Abstract
Background
The stomach-derived hormone ghrelin drives higher-order feeding processes related to food reward and food seeking via CNS signaling at its receptor (GHSR1A). The specific nuclei mediating these effects are only partially understood. Here, we use a rat model to examine whether ghrelin signaling in the ventral subregion of the hippocampus (VHPC), a brain substrate of recent interest in energy balance control, affects learned and motivational aspects of feeding behavior.
Methods
The effects of VHPC ghrelin administration were examined on feeding-relevant behavioral paradigms, including meal pattern analysis, operant lever pressing for sucrose, and conditioned stimulus-induced feeding. The intracellular signaling and downstream neuronal pathways stimulated by VHPC GHSR1A activation were assessed using immunoblot analysis and behavioral pharmacology.
Results
Ghrelin delivery to the VHPC, but not the dorsal hippocampus, increased food intake primarily by increasing meal frequency. Intra-VHPC ghrelin delivery also increased willingness to work for sucrose and increased spontaneous meal initiation in nondeprived rats following the presentation of a conditioned stimulus that previously signaled meal access when the rats were food restricted. The food intake enhancing effects of VHPC ghrelin were blocked by co-administration of a phosphoinositide 3-kinase (PI3K) inhibitor (LY294002). Immunoblot analyses provided complementary support for ghrelin activated PI3K-Akt signaling in the VHPC and revealed that this activation is blunted with high fat diet consumption. Other immunoblot results show that VHPC GHSR1A signaling activates downstream dopaminergic activity in the nucleus accumbens.
Conclusions
These findings illuminate novel neuronal and behavioral mechanisms mediating ghrelin's modulation of cognitive aspects of feeding control.
Keywords: GHSR, learning, memory, nucleus accumbens, reward, obesity, dopamine
Introduction
Ghrelin is synthesized by gastric endocrine cells and is the only known circulating hormone that increases feeding (1). CNS ghrelin signaling stimulates food intake by augmenting appetitive (e.g., food seeking) (2) and rewarding aspects of feeding (3), yet the neurons and the neural pathways mediating these effects are incompletely understood. Investigation of the specific nuclei mediating ghrelin's food intake regulatory effects has largely focused on hypothalamic [arcuate nucleus (ARC), paraventricular nucleus (PVH)] (4-7), caudal brainstem (nucleus tractus solitarius) (8, 9), and midbrain [ventral tegmental area (VTA)] (10-13) nuclei. The ghrelin receptor (GHSR1A) is also expressed in other brain regions, including the hippocampal formation (dentate gyrus and CA1/CA3 regions of the hippocampus) (14, 15). Circulating ghrelin reaches the hippocampus where it binds to neurons and promotes dendritic spine synapse formation and long-term potentiation (16). GHSR1A signaling in the hippocampus is functionally relevant to learning and memory function as genetic deletion of ghrelin (16) or its receptor (2) impairs hippocampal-dependent spatial memory paradigms, whereas direct administration of ghrelin to the hippocampus improves memory consolidation for the location of aversive reinforcement (17).
It is unknown whether ghrelin signaling in the hippocampus contributes to food intake and learned appetitive behaviors. The hippocampus is traditionally associated with visuospatial and declarative memory processes (18); however, several recent findings from humans and animal models also highlight this brain region in the control of food intake regulation [see (19-22) for reviews]. Anorectic control of feeding by the ventral subregion of the hippocampus (VHPC) (anterior hippocampus in primates), which monosynaptically projects to hypothalamic “feeding centers” (23), is directly supported by two recent reports: 1) neurotoxic VHPC lesions increase food intake and body weight in rats (24), and 2) VHPC delivery of the adipose tissue-derived hormone leptin suppresses food intake and learned behaviors related to food procurement (25). Here, we examine the hypothesis that the VHPC also contributes to the mediation of orexigenic (food intake stimulatory) aspects of feeding via ghrelin signaling. Results showed that VHPC GHSR1A stimulation potently increases feeding. The “higher-order” mechanisms (e.g., learned and motivational aspects of feeding) mediating these effects were assessed using various behavioral paradigms, including meal pattern analysis, willingness to work for palatable food [progressive ratio (PR) operant responding], and the initiation of meals induced by conditioned cues previously associated with food reward.
