. Journal of immunology. 1999;163(4):2081–2088. [PubMed] [Google Scholar]
- 50.Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infection and immunity. 2001;69(8):4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakao M, Konishi E. Proliferation of Toxoplasma gondii in human neutrophils in vitro. Parasitology. 1991;103(Pt 1):23–27. doi: 10.1017/s0031182000059242. [DOI] [PubMed] [Google Scholar]
- 52.Konishi E, Nakao M. Naturally occurring immunoglobulin M antibodies: enhancement of phagocytic and microbicidal activities of human neutrophils against Toxoplasma gondii. Parasitology. 1992;104(Pt 3):427–432. doi: 10.1017/s003118200006368x. [DOI] [PubMed] [Google Scholar]
- 53.Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, Striepen B, Robey EA. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29(3):487–496. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abi Abdallah DS, Lin C, Ball CJ, King MR, Duhamel GE, Denkers EY. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infection and immunity. 2012;80(2):768–777. doi: 10.1128/IAI.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egan CE, Sukhumavasi W, Bierly AL, Denkers EY. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunol Res. 2008;40(1):35–48. doi: 10.1007/s12026-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 56.Alexander J, Scharton-Kersten TM, Yap G, Roberts CW, Liew FY, Sher A. Mechanisms of innate resistance to Toxoplasma gondii infection. Philos Trans R Soc Lond B Biol Sci. 1997;352(1359):1355–1359. doi: 10.1098/rstb.1997.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201(11):1761–1769. doi: 10.1084/jem.20050054. jem.20050054 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benevides L, Milanezi CM, Yamauchi LM, Benjamim CF, Silva JS, Silva NM. CCR2 receptor is essential to activate microbicidal mechanisms to control Toxoplasma gondii infection in the central nervous system. Am J Pathol. 2008;173(3):741–751. doi: 10.2353/ajpath.2008.080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aldebert D, Durand F, Mercier C, Brenier-Pinchart MP, Cesbron-Delauw MF, Pelloux H. Toxoplasma gondii triggers secretion of interleukin-12 but low level of interleukin-10 from the THP-1 human monocytic cell line. Cytokine. 2007;37(3):206–211. doi: 10.1016/j.cyto.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Dunay IR, Sibley LD. Monocytes mediate mucosal immunity to Toxoplasma gondii. Curr Opin Immunol. 2010;22(4):461–466. doi: 10.1016/j.coi.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borges JS, Johnson WD., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. The Journal of experimental medicine. 1975;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson CB, Remington JS. Activity of human blood leukocytes against Toxoplasma gondii. The Journal of infectious diseases. 1979;140(6):890–895. doi: 10.1093/infdis/140.6.890. [DOI] [PubMed] [Google Scholar]
- 63.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185(7):1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gazzinelli RT, Bala S, Stevens R, Baseler M, Wahl L, Kovacs J, Sher A. HIV infection suppresses type 1 lymphokine and IL-12 responses to Toxoplasma gondii but fails to inhibit the synthesis of other parasite-induced monokines. Journal of immunology. 1995;155(3):1565–1574. [PubMed] [Google Scholar]
- 65.Hammouda NA, Rashwan EA, Hussien ED, Abo el-Naga I, Fathy FM. Measurement of respiratory burst of TNF and IL-1 cytokine activated murine peritoneal macrophages challenged with Toxoplasma gondii. J Egypt Soc Parasitol. 1995;25(3):683–691. [PubMed] [Google Scholar]
- 66.Halonen SK, Chiu F, Weiss LM. Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infection and immunity. 1998;66(10):4989–4993. doi: 10.1128/iai.66.10.4989-4993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hunter CA, Chizzonite R, Remington JS. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. Journal of immunology. 1995;155(9):4347–4354. [PubMed] [Google Scholar]
- 68.Shibuya K, Robinson D, Zonin F, Hartley SB, Macatonia SE, Somoza C, Hunter CA, Murphy KM, O’Garra A. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. Journal of immunology. 1998;160(4):1708–1716. [PubMed] [Google Scholar]
- 69.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunological reviews. 2010;234(1):90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 70.Johnson LL, VanderVegt FP, Havell EA. Gamma interferon-dependent temporary resistance to acute Toxoplasma gondii infection independent of CD4+ or CD8+ lymphocytes. Infection and immunity. 1993;61(12):5174–5180. doi: 10.1128/iai.61.12.5174-5180.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sher A, Oswald IP, Hieny S, Gazzinelli RT. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. Journal of immunology. 1993;150(9):3982–3989. [PubMed] [Google Scholar]
- 72.Denkers EY, Gazzinelli RT, Martin D, Sher A. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. The Journal of experimental medicine. 1993;178(5):1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hauser WE, Jr, Sharma SD, Remington JS. Natural killer cells induced by acute and chronic toxoplasma infection. Cell Immunol. 1982;69(2):330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- 74.Kang H, Suzuki Y. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infection and immunity. 2001;69(5):2920–2927. doi: 10.1128/IAI.69.5.2920-2927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perona-Wright G, Mohrs K, Szaba FM, Kummer LW, Madan R, Karp CL, Johnson LL, Smiley ST, Mohrs M. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell host & microbe. 2009;6(6):503–512. doi: 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Subauste CS, Dawson L, Remington JS. Human lymphokine-activated killer cells are cytotoxic against cells infected with Toxoplasma gondii. The Journal of experimental medicine. 1992;176(6):1511–1519. doi: 10.1084/jem.176.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hauser WE, Jr, Tsai V. Acute toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. Journal of immunology. 1986;136(1):313–319. [PubMed] [Google Scholar]
