Abstract
Background
Osteogenesis Imperfecta (OI) is a genetic disorder of connective tissue matrix. OI is caused by mutations that affect type I collagen. The hearing loss in OI is characterized by onset in early adulthood and can be conductive, sensorineural or mixed.
Objectives
To describe the temporal bone histopathology in 9 individuals with OI.
Material and Methods
Four adult, one pediatric, and four infant specimens were identified. Temporal bones were removed at autopsy and studied by light microscopy.
Results
All adults and one pediatric specimen showed otosclerotic lesions. The findings included examples of clinical, histologic and cochlear otosclerosis. The temporal bones of infants showed delayed ossification of the endochondral layer of bone and of the ossicles. There were no infant specimens with otosclerotic lesions.
Conclusions
Hearing loss in OI may be the result of clinical or cochlear otosclerosis. Fracture or atrophy of the ossicles may also be present in OI. A third unidentified mechanism of hearing loss may lead to cochlear degeneration. The described findings of otosclerotic lesions have implications for the observed heterogeneity of hearing loss patterns and for the surgical management of hearing loss in OI.
Introduction
Osteogenesis Imperfecta (OI) is a genetic disorder of connective tissue matrix characterized by reduced bone mass and strength. Heterogeneous in presentation, the clinical hallmarks of this disorder include susceptibility to bone fracture, deformation and growth abnormalities.
OI is classified into types based on phenotype and genotype. The most commonly used clinical classification includes a mild type I, lethal type II, severe type III and moderate type IV (1). The most frequent types of OI encountered by otolaryngologists are caused by mutations in the COL1A1 or COL1A2 gene transmitted in an autosomal dominant pattern. Over 1,500 mutations leading to OI have been described. These genes encode pro alpha 1 and 2 chains of type I collagen. An autosomal recessive form of OI is caused by gene defects whose products interact with type I collagen (2).
Up to 50% of OI patients present with hearing loss. The hearing loss often begins between the second and fourth decade of life and can be conductive, sensorineural or mixed (3,4,5,6). Clinical and histological data show that individuals with OI can develop otosclerotic like lesions in the temporal bone and otosclerotic lesions are associated with conductive, sensorineural, and mixed hearing loss. The association of otosclerosis with OI suggests that quantitative and/or qualitative defects in type I collagen may lead to abnormal remodeling in the temporal bone.
Histological findings of temporal bones with osteogenesis imperfecta have been described previously (7, 8, 9, 10, 11). The findings include delayed patterns of ossification in type II OI (infant – lethal form) and otosclerotic lesions in non-type II OI. To date, there have been no reports of type II OI with otosclerotic lesions and no reports of non type II OI without such lesions. We examined all available temporal bones within the National Temporal Bone Registry in patients with OI to determine the histopathologic features present and to correlate the results with available clinical history. We hypothesized that all infants who died of OI (putative type II cases) will display findings of delayed endochondral ossification and adults who survived infancy (putative non-type II cases) will display signs of otosclerosis. Otosclerotic lesions may account for the clinical heterogeneity of hearing loss patterns observed within the same OI genotypes.
Materials and Methods
Nine available cases of OI were identified through the National Temporal Bone Registry database. Clinical histories and audiometric information was examined when available. The temporal bones of four adults with a clinical diagnosis of type I, one pediatric case with type III, and four infants with type II.
The temporal bones were removed at autopsy and processed for light microscopy. They were decalcified in EDTA and processed for celloidin embedding. Serial sectioning of the axial or coronal plane was performed at a width of 20 um and every tenth section was stained with hematoxylin and eosin. All available sections were examined by light microscopy. All temporal bones were compared with normal age-matched controls.
Results
Osteogenesis Imperfecta type I
Case 1
History
This is an adult patient with the diagnosis of osteogenesis imperfecta type I, there was no additional clinical history.