We also examine the downstream neuronal pathways and intracellular signaling mechanisms mediating VHPC ghrelin effects on food intake. VHPC neurons project directly to the nucleus accumbens (NAcc) of the mesolimbic reward system (MRS) (26, 27). Central (ICV) administration of ghrelin elevates dopaminergic activity in the NAcc (28). Present experiments employ protein immunoblot analyses to examine the hypothesis that VHPC GHSR1A stimulation influences downstream catecholamine signaling in the NAcc. We also examine the intracellular signaling pathways mediating food intake elevations by VHPC ghrelin signaling. Recent findings show that ghrelin activates the phosphoinositide 3-kinase (PI3K)-Akt intracellular signaling in neurons (29, 30). Unknown is whether feeding effects driven by VHPC GHSR1A stimulation require PI3K-Akt signaling.
Materials and Methods
Animals and Drugs
Adult male Sprague-Dawley rats (Charles River; 300-500g during experimental procedures) housed individually under a 12h light/dark cycle had ad libitum access to chow (LabDiet; 5001) and water except where noted. All procedures conformed to and received approval from The UPenn Animal Care and Use Committee.
Ghrelin (Bachem) was dissolved in artificial cerebrospinal fluid (aCSF); the PI3K inhibitor LY294002 (EMD Millipore) was dissolved in DMSO. Volumes for injections were 100nl/hemisphere for parenchymal (via Harvard Apparatus infusion pump) and 1μl for ICV.
Cannula implantation
Under ketamine (90mg/kg), xylazine (2.7mg/kg), and acepromazine (0.64mg/kg) anesthesia and analgesia (Metacam 2mg/kg), guide cannulae (Plastics One; 26-guage) cemented to the skull using jewelers screws were implanted at the following coordinates for VHPC placement: 4.9mm A/P, 4.8mm M/L, 6.1mm D/V; for DHPC placement: 3.5mm A/P, 2.5mm M/L, 2.0mm D/V; for lateral ICV placement: 0.9mm A/P, + 1.6mm, M/L, 2.8mm, D/V. Injectors for drug administration projected 2mm beyond guide cannula for VHPC and ICV injections and 1mm for DHPC. Cannula placements for VHCP and DHPC were assessed postmortem through anatomical verification of the position of 100nl pontamine sky blue injections in coronal sections. Only animals with ink observed within the targeted region (VHPC CA regions) were included in data analyses. A representative VHPC injection site is shown in Figure S1 (see Supplement). The number of animals excluded based on incorrectly targeted cannula ranged between 0-2 for each experiment. Anatomical positions of lateral ICV injection sites were evaluated 1wk post-surgery by measurement of the cytoglucopenia-induced sympatho-adrenal mediated glycemic effect resulting from 210μg (2μl) of 5-thio-D-glucose (31, 32).
Signaling Analysis
Tissue collection
VHPC (CA regions) and NAcc tissue from ad libitum chow fed rats was prepared as described (31, 33). Briefly, following pharmacological treatments rats were sacrificed by decapitation. As previously described (34), brains were rapidly removed and bilateral tissue punches were taken from the VHPC and NAcc using stainless steel tubing (inside diameter 2.3mm) from 2mm coronal brain block sections. Tissue was flash frozen in isopentane and stored at -80°C.