- 78.Persson CM, Lambert H, Vutova PP, Dellacasa-Lindberg I, Nederby J, Yagita H, Ljunggren HG, Grandien A, Barragan A, Chambers BJ. Transmission of Toxoplasma gondii from infected dendritic cells to natural killer cells. Infection and immunity. 2009;77(3):970–976. doi: 10.1128/IAI.00833-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Combe CL, Curiel TJ, Moretto MM, Khan IA. NK cells help to induce CD8(+)-T-cell immunity against Toxoplasma gondii in the absence of CD4(+) T cells. Infection and immunity. 2005;73(8):4913–4921. doi: 10.1128/IAI.73.8.4913-4921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guan H, Moretto M, Bzik DJ, Gigley J, Khan IA. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J Immunol. 2007;179(1):590–596. doi: 10.4049/jimmunol.179.1.590. 179/1/590 [pii] [DOI] [PubMed] [Google Scholar]
- 81.Goldszmid RS, Bafica A, Jankovic D, Feng CG, Caspar P, Winkler-Pickett R, Trinchieri G, Sher A. TAP-1 indirectly regulates CD4+ T cell priming in Toxoplasma gondii infection by controlling NK cell IFN-gamma production. The Journal of experimental medicine. 2007;204(11):2591–2602. doi: 10.1084/jem.20070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164(5):2629–2634. doi: 10.4049/jimmunol.164.5.2629. ji_v164n5p2629 [pii] [DOI] [PubMed] [Google Scholar]
- 83.Johnson LL, Sayles PC. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infection and immunity. 2002;70(1):185–191. doi: 10.1128/IAI.70.1.185-191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Denkers EY, Yap G, Scharton-Kersten T, Charest H, Butcher BA, Caspar P, Heiny S, Sher A. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. Journal of immunology. 1997;159(4):1903–1908. [PubMed] [Google Scholar]
- 85.Wilson EH, Hunter CA. Immunodominance and recognition of intracellular pathogens. The Journal of infectious diseases. 2008;198(11):1579–1581. doi: 10.1086/593020. [DOI] [PubMed] [Google Scholar]
- 86.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, Shastri N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol. 2008;9(8):937–944. doi: 10.1038/ni.1629. ni.1629 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.John B, Weninger W, Hunter CA. Advances in imaging the innate and adaptive immune response to Toxoplasma gondii. Future Microbiol. 2010;5(9):1321–1328. doi: 10.2217/fmb.10.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. Jama. 1984;252(7):913–917. [PubMed] [Google Scholar]
- 89.Lutjen S, Soltek S, Virna S, Deckert M, Schluter D. Organ- and disease-stage-specific regulation of Toxoplasma gondii-specific CD8-T-cell responses by CD4 T cells. Infection and immunity. 2006;74(10):5790–5801. doi: 10.1128/IAI.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. Journal of immunology. 1992;149(1):175–180. [PubMed] [Google Scholar]
- 91.Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infection and immunity. 2000;68(3):1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andrade RM, Portillo JA, Wessendarp M, Subauste CS. CD40 signaling in macrophages induces activity against an intracellular pathogen independently of gamma interferon and reactive nitrogen intermediates. Infection and immunity. 2005;73(5):3115–3123. doi: 10.1128/IAI.73.5.3115-3123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Subauste CS, Wessendarp M. CD40 restrains in vivo growth of Toxoplasma gondii independently of gamma interferon. Infection and immunity. 2006;74(3):1573–1579. doi: 10.1128/IAI.74.3.1573-1579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Subauste CS, Andrade RM, Wessendarp M. CD40-TRAF6 and autophagy-dependent anti-microbial activity in macrophages. Autophagy. 2007;3(3):245–248. doi: 10.4161/auto.3717. [DOI] [PubMed] [Google Scholar]
- 95.Portillo JA, Okenka G, Reed E, Subauste A, Van Grol J, Gentil K, Komatsu M, Tanaka K, Landreth G, Levine B, Subauste CS. The CD40-autophagy pathway is needed for host protection despite IFN-Gamma-dependent immunity and CD40 induces autophagy via control of P21 levels. PloS one. 2010;5(12):e14472. doi: 10.1371/journal.pone.0014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annual review of immunology. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 97.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 98.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. Journal of immunology. 1999;162(6):3256–3262. [PubMed] [Google Scholar]
- 99.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. Journal of immunology. 2003;171(10):5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 100.Villegas EN, Lieberman LA, Mason N, Blass SL, Zediak VP, Peach R, Horan T, Yoshinaga S, Hunter CA. A role for inducible costimulator protein in the CD28- independent mechanism of resistance to Toxoplasma gondii. J Immunol. 2002;169(2):937–943. doi: 10.4049/jimmunol.169.2.937. [DOI] [PubMed] [Google Scholar]
- 101.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection. J Immunol. 2008;180(9):5935–5945. doi: 10.4049/jimmunol.180.9.5935. 180/9/5935 [pii] [DOI] [PubMed] [Google Scholar]
- 102.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. 19/1/23 [pii] [DOI] [PubMed] [Google Scholar]
- 103.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, Otsuka H, Hijikata A, Watanabe T, Ohara O, Kaisho T, Malissen B. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35(6):958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 105.McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J Immunol. 2004;173(4):2632–2640. doi: 10.4049/jimmunol.173.4.2632. 173/4/2632 [pii] [DOI] [PubMed] [Google Scholar]
