Abstract
The de-epoxidation of violaxanthin to antheraxanthin (Anth) and zeaxanthin (Zeax) in the xanthophyll cycle of higher plants and the generation of nonphotochemical fluorescence quenching in the antenna of photosystem II (PSII) are induced by acidification of the thylakoid lumen. Dicyclohexylcarbodiimide (DCCD) has been shown (a) to bind to lumen-exposed carboxy groups of antenna proteins and (b) to inhibit the pH-dependent fluorescence quenching. The possible influence of DCCD on the de-epoxidation reactions has been investigated in isolated pea (Pisum sativum L.) thylakoids. The Zeax formation was found to be slowed down in the presence of DCCD. The second step (Anth → Zeax) of the reaction sequence seemed to be more affected than the violaxanthin → Anth conversion. Comparative studies with antenna-depleted thylakoids from plants grown under intermittent light and with unstacked thylakoids were in agreement with the assumption that binding of DCCD to antenna proteins is probably responsible for the retarded kinetics. Analyses of the DCCD-induced alterations in different antenna subcomplexes showed that Zeax formation in the PSII antenna proteins was predominantly influenced by DCCD, whereas Zeax formation in photosystem I was nearly unaffected. Our data support the suggestion that DCCD binding to PSII antenna proteins is responsible for the observed alterations in xanthophyll conversion.
The light-dependent and reversible decrease of the Viol content of green leaves was discovered about 40 years ago (Sapozhnikov et al., 1957). Yamamoto et al. (1962) showed that these processes are based on the cyclic conversion of Viol to Zeax via the intermediate Anth. During the last decade this xanthophyll cycle has become more important due to the proposed function of the two de-epoxidized forms, Anth and Zeax, in nonradiative dissipation of excess excitation energy in the antennae of the photosynthetic apparatus (for recent reviews, see Pfündel and Bilger, 1994; Demmig-Adams and Adams, 1996; Horton et al., 1996; Yamamoto and Bassi, 1996; Gilmore, 1997).
The light dependence of the Viol de-epoxidation reflects the light-induced acidification of the thylakoid lumen (Hager, 1966). Recently, the VDE has been identified as a nuclear-encoded, lumen-localized 43-kD protein (Arvidsson et al., 1996; Bugos and Yamamoto, 1996; Rockholm and Yamamoto, 1996). The enzyme requires ascorbate as a cofactor (Hager, 1966) and shows a pH optimum of about 5.2 (Hager, 1969). Activation of the VDE seems to be accompanied by the binding of the VDE to the membrane (Yamamoto, 1985; Hager and Holocher, 1994; Bratt et al., 1995).
Thermal dissipation of excess excitation energy is important for the protection of plants against photooxidative damage of the photosynthetic apparatus under light-stress conditions. Two main mechanisms, the energy or pH-dependent mechanism, qE (e.g. Horton et al., 1996), and photoinhibition (Aro et al., 1993; Osmond, 1994), contribute to these processes. There is a large amount of experimental evidence that Zeax and Anth are involved in both mechanisms (for review, see Demmig-Adams and Adams, 1992; Horton et al., 1996; Yamamoto and Bassi, 1996; Gilmore, 1997). This action might originate from the ability of these two xanthophylls to trap energy from a singlet excited 1Chl a molecule (Frank et al., 1994), although energy transfer from Chl a to either Zeax or Anth, along with qE and photoinhibitory process, has not been proven experimentally.
Like Viol de-epoxidation, the qE component of energy dissipation is regulated by the lumen pH. The molecular mechanism by which the lumen pH controls the formation and relaxation of qE is still unclear. Horton and co-workers favor the idea that protonation of carboxy groups of PSII Chl a/b antenna proteins induces conformational changes in the PSII antenna, which in turn generate energy-quenching centers (Horton et al., 1991, 1996). One piece of evidence for this hypothesis was the observation that DCCD, which is known to bind to carboxy groups that are involved in proton binding, inhibits the generation of qE (Ruban et al., 1992). The DCCD-binding carboxy groups in Lhcb5 and Lhcb4 have thus been proposed to function as a sensor of the lumenal pH in regulation of the qE quenching of excitation energy in these proteins (Walters et al., 1994; Horton et al., 1996). DCCD-binding residues have been identified in Lhcb5 (Walters et al., 1996) and Lhcb4 (Pesaresi et al., 1997). According to the model of the folding of Lhcb1/2 derived from the crystal structure (Kühlbrandt et al., 1994), the DCCD-binding residues are indeed at positions that can be expected to have contact with the thylakoid lumen. Similar positions have been proposed for DCCD-binding residues in other antenna proteins (Jahns and Junge, 1990).