Immunoblotting
Lysates were subjected to SDS-PAGE and transferred to PVDF membranes for immunoblot analysis as previously described (31, 35). Immunoreactivity was visualized using enhanced chemiluminescence (BioRad; Chemidoc XRS). Phosphorylated PI3K p85 (Tyr458) and PI3K p85 antibodies (Cell Signaling) were used to evaluate PI3K activity normalized to total PI3K. Phosphorylated AKT (Ser471) (Cell Signaling) and Anti-Akt (Pierce Antibodies) antibodies were used to evaluate Akt activity normalized to total Akt. Phosphorylated p44/42 MAPK antibody (Thr202/Tyr204) was used to assess MAPK signaling normalized to total p44/42 MAPK (Cell Signaling). Phosphorylated tyrosine hydroxylase (pTH) antibodies (Cell Signaling) were used to evaluate TH activity normalized to total TH. Blots were quantified using densitometry analysis in NIH software (Image J).
Procedures
Experiment 1: Food intake following VHPC and DHPC ghrelin
Rats with either VHPC (n=12) or DHPC (n=12) cannulae were given bilateral injections of 0, 7.5, 75, or 750pmol ghrelin (total doses: 15, 150, 1500pmol) immediately before light onset. Treatments were separated by 2-3 days following a counterbalanced within-subjects design. Chow intake was recorded at 1h, 3h, and 5h (spillage accounted for).
Ghrelin dose selection for Experiments 1 and 2 was based on the literature. Previous studies show that parenchymal ghrelin doses of approximately 300pmol appear to be required for intake effects when delivered to various hypothalamic nuclei (lateral hypothalamus, anterior hypothalamus) (4). Following administration of ghrelin to the NAcc and VTA, 100pmol (36) and 150pmol (12) appear to be required for increasing intake, respectively. Lower doses of ghrelin are effective for increasing feeding when delivered to the NTS (8) or the ARC (4) (10 or 30pmol, respectively).
Experiment 2: VHPC ghrelin effects on meal pattern parameters
VHPC injections (0, 75 or 150pmol ghrelin) were given to rats (n=13) immediately before light onset using a within-subjects design. Cumulative intake was measured with an automated feeding system (DiaLog Instruments). Individually housed rats had access to a food cup on a load cell circuit that communicated with an interface and computer with customized software (LabVIEW, National Instruments). The weight of the food cup was measured every 10sec, enabling assessment of meal parameters. Meals were defined as an episode of feeding in which at least 0.25g was ingested, with meal termination criterion as the beginning of a pause in ingestion of at least 10min (37). Data were objectively calculated using a custom Microsoft Excel macro.
Experiment 3: Operant responding (PR schedule) for sucrose following VHPC ghrelin
Rats (n=6) were given operant lever press training for sucrose reinforcement as previously described (38). Rats were given daily chow rations to maintain ~85% of an ad libitum body weight established before training. Training was carried out over six days with a 1hr session each day in conditioning boxes (Med Associates; MedPC IV software). During the first 2 days a fixed ratio (FR1) autoshaping procedure was employed (each lever press earned a 45mg sucrose pellet; a free sucrose pellet dispensed every 600sec that elapsed without reinforcement). The animals then received 2 days of FR1 schedule with no autoshaping component and then 2 days of FR3 training. For all procedures the right lever was the “active” lever; a left “inactive” lever served as a control for nonconditioned elevations in responding.
The rats were given two tests (within-subjects design, separated by 2 days) using a PR reinforcement schedule. VHPC injections (vehicle or 150pmol ghrelin) were given 1hr before each test session. The response requirement of the PR schedule increased progressively as previously described (38). The breakpoint for each animal was defined as the final completed requirement that preceded a 20min period without earning a reinforcer.
Experiment 4: Stimulus-induced feeding by VHPC ghrelin
Previous studies show that discrete stimuli (lights, tones) previously paired with meal access when rats were food restricted would later stimulate increased eating when the rats were food-sated (39-43). We hypothesized that VHPC ghrelin signaling would increase this type of “cue-potentiated feeding”. We developed a paradigm [modified from (44)] in which discrete cues were paired with meal access in food-deprived rats (Stimulus+); the presentation of another discrete cue had no consequence (Stimulus-). The paradigm was designed to be below threshold for cue-potentiated feeding at baseline (i.e., weak effect of cues on feeding in the absence of pharmacological stimulation).
