Abstract
Objective
(1) Describe the association between hearing loss and dysfunction of each of the five vestibular end-organs – the horizontal, superior and posterior semicircular canals, saccule and utricle – in older individuals. (2) Evaluate whether hearing loss and vestibular end-organ deficits share any risk factors.
Study design
Cross-sectional study.
Setting
Academic medical center.
Patients
Fifty-one individuals age ≥70.
Interventions
Audiometry, head-thrust dynamic visual acuity (htDVA), sound-evoked cervical vestibular-evoked myogenic potential (cVEMP) and tap-evoked ocular VEMP (oVEMP).
Main Outcome Measures
Audiometric pure-tone averages (PTA), htDVA LogMAR scores as a measure of semicircular canal function in each canal plane, and cVEMP and oVEMP amplitudes as a measure of saccular and utricular function, respectively.
Results
We observed a significant correlation between hearing loss at high frequencies and reduced cVEMP amplitudes (or reduced saccular function; r = −0.37, p < 0.0001) in subjects age ≥70. In contrast, hearing loss was not associated with oVEMP amplitudes (or utricular function), or htDVA LogMAR scores (or semicircular canal function) in any of the canal planes. Age and noise exposure were significantly associated with measures of both cochlear and saccular dysfunction.
Conclusion
The concomitant decline in cochlear and saccular function associated with aging may reflect their common embryologic origin in the pars inferior of the labyrinth. Noise exposure appears to be related to both saccular and cochlear dysfunction. These findings suggest a potential benefit of screening individuals with presbycusis – particularly those with significant noise exposure history – for saccular dysfunction, which may contribute to fall risk in the elderly.
Keywords: vestibular, age-related hearing loss, noise-induced hearing loss, presbycusis, VEMP, utricle, saccule, otolith, DVA, fall
Introduction
The inner ear consists of auditory and vestibular structures responsible for hearing and balance function. It has been well described that both systems experience age-related changes causing functional decline that is considered part of the normal aging process. However, auditory and vestibular dysfunction can have severe consequences. The older adult with hearing impairment is more limited in verbal communication, with effects on productivity, quality of life, cognitive and emotional status,1–7 while the individual with balance dysfunction is more prone to suffer from dizziness and has an increased risk for falls, a major public health problem.8,9
Furthermore, deterioration of these inner ear functions is fairly common. In the US, 16% of the adult population suffers from hearing impairment. This proportion reaches 49% between ages 60–6910 and 63% in adults over 70 years old.11 Similarly, the prevalence of balance dysfunction is approximately 35% individuals over age of 40 and up to 69% in adults over age 70.8
While studies of aging effects on auditory and vestibular function continue to grow, only a few investigations have evaluated the relationship of the functional decline in these two systems.12–17 The primary aim of the present study was to investigate the association between hearing loss and deficits in each of the five vestibular end-organs – the horizontal, superior and posterior semicircular canals, saccule and utricle – in older individuals. Previously identified risk factors for hearing loss include race, gender, noise exposure, smoking and medical comorbidities such as hypertension, stroke and diabetes.8,10,18–23 Our second aim was to evaluate whether any of the known risk factors for hearing loss are similarly risk factors for the functional deterioration of the vestibular end-organs.
Methods
Subjects
We performed a cross-sectional study at a tertiary care academic medical center. Study subjects were recruited from a registry of older individuals interested in participating in clinical studies as well as from outpatient geriatrics clinics. Subjects were age 70 and over given the high prevalence of vestibular dysfunction in this age group. Individuals were excluded if they could not participate in study procedures due to blindness, poor neck range of motion or cervical spine instability (for htDVA and cVEMP testing). Subjects were also excluded if they had a history of diabetes mellitus, given prior data suggesting a significant association between diabetes mellitus and vestibular dysfunction, which could confound the effects of normative aging on the auditory and vestibular system.8 This study was approved by the Johns Hopkins Medicine Institutional Review Board and all participants provided informed consent.
Demographic and Hearing-related Variables
Data on patient age, gender, race and educational level were collected based on prior work showing significant associations between these factors and both auditory and vestibular dysfunction.8,10 Additional information regarding medical comorbidities such as hypertension, smoking history and noise exposure was also obtained. Hypertension was defined as self-reported physician diagnosis or use of antihypertensive medication. Smoking history included the number of years smoked and number of cigarette packs smoked per day. Pack-years of smoking were computed, and participants were divided into smoking categories (nonsmoker, <20 pack-years of smoking, and ≥20 pack-years of smoking).
We defined noise exposure based on both subjective and objective parameters. For the subjective determination of noise exposure, participants were queried about the presence of recreational, weapon or occupational noise exposure using standard questions.21 Exposure to recreational noise was determined by a question asking if the subject had ever been exposed outside of their occupation to loud noise (e.g., power tools or loud music) for an average of at least once a month for 1 year. Exposure to weapon noise was defined as exposure outside of their occupation to the noise of a firearm an average of at least once a month for 1 year. Exposure to occupational noise was defined as noise exposure in the workplace (requiring speaking in a raised voice) for at least 3 months. An affirmative answer to any of the noise categories was enough to determine subjective noise exposure. Objective noise exposure was based on audiometric configuration typical of noise induced hearing loss, defined by thresholds at 4, 6, and 8kHz ≥ 25dB and a 10 dB recovery of hearing thresholds at 8kHz (i.e. 10 dB better than the average thresholds at 4–6kHz).24,25 Participants were considered to have a history of noise exposure only if one of the subjective measures AND the objective audiometric measure were both positive.
