Abstract
Hypothesis
Adult mesenchymal stem cells (MSCs) can be converted into hair cell-like cells by transdetermination.
Background
Given the fundamental role sensory hair cells play in sound detection and the irreversibility of their loss in mammals, much research has focused on developing methods to generate new hair cells as a means of treating permanent hearing loss. Although MSCs can differentiate into multiple cell lineages, no efficient means of reprogramming them into sensory hair cells exists. Earlier work has shown that the transcription factor Atoh1 is necessary for early development of hair cells, but it is not clear whether Atoh1 can be used to convert MSCs into hair cells.
Methods
Clonal MSC cell lines were established and reprogrammed into hair cell-like cells by a combination of protein transfer, adenoviral based gene transfer and co-culture with neurons. During transdetermination, inner ear molecular markers were analyzed by RT-PCR, and cell structures were examined by immunocytochemistry.
Results
Atoh1 overexpression in MSCs failed to convert MSCs into hair cell-like cells, suggesting that the ability of Atoh1 to induce hair cell differentiation is context dependent. Because Atoh1 overexpression successfully transforms VOT-E36 cells into hair cell-like cells, we modified the cell context of MSCs by performing a total protein transfer from VOT-E36 cells prior to overexpressing Atoh1. The modified MSCs were transformed into hair cell-like cells and attracted contacts from spiral ganglion neurons in a co-culture model.
Conclusion
We established a new procedure, consisting of VOT-E36 protein transfer, Atoh1 overexpression, and co-culture with spiral ganglion neurons, which can transform MSCs into hair cell-like cells.
Keywords: adult stem cells, differentiation, sensory neurons, hearing loss
INTRODUCTION
Permanent hearing loss is the most common sensory disorder in humans, and represents a serious and prevalent health concern (1). This affliction is particularly common among the elderly, as about half of those over age 75 have age-related hearing loss (2). Permanent hearing loss is usually caused by the degeneration of hair cells and spiral ganglion neurons (SGNs) due to aging, noise exposure, ototoxic drugs, or bacterial and viral infections (3). Because loss of hair cells is irreversible in mammals (4–8), the development of effective methods to generate new hair cells would be of great therapeutic value.
As the linchpin for our sense of hearing, hair cells have been the subject of intensive research. Two main approaches for generating new hair cells have emerged from many studies on this subject. One approach has sought to induce hair cell-like cells by modifying the extracellular environment of embryonic stem (ES) cells, induced pluripotent stem (iPS) cells, and cells isolated from the inner ear (6, 9–12). Impressively, these studies have successfully induced hair cell-like cells that respond to mechanical stimulation (10). This stepwise differentiation method to induce hair cell-like cells from ES or iPS cells is effective but labor and time intensive (9, 10). The other principal approach has sought to obtain hair cell-like cells through Atoh1 overexpression induced transdetermination, a direct intrinsic modification. Transdetermination is a one form of reprogramming that involves direct fate switching of committed, but not yet fully differentiated, progenitor cells. Atoh1 is a basic helix-loop-helix (bHLH) transcription factor essential for development of hair cells (13–15). Overexpression of Atoh1 in differentiated nonsensory cells of the cochlea can induce hair cell-like cells in vitro and in vivo (16–18). The nonsensory epithelial cells reprogrammed in vivo attract innervations from SGNs and result in improved hearing thresholds (19, 20). This successful transdetermination of nonsensory cells into hair cell-like cells suggests that other progenitor cells, such as adult stem cells, could also be converted into hair cells by overexpressing Atoh1.
MSCs are adult stem cells derived mainly from bone marrow or adipose tissue. Patient specific mesenchymal stem cells (MSCs) are readily available and could be therapeutically useful if they could be converted into hair cells. Due to the lack of a definitive molecular marker, the exact origin and the cellular identity of MSCs remain elusive, although a perivascular localization of these cells is generally accepted (21, 22). Molecular markers for hair cells were induced in bone marrow-derived MSCs with or without Atoh1 overexpression (23). Because adipose tissue is endowed with an abundance of blood vessels and MSCs can be easily isolated from adipose (24), we chose to test whether adipose-derived MSCs could be reprogrammed into hair cell-like cells by Atoh1 overexpression. Primary adipose stem cell cultures contain a heterogeneous mixture of endothelial cells, including smooth muscle cells, pericytes, fibroblasts, mast cells, and preadipocytes (25). Thus, we established three single-cell derived adult stem cell lines from mouse MSCs. Next, we overexpressed Atoh1 in these cells and demonstrated that this treatment led to an incomplete reprogramming of MSCs. By combining Atoh1 overexpression with protein transfer from an otic epithelial cell line, we reprogrammed these cells into hair cell-like cells that expressed hair cell-specific molecular markers. These modified cells attracted contacts from spiral ganglion neurons (SGNs). Thus, these results reveal a new transdetermination mechanism to derive hair cells.
MATERIALS AND METHODS
Isolation and culture of adult adipose stem cells
The methods for isolation and culture of adult adipose stem cells were similar to those in previous reports (24). Briefly, subcutaneous or adipose fat from C57BL/6J mice was washed in PBS twice and minced into small particles. The minced tissue was digested in collagenase (Gibco, final concentration 0.5 g/100 ml) for 45 minutes at 37°C. After centrifuging to collect cells, possible blood cells in the sample were eliminated using RBC lysis buffer (3.735 g NH4Cl and 85 mg Tris-HCl in 500 ml water). The remaining cells were plated in DMEM-Low glucose (1 g/L) supplemented with 10% FBS, 1% Pen/Strep, and FGF2 (10 ng/ml, Gibco). For single-cell derivation, we diluted and split cells into a 96 well plate (about 0.5- 1 cell/well), and confirmed one cell per well under a microscope. The cells were cultured until colonies formed (about 2 weeks).