Rats (n=13) were maintained on a high fat (HF) (60% kcal fat; Research Diets D12492) for 5d before training. All training and testing procedures took place during the dark cycle. 10 training days were given where they received 5 meals (HF diet) distributed across the first 8hr of the dark cycle. The total kcal of the five meals was equal to 70% of an ad libitum 24hr intake established before training for each rat. On half of the training days the rats received 5 presentations of a 2.5-minute auditory/visual stimulus compound (Stimulus+) followed immediately by meal access. For the other half, a different auditory/visual stimulus compound (Stimulus-) was presented 5 times and the 5 meals were delivered at random times. The two stimuli were 1) a 2.5-min 1500hz tone combined with a dim light coming from one side of the room, and 2) a 2.5-min white noise combined with a dim light coming from the other side of the room. The order of training days and stimulus assignments were counterbalanced.
After training the rats were returned to ad libitum HF diet feeding. Cue-potentiated feeding was determined as a meal initiated within 3min of stimulus onset (within 30sec of stimulus offset). The rats were housed in the automated feeding apparatuses (described above) so that meal initiation could be determined with temporal specificity in relation to stimuli presentation. To confirm that this paradigm was subthreshold for cue-potentiated feeding at baseline, stimulus tests with 5 presentations of each stimulus were conducted on days 5, 6, and 7 of ad libitum feeding. These tests revealed no difference between the number of meals that followed Stimulus+ vs. Stimulus– (data not shown). A pharmacological test was then given on days 9 and 15 of ad libitum feeding where the rats were given VHPC ghrelin (150pmol) or vehicle injections (order counterbalanced) immediately before dark onset. The rats were then given 5 presentations of each stimulus across the subsequent 6h.
Experiment 5
Experiment 5a: Ghrelin-induced VHPC PI3K-Akt signaling
Rats (n=26) were maintained on chow or a “Western diet” (41% kcal from fat; Research Diets D12079B) for four weeks. The rats from each diet group were subdivided (matched for body weight within each diet group) to receive lateral ICV ghrelin [3nmol; dose selected to be effective for robustly increasing intake following ICV delivery (45)] or vehicle injections 60min before VHPC tissue harvest. Immunoblot analysis (PI3K, Akt and p44/42 MAPK) was carried out as described above.
Experiment 5b: Requirement of PI3K-Akt signaling for VHPC ghrelin-stimulated feeding
Using a four-treatment within-subjects design, rats (n=13) received 2 sets of bilateral VHPC injections on each treatment day (injections ~30min apart; treatments separated by 2-3 days). The first injection was the PI3K inhibitor LY294002 (0.2nmol) or its vehicle, whereas the second injection (immediately before light onset) was ghrelin (150pmol) or its vehicle.
Experiment 6: VHPC ghrelin effects on NAcc catecholamine signaling
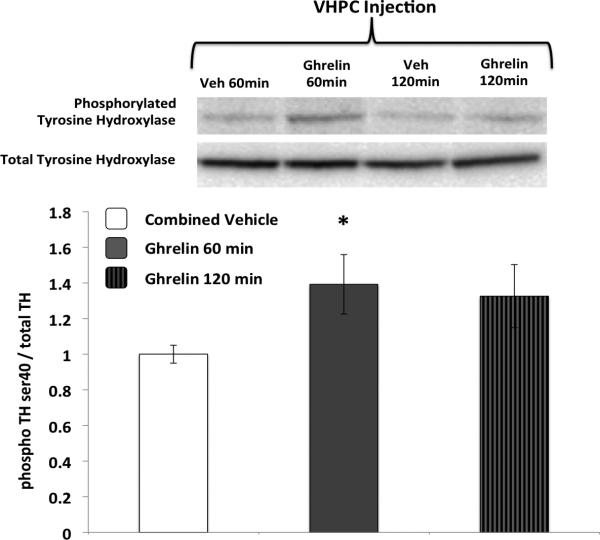
Rats (n=18) were divided into four groups (4-6/group) to receive VHPC vehicle or ghrelin (150pmol) injections either 120min or 60min before tissue harvest. These time points where chosen based on previous work demonstrating increased NAcc DA signaling following ghrelin administered to the VTA (46). NAcc tissue harvest and immunoblot analysis (pTH/TH) were carried out as described above. Previous research has utilized immunoblot pTH analysis to assess dopaminergic NAcc signaling (47, 48).