- 106.Lang C, Algner M, Beinert N, Gross U, Luder CG. Diverse mechanisms employed by Toxoplasma gondii to inhibit IFN-gamma-induced major histocompatibility complex class II gene expression. Microbes Infect. 2006;8(8):1994–2005. doi: 10.1016/j.micinf.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 107.Goldszmid RS, Sher A. Processing and presentation of antigens derived from intracellular protozoan parasites. Curr Opin Immunol. 2010;22(1):118–123. doi: 10.1016/j.coi.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldszmid RS, Coppens I, Lev A, Caspar P, Mellman I, Sher A. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206(2):399–410. doi: 10.1084/jem.20082108. jem.20082108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koshy AA, Fouts AE, Lodoen MB, Alkan O, Blau HM, Boothroyd JC. Toxoplasma secreting Cre recombinase for analysis of host-parasite interactions. Nat Methods. 2010;7(4):307–309. doi: 10.1038/nmeth.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Subauste CS, de Waal Malefyt R, Fuh F. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. Journal of immunology. 1998;160(4):1831–1840. [PubMed] [Google Scholar]
- 111.Subauste CS, Wessendarp M. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-gamma. Journal of immunology. 2000;165(3):1498–1505. doi: 10.4049/jimmunol.165.3.1498. [DOI] [PubMed] [Google Scholar]
- 112.Sabin AB, Feldman HA. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma) Science. 1948;108(2815):660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- 113.Correa D, Canedo-Solares I, Ortiz-Alegria LB, Caballero-Ortega H, Rico-Torres CP. Congenital and acquired toxoplasmosis: diversity and role of antibodies in different compartments of the host. Parasite immunology. 2007;29(12):651–660. doi: 10.1111/j.1365-3024.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 114.Remington JS, Miller MJ, Brownlee I. IgM antibodies in acute toxoplasmosis. II. Prevalence and significance in acquired cases. J Lab Clin Med. 1968;71(5):855–866. [PubMed] [Google Scholar]
- 115.Remington JS. The present status of the IgM fluorescent antibody technique in the diagnosis of congenital toxoplasmosis. J Pediatr. 1969;75(6):1116–1124. doi: 10.1016/s0022-3476(69)80366-5. [DOI] [PubMed] [Google Scholar]
- 116.Remington JS, Thulliez P, Montoya JG. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol. 2004;42(3):941–945. doi: 10.1128/JCM.42.3.941-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erbe DV, Pfefferkorn ER, Fanger MW. Functions of the various IgG Fc receptors in mediating killing of Toxoplasma gondii. Journal of immunology. 1991;146(9):3145–3151. [PubMed] [Google Scholar]
- 118.Hammouda NA, Abo el-Naga I, Hussein ED, Rashwan EA. Opsonization and intracellular killing of Toxoplasma gondii by human mononuclear phagocytes. J Egypt Soc Parasitol. 1995;25(1):11–17. [PubMed] [Google Scholar]
- 119.Vercammen M, Scorza T, El Bouhdidi A, Van Beeck K, Carlier Y, Dubremetz JF, Verschueren H. Opsonization of Toxoplasma gondii tachyzoites with nonspecific immunoglobulins promotes their phagocytosis by macrophages and inhibits their proliferation in nonphagocytic cells in tissue culture. Parasite immunology. 1999;21(11):555–563. doi: 10.1046/j.1365-3024.1999.00256.x. [DOI] [PubMed] [Google Scholar]
- 120.Suzuki M, Tsunematsu Y. Studies on the accessory factor for the toxoplasma dye test: essential role of complement. The Journal of parasitology. 1971;57(4):924–925. [PubMed] [Google Scholar]
- 121.Schreiber RD, Feldman HA. Identification of the activator system for antibody to Toxoplasma as the classical complement pathway. The Journal of infectious diseases. 1980;141(3):366–369. doi: 10.1093/infdis/141.3.366. [DOI] [PubMed] [Google Scholar]
- 122.Johnson LL, Gibson GW, Sayles PC. CR3-dependent resistance to acute Toxoplasma gondii infection in mice. Infect Immun. 1996;64(6):1998–2003. doi: 10.1128/iai.64.6.1998-2003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zaretsky AG, Silver JS, Durham A, Ware CF, Hunter CA. Infection with Toxoplasma gondii alters lymphotoxin expression associated with changes in splenic architecture. Infect Immun. 2012 doi: 10.1128/IAI.00333-12. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.John B, Harris TH, Tait ED, Wilson EH, Gregg B, Ng LG, Mrass P, Roos DS, Dzierszinski F, Weninger W, Hunter CA. Dynamic Imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 2009;5(7):e1000505. doi: 10.1371/journal.ppat.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5(11):853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 126.Benedict CA, De Trez C, Schneider K, Ha S, Patterson G, Ware CF. Specific remodeling of splenic architecture by cytomegalovirus. PLoS pathogens. 2006;2(3):e16. doi: 10.1371/journal.ppat.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cadman ET, Abdallah AY, Voisine C, Sponaas AM, Corran P, Lamb T, Brown D, Ndungu F, Langhorne J. Alterations of splenic architecture in malaria are induced independently of Toll-like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infection and immunity. 2008;76(9):3924–3931. doi: 10.1128/IAI.00372-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(18):8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Racine R, Jones DD, Chatterjee M, McLaughlin M, Macnamara KC, Winslow GM. Impaired germinal center responses and suppression of local IgG production during intracellular bacterial infection. Journal of immunology. 2010;184(9):5085–5093. doi: 10.4049/jimmunol.0902710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nature immunology. 2008;9(6):667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 131.St John AL, Abraham SN. Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines. Nat Med. 2009;15(11):1259–1265. doi: 10.1038/nm.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parker SJ, Roberts CW, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991;84(2):207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gigley JP, Fox BA, Bzik DJ. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J Immunol. 2009;182(2):1069–1078. doi: 10.4049/jimmunol.182.2.1069. 182/2/1069 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khan IA, Smith KA, Kasper LH. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. Journal of immunology. 1988;141(10):3600–3605. [PubMed] [Google Scholar]