Differentiation of single-cell derived cell lines
For adipogenesis, cells were cultured for two weeks in adipogenic medium, which consisted of DMEM supplemented with 10% FBS, 1 μm Dexamethasone, 0.5 mM Isobuthylmethylxanthine, and 10 μg/ml insulin. Oil Red O stain, an established lipid dye, was used to identify adipose cells. For chondrogenesis, cells were cultured for four weeks in chondrogenic medium, which consisted of DMEM supplemented with 1% FBS, 110 μg/ml sodium pyruvate, 0.15 mM ascorbate-2-phosphate, 100 nM dexamethasone, 1% ITS (insulin, transferrin, and sodium selenite), and 10 ng/ml TGF. Alcian Blue was used to identify the presence of sulfated proteoglycans characteristic of chondrogenesis. For osteogenesis, cells were cultured for four weeks in osteogenic medium, which consisted of DMEM supplemented with 10% FBS, 10 mM glycerophosphate, 0.15 mM ascorbic acid, 10 nM vitamin D3, and 10 nM dexamethasone. Alizarin Red was used to identify high extracellular calcium accumulation typical of osteogenic differentiation.
Protein transfer
Culture of mouse VOT-E36 cells was performed as described in our previous reports (18, 26). After being induced at 39°C without γ–interferon for four days (the T-antigen mutant, tsA58, is inactivated at 39°C), VOT-E36 cells were washed sequentially in PBS and cell lysis buffer (100 mM HEPES, pH 8.2, 50 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol, and protease inhibitors), then sedimented at 750 rpm. The collected cells were resuspended in 1 volume of cold cell lysis buffer, and incubated for 30–45 minutes on ice. The cells were sonicated on ice using a 3 mm diameter probe. The lysate was sedimented at 12,000 rpm for 15 minutes at 4°C to pellet the coarse material. The supernatant was aliquoted and frozen in liquid nitrogen until the protein transfer. For protein transfer by Streptolysin O (SLO; Sigma), adult adipose stem cells were grown to confluence, trypsinized, and washed twice in cold PBS and once in cold HBSS. 20,000 cells in 14 μl of HBSS were mixed with 4.6 μl SLO (100 μg/ml) to open up the cell membranes. Samples were incubated for one hour at 37°C and collected by centrifugation. The recovered cells were incubated with either 50μg VOT-E36 cell lysate or 50μg boiled VOT-E36 cell lysate in 20 μl medium containing an ATP-regenerating system. Cells were incubated for 30 minutes at 37°C and then plated for recovery.
Adenoviral Infection
A replication-incompetent adenovirus expressing Atoh1 was constructed and used as previously described (18). Following protein transfer and recovery, cells were incubated in adenoviral supernatant for three hours before being washed and cultured in neurobasal medium (Gibco) enriched with N2 supplement (1%, Gibco), 73 μg/ml L-glutamate, 4.2 mg/ml L-glutamic acid, and 1 mg/ml FCS. All procedures were performed at Biosafety Level 2, which has been approved by the Institutional Biological and Chemical Safety Committee at Washington University in St. Louis.
Co-culture with SGNs
E16 C57BL/6J mice were decapitated after being cooled down on ice, and both inner ears were removed from the base of the cranium. Cochleae were extracted from the outer bony labyrinth. The outer ligament/stria vascularis and organ of Corti were dissected away from the central core of the cochlea that contains the spiral ganglion. The ganglion neurons were mechanically dissociated and seeded into the dishes cultured with the modified adult adipose stem cells.
Immunocytochemistry
For immunofluorescence staining, cells were cultured on cover slips and fixed for 15 minutes in 4% paraformaldehyde. Inhibition of non-specific binding and permeabilization steps were performed by incubating the cover slips for one hour in PBS+Block (1X PBS, 5% serum, and 0.1% Triton X-100). Primary and secondary antibodies (fluorescein- or cyanine 3-conjugated, Jackson Immunoresearch Laboratory, West Grove, PA) were incubated overnight and for two hours, respectively, in PBS+Block. Nuclei were stained with a 1 μg/ml solution of Hoechst 33242 (Sigma, MO). Cover slips were mounted with the anti-fading agent (Vectashield, Vector Laboratories, USA). Cells were visualized using a Zeiss fluorescence microscope or a Leica TCS 4D confocal laser scanning microscope equipped with a krypton/argon mixed gas laser.
Reverse Transcriptase-Polymerase Chain Reaction Assay
Total RNA was extracted using the SV Total RNA Isolation kit (Promega, Madison, WI). For reverse transcription, we used the RETROscript kit (Ambion, Austin, TX). RT-PCR was performed on a PCR machine (Eppendorf, Germany). The exact sequence of each primer is listed in the supplementary information.