Statistical Analysis
All statistical analyses employed repeated measures analysis of variance (ANOVA), except for Experiments 5a and 6 (one-way ANOVA). Newman Keuls posthoc tests were used to compare individual treatments for all experiments that involved more than two treatments. Alphalevel for significance was 0.05. Statistical analyses were conducted using Statsoft software (Statistica V10).
Results
Experiment 1
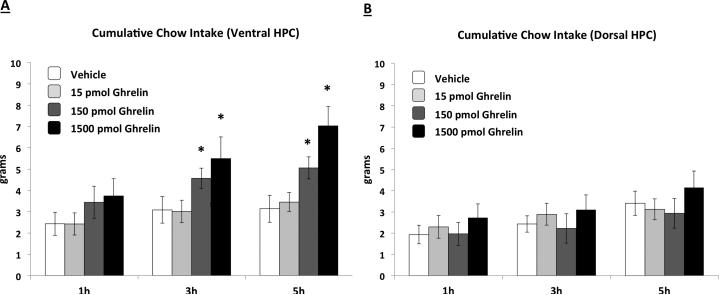
Ghrelin delivered to the VHPC significantly increased food intake at 3hr and 5hr compared to vehicle injection for the two higher doses (Figure 1a) (ps vs. vehicle <0.05). DHPC ghrelin injections had no effect on food intake for all doses examined (Figure 1b).
Figure 1.
Cumulative chow intake following VHPC (Fig. 1A) or DHPC (Fig. 1B) administration of ghrelin. VHPC, but not DHPC ghrelin delivery stimulated food intake relative to vehicle treatment. Data are mean ± SEM, * = p < 0.05 vs. vehicle.
Experiment 2
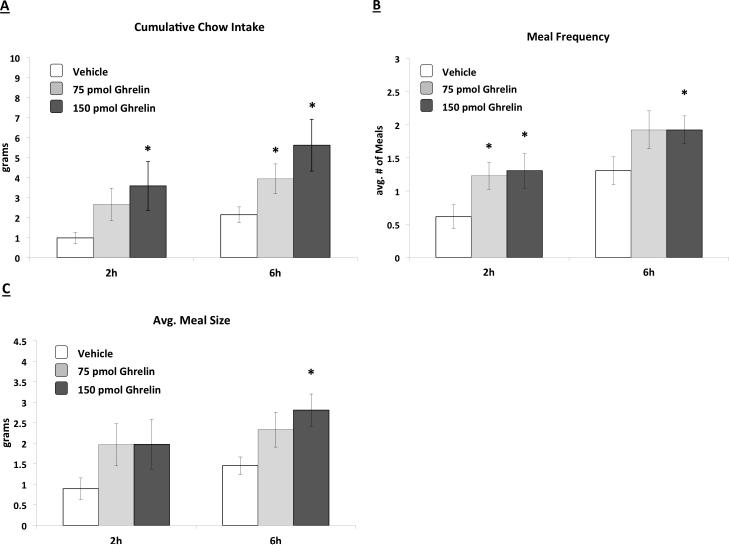
VHPC ghrelin injections increased cumulative food intake for both the 75pmol and the 150pmol doses (Figure 2A). This increased feeding appeared to be based on increased meal frequency for both doses (Figure 2B) (ps<0.05 vs. vehicle), whereas only the 150pmol dose significantly increased meal size relative to vehicle treatment (Figure 2C).