- 135.Frickel EM, Sahoo N, Hopp J, Gubbels MJ, Craver MP, Knoll LJ, Ploegh HL, Grotenbreg GM. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J Infect Dis. 2008;198(11):1625–1633. doi: 10.1086/593019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel EM, Gubbels MJ, Ploegh HL, Yap GS. Differential regulation of effector- and central-memory responses to Toxoplasma gondii Infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog. 2010;6(3):e1000815. doi: 10.1371/journal.ppat.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kwok LY, Lutjen S, Soltek S, Soldati D, Busch D, Deckert M, Schluter D. The induction and kinetics of antigen-specific CD8 T cells are defined by the stage specificity and compartmentalization of the antigen in murine toxoplasmosis. J Immunol. 2003;170(4):1949–1957. doi: 10.4049/jimmunol.170.4.1949. [DOI] [PubMed] [Google Scholar]
- 138.Pepper M, Dzierszinski F, Crawford A, Hunter CA, Roos D. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infect Immun. 2004;72(12):7240–7246. doi: 10.1128/IAI.72.12.7240-7246.2004. 72/12/7240 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gregg B, Dzierszinski F, Tait E, Jordan KA, Hunter CA, Roos DS. Subcellular antigen location influences T-cell activation during acute infection with Toxoplasma gondii. PloS one. 2011;6(7):e22936. doi: 10.1371/journal.pone.0022936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chtanova T, Han SJ, Schaeffer M, van Dooren GG, Herzmark P, Striepen B, Robey EA. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31(2):342–355. doi: 10.1016/j.immuni.2009.06.023. S1074-7613(09)00326-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dzierszinski F, Pepper M, Stumhofer JS, LaRosa DF, Wilson EH, Turka LA, Halonen SK, Hunter CA, Roos DS. Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells. Infect Immun. 2007;75(11):5200–5209. doi: 10.1128/IAI.00954-07. IAI.00954-07 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gubbels MJ, Striepen B, Shastri N, Turkoz M, Robey EA. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infection and immunity. 2005;73(2):703–711. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin ML, Zhan Y, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 2008;86(4):353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 144.Denkers EY, Scharton-Kersten T, Barbieri S, Caspar P, Sher A. A role for CD4+ NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. The Journal of experimental medicine. 1996;184(1):131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jordan KA, Wilson EH, Tait ED, Fox BA, Roos DS, Bzik DJ, Dzierszinski F, Hunter CA. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to Toxoplasma gondii. Infection and immunity. 2009;77(9):3894–3901. doi: 10.1128/IAI.00024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4(8):595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 147.Yamamoto M, Takeda K. Inhibition of ATF6beta-dependent host adaptive immune response by a Toxoplasma virulence factor ROP18. Virulence. 2012;3(1) doi: 10.4161/viru.3.1.18340. 18340 [pii] [DOI] [PubMed] [Google Scholar]
- 148.Jordan KA, Dupont CD, Tait ED, Liou HC, Hunter CA. Role of the NF-kappaB transcription factor c-Rel in the generation of CD8+ T-cell responses to Toxoplasma gondii. Int Immunol. 2010;22(11):851–861. doi: 10.1093/intimm/dxq439. dxq439 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, David C, Remington JS, McLeod R. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85(3):419–428. [PMC free article] [PubMed] [Google Scholar]
- 150.Suzuki Y, Joh K, Kwon OC, Yang Q, Conley FK, Remington JS. MHC class I gene(s) in the D/L region but not the TNF-alpha gene determines development of toxoplasmic encephalitis in mice. J Immunol. 1994;153(10):4649–4654. [PubMed] [Google Scholar]
- 151.Rosenberg CS, Martin DL, Tarleton RL. CD8+ T cells specific for immunodominant trans-sialidase epitopes contribute to control of Trypanosoma cruzi infection but are not required for resistance. Journal of immunology. 2010;185(1):560–568. doi: 10.4049/jimmunol.1000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Deckert-Schluter M, Bluethmann H, Rang A, Hof H, Schluter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. Journal of immunology. 1998;160(7):3427–3436. [PubMed] [Google Scholar]
- 153.Yap GS, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. Journal of immunology. 1998;160(3):1340–1345. [PubMed] [Google Scholar]
- 154.Schluter D, Kwok LY, Lutjen S, Soltek S, Hoffmann S, Korner H, Deckert M. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. Journal of immunology. 2003;170(12):6172–6182. doi: 10.4049/jimmunol.170.12.6172. [DOI] [PubMed] [Google Scholar]
- 155.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. The Journal of experimental medicine. 1983;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adams LB, Hibbs JB, Jr, Taintor RR, Krahenbuhl JL. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. Journal of immunology. 1990;144(7):2725–2729. [PubMed] [Google Scholar]
- 157.Koide M, Kawahara Y, Tsuda T, Yokoyama M. Cytokine-induced expression of an inducible type of nitric oxide synthase gene in cultured vascular smooth muscle cells. FEBS Lett. 1993;318(3):213–217. doi: 10.1016/0014-5793(93)80514-u. [DOI] [PubMed] [Google Scholar]