Histopathology
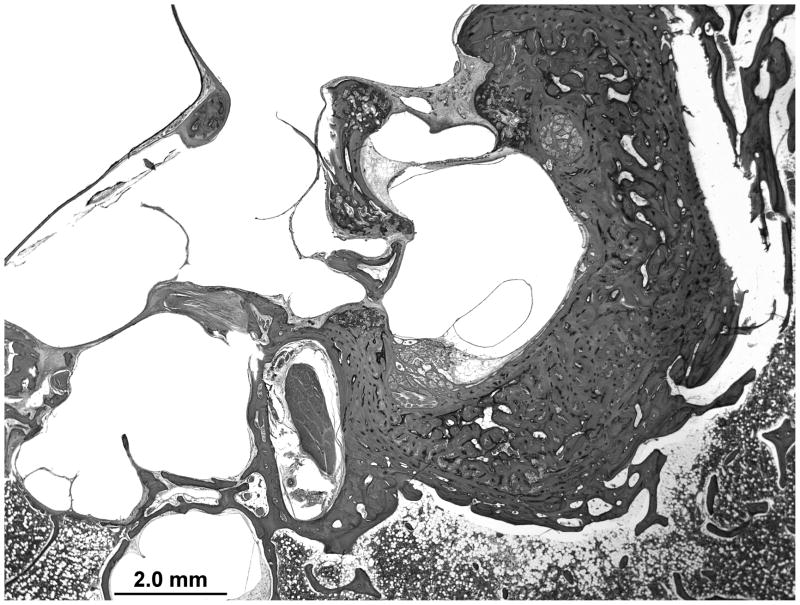
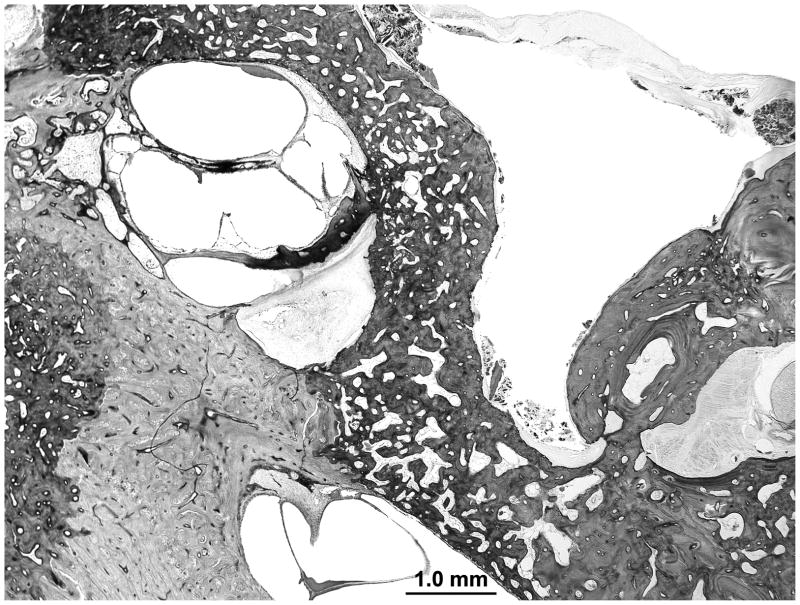
The cytoarchitecture of the cochlea is normal. There is an otosclerotic focus at the oval window and a second focus at the round window niche. There is no fixation of the stapes footplate or obliteration of the round window membrane. A third otosclerotic focus surrounds the internal auditory canal. The contralateral temporal bone has a single otosclerotic focus surrounding the internal auditory canal with an area of cavitation (Figure 1).
Figure 1.
A midmodiolar section of the left temporal bone. An otosclerotic lesion is seen on both sides of the internal auditory canal. Inferiorly the lesion shows an area of cavitation.