RESULTS
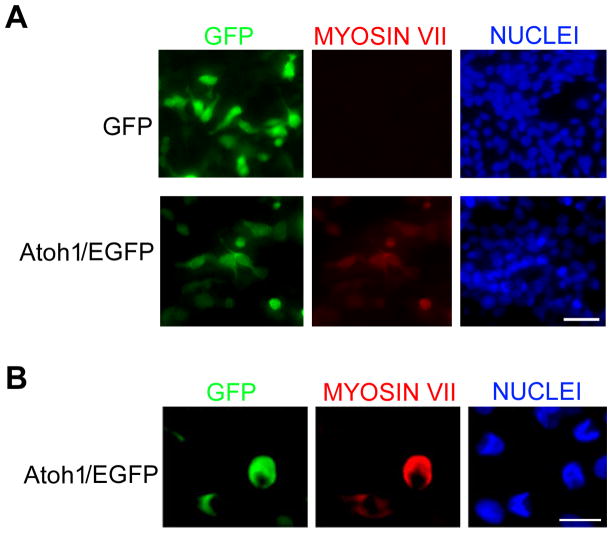
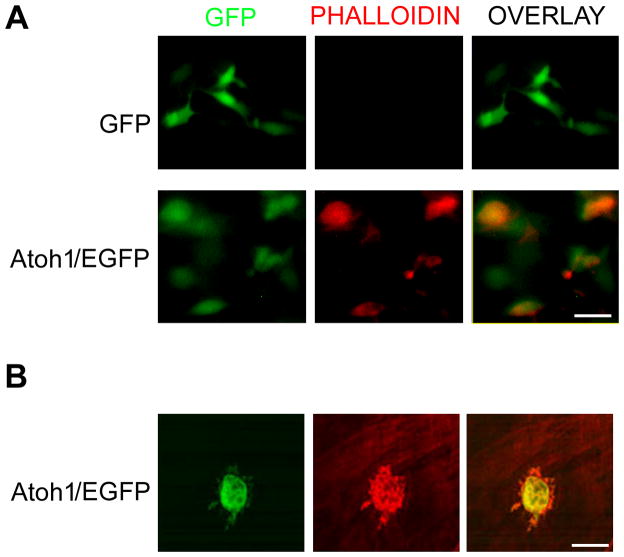
To test whether adult MSCs could be reprogrammed into cells similar to sensory hair cells, we transfected primary MSC cultures with an adenovirus expressing Atoh1 fused to the amino-terminus of enhanced green fluorescent protein (EGFP). Two days after Atoh1 overexpression, we used the Stereo Investigator software (MicroBrightField, Inc.) to examine five random fields per dish. All EGFP-positive cells expressed Myosin VII (seven replicate experiments), a hair cell marker (Fig. 1) (11). In addition, using phalloidin staining, we showed that cellular actin was reorganized after Atoh1 overexpression (Fig. 2A); this may be the cause of protrusions seen at the cell surface revealed by confocal imaging (Fig. 2B). Approximately 82.5% (SD+/−4.52, four replicate experiments) of Atoh1 overexpressing cells, but fewer control cells (0.3%, SD+/−0.02; six replicate experiments), had surface protrusions.
Figure 1.
Induction of hair cell specific markers in primary adult mouse adipose-derived stem cells overexpressing Atoh1. A, Immunofluorescent imaging of MSCs infected adenovirus expressing EGFP only or Atoh1/EGFP. Scale bar is 50 μm. B, Confocal immunofluorescent imaging of MSCs infected adenovirus expressing Atoh1/EGFP. Scale bar is 10 μm.
Figure 2.
Actin reorganization in primary adult mouse adipose-derived stem cells overexpressing Atoh1. A, Immunofluorescent imaging of MSCs infected adenovirus expressing EGFP only or Atoh1-EGFP. Scale bar is 50 μm. B, Confocal immunofluorescent imaging of MSCs infected adenovirus expressing Atoh1-EGFP. Scale bar is 10 μm.
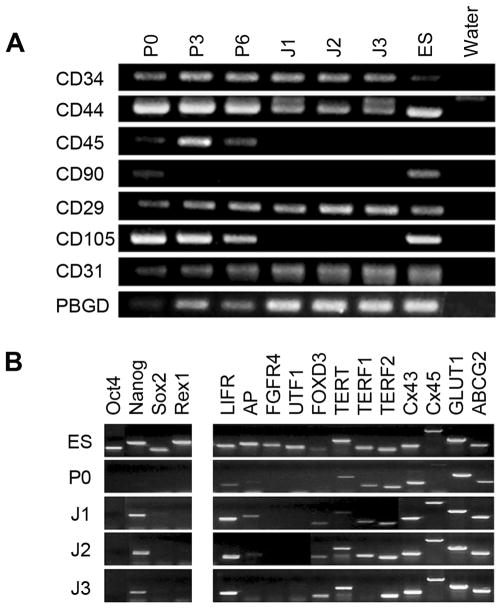
Consistent with previous studies showing a heterogeneous mixture of cell types in primary MSC cultures (25), we observed various cellular morphologies in our primary cultures (Fig. 3A). We posited that the incomplete transdifferentiation could be at least partially due to interactions among a heterogeneous cell population in this culture. To overcome this problem, we established clonal cell lines from our primary cultures. Following two passages of the primary cultures, single cell-derived clones were derived by serial dilutions in 96-well plates. Three healthy single clones were selected and expanded for characterization and in vitro differentiation. All three single cell-derived clones showed homogenous fibroblast-like morphological characteristics, and they all had similar growth curves (Fig. 3B, 3C). To further examine these clonal cell lines, we used RT-PCR to analyze the expression of surface markers in each line (Fig. 4A). These cell lines expressed three molecular markers of adipose-derived stem cells, CD34, CD44, and CD29 (Itgb1), but lacked two other markers, CD90 (Thy1) and CD105 (Eng). The absence of CD45 (Ptprc), which was present in the primary (P0), the third (P3), and the sixth (P6) passages of the original adipose cell cultures, indicates that these cell lines were not derived from hematopoietic cells. Interestingly, these cell lines also expressed CD31 (Pecam1), a molecular marker specific to vascular endothelial cells. Together, these data strongly suggested that these cells were of endothelial origin, possibly from blood vessels in the adipose tissue.