- 158.Langermans JA, Van der Hulst ME, Nibbering PH, Hiemstra PS, Fransen L, Van Furth R. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. Journal of immunology. 1992;148(2):568–574. [PubMed] [Google Scholar]
- 159.Chao CC, Anderson WR, Hu S, Gekker G, Martella A, Peterson PK. Activated microglia inhibit multiplication of Toxoplasma gondii via a nitric oxide mechanism. Clin Immunol Immunopathol. 1993;67(2):178–183. doi: 10.1006/clin.1993.1062. [DOI] [PubMed] [Google Scholar]
- 160.Jun CD, Kim SH, Soh CT, Kang SS, Chung HT. Nitric oxide mediates the toxoplasmastatic activity of murine microglial cells in vitro. Immunol Invest. 1993;22(8):487–501. doi: 10.3109/08820139309084178. [DOI] [PubMed] [Google Scholar]
- 161.Zhao Y, Ferguson DJ, Wilson DC, Howard JC, Sibley LD, Yap GS. Virulent Toxoplasma gondii evade immunity-related GTPase-mediated parasite vacuole disruption within primed macrophages. Journal of immunology. 2009;182(6):3775–3781. doi: 10.4049/jimmunol.0804190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem. 2001;276(48):44527–44533. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hayashi S, Chan CC, Gazzinelli R, Roberge FG. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;156(4):1476–1481. [PubMed] [Google Scholar]
- 164.Chakravortty D, Hensel M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003;5(7):621–627. doi: 10.1016/s1286-4579(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 165.Taylor GA, Jeffers M, Largaespada DA, Jenkins NA, Copeland NG, Woude GF. Identification of a novel GTPase, the inducibly expressed GTPase, that accumulates in response to interferon gamma. J Biol Chem. 1996;271(34):20399–20405. doi: 10.1074/jbc.271.34.20399. [DOI] [PubMed] [Google Scholar]
- 166.Howard JC, Hunn JP, Steinfeldt T. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr Opin Microbiol. 2011;14(4):414–421. doi: 10.1016/j.mib.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 167.Taylor GA, Collazo CM, Yap GS, Nguyen K, Gregorio TA, Taylor LS, Eagleson B, Secrest L, Southon EA, Reid SW, Tessarollo L, Bray M, McVicar DW, Komschlies KL, Young HA, Biron CA, Sher A, Vande Woude GF. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc Natl Acad Sci U S A. 2000;97(2):751–755. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, Woude GF, Sher A, Taylor GA. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. The Journal of experimental medicine. 2001;194(2):181–188. doi: 10.1084/jem.194.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pawlowski N, Khaminets A, Hunn JP, Papic N, Schmidt A, Uthaiah RC, Lange R, Vopper G, Martens S, Wolf E, Howard JC. The activation mechanism of Irga6, an interferon-inducible GTPase contributing to mouse resistance against Toxoplasma gondii. BMC Biol. 2011;9:7. doi: 10.1186/1741-7007-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, Fox BA, Bzik DJ, Taylor GA, Turk BE, Lichti CF, Townsend RR, Qiu W, Hui R, Beatty WL, Sibley LD. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell host & microbe. 2010;8(6):484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS pathogens. 2005;1(3):e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. The Journal of experimental medicine. 2006;203(9):2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hunn JP, Koenen-Waisman S, Papic N, Schroeder N, Pawlowski N, Lange R, Kaiser F, Zerrahn J, Martens S, Howard JC. Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. Embo J. 2008;27(19):2495–2509. doi: 10.1038/emboj.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, Schmidt RE, Mizushima N, Deretic V, Sibley LD, Virgin HW. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell host & microbe. 2008;4(5):458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS pathogens. 2009;5(2):e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Khaminets A, Hunn JP, Konen-Waisman S, Zhao YO, Preukschat D, Coers J, Boyle JP, Ong YC, Boothroyd JC, Reichmann G, Howard JC. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol. 2010;12(7):939–961. doi: 10.1111/j.1462-5822.2010.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Steinfeldt T, Konen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, Hunn JP, Howard JC. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 2010;8(12):e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Melzer T, Duffy A, Weiss LM, Halonen SK. The gamma interferon (IFN-gamma)-inducible GTP-binding protein IGTP is necessary for toxoplasma vacuolar disruption and induces parasite egression in IFN-gamma-stimulated astrocytes. Infection and immunity. 2008;76(11):4883–4894. doi: 10.1128/IAI.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Virreira Winter S, Niedelman W, Jensen KD, Rosowski EE, Julien L, Spooner E, Caradonna K, Burleigh BA, Saeij JP, Ploegh HL, Frickel EM. Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PloS one. 2011;6(9):e24434. doi: 10.1371/journal.pone.0024434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332(6030):717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 181.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Murray HW, Szuro-Sudol A, Wellner D, Oca MJ, Granger AM, Libby DM, Rothermel CD, Rubin BY. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infection and immunity. 1989;57(3):845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Daubener W, Remscheid C, Nockemann S, Pilz K, Seghrouchni S, Mackenzie C, Hadding U. Anti-parasitic effector mechanisms in human brain tumor cells: role of interferon-gamma and tumor necrosis factor-alpha. Eur J Immunol. 1996;26(2):487–492. doi: 10.1002/eji.1830260231. [DOI] [PubMed] [Google Scholar]