The xanthophyll-cycle pigments are bound by Chl a/b antenna proteins of both photosystems (Bassi et al., 1993; Ruban et al., 1994; Lee and Thornber, 1995). Because of the close relation of the generation of qE and the de-epoxidation of Viol, it might be expected that DCCD binding to antenna proteins might also interfere with the xanthophyll conversion. We tested this possibility by investigating the influence of DCCD on the de-epoxidation reactions of the xanthophyll cycle.
MATERIALS AND METHODS
Pea (Pisum sativum L. cv Kleine Rheinländerin) plants were grown in a climate chamber in a 14-h light/10-h dark cycle (control plants) or in a 2-min light/118-min dark cycle (IML plants). Illumination was performed with white light at a photon flux density of 100 μmol m−2 s−1. Leaves of 12- to 14-d-old control plants and 12-d-old IML plants were used for all experiments. Control plants were harvested at the end of the dark period.
Thylakoid Preparation
Preparation of thylakoids was based on the method of Jensen and Bassham (1966) with the modifications described by Krause et al. (1985). Unstacked thylakoids were prepared under LS conditions according to the method of Polle and Junge (1986).
Incubation with DCCD
Incubation of thylakoids with DCCD was carried out at room temperature in the dark. Thylakoids equivalent to 50 μg/mL Chl were suspended in a medium containing 0.33 m sorbitol, 5 mm MgCl2, 5 mm NaCl, 1 mm KH2PO4, and 40 mm Hepes/NaOH, pH 7.8. For unstacked, LS thylakoids, MgCl2 was omitted from the medium. DCCD was added from an ethanolic stock solution (100 mm) to give a final concentration of 100 μm. For controls, the same amount of ethanol was added to the samples. After 10 min of incubation, thylakoids were spun down by centrifugation for 5 min at 2000g and used immediately for the de-epoxidation experiments.
De-Epoxidation Conditions
For de-epoxidation, control thylakoids (40 μg Chl/mL) or IML thylakoids (20 μg Chl/mL) were suspended in 0.33 m sorbitol, 5 mm MgCl2, 5 mm NaCl, and 50 mm Mes/NaOH, pH 5.2. De-epoxidation was started by addition of 20 mm ascorbate from a 1 m stock solution. All experiments were carried out in the dark at room temperature under gentle stirring of the samples. At different times 1-mL aliquots were taken, centrifuged for 2 min at 2000g, and prepared for pigment analyses.
IEF
Nondenaturing IEF was carried out following the protocol of Ruban et al. (1994) with the modifications for the use of thylakoids as starting material as described in Färber et al. (1997). The procedure resulted in formation of 11 distinct green bands. Each band was carefully collected using a spatula. The pigment-protein complexes were eluted from the gel in a PEGG Elution column (Pharmacia) using a buffer containing 10 mm Hepes, pH 7.6, and 0.06% (w/v) n-dodecyl-β-d-maltoside. Bands 1 to 4 contained the major component of the PSII antenna, Lhcb1–3, and were collected together as fraction I. Fraction II was composed of band 5 and contained a mixture of Lhcb5 and Lhcb6. Band 6 contained Lhcb4 and PSII reaction-center core proteins and was isolated as fraction III. The final five bands, 7 to 11, contained the PSI antenna proteins, Lhca1–4, together with the PSI reaction center core, and were again collected together as fraction IV (Färber et al., 1997).
Pigment Analysis
Pigment analysis was carried out by HPLC as described elsewhere (Färber et al., 1997). For thylakoid samples, membranes were collected by a 5-min centrifugation at 2000g and pigments were extracted with acetone, yielding a final acetone concentration of about 80%. Proteins were spun down by centrifugation. Pigment extracts were either used directly for HPLC analysis or stored for up to 2 d at −20°C.
For IEF samples, pigments were extracted with diethyl ether by mixing 0.5 mL of sample, 0.5 mL of ethanol, 1 mL of diethyl ether, and 0.25 mL of water in a tube. The upper phase was collected, whereas the lower phase was washed again with 1 mL of ether. The collected upper phases were dried under N2 and stored at −20°C until resuspension in acetone for HPLC analysis.
RESULTS
Influence of DCCD on the De-Epoxidation Kinetics
The effect of DCCD on the de-epoxidation reactions in pea thylakoids is shown in Figure 1. According to former studies (Jahns et al., 1988; Ruban et al., 1992), a molar ratio of DCCD:Chl (2:1) was chosen for the incubation, which is known to cause minimal inhibition of electron transport, but significant effects on proton release from water oxidation (Jahns et al., 1988) and on qE (Ruban et al., 1992). A possible uncoupling effect of DCCD (Jahns et al., 1988; Ruban et al., 1992) was not critical for these experiments, since no transmembrane pH gradient was present when de-epoxidation was induced in the dark by low pH and addition of ascorbate.