Audiometry
In order to assess auditory function, pure tone threshold audiometry was performed in a sound-proofed room by a licensed audiologist (R.E.D.). Instrumentation included a Madsen Auricle audiometer with standard TDH-39 headphones. Equipment was calibrated according to the American National Standard Specifications for Audiometers (ANSI S3.6-1969). The test environment met the criteria for background noise in audiometric rooms as specified by the American National Standard Criteria for Permissible Ambient Noise during Audiometric Testing (ANSI S3.1-1977).
Air-conduction thresholds were determined for each ear from 0.5 to 8 kHz over an intensity range of −10 to 120 dB. Outcome parameters were defined as the pure-tone average (PTA) at different frequencies: speech-frequency was defined as PTA at 0.5, 1, 2, and 4 kHz; high-frequency was calculated from the PTA between 4 and 8kHz; and low-frequency was calculated from the PTA between 0.25 to 1kHz. Hearing loss was defined for each of these three frequency-specific categories as a PTA of 25 dB or greater. One participant did not undergo audiometry because the testing facility was unavailable.
Vestibular physiologic testing
All participants underwent comprehensive vestibular physiologic testing, including head thrust dynamic visual acuity (htDVA), cervical and ocular vestibular evoked myogenic potential (c-, and oVEMP) testing. Vestibular testing procedures have previously been described in detail.26
HtDVA testing
We used head thrust dynamic visual acuity (htDVA) testing to evaluate semicircular canal function (DVA-Test Micromedical Technologies; IL, USA).27 Briefly, the participant was seated 8 ft (2.4 meters) in front of a high resolution 18.1-inch monitor. Participants were instructed not to wear their glasses or contact lenses during DVA testing given difficulties consistently viewing through the corrective lenses during head movement. Static visual acuity was measured first, by repeatedly displaying a single optotype (the letter C, randomly rotated each trial by 0, 90, 180 or 270°) on the monitor. Participants viewed three optotypes per acuity level, with optotype size then being decremented in steps equivalent to a visual acuity change of 0.1 LogMAR (log10X, where X = the minimum angle resolved, in arcmin, with 1 arcmin = 1/60°). The better one’s visual acuity, the lower one’s LogMAR score, with LogMAR = –0.3, 0, 0.3, 0.7, and 1.0, corresponding to Snellen visual acuity of 20/10, 20/20, 20/40, 20/100, and 20/200, respectively.
Static visual acuity was scored at the lowest acuity level where the participant was able to correctly identify all three optotypes. For the dynamic component of the test, a single-axis rate sensor was positioned on the subject’s head so that the sensor’s axis of maximum sensitivity aligned with that of the semicircular canal being tested. Passive (manually-imposed) head thrusts were delivered within a semicircular canal-pair plane in a random direction.
During each head thrust, the optotype was displayed when head velocity, sensed by the rate sensor, was between 120 and 180°/s for more than 40 ms. The optotype flashed on the monitor for up to 85 ms (equivalent to approximately a 9–13.5° head rotation). The test began at each individual’s static visual acuity and then increased in steps equivalent to a visual acuity change of 0.1 LogMAR (given that dynamic visual acuity is poorer than static visual acuity), until the subject was able to identify three optotypes at a given level. The DVA test score was calculated by subtracting the static visual acuity LogMAR score from the htDVA LogMAR score. One participant did not undergo htDVA testing due to concerns with neck discomfort (not the same participant who did not undergo audiometry).
CVEMP testing
Participants underwent cervical vestibular-evoked myogenic potential (cVEMP) testing in response to air-conducted sound to assess saccular function.28–30 They were positioned supine with their upper body elevated at a 30-degree angle from horizontal. The neck was actively flexed by the participant during cVEMP stimulation and recording to provide tonic background muscle activity. Air-conducted 500 Hz (125 dB SPL) tone bursts of positive polarity, with a linear envelope (1 ms rise/fall time, 2 ms plateau), at a repetition rate of 5 pulses per second were delivered monaurally via intra-auricular speakers. This stimulus has provided good reliability in eliciting VEMPs.31,32 CVEMPs were recorded from an electrode montage consisting of a non-inverting electrode placed at the midpoint of the ipsilateral sternocleidomastoid muscle belly, an inverting electrode placed on the sternoclavicular junction, and a ground electrode placed on the manubrium sterni. The responses to 100 stimuli were averaged. The first positive and negative peaks that occurred between 13 and 23 ms after stimulus onset were designated p13 and n23, respectively. The raw peak-to-peak amplitude was calculated as the sum of the p13 and the n23 amplitudes. The corrected peak-to-peak amplitude was calculated by dividing the raw peak-to-peak amplitude by the rectified background EMG activity recorded during the 10-ms interval before stimulus onset. The corrected p13 amplitude (referred to hereafter as the p13 amplitude) was calculated in a similar manner. This correction factor accounts for the variable tonic muscle tone that affects cVEMP amplitudes.31,33
OVEMP testing
To evaluate utricular function, tap-evoked ocular VEMP (oVEMP) testing was performed.30,34–36 Participants were instructed to maintain a 30-degree upgaze while they lay supine with their upper bodies elevated at a 30-degree angle from horizontal. “Mini taps” were manually delivered by a reflex hammer at the Fz cranial site (in the midline at the hairline, 30% of the distance between the inion and nasion).34 For oVEMPs the electrode montage included a noninverting electrode centered 5 mm beneath the pupil, an inverting electrode centered 2 cm below the noninverting electrode, and a ground electrode placed on the manubrium sterni. The responses to 50 stimuli were averaged. Before testing with tap stimulation, 20° vertical saccades were recorded from both eyes. If the signal change showed > 25% asymmetry, the electrodes were replaced. The n10 potential was identified as the first negative peak in the waveform, and occurred 7–11 ms after stimulus onset. The n10 amplitude was measured at the maximum negative voltage of the n10 potential.31
Statistical analysis
Bivariate associations between PTA (high-frequency, low frequency and speech frequency) and measurements of vestibular end-organ function were analyzed using Pearson’s correlation.