Case 2
History
This is an adult patient with the diagnosis of osteogenesis imperfecta type I, there was no additional clinical history
Histopathology
The cytoarchitecture of the cochlea is normal. There is a focus of otosclerosis filling the oval window. The crura of the stapes are not connected to the footplate (Figure 2). A thin membrane surrounds the distal aspect of the crura suggesting that the separation from the foot plate was pre-mortem and not an artifact of specimen retrieval.
Figure 2.
The right temporal bone shows an otosclerotic lesion obliterating the oval window. The crura of the stapes are separated from the footplate. There is a mucous membrane surrounding the distal aspect of the crura indicating that this is not an artifact of specimen retrieval.
Case 3
History
The patient had a history of multiple fractures including hip, ankles, shoulders and ribs. She had blue sclera. She had a 25 year history of progressive sensorineural hearing loss. An audiogram 2 years prior to death shows a symmetric sensorineural hearing loss with pure tone thresholds between 70 and 90dB. She died at the age of 88 of myocardial infarction.
Histopathology
There are degenerative changes involving the organ of Corti and spiral ganglion cells. There is atrophy of the stria vascularis. There is a focus of otosclerosis in both ears anterior to the oval window that does not fix the footplate (Figure 3). The ossicles are normal in appearance.
Figure 3.
The right temporal bone with an otosclerotic lesion anterior to the oval window. There is no fixation of the stapes.
Case 4
History
This patient has been previously reported (8). She had blue sclera and a history of multiple non-deforming fractures. She had an asymmetric mixed hearing loss and ultimately underwent stapedotomy in both ears that was followed by a progressive profound sensorineural hearing loss. She died from a cerebral vascular accident.
Histopathology
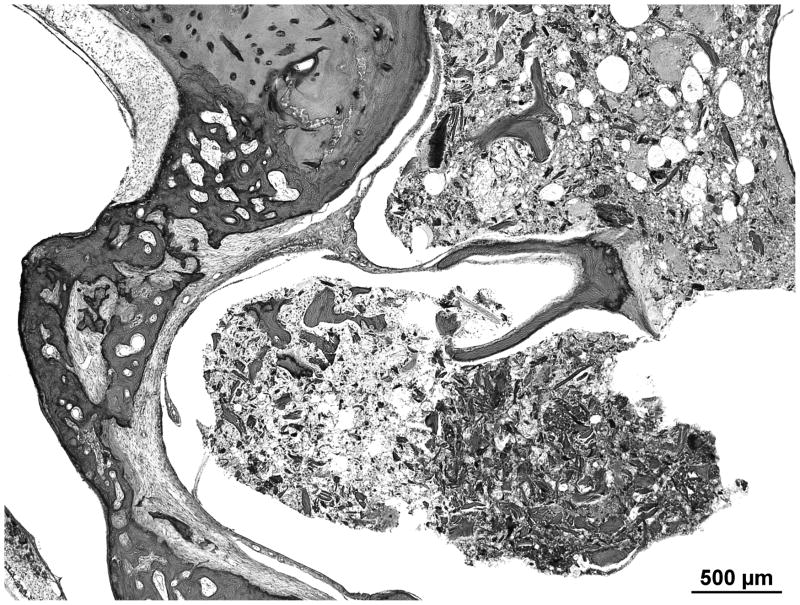
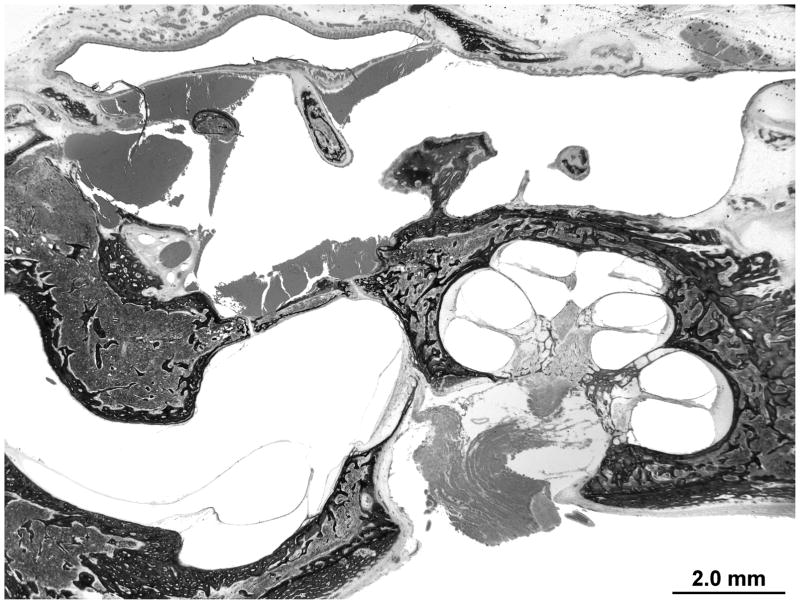
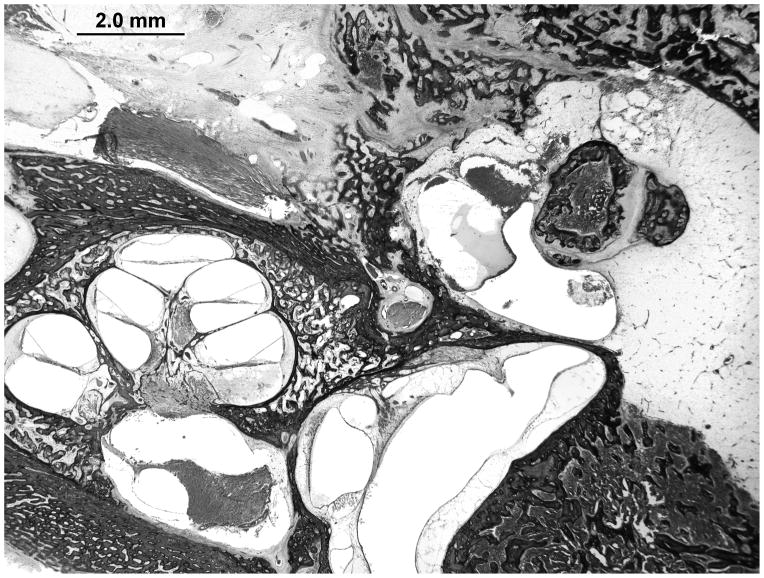
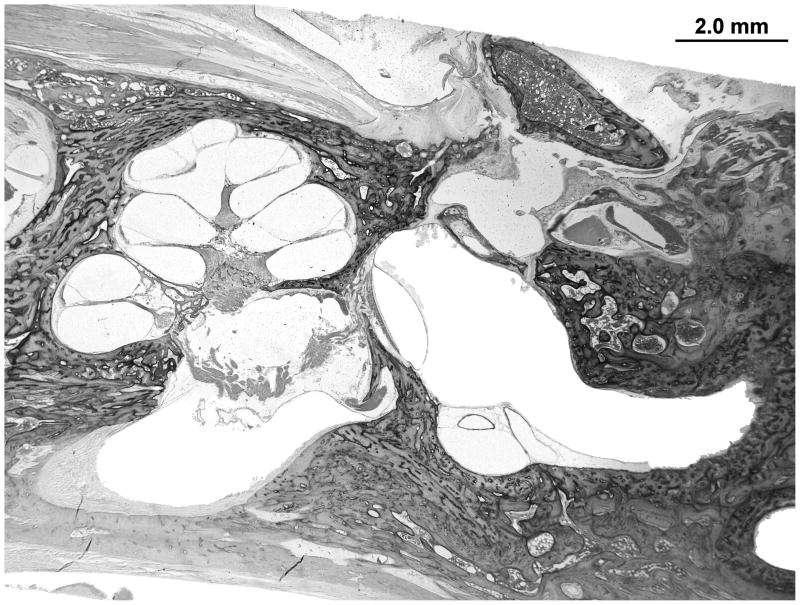
There is advanced postmortem autolysis. There is evidence of a prior stapedotomy with otosclerosis fixating the footplate. Otosclerotic changes surround the entire cochlea. The round window is obliterated with new bone formation in the scala tympani (Figures 4 and 5). There is hyalinization of the stria vascularis. There is cavitation of bone medial to the basal turn of the cochlea. There is too much autolysis to make a determination of hydrops. The endolymphatic duct is patent but is surrounded by otosclerotic bone.