Figure 3.
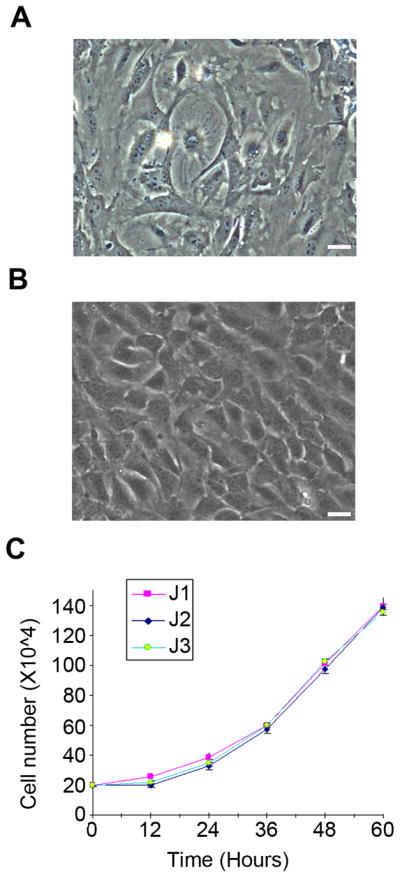
Morphology and growth kinetics of adult adipose-derived cells. A, Phase-contrast image showing heterogeneous morphologies of primary adult adipose-derived cells. B, Phase-contrast image of one single-cell derived cell line, J3, from mouse adipose tissue. Scale bars are 10 μm. C, Growth kinetics of clonal cell lines derived from adult mouse adipose tissue (J1, J2, and J3).
Figure 4.
Molecular characterization of three single cell-derived cell lines. A, RT-PCR analysis of surface markers in primary adult adipose-derived omental cells (ASOC) at passages 0 (P0), 3 (P3), and 6 (P6), clonally derived J1, J2, and J3 cells, and ES cells. B, RT-PCR analysis of stem cell markers (left panel) and undifferentiated cell markers (right panel) in ES, AOSC, J1, J2, and J3 cells.
We used RT-PCR to detect a panel of known stem cell molecular markers (Oct-4, Nanog, Sox-2, and Rex-1) and compare these cell lines to different passages of primary cultures and ES cells (Fig. 4B, left panel). Nanog was the only marker expressed in these cell lines. Next, we tested the cell lines by assaying expression of a panel of 12 molecular makers of undifferentiated cells. A majority of these markers were present in these three cell lines (9 out of 12 markers). One line (J1) expressed 10 of 12 markers (Fig. 4B, right panel). Another line (J3) did not express Terf1, an important marker for pluripotent cells. Clearly, all three cell lines had certain molecular markers for stem cells but were not the same as ES cells. Interestingly, the molecular characterization revealed similar expression patterns for the three lines.
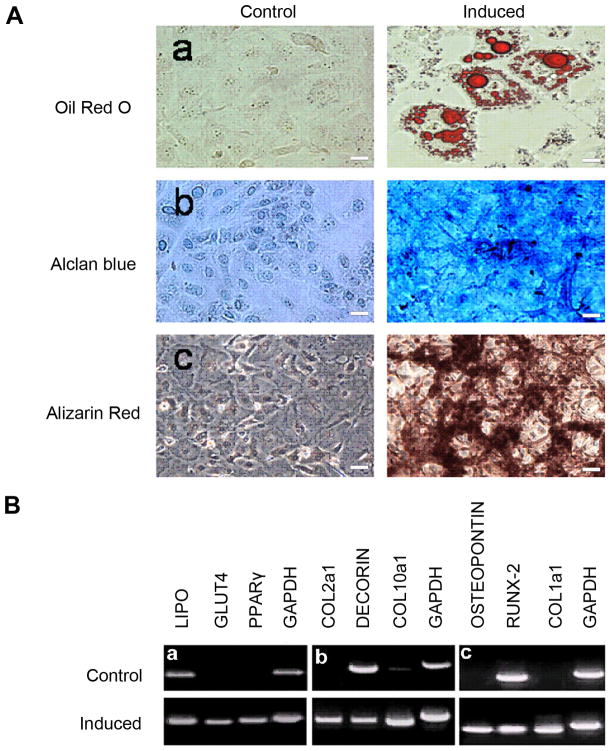
To address the critical issue of whether these cells were adult stem cells, we tested their ability to differentiate into various mesoderm derived cell types. We passed each line for more than 50 generations and treated them with three different induction media. Because results were similar among all three lines, only the data from the J1 line is presented here with three replicated experiments for each differentiation condition (Fig. 5). After two weeks of culture in adipogenic medium, a small percentage of cells (2.67%, SD+/−1.62, three replicate experiments) were positive for Oil Red O staining, an established lipid dye for adipose cells. Following four weeks of culture in chondrogenic medium most cells were positive for Alcian Blue, indicating the presence of sulfated proteoglycans characteristic of chondrogenesis. After four weeks of culture in osteogenic medium, many cells were labeled with Alizarin Red, indicating high extracellular calcium accumulation, which is typical of osteogenic differentiation (Fig. 5A). Because of their diffuse staining patterns, no counting of positive cells was obtained for both chondrogenesis and osteogenic differentiation of MSCs. However, these histological changes were well-correlated with molecular characterizations of the treated cells. Specific molecular markers for each differentiation lineage were appropriately induced (Fig. 5B). In short, the data showed that each of three adult mesoderm stem cell lines could differentiate into three different mesoderm-derived cell types. Thus, all three clonal cell lines were MSCs.