Vestibular test results stratified by previously defined risk factors for age-related hearing loss were analyzed with Mann-Whitney U tests. Hearing thresholds were also analyzed based on the presence of risk factors using t-tests, given their normal distribution. Multivariate analyses were performed to assess the association between corrected cVEMP amplitude and high frequency PTA adjusting for demographic characteristics. Data from the right and left sides were considered individually for a total of 102 ears. All results were considered significant at the P < 0.05 level. SPSS version 18 (IBM SPSS, Chicago, IL) was used for statistical analyses.
Results
Fifty-one participants were included in this study. Males comprised 49% of subjects and females 51%, Forty subjects (78%) were age 70–80, and 11 (22%) were above age 80. 88% of subjects were white and 12% were black, and a majority of subjects had greater than a high school education (Table 1). In terms of medical comorbidities, 28% of subjects were heavy smokers and 63% had a history of hypertension (Table 1). Twelve percent of participants met criteria for noise exposure (Table 1).
Table 1.
Subjects demographics and medical comorbidities
| No. (%) Participants | |
|---|---|
| Demographic Characteristics | |
| Gender | |
| Male | 25 (49%) |
| Female | 26 (51%) |
| Age | |
| 70–80 | 40 (78%) |
| > 80 | 11 (22%) |
| Race | |
| White | 45 (88%) |
| Black | 6 (12%) |
| Education | |
| High school or less | 17 (33%) |
| More than high school | 34 (66%) |
| Medical Comorbidities | |
| Smoking | |
| Nonsmoker | 26 (51%) |
| <20 pack-years | 11 (22%) |
| ≥20 pack-years | 14 (27%) |
| Hypertension | |
| No | 19 (37%) |
| Yes | 32 (63%) |
| Noise exposure1 | |
| No | 38 (74%) |
| Yes | 6 (12%) |
Data missing in 7 participants
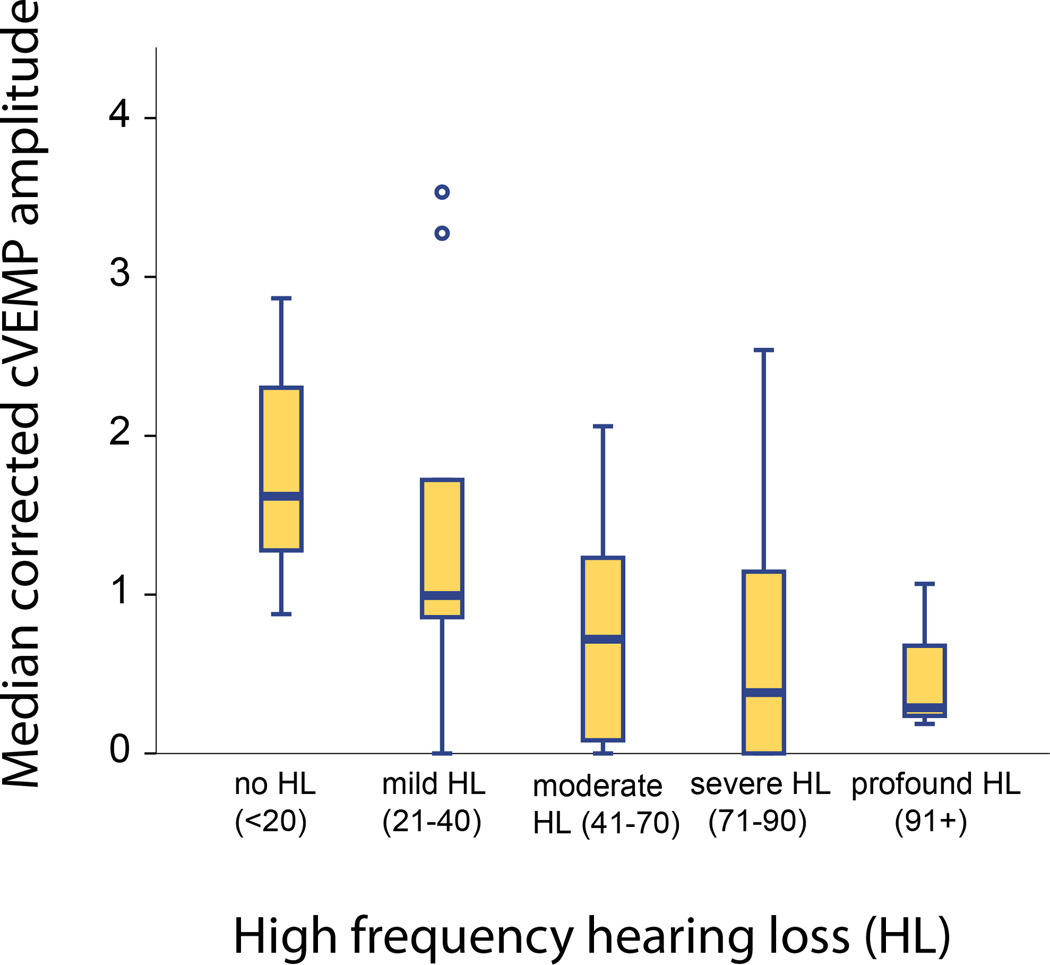
Hearing loss was significantly correlated with reduced saccular function, represented by reduced corrected cVEMP amplitudes for air-conducted sound (ACS). This relationship was more statistically significant when considering high-frequency PTA (r = −0.37, p<0.001, Figure 1) compared to speech frequency (r = −0.33, p=0.001) or low-frequency PTA (r = −0.234, p=0.019)(Table 2). Five ears showed normal hearing thresholds (≤20) in the high frequencies. Of these, cVEMP responses were present in all ears. Given that the association between saccular dysfunction and high-frequency hearing loss was strongest, we consider high-frequency hearing loss in subsequent analyses.
Figure 1.
Corrected cVEMP amplitude as a function of high frequency hearing loss.
Table 2.
Mean speech and high frequency PTA and correlations with vestibular physiologic tests in study population
| Hearing level | N=100 Mean PTA (SD) |
Correlations | ||||
|---|---|---|---|---|---|---|
| cVEMP | oVEMP | Hor DVA |
Sup DVA |
Post DVA |
||