Figure 4.
The right temporal bone with otosclerosis surrounding the cochlea. There is significant post mortem autolysis. Hyalinization of the spiral ligament is evident in the mid turn of the cochlea. There is cavitation of the otosclerosis inferior to the basal turn of the cochlea, a potential source for electrode migration during cochlear implantation. The silhouette of a stapes prosthesis is seen traversing a fixed footplate.
Figure 5.
A magnified view of the the ipsilateral round window niche shows complete obliteration and new bone formation within the scala tympani.
Osteogenesis Imperfecta Type III
Case 5
History
This 9 year old patient with type III OI had blue sclera and a history of multiple fractures with shortened and deformed extremities. He had a barrel shaped chest and died of acute necrotizing pneumonia. There was no documented history of hearing loss.
Histopathology
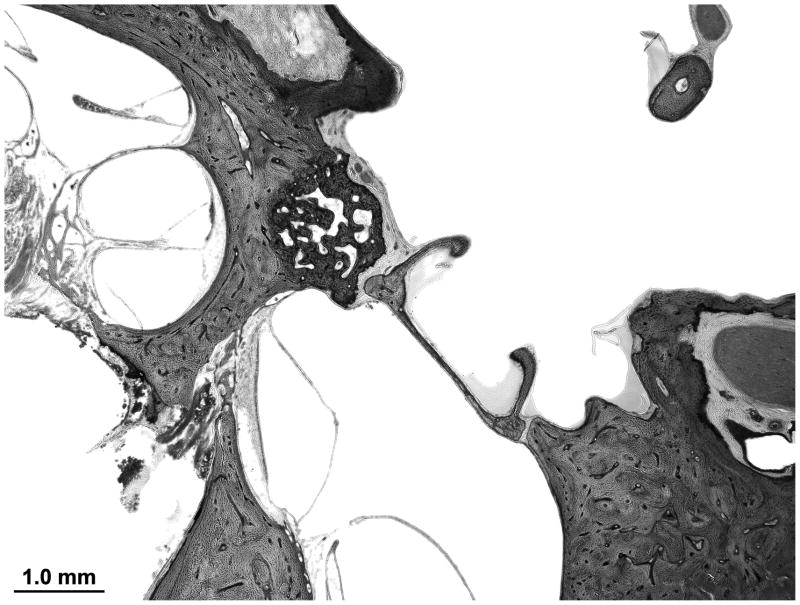
The organ of corti, utricular and saccular macula and crista appear normal. The ossicles are intact. There is a non-obliterating focus of otosclerosis at the round window niche (Figure 6).
Figure 6.
The left ear demonstrates a non-obliterating focus of otosclerosis at the round window niche. The tympanic facial nerve is dehiscent.
Osteogenesis Imperfecta type II
Case 6
History
This infant was born term with multiple fractures. He died postnatal day 6.
Histopathology
Hair cells, spiral ganglion cells and the stria vascularis appear normal. The ossicles show an increased amount of marrow compared to age matched controls. The stapes footplate is split in 2 layers by bone marrow. There is marked thinning of the crura of the stapes.
Case 7
History
This infant died 1 hour and 19 minutes after birth from respiratory insufficiency. Autopsy revealed foreshortened limbs, a soft cranial vault, blue sclera and hypoplastic thorax with pulmonary hypoplasia. X-rays revealed multiple fractures of the ribs and extremities.