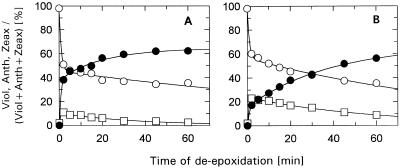
Figure 1.
Time course of de-epoxidation in isolated control thylakoids. Thylakoids (50 μg Chl/mL) were incubated for 10 min in the dark and at room temperature in the absence (A) or in the presence (B) of 100 μm DCCD. The relative portions (mol%) of the xanthophyll-cycle pigments Viol (○), Anth (□), and Zeax (•) are plotted. The sum of the three pigments remained constant during the time course of the experiments. The data represent the mean value of three independent experiments. sd was in the range of 5 to 7% of the respective values for Viol and Zeax, and up to 20% of the respective values for Anth.
In the absence of DCCD (Fig. 1A), the well-known kinetics of de-epoxidation reported in other in vitro and in vivo studies (Hager, 1967; Siefermann and Yamamoto, 1974; Pfündel and Dilley, 1993; Jahns, 1995; Härtel et al., 1996) were confirmed. In agreement with the data of Pfündel and Dilley (1993), the formation of Zeax followed biphasic kinetics, with about one-third of the Zeax being formed with the slower kinetics.
After incubation of the thylakoids with DCCD, the de-epoxidation was significantly slowed down (Fig. 1B). However, the final extent of de-epoxidation (i.e. the maximum de-epoxidation state) and thus the Viol availability were not affected by this treatment. Also, the total amount of xanthophyll-cycle pigments remained constant throughout the time course of the experiment. DCCD seemed to influence mainly the kinetics of the second step of the reaction sequence, Anth → Zeax. This can be concluded from Figure 1 because the reduction of the rapidly formed Zeax (from about 40% to less than 20%) was more than twice as high as the reduction of the rapidly converted Viol (from 50 to about 40%). The somewhat slowed-down Viol → Anth de-epoxidation may be sufficiently explained by the transient accumulation of Anth (attributable to the retarded Anth → Zeax conversion). Fitting the sum of two exponentials to the data (Table I) indicated that DCCD altered the proportion of the two phases of the Anth → Zeax reaction, but not the rate constants of each. Thus, the fast phase of Anth → Zeax de-epoxidation seems to be converted into the slow one in the presence of DCCD.
Table I.
Kinetic parameters of the second step (Anth → Zeax) of de-epoxidation
| Parameter | Control Plants
|
IML Plants
|
||
|---|---|---|---|---|
| − DCCD | + DCCD | − DCCD | + DCCD | |
| k1 (min−1) | 1.36 | 1.32 | 0.94 | 1.05 |
| A1 (%) | 38.3 | 15.6 | 62.5 | 38.1 |
| k2 (min−1) | 0.05 | 0.03 | 0.06 | 0.06 |
| A2 (%) | 26.1 | 50.6 | 30.5 | 52.9 |
| Δ Zeax (t = ∞) | 64.4 | 66.2 | 93.0 | 91.0 |
Rate constants (k1, k2) and amplitudes (A1, A2) of the two phases of Zeax formation were determined by fitting the data from Figure 1 (control plants) and Figure 2 (IML plants) as the sum of two exponentials. The total degree of Zeax formation (in percent of the total pool of xanthophyll-cycle pigments) is given by the sum of the amplitudes of both phases, Δ Zeax (t = ∞). Although ignoring the influence of the first step (Viol → Anth) of de-epoxidation on the kinetics of the second step, this procedure is a reliable approximation for the quantitative description of our data (compare also Härtel et al., 1996).
DCCD has been shown to bind to nearly all Chl a/b antenna proteins of both photosystems (Jahns and Junge, 1990; Walters et al., 1994). In addition, only binding of DCCD to subunits c (Sigrist-Nelson et al., 1978) and β (Shoshan and Selman, 1980) of the coupling factor0/coupling factor1 ATPase has been reported so far for thylakoid membranes. Since antenna proteins also contain the xanthophyll-binding sites, it is reasonable to assume that the effect of DCCD on the de-epoxidation kinetics is attributable to binding to antenna proteins. We tested this hypothesis with IML thylakoids, which are known to have drastically reduced amounts of antenna proteins.