- 184.Daubener W, Spors B, Hucke C, Adam R, Stins M, Kim KS, Schroten H. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infection and immunity. 2001;69(10):6527–6531. doi: 10.1128/IAI.69.10.6527-6531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 186.Sibley LD, Messina M, Niesman IR. Stable DNA transformation in the obligate intracellular parasite Toxoplasma gondii by complementation of tryptophan auxotrophy. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(12):5508–5512. doi: 10.1073/pnas.91.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Divanovic S, Sawtell NM, Trompette A, Warning JI, Dias A, Cooper AM, Yap GS, Arditi M, Shimada K, Duhadaway JB, Prendergast GC, Basaraba RJ, Mellor AL, Munn DH, Aliberti J, Karp CL. Opposing biological functions of tryptophan catabolizing enzymes during intracellular infection. J Infect Dis. 2012;205(1):152–161. doi: 10.1093/infdis/jir621. jir621 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16(4):354–359. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. Journal of immunology. 1993;151(7):3672–3681. [PubMed] [Google Scholar]
- 190.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. Journal of immunology. 2003;171(11):6052–6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 191.Li ZY, Manthey CL, Perera PY, Sher A, Vogel SN. Toxoplasma gondii soluble antigen induces a subset of lipopolysaccharide-inducible genes and tyrosine phosphoproteins in peritoneal macrophages. Infection and immunity. 1994;62(8):3434–3440. doi: 10.1128/iai.62.8.3434-3440.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schluter D, Meyer T, Strack A, Reiter S, Kretschmar M, Wiestler OD, Hof H, Deckert M. Regulation of microglia by CD4+ and CD8+ T cells: selective analysis in CD45-congenic normal and Toxoplasma gondii-infected bone marrow chimeras. Brain Pathol. 2001;11(1):44–55. doi: 10.1111/j.1750-3639.2001.tb00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schluter D, Kaefer N, Hof H, Wiestler OD, Deckert-Schluter M. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am J Pathol. 1997;150(3):1021–1035. [PMC free article] [PubMed] [Google Scholar]
- 194.Chang HR, Grau GE, Pechere JC. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology. 1990;69(1):33–37. [PMC free article] [PubMed] [Google Scholar]
- 195.Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. The Journal of experimental medicine. 1999;189(7):1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao Y, Wilson D, Matthews S, Yap GS. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infection and immunity. 2007;75(10):4799–4803. doi: 10.1128/IAI.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Subauste CS. CD40 and the immune response to parasitic infections. Semin Immunol. 2009;21(5):273–282. doi: 10.1016/j.smim.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Subauste CS, Wessendarp M, Sorensen RU, Leiva LE. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. Journal of immunology. 1999;162(11):6690–6700. [PubMed] [Google Scholar]
- 199.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264(5159):703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 200.Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184(2):597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Egan CE, Cohen SB, Denkers EY. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liesenfeld O, Nguyen TA, Pharke C, Suzuki Y. Importance of gender and sex hormones in regulation of susceptibility of the small intestine to peroral infection with Toxoplasma gondii tissue cysts. J Parasitol. 2001;87(6):1491–1493. doi: 10.1645/0022-3395(2001)087[1491:IOGASH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 203.Egan CE, Craven MD, Leng J, Mack M, Simpson KW, Denkers EY. CCR2-dependent intraepithelial lymphocytes mediate inflammatory gut pathology during Toxoplasma gondii infection. Mucosal Immunol. 2009;2(6):527–535. doi: 10.1038/mi.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Egan CE, Maurer KJ, Cohen SB, Mack M, Simpson KW, Denkers EY. Synergy between intraepithelial lymphocytes and lamina propria T cells drives intestinal inflammation during infection. Mucosal Immunol. 2011;4(6):658–670. doi: 10.1038/mi.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ronet C, Darche S, Leite de Moraes M, Miyake S, Yamamura T, Louis JA, Kasper LH, Buzoni-Gatel D. NKT cells are critical for the initiation of an inflammatory bowel response against Toxoplasma gondii. J Immunol. 2005;175(2):899–908. doi: 10.4049/jimmunol.175.2.899. 175/2/899 [pii] [DOI] [PubMed] [Google Scholar]
- 206.Khan IA, Thomas SY, Moretto MM, Lee FS, Islam SA, Combe C, Schwartzman JD, Luster AD. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS pathogens. 2006;2(6):e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li W, Buzoni-Gatel D, Debbabi H, Hu MS, Mennechet FJD, Durell BG, Noelle RJ, Kasper LH. CD40/CD154 ligation is required for the development of acute ileitis following oral infection with an intracellular pathogen in mice. Gastroenterology. 2002;122(3):762–773. doi: 10.1053/Gast.2002.31888. [DOI] [PubMed] [Google Scholar]
- 208.Vossenkamper A, Struck D, Alvarado-Esquivel C, Went T, Takeda K, Akira S, Pfeffer K, Alber G, Lochner M, Forster I, Liesenfeld O. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur J Immunol. 2004;34(11):3197–3207. doi: 10.1002/eji.200424993. [DOI] [PubMed] [Google Scholar]
- 209.Munoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Holscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. The Journal of experimental medicine. 2009;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liesenfeld O, Kang H, Park D, Nguyen TA, Parkhe CV, Watanabe H, Abo T, Sher A, Remington JS, Suzuki Y. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 1999;21(7):365–376. doi: 10.1046/j.1365-3024.1999.00237.x. pim237 [pii] [DOI] [PubMed] [Google Scholar]