Histopathology
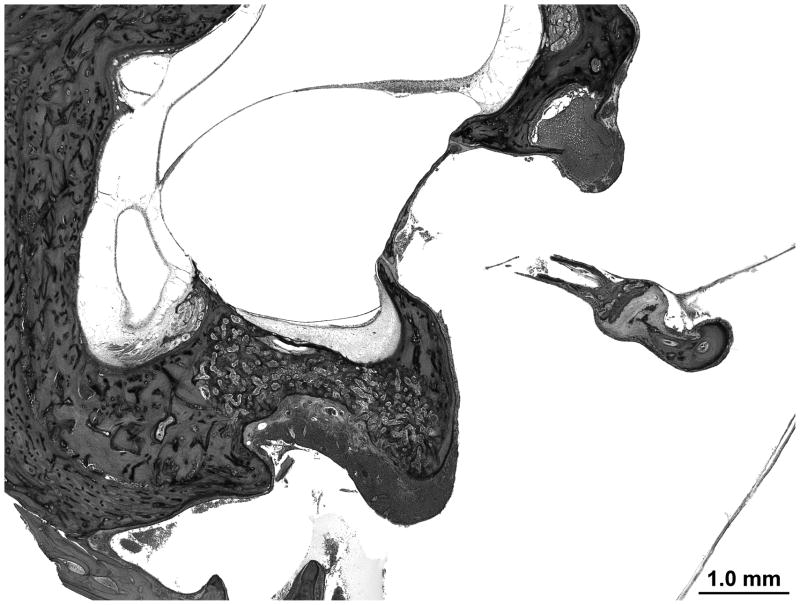
The organ of Corti, utricular macula, saccular macula and crista appear normal. The endochondral layer is less ossified than age matched controls. The ossicles are poorly ossified and have a high content of marrow including the stapes footplate which is separated into two bony layers by marrow (Figure 7).
Figure 7.
The endochondral layer of bone is not fully ossified. The stapes foot plate is split in two layers by bone marrow.
Case 8
History
This newborn died 2 1/2 hours after birth. He was diagnosed with osteogenesis imperfecta, the details of the clinical history are unavailable.
Histopathology
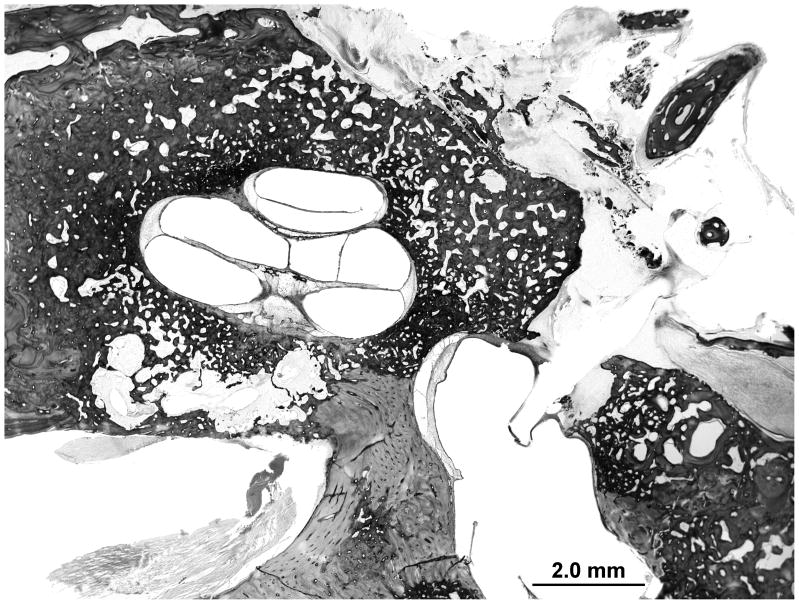
The endochondral layer is less ossified than expected for age-matched controls. A large amount of marrow is observed in the body of the incus and head of the malleus (Figure 8). The stapes footplate is split in 2 layers separated by a thin layer of marrow. The organ of Corti shows signs of postmortem autolysis with otherwise normal cytological structures.