Influence of DCCD on the De-Epoxidation in IML Thylakoids
The influence of DCCD on the time course of pigment conversion in IML thylakoids is shown in Figure 2. The increased Viol to Zeax conversion (i.e. the much higher maximum de-epoxidation state) in IML thylakoids in comparison with control thylakoids corroborates the findings of former in vivo studies with IML plants (Jahns, 1995; Härtel et al., 1996). Apart from this point, however, the de-epoxidation reactions in the absence of DCCD (Fig. 2A) were similar to control thylakoids. Incubation with DCCD (Fig. 2B) showed a qualitatively similar but less-pronounced effect, as shown in Figure 1B. The relative portion of the fast phase of Zeax formation was only reduced from about 65% in the absence of DCCD to about 40% in the presence of DCCD (in comparison to 20% in control thylakoids). Again, the rate constants for both phases of the Anth → Zeax step were not altered remarkably by incubation with DCCD (Table I).
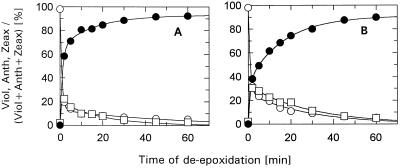
Figure 2.
Time course of de-epoxidation in isolated IML thylakoids. Thylakoids (50 μg Chl/mL) were incubated for 10 min in the dark and at room temperature in the absence (A) or in the presence (B) of 100 μm DCCD. The relative portions of the xanthophyll-cycle pigments Viol (○), Anth (□), and Zeax (•) are plotted. The sum of the three pigments remained constant during the time course of the experiments. The data represent the mean value of three independent experiments. sd was in the range of 2 to 10% of the respective values for Viol and Zeax, and up to 30% of the respective values for Anth.
The reduced influence of DCCD in IML thylakoids is therefore in agreement with the assumption that binding of DCCD to antenna proteins is responsible for the altered de-epoxidation kinetics. The remaining effect that was observable with IML thylakoids might result from binding of DCCD to Lhcb5, the only Chl a/b-binding protein, which is present to a normal extent in IML thylakoids (Jahns and Krause, 1994; Król et al., 1995).
It is worth mentioning that any effects of DCCD on proton release from water oxidation or uncoupling can be neglected, since the de-epoxidation is induced in the dark by low pH/ascorbate and, thus, neither water-splitting activity nor a transmembrane pH gradient occur with our experimental setup.
DCCD Incubation in LS Media
Earlier flash spectrophotometrical studies have shown that DCCD inhibits proton release into the thylakoid lumen at the donor side of PSII (Jahns et al., 1988). It is interesting that incubation with DCCD in the absence of divalent cations (LS conditions) abolished this effect (Jahns et al., 1988) and also reduced the binding of DCCD to antenna proteins (Jahns and Junge, 1990). This was used in the following to test whether DCCD binding to antenna proteins might be responsible for the observed alterations of the de-epoxidation reactions.
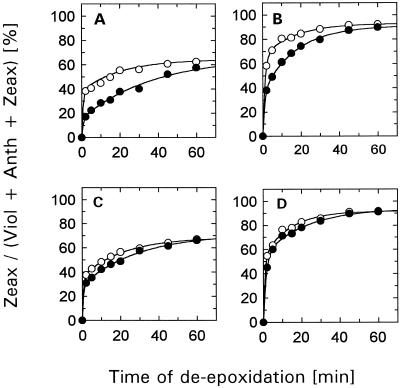
The result for both types of thylakoids is illustrated in Figure 3. For a better comparison, only the changes of the Zeax content are shown. Obviously, the effect of DCCD on the de-epoxidation kinetics was suppressed when thylakoids of either type were incubated with DCCD under LS conditions. Similar to the generally reduced effect of DCCD in antenna-depleted IML thylakoids, this result supports the suggestion that DCCD binding to antenna proteins is responsible for the retarded de-epoxidation in DCCD-treated control HS thylakoids.
Figure 3.
Time course of de-epoxidation in control (A and C) and IML (B and D) thylakoids. Thylakoids (50 μg Chl/mL) were incubated for 10 min in the dark and at room temperature in the absence (○) or in the presence (•) of 100 μm DCCD. Incubation with DCCD was performed either under HS (A and B) or LS (C and D) conditions. Only the relative portion of Zeax is plotted. The data represent the mean value of three independent experiments. sd was in the range of 3 to 10% of the respective values.
Unstacking of thylakoid membranes has been shown to influence de-epoxidation kinetics and Viol availability (Färber and Jahns, 1998). Therefore, it should be noted that for both incubation conditions (LS and HS) the respective de-epoxidation experiment has been performed under HS conditions. This procedure excludes that unstacking of the membranes or the different surface charge of the thylakoid membrane distorts the DDCD-induced alterations.