Figure 5.
Pluripotency of single cell-derived MSC line J1. A, induction of adipogenesis (a), chondrogenesis (b), and osteogenesis (c) in J1 cells. B, RT-PCR analysis of molecular markers for adipogenesis (a), chondrogenesis (b), and osteogenesis (c) in J1 cells. Scale bars are 10 μm.
In order to reprogram MSCs into hair cells, we reasoned that Atoh1 should activate molecular cascades important for hair cell differentiation. Focusing on one line (J3) for subsequent studies, we first determined the expression patterns of key hair cell differentiation markers in MSCs three (D3) and six days (D6) after Atoh1 overexpression. We found that Atoh1 overexpression induced key markers Bmp7, Pax2, Jagged1, p27Kip, Espin and Myosin VIIa (Fig. 6). Pax2 and BMP7 are early otic vesicle markers, Jagged-1 is a mature sensory epithelia marker, p27Kip is a marker of developing sensory epithelia and supporting cells, and Myosin VIIa and Espin are mature hair cell markers. These results showed that Atoh1 activated molecular cascades for hair cell differentiation and that the expression of Terf1 was not required for this transdetermination. However, as with earlier experiments, functional calcium imaging of these modified cells failed to detect any response to sound-induced mechanical stimulations (data not shown). Thus, Atoh1 overexpression was insufficient to completely convert MSCs into hair cell-like cells.
Figure 6.
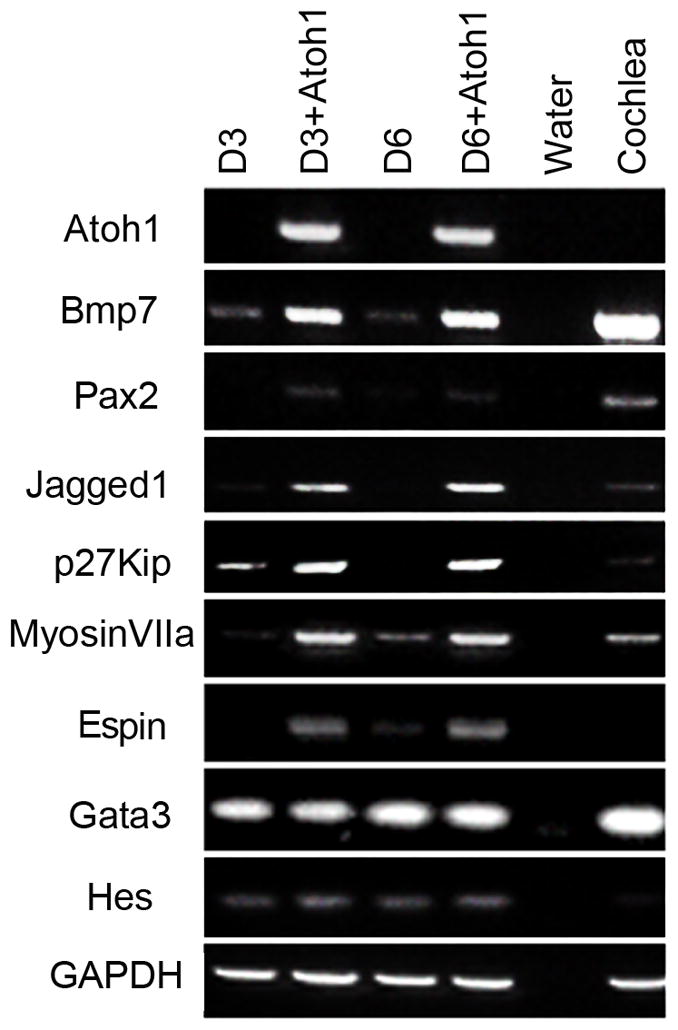
Characterization of MSCs (J3) overexpressing Atoh1. RT-PCR analysis of cochlear markers in J3 cells at day 3 (D3) and day 6 (D6) with and without Atoh1 overexpression. Negative (water) and positive (4 month old cochleae) detection controls are included.
Having repeatedly failed to convert MSCs to hair cell-like cells by Atoh-1 overexpression, we realized that reprogramming of MSCs into hair cells might depend on specific cell context because previous studies have clearly shown that Atoh1 expression is critical for the differentiation of multiple neuronal types, not just hair cells (27–29). However, molecular regulatory mechanisms underlying this specific cell context for hair cell are still unknown. Our previous work showed that VOT-E36 cells, a stable line of otic epithelial cells derived from the ventral otocyst, are able to sense sound-induced mechanical stimulation after Atoh1 overexpression and SGN co-culture (18).