- 211.Cavalcanti MG, Mesquita JS, Madi K, Feijo DF, Assuncao-Miranda I, Souza HS, Bozza MT. MIF participates in Toxoplasma gondii-induced pathology following oral infection. PLoS One. 2011;6(9):e25259. doi: 10.1371/journal.pone.0025259. PONE-D-11–01622 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nickdel MB, Lyons RE, Roberts F, Brombacher F, Hunter CA, Alexander J, Roberts CW. Intestinal pathology during acute toxoplasmosis is IL-4 dependent and unrelated to parasite burden. Parasite Immunology. 2004;26(2):75–82. doi: 10.1111/j.0141-9838.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 213.Nickdel MB, Roberts F, Brombacher F, Alexander J, Roberts CW. Counter-protective role for interleukin-5 during acute Toxoplasma gondii infection. Infect Immun. 2001;69(2):1044–1052. doi: 10.1128/IAI.69.2.1044-1052.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Roberts CW, Ferguson DJ, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infection and immunity. 1996;64(3):897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Gobel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. Journal of immunology. 2006;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 216.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Gobel UB, Bereswill S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007;56(7):941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Israelski DM, Araujo FG, Conley FK, Suzuki Y, Sharma S, Remington JS. Treatment with anti-L3T4 (CD4) monoclonal antibody reduces the inflammatory response in toxoplasmic encephalitis. Journal of immunology. 1989;142(3):954–958. [PubMed] [Google Scholar]
- 218.Reichmann G, Villegas EN, Craig L, Peach R, Hunter CA. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. Journal of immunology. 1999;163(6):3354–3362. [PubMed] [Google Scholar]
- 219.Vollmer TL, Waldor MK, Steinman L, Conley FK. Depletion of T-4+ lymphocytes with monoclonal antibody reactivates toxoplasmosis in the central nervous system: a model of superinfection in AIDS. J Immunol. 1987;138(11):3737–3741. [PubMed] [Google Scholar]
- 220.Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. 2005;165(1–2):63–74. doi: 10.1016/j.jneuroim.2005.04.018. S0165-5728(05)00176-1 [pii] [DOI] [PubMed] [Google Scholar]
- 221.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157(2):798–805. [PubMed] [Google Scholar]
- 222.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 223.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 224.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. Journal of immunology. 1992;148(6):1792–1796. [PubMed] [Google Scholar]
- 225.Hunter CA, Abrams JS, Beaman MH, Remington JS. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: importance of T-cell-independent regulation of resistance to T. gondii. Infection and immunity. 1993;61(10):4038–4044. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Khan IA, Matsuura T, Kasper LH. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite immunology. 1995;17(4):185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 227.Sher A, Gazzinelli RT, Oswald IP, Clerici M, Kullberg M, Pearce EJ, Berzofsky JA, Mosmann TR, James SL, Morse HC., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunological reviews. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 228.Deckert-Schluter M, Buck C, Weiner D, Kaefer N, Rang A, Hof H, Wiestler OD, Schluter D. Interleukin-10 downregulates the intracerebral immune response in chronic Toxoplasma encephalitis. J Neuroimmunol. 1997;76(1–2):167–176. doi: 10.1016/s0165-5728(97)00047-7. S0165-5728(97)00047-7 [pii] [DOI] [PubMed] [Google Scholar]
- 229.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204(2):273–283. doi: 10.1084/jem.20062175. jem.20062175 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Neyer LE, Grunig G, Fort M, Remington JS, Rennick D, Hunter CA. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infection and immunity. 1997;65(5):1675–1682. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, Stenzel W, Gruber AD, Krieg T, Rajewsky K, Muller W. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. The Journal of experimental medicine. 2004;200(10):1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14(5):361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 233.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19(5):645–655. doi: 10.1016/s1074-7613(03)00300-5. S1074761303003005 [pii] [DOI] [PubMed] [Google Scholar]
- 234.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8(12):1363–1371. doi: 10.1038/ni1537. ni1537 [pii] [DOI] [PubMed] [Google Scholar]
- 235.Hirahara K, Kamran G, Yang X, Takahashi H, Laurence A, Vahedi G, Sciume G, O’Hara Hall A, Dupont CD, Francisco LM, Chen Q, Tanaka M, Kanno Y, Sun H, Sharpe AH, Hunter CA, O’Shea JJ. IL-27 priming of T cells controls IL-17-production in trans via induction of PD-L1. Immunity. 2012 doi: 10.1016/j.immuni.2012.03.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–945. doi: 10.1038/ni1376. ni1376 [pii] [DOI] [PubMed] [Google Scholar]