Xanthophyll Conversion in Different Antenna Subcomplexes
The xanthophyll-cycle pigments are distributed heterogeneously among the different antenna subcomplexes, and the degree of pigment conversion is variable in different antenna proteins (Bassi et al., 1993; Ruban et al., 1994; Lee and Thornber, 1995; Färber et al., 1997). Under the assumption that DCCD binding to antenna proteins caused the reduced de-epoxidation, one might expect that this action is possibly restricted to distinct antenna subcomplexes.
We analyzed the Zeax content of antenna subcomplexes that were separated by IEF after 10 min of de-epoxidation in the absence or in the presence (LS and HS incubation) of DCCD (Fig. 4). At this incubation time, a high de-epoxidation state should already be established along with maximal differences between DCCD-treated and control thylakoids (compare with Fig. 3A).
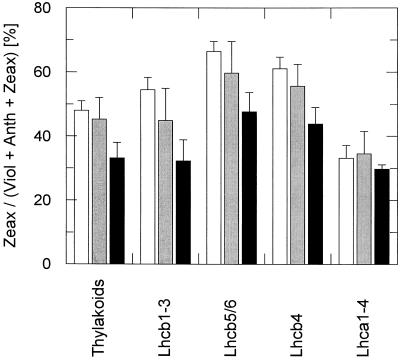
Figure 4.
Zeax content in different antenna subcomplexes. Thylakoids (50 μg Chl/mL) were incubated for 10 min in the dark and at room temperature in the absence (white columns) or in the presence (gray and black columns) of 100 μm DCCD. Incubation with DCCD was performed either under LS (gray columns) or HS (black columns) conditions. Different antenna subcomplexes were separated by IEF. The distinct antenna proteins were assigned to the different fractions as in Färber et al. (1997). Only the relative Zeax content of each fraction is plotted. The data represent the mean value of three to five independent experiments. sd is indicated by the respective bars.
The data obtained with control thylakoids (Fig. 4, white columns) confirmed the recently described differences in the Zeax content of the four fractions Lhcb1–3, Lhcb5/6, Lhcb4, and Lhca1–4 (Färber et al., 1997). The effect of DCCD on the de-epoxidation kinetics under LS and HS conditions (Fig. 3, A and C), however, was restricted to xanthophylls that are associated with PSII antenna proteins. In the fraction containing the PSI antenna only marginal differences in the Zeax content were detectable in the absence and in the presence of DCCD (Fig. 4). This implies that DCCD binding specifically to PSII antenna proteins is responsible for the altered de-epoxidation kinetics shown in Figure 1B.
DISCUSSION
Our experiments show that DCCD slows down the de-epoxidation kinetics of the xanthophyll cycle. This action did not have a general effect on VDE activity, but was more pronounced in the second step of the reaction, the Anth → Zeax conversion, and restricted to the xanthophylls that are associated with the PSII antenna proteins, Lhcb1–6. It is tempting to speculate that DCCD binding to these proteins is responsible for the observed alterations, particularly because any other possibly unspecific effect of DCCD can be expected to affect both steps (Viol → Anth and Anth → Zeax) of de-epoxidation to a similar extent and also the conversion of pigments that are associated with PSI antenna proteins. These interesting features may provide new insight into the reaction mechanism of the pigment conversion in the xanthophyll cycle.
So far it is unknown to what extent the pigments are bound to proteins during the de-epoxidation reaction. Although after isolation of pigment-protein complexes under nondenaturing conditions most of the xanthophylls were found to be associated with proteins (Bassi et al., 1993; Ruban et al., 1994; Lee and Thornber, 1995; Färber et al., 1997), it cannot be ruled out that conversion of free pigment occurs in the surrounding lipid phase (as discussed by Rockholm and Yamamoto, 1996). For the latter case, it would not be easy to explain why DCCD binding to antenna proteins influences pigment conversion, therefore, our data support the hypothesis of protein-bound pigment conversion.
DCCD is known to bind to carboxy groups that are involved in proton binding or translocation (Azzi et al., 1984). In antenna proteins, carboxy groups that are located near the thylakoid lumen surface have been proposed (Jahns and Junge, 1990) or identified (Walters et al., 1996; Pesaresi et al., 1997) as DCCD-binding sites. The fact that only those pigments that are associated with PSII antenna proteins were affected by DCCD (Fig. 4) implies that the DCCD-binding sites in these proteins are in close contact with either the pigment-binding sites of the antenna proteins or the active site of the VDE.