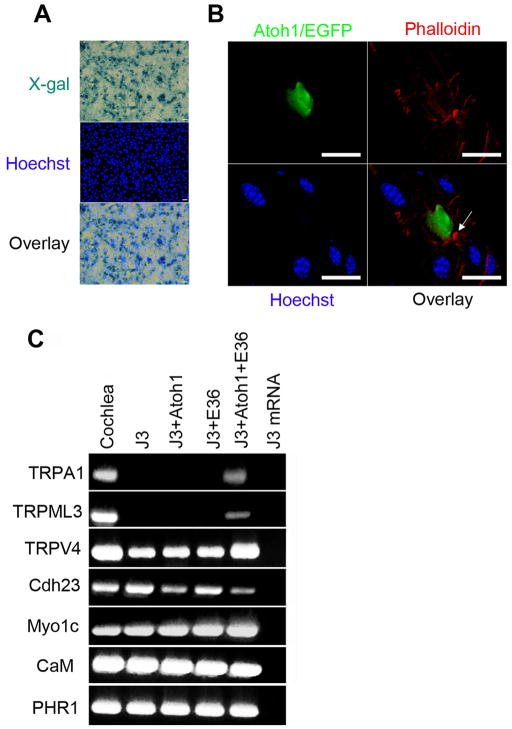
Building on earlier work, which showed that cell fate can be altered by direct transfer of total cellular protein (30–33), we tested whether a direct transfer of total proteins from VOT-E36 cells could modify the cell context of these MSCs and allow Atoh1 overexpression to convert them into hair cell-like cells. We first tested the Streptolysin O mediated protein transfection method with β-galactosidase to determine the efficiency of this procedure (Fig. 7A). Using the same systematic random sampling method (Stereo Investigator Software, MicroBrightField, Inc.), we compared the ratio of cells positive for X-gal staining relative to cells positive for Hoechst (a total cell number under the same visual field). We found that the protein transfer efficiency was consistently over 50%, although detailed statistical analysis was not performed.
Figure 7.
Characterization of single-cell derived MSCs (J3) after protein transfer from VOT-E36 cells and overexpression of Atoh1. A, Staining for X-gal and Hoechst showing the β-galactosidase protein transfer to MSCs (J3). B, Confocal imaging of one MSC overexpressing Atoh1 after VOT-E36 protein transfers (once per day for two days). The cultures were stained with Phalloidin (red) and Hoechst (blue). The arrow indicates a hair bundle-like structure. Scale bars are 10 μm. C, RT-PCR analysis of mature hair cell markers in cochlea, MSCs (J3), MSCs overexpressing Atoh1 (J3+Atoh1), MSCs with VOT-E36 protein transfer (J3+E36), MSCs with VOT-E36 protein transfer and Atoh1 overexpression (J3+Atoh1+E36), and no RT control
After establishing the method, we transferred total protein from VOT-E36 cells to MSCs and subsequently infected these cells with the adenovirus expressing Atoh1. Following these treatments, the modified cells showed no hair bundles and were still unable to respond to sound-induced mechanical stimulation (data not shown). Next, we increased the protein transfer time to two days (one transfer per day) before Atoh1 adenoviral infection. After this change, phalloidin staining showed focal accumulations of F-actin and protrusions similar to hair cell bundles (Fig. 7B).
We compared the expression pattern of hair cell markers in MSCs under four conditions: (1) no treatments, (2) Atoh1 overexpression, (3) VOT-E36 protein transfers (twice), and (4) a combination of (2) and (3). We found TRPV4, Cdh23, Myo1C, CaM and PHR1 expression in all four groups in three independent experiments. TRPA1 and TRPML3 were only induced in group 4 (Fig. 7C). Two important caveats were noted for this finding. First, recent studies have excluded these three TRP channels as possible mechanotransduction channels; nevertheless, all of them are present in hair cells and play important roles for hair cell functions (for recent reviews, 34,35). Second, this experiment lacked a proper negative control because our attempts to use boiled protein lysate induced cell death, although previous studies showed no contaminating DNA and RNA in protein lysates isolated using the same method (30–33).
Next, we examined possible interactions between modified MSCs and co-cultured SGNs. By using calretinin to label SGNs in the co-culture, we discovered that some modified MSCs had obvious contacts with SGNs, although the number was relatively low (0.99%, SD+/−0.21, three replicate experiments). A representative example is shown in Figure 8. A single terminal-like structure was present on this transfected cell. Importantly, the confocal image was focused on the surface of a transfected cell overexpressing ATOH1 and EGFP; thus, its nucleus was not visible at this level, but was confirmed in another focal plane (data not shown).
Figure 8.
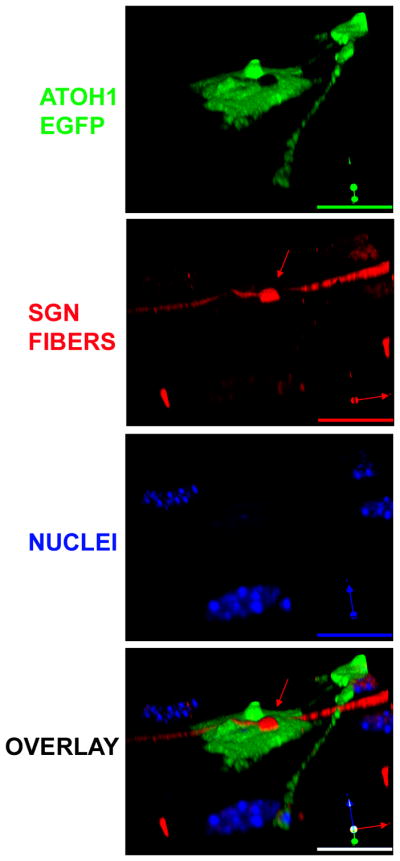
Physical contacts between SGN and modified adult stem cells. Confocal images of immunofluorescent staining for SGN terminals (calretinin, red), nuclei (Hoechst, blue), and Atoh1 overexpression (green). The red arrows point to the terminal-like SGN contact. Next to the contact, there was a hair-bundle like structure. Scale bars are 10 μm.