Figure 8.
The right temporal bone shows delayed ossification of the endochondral layer. The incus and malleus have a larger than expected amount of marrow.
Case 9
History
This is a 2 year old patient with blue sclera and a diagnosis of OI with no other available clinical history.
Histopathology
The endochondral layer appears well ossified. There is a greater degree of marrow in the body of the incus and head of the malleus than was seen in controls. The stapes footplate is split in two bony layers but there is no evidence of marrow (Figure 9). The cytological structures of the cochlea are normal.
Figure 9.
The endochondral layer is ossified. The stapes foot plate remains split in two layers. The body of the incus is filled with marrow.
Discussion
Our central hypothesis that adults with type I OI display signs of otosclerosis and infants who died from type II OI display abnormalities of endochondral ossification was supported by the histologic findings among the nine cases we identified. All four adults with OI had histologic evidence of otosclerosis. This was also observed in a 9 year old child with type III OI. The four infants with type II OI had decreased endochondral ossification compared with age matched controls. We hypothesize that in adults abnormal type I collagen results in increased bony turnover in the otic capsule which ultimately results in the development of otosclerosis. Further investigation is warranted to evaluate the cause of the variability observed in the location of otosclerotic foci in OI.
The reported prevalence of hearing loss in OI approximates 50% (2). Hearing loss occurs in conductive, sensorineural, and mixed patterns. A recent clinical study of 41 adult type I OI patients with COL1A1 and COL1A2 mutations found no correlation between genotype and auditory phenotype. Moreover, there was no intra-familial correlation to the pattern of hearing loss (3). This observation has been replicated in other studies, where a genotype-auditory phenotype correlate and an association with other risk factors for hearing loss – including otitis media, skull fracture and noise exposure – were not identified (4).
In the current study, there was limited availability of clinical audiometric data. Among the four adult type I OI patients, three had clinically confirmed hearing loss or histologic evidence to suggest clinically relevant hearing loss. One patient had a documented mixed hearing loss, one patient had documented sensorineural hearing loss, and one patient did not have an available audiogram but histopathology revealed an obliterated footplate indicating at minimum a conductive hearing loss. There was no history or histopathologic correlate of hearing loss in the other patient. All adults with type I OI had histologic evidence of otosclerosis. The presentation of otosclerotic lesions included patterns of histologic otosclerosis (a lesion without fixation of the footplate or known hearing loss – cases 1and 3), clinical otosclerosis (fixation of the footplate with conductive hearing loss – case 2 and 4) and cochlear otosclerosis (sensorineural hearing loss without stapes fixation where hyalinization of the stria vascularis is thought to induce sensorineural hearing loss (12)– case 4). Separation of the stapes supra-structure from a fixed footplate (case 2) was also observed and would result in a conductive hearing loss. This may have resulted from bony fracture or atrophy.
These histopathologic findings highlight three potential etiologies of clinically relevant hearing loss in osteogenesis imperfecta. The first includes otosclerosis mediated conductive, mixed and sensorineural hearing loss. The second mechanism is fracture or atrophy of the stapes. This fracture or atrophy could be related to osteogenesis imperfecta through weakened bone and fixation of the stapes from otosclerosis or could potentially develop from abnormal bone turnover. A third mechanism, primary neural degeneration, was raised by one case (case 3). COL1A2 is expressed in moderate to high levels in membranous cochlear cells (13) the function of which remains unknown. Dismorphic collagen expression within the cochlea could be responsible for the neural degeneration noted.