The restriction of the DCCD effect to xanthophylls associated with PSII antenna proteins does not necessarily indicate a specific function of the DCCD-binding carboxy groups for the de-epoxidation, but could simply be due to the high-protein (and high-pigment) concentration in the grana region of the membranes. The altered kinetics could then originate from a higher DCCD:lipid ratio in the grana stacks that might disturb the interaction of the pigments with the VDE. This explanation would also explain the diminished or abolished DCCD effect in unstacked IML thylakoids and (also unstacked) LS thylakoids, respectively, and would therefore be in agreement with pigment conversion in the lipid phase. In this case, however, one would expect that both steps of the de-epoxidation (Viol → Anth and Anth → Zeax) are affected to a similar extent.
One of the most intriguing mechanistic problems of the de-epoxidation reaction is how the two epoxy groups of Viol (which are most likely arranged at opposite sides of the membrane) are enzymatically converted by an enzyme that is located exclusively at the lumen side. In principle, this may be achieved by a flip-flop of the pigment molecule or by a membrane arm of the VDE that has access to both epoxy groups of the carotenoid (see also Gilmore, 1997). In either case, conformational changes (of the pigment or the protein) have to be postulated to allow for an interaction of the second epoxy group of the carotenoid with the active site of the protein. The more-pronounced retardation by DCCD of the Anth → Zeax step of the de-epoxidation might indicate that these conformational changes are hampered by DCCD and could be an argument for a role of the DCCD-binding amino acids in pigment conversion.
Assuming a flip-flop of the pigment molecule during conversion of both epoxy groups, it seems more likely that this turn of the molecule is catalyzed by a protein rather than occurring spontaneously in the lipid phase of the membrane. For de-epoxidation, binding of xanthophylls to antenna proteins seems to be dispensable, since de-epoxidation in IML plants (and also from other Chl b-deficient or Chl b-less plants) is nearly unchanged compared with normally developed plants (Jahns, 1995; Härtel et al., 1996). Thus, any protein-mediated flip-flop of Anth should then be facilitated by the VDE. The ability of the isolated (or partially purified) VDE to convert Viol to Zeax in the absence of thylakoid membranes and, thus, of any integral membrane protein (Hager and Perz, 1970; Yamamoto and Higashi, 1978; Arvidsson et al., 1996; Rockholm and Yamamoto, 1996) further supports this point of view. However, these features are also fully compatible with the assumption that a membrane arm of the VDE provides access to the two epoxy groups of Viol and that no flip-flop of the carotenoid is required.
The slower kinetics to which the Anth → Zeax conversion was apparently switched by DCCD was already visible in untreated thylakoids, although this slower portion was much less pronounced in this case (Fig. 1). The slow kinetics of the Anth → Zeax conversion has also been described by Pfündel and Dilley (1993), but was not detected in the work by Siefermann and Yamamoto (1974), perhaps due to the relatively short-time interval of 15 min used in the latter study. Pfündel and Dilley (1993) explained the two phases of Zeax formation by two different Viol pools. However, the relative increase of the slower portion at a higher pH shown by these authors might alternatively indicate that the slow conversion rate is based on another pH-dependent process. This could be the binding of the VDE to the membrane, which is thought to be a pH-dependent process (Hager and Holocher, 1994; Bratt et al., 1995). Thus, the DCCD-induced changes might then simply be understood as an altered binding behavior of the VDE to the membrane, induced by the presence of DCCD. This could point to an interaction of the DCCD-binding carboxy group and the VDE along with binding to the membrane, and may reflect the importance of a protonable residue (which is blocked by binding of DCCD) for VDE binding to the membrane.
Abbreviations:
- Anth
antheraxanthin
- Chl
chlorophyll
- DCCD
dicyclohexylcarbodiimide
- HS
high-salt
- IML
intermittent-light
- LS
low-salt
- qE
pH-dependent Chl fluorescence quenching
- VDE
violaxanthin de-epoxidase
- Viol
violaxanthin
- Zeax
zeaxanthin
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. SFB 189, TP B13).