Figure 2.
Cumulative chow intake (Fig. 2A), average meal frequency (Fig. 2B), and average meal size (Fig. 2C) following VHPC ghrelin delivery. Both 75 and 150pmol ghrelin increased cumulative food intake and meal frequency; 150pmol ghrelin also increased average 6hr meal size relative to vehicle treatment. Data are mean ± SEM, * = p < 0.05 vs. vehicle.
Experiment 3
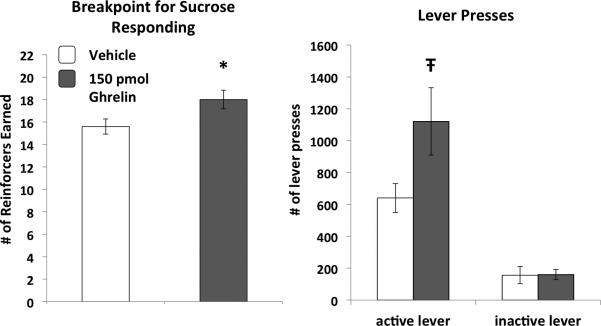
As shown in Figure 3, VHPC ghrelin increased breakpoint responding for sucrose during the PR test relative to vehicle treatment (p<0.05). This effect was based on elevated active lever pressing, whereas pressing of the inactive lever was not influenced by VHPC ghrelin.
Figure 3.
VHPC ghrelin increased breakpoint operant responding for sucrose in a progressive ratio reinforcement test. No ghrelin treatment-based differences in lever pressing were observed for the inactive lever. Data are mean ± SEM, * = p < 0.05 vs. vehicle, Ŧ = p < 0.07 vs. vehicle.
Experiment 4
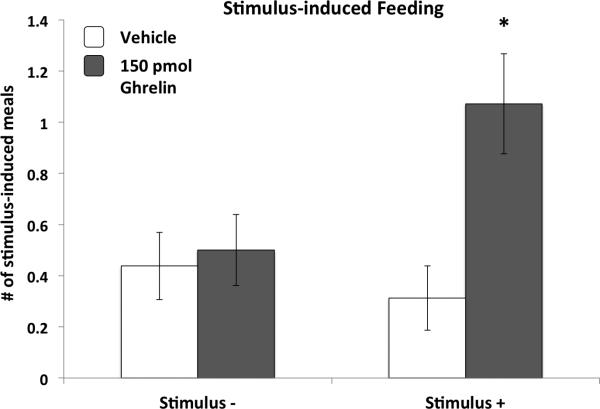
Consistent with the results of the cue-potentiated feeding tests that were conducted before the VHPC ghrelin test, there was no baseline (following vehicle administration) cue-potentiated feeding effect. However, relative to vehicle treatment, VHPC ghrelin (150pmol) significantly elevated the number of meals that followed presentation of the Stimulus+, but not following the Stimulus– (p<0.05 for ghrelin Stimulus+ vs. all 3 other treatments). Analysis of the average size of each stimulus-induced meal revealed no significant differences with regards to stimulus or drug (data not shown).