| Low frequency | 25.2 (15.1) | r=−0.23 p=0.019 |
r=−0.10 p=0.313 |
r=0.09 p=0.385 |
r=0.10 p=0.312 |
r=0.13 p=0.187 |
| Speech frequency |
34.2 (14.3) | r=−0.33 p=0.001 |
r=−0.06 p=0.582 |
r=0.09 p=0.384 |
r=0.02 p=0.849 |
r=0.07 p=0.465 |
| High frequency | 62.6 (19) | r=−0.37 p<0.0001 |
r=0.04 p=0.683 |
r=0.13 p=0.208 |
r=−0.05 p=0.600 |
r=0.09 p=0.399 |
Hor: horizontal; Sup: superior; Post: posterior
Hearing loss was not associated with utricular dysfunction represented by low oVEMP amplitudes nor with semicircular canal dysfunction represented by poor htDVA LogMAR scores in any of the semicircular canal planes (Table 2).
Given that the saccular measure (cVEMP for ACS) was the only measure associated with hearing loss, we evaluated for associations between corrected cVEMP amplitudes and previously identified ARHL risk factors, including demographic characteristics and medical comorbidities, in bivariate analyses (Table 3). Consistent with prior studies, we observed that older age, male gender, white race, smoking and a history of noise exposure were significantly associated with high frequency hearing loss (p<0.010). However, only older age, smoking history and noise exposure had a significant influence on cVEMP amplitudes in bivariate analyses (p<0.0001, p=0.028 and p=0.004 respectively; Table 3). Increasing age and a history of noise exposure were associated with reduced saccular function (corrected cVEMP amplitudes). Participants with a smoking history of <20 pack-years had significantly higher cVEMP amplitudes and lower high frequency PTA than non-smokers and heavy smokers (p=0.028 and 0.033 respectively). It should be noted that participants who smoked <20 pack-years were younger than the non-smokers and heavy smokers; this apparent effect of smoking is thus reversed with adjustment for age in multivariate analyses below. To determine whether the association between high frequency hearing loss and saccular dysfunction may be due to their shared risk factors, we performed multivariate analyses adjusting for age, race, gender, smoking history and noise exposure (Table 4). We observed that high frequency PTA was significantly associated with cVEMP amplitude even after adjustment for shared risk factors: each dB increase in high frequency PTA was associated with a 0.01 decrease in cVEMP amplitude (p=0.040; Table 4). We also observed that noise exposure maintained a significant association with cVEMP amplitudes independent of its effect on hearing loss: noise exposure was associated with a decline in corrected cVEMP amplitude of 0.52 (p=0.043; Table 4). Smoking history did not have a significant independent association with cVEMP amplitude in multivariate analyses. Of note, noise exposure was not associated with oVEMP amplitudes or LogMAR scores in any of the canal planes (data not shown).
Table 3.