DISCUSSION
To reprogram MSCs into hair cell-like cells, we established a new procedure, consisting of VOT-E36 protein transfers, Atoh1 overexpression, and SGN co-culture. Modified cells express genes present in hair cells, show actin reorganization with structures similar to hair bundles, and are contacted by SGNs. Thus, our data provide convincing evidence for the transdetermination of single-cell derived MSCs into hair cell-like cells.
An ideal source of autologous stem cells is adipose tissue, which, similar to bone marrow, is rich in MSCs (22). The clonally derived MSC lines that we developed from adipose tissue have similar morphologies and rates of self-renewal, and are equivalently pluripotent. However, they are distinct from adipose omental stem cells (AOSCs). In AOSCs, the surface markers CD29, CD34, CD44, CD90, and CD105 are expressed, while the endothelial marker CD31 and the hematopoietic marker CD45 are not expressed (36–38). In our cell lines, CD29, CD34, and CD44 are expressed, but the other two markers, CD90 and CD105, are not. In addition, CD31 is expressed in our cell lines, but CD45 is absent. The co-expression of CD31 and CD34 is used to identify endothelial progenitor cells (22, 38–41). Overall, this expression pattern of surface markers suggests that our cell lines are most similar to MSCs and are close to the lineage of adult endothelial cells. This leads us to believe that these single-cell derived cell lines may have made an incomplete endothelial-to-mesenchymal transition (42).
In normal development, MSCs are derived from mesoderm and hair cells are derived from ectoderm. Thus, direct reprogramming between divergent lineages might not be possible. For example, careful experiments clearly demonstrated that early claims of direct “transdifferentiation” of blood cells into neurons, cardiomyocytes, and liver cells, or muscle and neural cells into blood cells can be mostly attributed to cell fusion instead of cell conversion (43). However, transcription factor-induced reprogramming of differentiated cells into other specialized or pluripotent cells has been demonstrated repeatedly (for review, 44, 45).
The reprogramming process can be further divided into pluripotent reprogramming (converting specialized cells into pluripotent stem cells) and lineage reprogramming (directly conversion between different cell lineages). Lineage reprogramming can be further divided into transdifferentiation (direct conversion of mature cells between different lineage) and transdetermination (conversion of progenitor cells between different lineages) (44, 45). In this study, we used transdetermination to convert MSC into hair cell-like cells by Atoh1 overexpression.
Atoh1 overexpression in MSCs activates molecular cascades necessary for hair cell differentiation and induces molecular markers of sensory epithelial cells, but it cannot completely convert MSCs into hair cells. The combination of total protein extract transfer from VOT-E36 otic epithelial cells and Atoh1 overexpression represents a novel method to convert MSCs into hair cell-like cells. Because the protein extract transfer itself does not induce the unique molecular markers we examined (Fig. 7C) and our boiled protein control did not work, at present, we do not know what factors in the protein extract contributed to this conversion. In addition, factors provided by SGN co-culture were required to convert MSCs into hair cell-like cells. Future studies should focus on parsing out the specific factors that lead to the induction of hair cell-like cells. In addition, the current low transdetermination rate, compounded with variability in SGN co-cultures, makes it difficult to perform detailed functional characterization of the modified cells. Patch clamp studies of mechanotransduction channels for this study were unsuccessful, primarily due to the scarcity of cells demonstrating a functional response. These data suggest that it may be beneficial to derive hair cells by both lineage and pluripotent reprogramming. Because we have recently identified a two-step induction process for iPS cells (46), we will explore whether hair cell-like cells can be more efficiently derived from partially reprogrammed iPS cells.
In short, this study demonstrates the feasibility of reprogramming adult MSCs into hair cell-like cells using a new method. Because adult adipose tissue is easy to obtain and MSCs from adipose are readily isolated, our findings identify another potential source from which specific sensory neurons, such as hair cells, may be derived. Transdetermination by total protein transfer in combination with a lineage specific transcription factor expression also represents a novel intrinsic method for deriving specific cell types that could prove useful for future applications of stem cell therapies for age-related degenerative diseases, such as presbycusis.
Supplementary Material
Acknowledgments
We thank Kyunghee Choi, Albert S.B. Edge, and Mark Warchol for helpful discussions. We are also grateful for funding from the Fay and Carl Simons Center for the Biology of Hearing and Deafness.
Footnotes
Potential conflict of interest
None of the authors have actual or potential conflicts of interest.