LITERATURE CITED
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Arvidsson P-O, Bratt CE, Carlsson M, Åkerlund H-E. Purification and identification of the violaxanthin deepoxidase as a 43 kDa protein. Photosynth Res. 1996;49:119–129. doi: 10.1007/BF00117662. [DOI] [PubMed] [Google Scholar]
- Azzi A, Casey RP, Nalecz MJ. The effect of N,N′-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta. 1984;768:209–226. doi: 10.1016/0304-4173(84)90017-x. [DOI] [PubMed] [Google Scholar]
- Bassi R, Pineau B, Dainese P, Marquardt J. Carotenoid-binding proteins of photosystem II. Eur J Biochem. 1993;212:297–303. doi: 10.1111/j.1432-1033.1993.tb17662.x. [DOI] [PubMed] [Google Scholar]
- Bratt CE, Arvidsson P-O, Carlsson M, Åkerlund H-E. Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth Res. 1995;45:169–175. doi: 10.1007/BF00032588. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Yamamoto HY. Molecular cloning of violaxanthin de-epoxidase from romaine lettuce and expression in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:6320–6325. doi: 10.1073/pnas.93.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW. Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Demmig-Adams B, Adams WW. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- Färber A, Jahns P. The xanthophyll cycle of higher plants: influence of antenna size and membrane organization. Biochim Biophys Acta. 1998;1363:47–58. doi: 10.1016/s0005-2728(97)00093-5. [DOI] [PubMed] [Google Scholar]
- Färber A, Young AJ, Ruban AV, Horton P, Jahns P. Dynamics of xanthophyll cycle activity in different antenna subcomplexes in the photosynthetic membranes of higher plants. The relationship between zeaxanthin conversion and non-photochemical fluorescence quenching. Plant Physiol. 1997;115:1609–1618. doi: 10.1104/pp.115.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank HA, Cua A, Chynwat V, Young A, Gosztola D, Wasielewski MR. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth Res. 1994;41:389–395. doi: 10.1007/BF02183041. [DOI] [PubMed] [Google Scholar]
- Gilmore AM. Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant. 1997;99:197–209. [Google Scholar]
- Hager A. Die Zusammenhänge zwischen lichtinduzierten Xanthophyll-Umwandlungen und der Hill-Reaktion. Ber Dtsch Bot Ges. 1966;79:94–107. [Google Scholar]
- Hager A. Untersuchungen über die lichtinduzierten reversiblen Xanthophyllumwandlungen an Chlorella und Spinacia. Planta. 1967;74:148–172. doi: 10.1007/BF00388326. [DOI] [PubMed] [Google Scholar]
- Hager A. Lichtbedingte pH-Erniedrigung in einem Chloroplastenkompartiment als Ursache der enzymatischen Violaxanthin-Zeaxanthin-Umwandlung; Beziehungen zur Photophosphorylierung. Planta. 1969;89:224–243. doi: 10.1007/BF00385028. [DOI] [PubMed] [Google Scholar]
- Hager A, Holocher K. Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta. 1994;192:581–589. [Google Scholar]
- Hager A, Perz H. Veränderung der Lichtabsorption eines Carotinoids im Enzym (De-epoxidase)-Substrat(Violaxanthin)-Komplex. Planta. 1970;93:314–322. doi: 10.1007/BF00384105. [DOI] [PubMed] [Google Scholar]
- Härtel H, Lokstein H, Grimm B, Rank B. Kinetic studies on the xanthophyll cycle in barley leaves. Influence of antenna size and relations to nonphotochemical chlorophyll fluorescence quenching. Plant Physiol. 1996;110:471–482. doi: 10.1104/pp.110.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Rees D, Pascal AA, Noctor G, Young AJ. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll protein complex. FEBS Lett. 1991;292:1–4. doi: 10.1016/0014-5793(91)80819-o. [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Jahns P. The xanthophyll cycle in intermittent light grown pea plants. Possible functions of chlorophyll a/b binding proteins. Plant Physiol. 1995;108:149–156. doi: 10.1104/pp.108.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns P, Junge W. Dicyclohexylcarbodiimide-binding proteins related to the short circuit of the proton-pumping activity of photosystem II: identified as light-harvesting chlorophyll-a/b-binding proteins. Eur J Biochem. 1990;193:731–736. doi: 10.1111/j.1432-1033.1990.tb19393.x. [DOI] [PubMed] [Google Scholar]
- Jahns P, Krause GH. Xanthophyll cycle and energy-dependent fluorescence quenching in leaves from pea plants grown under intermittent light. Planta. 1994;192:176–182. [Google Scholar]
- Jahns P, Polle A, Junge W. The photosynthetic water oxidase: its proton pumping activity is short-circuited within the protein by DCCD. EMBO J. 1988;7:589–594. doi: 10.1002/j.1460-2075.1988.tb02851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RG, Bassham JA. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci USA. 1966;56:1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause GH, Köster S, Wong SY. Photoinhibition of photosynthesis under anaerobic conditions studied with leaves and chloroplasts of Spinacia oleracea L. Planta. 1985;165:430–438. doi: 10.1007/BF00392242. [DOI] [PubMed] [Google Scholar]