The otopathology of OI has important implications for the treating otologic surgeon. Adult patients with type I OI and conductive or mixed hearing loss likely have clinical otosclerosis. For those that elect to undergo stapedotomy, the surgeon must evaluate the status of the round window because it may be obliterated due to otosclerosis in OI and round window obliteration may be responsible for failure of hearing improvement following stapedotomy. Patients with profound hearing loss may be candidates for cochlear implantation. Cavitary otosclerotic lesions are seen in OI and could represent an avenue for electrode misplacement. Pre-operative CT imaging may demonstrate these lesions and highlight patients at risk of this complication.
Conclusions
The predominant feature of the otopathology in adults with OI is lesions that are clinically indistinguishable from otosclerosis. These otosclerotic lesions may account for the variable presentation of hearing loss seen in adults with OI that can be conductive, sensorineural or mixed. The association of adult type I OI and otosclerosis has important clinical implications. The otopathology of infants with type II OI is characterized by delayed patterns of endochondral ossification. This finding has implications for the understanding of the process of normal otic capsule development and turnover.
Acknowledgments
NIDCD: IU24DC011943–01
References
- 1.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–16. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7:540–57. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartikka H, Kuurila K, Korkko J, et al. Lack of correlation between the type of COL1A1 or COL1A2 mutation and hearing loss in osteogenesis imperfecta patients. Hum Mutat. 2004;24:147–54. doi: 10.1002/humu.20071. [DOI] [PubMed] [Google Scholar]
- 4.Swinnen FK, Coucke PJ, De Paepe AM, et al. Osteogenesis Imperfecta: the audiological phenotype lacks correlation with the genotype. Orphanet J Rare Dis. 2011;6:88. doi: 10.1186/1750-1172-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuurila K, Kaitila I, Johansson R, Grenman R. Hearing loss in Finnish adults with osteogenesis imperfecta: a nationwide survey. Ann Otol Rhinol Laryngol. 2002;111:939–946. doi: 10.1177/000348940211101014. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen U. Hearing loss in patients with osteogenesis imperfecta. A clinical and audiological study of 201 patients. Scand Audiol. 1984;13:67–74. doi: 10.3109/01050398409043042. [DOI] [PubMed] [Google Scholar]
- 7.Bretlau P, Jorgensen MB. Otosclerosis and osteogenesis imperfecta. Arch Otolaryngol. 1969;90:4–10. doi: 10.1001/archotol.1969.00770030006004. [DOI] [PubMed] [Google Scholar]
- 8.Sando I, Myers D, Harada T, Hinojosa R, Myers EN. Osteogenesis imperfecta tarda and otosclerosis. A temporal bone histopathology report. Ann Otol Rhinol Laryngol. 1981 May-Jun;90(3 Pt 1):199–203. doi: 10.1177/000348948109000301. [DOI] [PubMed] [Google Scholar]
- 9.Nager GT. Osteogenesis imperfecta of the temporal bone and its relation to otosclerosis. Ann Otol Rhinol Laryngol. 1988;97:585–93. doi: 10.1177/000348948809700603. [DOI] [PubMed] [Google Scholar]
- 10.Berger G, Hawke M, Johnson A, Proops D. Histopathology of the temporal bone in osteogenesis imperfecta congenita: a report of 5 cases. Laryngoscope. 1985;95:193–9. doi: 10.1288/00005537-198502000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Zajtchuk JT, Lindsay JR. Osteogenesis imperfecta congenita and tarda: a temporal bone report. Ann Otol Rhinol Laryngol. 84:350–8. doi: 10.1177/000348947508400311. [DOI] [PubMed] [Google Scholar]
- 12.Parahy C, Linthicum FH., Jr Otosclerosis: relationship of spiral ligament hyalinization to sensorineural hearing loss. Laryngoscope. 1983;93:717–20. doi: 10.1288/00005537-198306000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Khetarpal U, Morton CC. COL1A2 and COL2A1 expression in temporal bone of lethal osteogenesis imperfecta. Arch Otolaryngol Head Neck Surg. 1993;119:1305–14. doi: 10.1001/archotol.1993.01880240041006. [DOI] [PubMed] [Google Scholar]