Mean high frequency PTA and median cVEMP amplitude by medical comorbidities
| Characteristics | N=50 N (%) |
High frequency PTA | N=51 N (%) |
cVEMP amplitude | ||
|---|---|---|---|---|---|---|
| dB | p-value | µV | p-value | |||
| DEMOGRAPHIC CHARACTERISTICS | ||||||
| Age | 0.001 | < 0.0001 | ||||
| 70–80 | 39 (78%) | 59.3 | 40 (78%) | 0.92 | ||
| > 80 | 11 (22%) | 74.5 | 11 (22%) | 0.09 | ||
| Gender | < 0.0001 | 0.634 | ||||
| Male | 25 (50%) | 71.5 | 25 (49%) | 0.70 | ||
| Female | 25 (50%) | 53.8 | 26 (51%) | 0.85 | ||
| Race | 0.003 | 0.929 | ||||
| White | 45 (90%) | 64.5 | 45 (88%) | 0.81 | ||
| Black | 5 (10%) | 45.6 | 6 (12%) | 0.70 | ||
| Education | 0.255 | 0.354 | ||||
| High school or less | 16 (32%) | 65.8 | 17 (33%) | 0.84 | ||
| More than high school | 34 (68%) | 61.1 | 34 (66%) | 0.73 | ||
| MEDICAL COMORBIDITIES | ||||||
| Smoking | 0.033 | 0.028 | ||||
| Nonsmoker | 26 (52%) | 58.4 | 26 (51%) | 0.61 | ||
| < 20 packyears | 10 (20%) | 46.7 | 11 (22%) | 1.25 | ||
| ≥ 20 packyears | 14 (28%) | 57.8 | 14 (27%) | 0.70 | ||
| Hypertension | 0.764 | 0.451 | ||||
| No | 19 (38%) | 63.4 | 19 (37%) | 0.98 | ||
| Yes | 31 (62%) | 62.2 | 32 (63%) | 0.70 | ||
| Noise exposure1 | ||||||
| No | 37 (74%) | 60.5 | 0.010 | 38 (76%) | 0.86 | 0.004 |
| Yes | 6 (12%) | 75.6 | 6 (12%) | 0.00 | ||
Data missing in 7 participants
Table 4.
Multivariate analysis of association between cVEMP amplitude and high frequency PTA
| Risk factor | cVEMP amplitude | |
|---|---|---|
| β (SE) | p-value | |
| Age | −0.03.7 (0.1) | 0.027 |
| Gender | −0.15 (0.17) | 0.386 |
| Race | −0.32 (0.26) | 0.215 |
| Noise exposure | −0.52 (0.25) | 0.043 |
| Smoking history | 0.065 (0.09) | 0.48 |
| High frequency PTA | −0.01 (0.005) | 0.040 |
Finally, we considered the association between frequency-specific auditory function and corrected cVEMP amplitudes. We observed the strongest correlation at 6000 Hz (r = − 0.414, p < 0.0001), a frequency typically associated with noise induced hearing loss.
Discussion
These data suggest that in older individuals, functional loss occurs concomitantly in the cochlea and the saccule. The cochlea is the inner ear organ that subserves auditory function, while the saccule is an otolith organ involved in vertical linear movement detection and sensing gravitational changes. Prior studies in animal models have also observed parallel declines in auditory and vestibular function;14–17,37 indeed, one study specifically reported combined auditory and gravity receptor aging in a strain of mice.17 However, no prior study has evaluated the function of the cochlea and each of the 5 vestibular end-organs in humans. Although we observed concurrent cochleosaccular dysfunction, we did not find any significant associations between aging of the cochlea and of the utricle or any of the semicircular canals.
A possible explanation for the association between cochlear and saccular function is the shared embryological origin of these structures. The cochlea and saccule arise from the pars inferior of the inner ear, after the three semicircular canals and utricle have already developed from the pars superior.38 The common embryology may result in anatomic and physiologic coupling of these organs. Indeed, Gussen (1980) suggested that presbycusis is an example of a cochleosaccular degenerative process whereby dislodged saccular otoconia reach the cochlea through the ductus reuniens and cochlear duct, thus also affecting the cochlear base and high frequency hearing thresholds.39 Other studies of human temporal bone specimens have also described otoconial loss from the saccule associated with cochleosaccular degeneration with increasing age.40–42 In addition, an experiment in the squirrel monkey showed independent endolymphatic circulation in the utricle and saccule which may explain why insults to the saccule may not affect the utricle and perhaps also the semicircular canals.43 The shared susceptibility of the cochlea and saccule has also been observed in other pathologic processes such as Meniere’s disease, which is typified histopathologically by cochleosaccular hydrops.44
While noise exposure is well recognized as a risk factor for hearing function, its possible negative effect on vestibular function is less well-characterized. McCabe and Lawrence (1958) showed that intense noise exposure in guinea pigs only affected the saccule within the vestibular labyrinth and spared the utricle and canals.45 More recently, Kumar et al (2010) noted saccular dysfunction based on cVEMP testing in individuals who suffered from noise-induced hearing loss.46 Sound transmission across the stapes footplate displaces the inner ear fluids, and excessive noise exposure may lead to a destruction of the saccular neuroepithelium which lies in proximity to the oval window.47 Acoustic trauma also leads to the generation of reactive oxygen species which may be toxic to the saccular macula. The pars superior structures may be shielded from both fluid shifts and noxious substances by the intervening membrane limitans.48,49 Our findings corroborate that noise exposure may be a significant risk factor for both auditory and saccular dysfunction. Furthermore, we extended the work of Kumar et al (2010) by evaluating all five peripheral vestibular end-organs. We observed as McCabe and Lawrence did that noise exposure was only significantly associated with saccular function; however, we cannot exclude the possibility of some noise-induced damage to the utricle or canals that is subclinical or compensated. Indeed a recent study in rats showed that utricular and semicircular canal afferents are responsive to click stimuli.50 Furthermore, in non-human primates, oculomotor signals recorded in response to acoustic clicks suggest origins from the utricle and horizontal canal.51 More work will be required to fully characterize the sound-sensitivity of the vestibular end-organ.