References
- 1.Cohen MMJ, Gorlin RJ, editors. Epidemiology, etiology and genetic patterns. New York: Oxford University Press; 1995. Hereditary Hearing Loss and its Syndromes; pp. 9–21. [Google Scholar]
- 2.Vital and Health Statistics (Series 10) Vol. 209. Hyattsville, MD: National Center for Health Statistics; 2002. Summary of Health Statistics for US adults: National Health Interview Survey, 1998. [PubMed] [Google Scholar]
- 3.Davis AC. Hearing disorders in the population: first phase findings of the MRC National Study of Hearing. In: Lutman M, Haggard M, editors. Hearing Science and Hearing Disorders. Vol. 35 New York: Academic Press; 1993. [Google Scholar]
- 4.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 5.Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 6.White PM, Doetzelhofer A, Lee YS, et al. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 7.Forge A, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- 8.Warchol ME, Lambert PR, Goldstein BJ, et al. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Roblin G, Liu H, et al. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oshima K, Shin K, Diensthuber M, et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Johnson SL, Marcotti W, et al. Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cells. 2009;27:1196–1204. doi: 10.1002/stem.62. [DOI] [PubMed] [Google Scholar]
- 13.Bermingham NA, Hassan BA, Price SD, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 14.Zine A, Aubert A, Qiu J, et al. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen P, Johnson J, Zoghbi H, et al. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- 16.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 17.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 18.Liu JJ, Shin JH, Hyrc KL, et al. Stem cell therapy for hearing loss: Math1 overexpression in VOT-E36 cells. Otol Neurotol. 2006;27:414–421. doi: 10.1097/00129492-200604000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto K, Ishimoto SI, Minoda R, et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubbels SP, Woessner DW, Mitchell JC, et al. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacic JC, Boehm M. Resident vascular progenitor cells: an emerging role for non-terminally differentiated vessel-resident cells in vascular biology. Stem Cell Res. 2009;2:2–15. doi: 10.1016/j.scr.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CS, Xin ZC, Deng CH, et al. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 23.Jeon SJ, Oshima K, Heller S, et al. Bone marrow mesenchymal stem cells are progenitors in vitro for inner ear hair cells. Mol Cell Neurosci. 2007;34:59–68. doi: 10.1016/j.mcn.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettersson P, Cigolini M, Sjöström L, et al. Cells in human adipose tissue developing into adipocytes. Acta Med Scan. 1984;215:447–451. doi: 10.1111/j.0954-6820.1984.tb17677.x. [DOI] [PubMed] [Google Scholar]
- 26.Helyer R, Cacciabue-Rivolta D, Davies D, et al. A model for mammalian cochlear hair cell differentiation in vitro: effects of retinoic acid on cytoskeletal proteins and potassium conductances. Eur J Neurosci. 2007;25:957–973. doi: 10.1111/j.1460-9568.2007.05338.x. [DOI] [PubMed] [Google Scholar]
- 27.Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371–379. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 28.Safford KM, Rice HE. Stem cell therapy for neurologic disorders: therapeutic potential of adipose-derived stem cells. Curr Drug Targets. 2005;6:57–62. doi: 10.2174/1389450053345028. [DOI] [PubMed] [Google Scholar]
- 29.Safford KM, Safford SD, Gimble JM, et al. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319–328. doi: 10.1016/j.expneurol.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Hakelien AM, Landsverk HB, Robl JM, et al. Reprogramming fibroblasts to express T-cell functions using cell extracts. Nat Biotechnol. 2002;20:460–466. doi: 10.1038/nbt0502-460. [DOI] [PubMed] [Google Scholar]
- 31.Hakelien AM, Gaustad KG, Collas P. Transient alteration of cell fate using a nuclear and cytoplasmic extract of an insulinoma cell line. Biochem Biophys Res Commun. 2004;316:834–841. doi: 10.1016/j.bbrc.2004.02.127. [DOI] [PubMed] [Google Scholar]
- 32.Hakelien AM, Gaustad KG, Collas P. Modulation of cell fate using nuclear and cytoplasmic extracts. Methods Mol Biol. 2006;325:99–114. doi: 10.1385/1-59745-005-7:99. [DOI] [PubMed] [Google Scholar]
- 33.Qin M, Tai G, Collas P, et al. Cell extract-derived differentiation of embryonic stem cells. Stem Cells. 2005;23:712–718. doi: 10.1634/stemcells.2004-0195. [DOI] [PubMed] [Google Scholar]
- 34.Cuajungco MP, Grimm C, Heller TRP channels as candidates for hearing and balance abnormalities in vertebrates. Biochim Biophys Acta. 2007;1772:1022–1027. doi: 10.1016/j.bbadis.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fettiplace R. Defining features of the hair cell mechanoelectrical transducer channel. Pflugers Arch. 2009;458:1115–1123. doi: 10.1007/s00424-009-0683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser GJ, Berkovitz BK, Graham A, et al. Gene deployment for tooth replacement in the rainbow trout (Oncorhynchus mykiss): a developmental model for evolution of the osteichthyan dentition. Evol Dev. 2006;8:446–457. doi: 10.1111/j.1525-142X.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- 37.Hwangbo S, Kim J, Her S, et al. Therapeutic potential of human adipose stem cells in a rat myocardial infarction model. Yonsei Med J. 2010;51:69–76. doi: 10.3349/ymj.2010.51.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto N, Akamatsu H, Hasegawa S, et al. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Yoder MC, Ingram DA. Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol. 2009;16:269–273. doi: 10.1097/MOH.0b013e32832bbcab. [DOI] [PubMed] [Google Scholar]
- 40.Yoder MC. Defining human endothelial progenitor cells. J Thromb Haemost. 2009;7(suppl 1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin G, Garcia M, Ning H, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arciniegas E, Frid MG, Douglas IS, et al. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 43.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell Stem Cell. 2008;3:382–388. doi: 10.1016/j.stem.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9:504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Lin Z, Perez P, Lei D, Xu J, Gao X, Bao J. Two-phase analysis of molecular pathways underlying induced pluripotent stem cell induction. Stem Cells. 2011:1963–74. doi: 10.1002/stem752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.