- 237.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19(5):657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 238.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168(1):158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, Koretzky G, Goldschmidt M, Wu GD, de Sauvage F, Miller HR, Saris CJ, Scott P, Hunter CA. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. Journal of immunology. 2004;173(9):5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 240.Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. Journal of immunology. 2004;173(12):7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 241.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. Journal of immunology. 2005;174(6):3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 242.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature immunology. 2006;7(9):929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 243.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 244.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. Journal of immunology. 2007;179(5):3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 245.Villarino AV, Artis D, Bezbradica JS, Miller O, Saris CJ, Joyce S, Hunter CA. IL-27R deficiency delays the onset of colitis and protects from helminth-induced pathology in a model of chronic IBD. International immunology. 2008;20(6):739–752. doi: 10.1093/intimm/dxn032. [DOI] [PubMed] [Google Scholar]
- 246.Troy AE, Zaph C, Du Y, Taylor BC, Guild KJ, Hunter CA, Saris CJ, Artis D. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. Journal of immunology. 2009;183(3):2037–2044. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Stumhofer JS, Hunter CA. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117(2):123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. The Journal of experimental medicine. 2002;196(9):1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38(23):7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- 250.Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391(11):1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- 251.Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nature immunology. 2002;3(1):76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 252.Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12(3):330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 253.Sanchez Y, de Rosado JD, Vega L, Elizondo G, Estrada-Muniz E, Saavedra R, Juarez I, Rodriguez-Sosa M. The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis. J Biomed Biotechnol. 2010;2010:505694. doi: 10.1155/2010/505694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yarovinsky F, Hieny S, Sher A. Recognition of Toxoplasma gondii by TLR11 prevents parasite-induced immunopathology. Journal of immunology. 2008;181(12):8478–8484. doi: 10.4049/jimmunol.181.12.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- 256.Mullarky IK, Szaba FM, Berggren KN, Kummer LW, Wilhelm LB, Parent MA, Johnson LL, Smiley ST. Tumor necrosis factor alpha and gamma interferon, but not hemorrhage or pathogen burden, dictate levels of protective fibrin deposition during infection. Infection and immunity. 2006;74(2):1181–1188. doi: 10.1128/IAI.74.2.1181-1188.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Johnson LL, Berggren KN, Szaba FM, Chen W, Smiley ST. Fibrin-mediated protection against infection-stimulated immunopathology. The Journal of experimental medicine. 2003;197(6):801–806. doi: 10.1084/jem.20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Reese ML, Boyle JP. Virulence without catalysis: how can a pseudokinase affect host cell signaling? Trends Parasitol. 2012;28(2):53–57. doi: 10.1016/j.pt.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 259.Fox BA, Bzik DJ. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature. 2002;415(6874):926–929. doi: 10.1038/415926a. [DOI] [PubMed] [Google Scholar]
- 260.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11(4 Suppl):S25–32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 261.Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, Liu AJ, Hunter CA. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Naute. 2012 doi: 10.1038/nature11098. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. Journal of immunology. 2000;165(2):628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 263.Pelloux H, Pernod G, Ricard J, Renversez TC, Ambroise-Thomas P. Interleukin-6 is secreted by human monocytes after stimulation with anti-Toxoplasma gondii sera. The Journal of infectious diseases. 1994;169(5):1181–1182. doi: 10.1093/infdis/169.5.1181. [DOI] [PubMed] [Google Scholar]
- 264.Fischer HG, Nitzgen B, Reichmann G, Hadding U. Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. Eur J Immunol. 1997;27(6):1539–1548. doi: 10.1002/eji.1830270633. [DOI] [PubMed] [Google Scholar]
- 265.Chou DB, Sworder B, Bouladoux N, Roy CN, Uchida AM, Grigg M, Robey PG, Belkaid Y. Stromal-derived IL-6 alters the balance of myeloerythroid progenitors during Toxoplasma gondii infection. J Leukoc Biol. 2012 doi: 10.1189/jlb.1011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nagineni CN, Detrick B, Hooks JJ. Toxoplasma gondii infection induces gene expression and secretion of interleukin 1 (IL-1), IL-6, granulocyte-macrophage colony-stimulating factor, and intercellular adhesion molecule 1 by human retinal pigment epithelial cells. Infection and immunity. 2000;68(1):407–410. doi: 10.1128/iai.68.1.407-410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jebbari H, Roberts CW, Ferguson DJ, Bluethmann H, Alexander J. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite immunology. 1998;20(5):231–239. doi: 10.1046/j.1365-3024.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 268.Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen TA, Dalrymple SA, Murray R, Remington JS. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infection and immunity. 1997;65(6):2339–2345. doi: 10.1128/iai.65.6.2339-2345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Browning J, Sizing I, Lawton P, Bourdon P, Rennert P, Majeau G, Ambrose C, Hession C, Miatkowski K, Griffiths D, Ngam-ek A, Meier W, Benjamin C, Hochman P. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J Immunol. 1997;159(7):3288–3298. [PubMed] [Google Scholar]
- 270.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 271.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annual review of immunology. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]