What are the clinical implications of the association between hearing loss and saccular dysfunction? Recent investigations in older adults have described that hearing loss is associated with an increased risk for falls.52–54 The present work suggests that hearing loss may be associated with fall risk in part due to its coexistence with vestibular (specifically, saccular) dysfunction, which is a known risk factor for falling.8,26,55 Thus, one implication of our findings is that older individuals found to have high-frequency sensorineural hearing loss should be screened for fall risks, and that screening may include cVEMP testing. Another implication is that older individuals with a history of noise exposure may require attention not only to the risk of hearing loss, but to the risk of balance dysfunction as well.
Individuals found to be at risk for falls can be referred to fall risk-reduction programs. The US Centers for Disease Control (CDC) note that at least 22 specific interventions for community-dwelling older adults have rigorous scientific evidence of effectiveness in reducing the risk of falls.56 However, the CDC’s guidelines do not presently include hearing loss as a risk factor that should trigger referral to a fall prevention program. Our results suggest that older individuals with high-frequency hearing loss may well be at increased risk for falls and may benefit from directed fall risk reduction measures such as updating prescription lenses, home environmental modifications and possibly vestibular physical therapy.
A limitation of this study is the inability to segregate central from peripheral influences on the results of the vestibular physiologic testing. For instance, the cVEMP test is an electromyographic response measured at the sternocleidomastoid muscle that is believed to evaluate a sacculo-collic reflex. It was originally suggested that the latency of the response was strongly influenced by central mechanisms,57–59 but it cannot be ruled out that a low amplitude may also be the result of a central influence on the signal. Interpretation of oVEMPs and htDVA LogMAR scores, and potentially audiometry as well, are also subject to this constraint. A further limitation of this study is the cross-sectional nature of the data and the hazards of attributing causality to the association found between noise exposure and saccular dysfunction. However, work in guinea pigs also suggests that noise exposure sufficient to damage the cochlea also damages saccular function.45
Conclusion
The concomitant decline in cochlear and saccular function associated with aging may reflect the common embryologic origin of both structures, which comprise the pars inferior of the labyrinth. Noise exposure appears to be related to both saccular and cochlear dysfunction. These findings suggest a potential benefit of screening older individuals with high-frequency hearing loss – especially in those who were exposed to intense noise – for saccular dysfunction, which may contribute to fall risk.
Acknowledgments
Authors are grateful for the support of the American Neurotological Society’s Silverstein Award and the Older Americans Independence Center Pilot Award (YA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The present work was accepted for Oral Presentation at the 2012 AOS Spring Meeting.
References
- 1.Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261(13):1916–1919. [PubMed] [Google Scholar]
- 2.Herbst KG, Humphrey C. Hearing impairment and mental state in the elderly living at home. Br Med J. 1980;281(6245):903–905. doi: 10.1136/bmj.281.6245.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gates GA, Cobb JL, Linn RT, Rees T, Wolf PA, D'Agostino RB. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg. 1996;122(2):161–167. doi: 10.1001/archotol.1996.01890140047010. [DOI] [PubMed] [Google Scholar]
- 4.Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 5.Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- 6.Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurootol. 2007;12(6):345–358. doi: 10.1159/000106478. [DOI] [PubMed] [Google Scholar]
- 7.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169(10):938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 9.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348(1):42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med. 2008;168(14):1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- 11.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(10):1131–1136. doi: 10.1093/gerona/glr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleeson M, Felix H. A comparative study of the effect of age on the human cochlear and vestibular neuroepithelia. Acta Otolaryngol Suppl. 1987;436:103–109. doi: 10.3109/00016488709124982. [DOI] [PubMed] [Google Scholar]
- 13.Enrietto JA, Jacobson KM, Baloh RW. Aging effects on auditory and vestibular responses: a longitudinal study. Am J Otolaryngol. 1999;20(6):371–378. doi: 10.1016/s0196-0709(99)90076-5. [DOI] [PubMed] [Google Scholar]