- Król M, Spangfort MD, Huner NPA, Öquist G, Gustafsson P, Jansson S. Chlorophyll a/b-binding proteins, pigment conversions, and early light-induced proteins in a chlorophyll b-less barley mutant. Plant Physiol. 1995;107:873–883. doi: 10.1104/pp.107.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W, Wang DN, Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- Lee AI, Thornber JP. Analysis of the pigment stoichiometry of pigment-protein complexes from barley (Hordeum vulgare). The xanthophyll cycle intermediates occur mainly in the light-harvesting complexes of photosystem I and photosystem II. Plant Physiol. 1995;107:565–574. doi: 10.1104/pp.107.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In NR Baker, JR Bowyer, eds, Photoinhibition of Photosynthesis: from Molecular Mechanisms to the Field. BIOS Scientific Publishers, Oxford, UK, pp 1–24
- Pesaresi P, Sandona D, Giuffra E, Bassi B. A single point mutation (E166Q) prevents dicyclohexylcarbodiimide binding to the photosystem II subunit CP29. FEBS Lett. 1997;402:151–156. doi: 10.1016/s0014-5793(96)01518-9. [DOI] [PubMed] [Google Scholar]
- Pfündel E, Bilger W. Regulation and possible function of the violaxanthin cycle. Photosynth Res. 1994;42:89–109. doi: 10.1007/BF02187121. [DOI] [PubMed] [Google Scholar]
- Pfündel EE, Dilley RA. The pH dependence of violaxanthin deepoxidation in isolated pea chloroplasts. Plant Physiol. 1993;101:65–71. doi: 10.1104/pp.101.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A, Junge W. The slow rate of proton consumption at the reducing side of photosystem I is limited by the rate of redox reactions of extrinsic electron acceptors, but not by a diffusion barrier for protons. Biochim Biophys Acta. 1986;848:274–278. [Google Scholar]
- Rockholm DC, Yamamoto HY. Violaxanthin de-epoxidase: purification of a 43-kilodalton lumenal protein from lettuce by lipid-affinity precipitation with monogalactosyldiacylglyceride. Plant Physiol. 1996;110:697–703. doi: 10.1104/pp.110.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Walters RG, Horton P. The molecular mechanism of the control of excitation energy dissipation in chloroplast membranes. FEBS Lett. 1992;309:175–179. doi: 10.1016/0014-5793(92)81089-5. [DOI] [PubMed] [Google Scholar]
- Ruban AV, Young AJ, Pascal AA, Horton P. The effects of illumination on the xanthophyll composition of the photosystem II light harvesting complex of spinach thylakoid membranes. Plant Physiol. 1994;104:227–234. doi: 10.1104/pp.104.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapozhnikov DI, Krasovskaya TA, Maevskaya AN. Change in the interrelationship of the basic carotenoids of the plastids of green leaves under the action of light. Dokl Akad Nauk USSR. 1957;113:465–467. [Google Scholar]
- Shoshan V, Selman BR. The interaction of N,N′-dicyclohexylcarbodiimide with chloroplast couping factor 1. J Biol Chem. 1980;255:384–389. [PubMed] [Google Scholar]
- Siefermann D, Yamamoto HY. Light-induced de-epoxidation of violaxanthin in lettuce chloroplasts. III. Reaction kinetics and effect of light intensity on de-epoxidase activity and substrate availability. Biochim Biophys Acta. 1974;357:144–150. doi: 10.1016/0005-2728(74)90119-4. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K, Sigrist H, Azzi A. Characterization of the dicyclohexylcarbodiimide binding protein isolated from chloroplast membranes. Eur J Biochem. 1978;92:9–14. doi: 10.1111/j.1432-1033.1978.tb12717.x. [DOI] [PubMed] [Google Scholar]
- Walters RG, Ruban AV, Horton P. Higher plant lightharvesting complexes LHCIIa and LHCIIc are bound by dicyclohexylcarbodiimide during inhibition of energy dissipation. Eur J Biochem. 1994;226:1063–1069. doi: 10.1111/j.1432-1033.1994.01063.x. [DOI] [PubMed] [Google Scholar]
- Walters RG, Ruban AV, Horton P. Identification of proton-active residues in a higher plant light-harvesting complex. Proc Natl Acad Sci USA. 1996;93:14204–14209. doi: 10.1073/pnas.93.24.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto HY. Xanthophyll cycles. Methods Enzymol. 1985;34:303–311. [Google Scholar]
- Yamamoto HY, Bassi R (1996) Carotenoids: localization and function. In DR Ort, CF Yocum, eds, Oxygenic Photosynthesis: The Light Reactions. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 539–563
- Yamamoto HY, Higashi RM. Violaxanthin de-epoxidase: lipid composition and substrate specificity. Arch Biochem Biophys. 1978;190:514–522. doi: 10.1016/0003-9861(78)90305-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto HY, Nakayama TOM, Chichester CO. Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys. 1962;97:168–173. doi: 10.1016/0003-9861(62)90060-7. [DOI] [PubMed] [Google Scholar]