- 14.Jones SM, Jones TA, Johnson KR, Yu H, Erway LC, Zheng QY. A comparison of vestibular and auditory phenotypes in inbred mouse strains. Brain Res. 2006;1091(1):40–46. doi: 10.1016/j.brainres.2006.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiga A, Nakagawa T, Nakayama M, et al. Aging effects on vestibulo-ocular responses in C57BL/6 mice: comparison with alteration in auditory function. Audiol Neurootol. 2005;10(2):97–104. doi: 10.1159/000083365. [DOI] [PubMed] [Google Scholar]
- 16.Jones SM, Robertson NG, Given S, Giersch AB, Liberman MC, Morton CC. Hearing and vestibular deficits in the Coch(−/−) null mouse model: comparison to the Coch(G88E/G88E) mouse and to DFNA9 hearing and balance disorder. Hear Res. 2011;272(1–2):42–48. doi: 10.1016/j.heares.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mock B, Jones TA, Jones SM. Gravity receptor aging in the CBA/CaJ strain: a comparison to auditory aging. J Assoc Res Otolaryngol. 2011;12(2):173–183. doi: 10.1007/s10162-010-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruickshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148(9):879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30(2):139–145. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otol Neurotol. 2010;31(9):1445–1450. doi: 10.1097/MAO.0b013e3181f2f035. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal Y, Niparko JK, Dobie RA. Estimating the effect of occupational noise exposure on hearing thresholds: the importance of adjusting for confounding variables. Ear Hear. 2010;31(2):234–237. doi: 10.1097/AUD.0b013e3181c6b9fd. [DOI] [PubMed] [Google Scholar]
- 22.Helzner EP, Patel AS, Pratt S, et al. Hearing sensitivity in older adults: associations with cardiovascular risk factors in the health, aging and body composition study. J Am Geriatr Soc. 2011;59(6):972–979. doi: 10.1111/j.1532-5415.2011.03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golz A, Westerman ST, Westerman LM, et al. The effects of noise on the vestibular system. Am J Otolaryngol. 2001;22(3):190–196. doi: 10.1053/ajot.2001.23428. [DOI] [PubMed] [Google Scholar]
- 24.Coles RR, Lutman ME, Buffin JT. Guidelines on the diagnosis of noise-induced hearing loss for medicolegal purposes. Clin Otolaryngol Allied Sci. 2000;25(4):264–273. doi: 10.1046/j.1365-2273.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- 25.McBride DI, Williams S. Audiometric notch as a sign of noise induced hearing loss. Occup Environ Med. 2001;58(1):46–51. doi: 10.1136/oem.58.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agrawal Y, Zuniga MG, Davalos-Bichara M, et al. Physiology of vestibular dysfunction and aging. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012 doi: 10.1097/MAO.0b013e3182a09ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schubert MC, Migliaccio AA, Della Santina CC. Dynamic visual acuity during passive head thrusts in canal planes. J Assoc Res Otolaryngol. 2006;7(4):329–338. doi: 10.1007/s10162-006-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57(2):190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todd NP, Cody FW, Banks JR. A saccular origin of frequency tuning in myogenic vestibular evoked potentials?: implications for human responses to loud sounds. Hear Res. 2000;141(1–2):180–188. doi: 10.1016/s0378-5955(99)00222-1. [DOI] [PubMed] [Google Scholar]
- 30.Yang TH, Liu SH, Young YH. Evaluation of guinea pig model for ocular and cervical vestibular-evoked myogenic potentials for vestibular function test. Laryngoscope. 2010;120(9):1910–1917. doi: 10.1002/lary.21056. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31(5):793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viciana D, Lopez-Escamez JA. Short tone bursts are better than clicks for cervical vestibular-evoked myogenic potentials in clinical practice. Eur Arch Otorhinolaryngol. 2012 doi: 10.1007/s00405-011-1912-4. [DOI] [PubMed] [Google Scholar]
- 33.Akin FW, Murnane OD, Tampas JW, Clinard CG. The Effect of Age on the Vestibular Evoked Myogenic Potential and Sternocleidomastoid Muscle Tonic Electromyogram Level. Ear Hear. 2011 doi: 10.1097/AUD.0b013e318213488e. [DOI] [PubMed] [Google Scholar]
- 34.Iwasaki S, McGarvie LA, Halmagyi GM, et al. Head taps evoke a crossed vestibulo-ocular reflex. Neurology. 2007;68(15):1227–1229. doi: 10.1212/01.wnl.0000259064.80564.21. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki S, Smulders YE, Burgess AM, et al. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol. 2008;119(9):2135–2147. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Manzari L, Burgess AM, Curthoys IS. Effect of bone-conducted vibration of the midline forehead (Fz) in unilateral vestibular loss (uVL). Evidence for a new indicator of unilateral otolithic function. Acta Otorhinolaryngol Ital. 2010;30(4):175. [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson NG, Jones SM, Sivakumaran TA, et al. A targeted Coch missense mutation: a knock-in mouse model for DFNA9 late-onset hearing loss and vestibular dysfunction. Hum Mol Genet. 2008;17(21):3426–3434. doi: 10.1093/hmg/ddn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulya AJ. Developmental Anatomy of the Temporal Bone and Skull Base. In: Gulya AJ, Minor LB, Poe DS, editors. Glasscock-Shambaugh Surgery of the Ear. Sixth edition ed. PMPH-USA; 2010. p. 9. [Google Scholar]
- 39.Gussen R. Saccule otoconia displacement into cochlea in cochleosaccular degeneration. Arch Otolaryngol. 1980;106(3):161–166. doi: 10.1001/archotol.1980.00790270025006. [DOI] [PubMed] [Google Scholar]
- 40.Johnsson LG. Degenerative changes and anomalies of the vestibular system in man. Laryngoscope. 1971;81(10):1682–1694. doi: 10.1288/00005537-197110000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Johnsson LG, Hawkins JE., Jr. Sensory and neural degeneration with aging, as seen in microdissections of the human inner ear. Ann Otol Rhinol Laryngol. 1972;81(2):179–193. doi: 10.1177/000348947208100203. [DOI] [PubMed] [Google Scholar]
- 42.Ross MD, Peacor D, Johnsson LG, Allard LF. Observations on normal and degenerating human otoconia. Ann Otol Rhinol Laryngol. 1976;85(3 pt 1):310–326. doi: 10.1177/000348947608500302. [DOI] [PubMed] [Google Scholar]
- 43.Igarashi M. Histopathological Findings After Experimental Saccular Destruction in the Squirrel Monkey. Laryngoscope. 1965;75:1048–1061. doi: 10.1288/00005537-196507000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Morita N, Kariya S, Farajzadeh Deroee A, et al. Membranous labyrinth volumes in normal ears and Meniere disease: a three-dimensional reconstruction study. Laryngoscope. 2009;119(11):2216–2220. doi: 10.1002/lary.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCabe BF, Lawrence M. The effects of intense sound on the non-auditory labyrinth. Acta Otolaryngol. 1958;49(2):147–157. doi: 10.3109/00016485809134738. [DOI] [PubMed] [Google Scholar]
- 46.Kumar K, Vivarthini CJ, Bhat JS. Vestibular evoked myogenic potential in noise-induced hearing loss. Noise Health. 2010;12(48):191–194. doi: 10.4103/1463-1741.64973. [DOI] [PubMed] [Google Scholar]
- 47.Wang YP, Young YH. Vestibular-evoked myogenic potentials in chronic noise-induced hearing loss. Otolaryngol Head Neck Surg. 2007;137(4):607–611. doi: 10.1016/j.otohns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Sohmer H. Sound induced fluid pressures directly activate vestibular hair cells: implications for activation of the cochlea. Clin Neurophysiol. 2006;117(5):933–934. doi: 10.1016/j.clinph.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Hara M, Kimura RS. Morphology of the membrana limitans. Ann Otol Rhinol Laryngol. 1993;102(8 Pt 1):625–630. doi: 10.1177/000348949310200811. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Tang X, Wei W, Mustain W, Xu Y, Zhou W. Click-evoked responses in vestibular afferents in rats. J Neurophysiol. 2011;106(2):754–763. doi: 10.1152/jn.00003.2011. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Simpson I, Tang X, Zhou W. Acoustic clicks activate both the canal and otolith vestibulo-ocular reflex pathways in behaving monkeys. J Assoc Res Otolaryngol. 2009;10(4):569–577. doi: 10.1007/s10162-009-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012;172(4):369–371. doi: 10.1001/archinternmed.2011.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez D, McCaul KA, Hankey GJ, et al. Falls, injuries from falls, health related quality of life and mortality in older adults with vision and hearing impairment--is there a gender difference? Maturitas. 2011;69(4):359–364. doi: 10.1016/j.maturitas.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Viljanen A, Kaprio J, Pyykko I, Sorri M, Koskenvuo M, Rantanen T. Hearing acuity as a predictor of walking difficulties in older women. J Am Geriatr Soc. 2009;57(12):2282–2286. doi: 10.1111/j.1532-5415.2009.02553.x. [DOI] [PubMed] [Google Scholar]
- 55.Herdman SJ, Blatt P, Schubert MC, Tusa RJ. Falls in patients with vestibular deficits. Am J Otol. 2000;21(6):847–851. [PubMed] [Google Scholar]
- 56.Stevens JA. A CDC Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults. 2nd, ed. ed. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. pp. 92–101. [Google Scholar]
- 57.Murofushi T, Shimizu K, Takegoshi H, Cheng PW. Diagnostic value of prolonged latencies in the vestibular evoked myogenic potential. Arch Otolaryngol Head Neck Surg. 2001;127(9):1069–1072. doi: 10.1001/archotol.127.9.1069. [DOI] [PubMed] [Google Scholar]
- 58.Eleftheriadou A, Deftereos SN, Zarikas V, et al. The diagnostic value of earlier and later components of Vestibular Evoked Myogenic Potentials (VEMP) in multiple sclerosis. J Vestib Res. 2009;19(1–2):59–66. doi: 10.3233/VES-2009-0342. [DOI] [PubMed] [Google Scholar]
- 59.Bandini F, Beronio A, Ghiglione E, Solaro C, Parodi RC, Mazzella L. The diagnostic value of vestibular evoked myogenic potentials in multiple sclerosis. J Neurol. 2004;251(5):617–621. doi: 10.1007/s00415-004-0378-3. [DOI] [PubMed] [Google Scholar]