Experiment 5
Experiment 5a
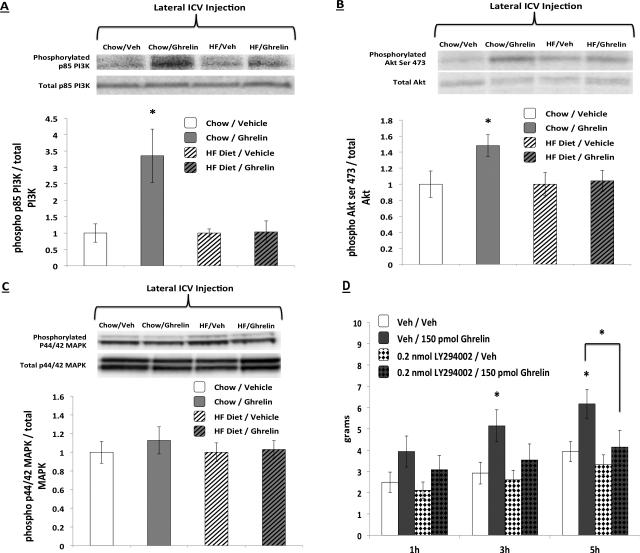
Comparison of the chow vehicle-treated group to the Western diet vehicle-treated group revealed no significant differences for PI3K, Akt, or p44/42 MAPK activation. Thus, data are expressed as % of the vehicle-treated groups separately for each diet group to better illustrate ghrelin-induced increased signaling within each diet group. ICV ghrelin (3nmol) significantly increased VHPC PI3K (Figure 5A) and Akt (Figure 5B) in chow-fed but not for Western diet-fed rats. p44/42 MAPK signaling in the VHPC was not elevated by ghrelin (Figure 5C). Chow-fed vehicle- and ghrelin-treated rats used for immunoblot analysis weighed 498.4 (+/-30.6) and 490.4 (+/-30.0) grams respectively. Western diet-fed vehicle-treated and ghrelin-treated rats weighed 592.9 (+/-27.1) and 585.9 (+/-8.6) grams respectively.
Figure 5.
Ghrelin (delivered lateral ICV) activated PI3K (Fig. 5A) and Akt (Fig. 5B) signaling in the VHPC in chow-fed, but not Western diet-fed rats, whereas p44/42 MAPK signaling was not elevated by ghrelin (Fig. 5C). The food intake stimulatory effects of VHPC ghrelin were blunted with co-administration of the PI3K inhibitor LY294002 at 3hr and 6hr after injections (Fig. 5D). Data are mean ± SEM, * = p < 0.05 vs. vehicle.
Experiment 5b
Ghrelin-stimulated food intake at 3hr and 5hr after injections was blocked with pretreatment of the PI3K inhibitor LY294002 (Figure 5D). At 3hr, DMSO/ghrelin treatment increased food intake relative to DMSO/aCSF treatment (p<0.05), whereas LY294002/ghrelin treatment was not significantly different from any other treatment. At 5hr, DMSO/ghrelin treatment produced significantly greater food intake compared to all other treatments (ps<0.05); a significant drug interaction was also obtained at 5hr (p<0.05).
Experiment 6
pTH levels (relative to total TH) were not significantly different between the two vehicle groups (60 and 120min); thus, the vehicle groups were combined for subsequent analyses and for Figure 6. Ghrelin (150pmol) injected in the VHPC significantly increased pTH in the NAcc at 60min after injections (p<0.05) (Figure 6).
Figure 6.
VHPC ghrelin administration increased phosphorylated TH in the NAcc at 60min after injections. Data are mean ± SEM, * = p < 0.05 vs. vehicle.
Discussion
Research examining energy balance control by CNS ghrelin signaling has focused primarily on brain regions traditionally associated with homeostatic aspects of food intake (e.g., hypothalamus, caudal brainstem) and more recently on MRS nuclei such as the VTA (12, 13, 36) and the NAcc (13, 36). Here, we establish the VHPC, a brain region linked with emotional and motivational memory processes, as a novel site regulating learned and rewarding orexigenic aspects of feeding by ghrelin signaling. Ghrelin administration to the VHPC potently stimulated food intake in rats, the latency of which was similar to previous studies administering ghrelin ICV (49, 50). On the other hand, ghrelin administration to the DHPC, an area associated with the control of learning/memory function related to visuospatial processing (51, 52), was without effect on feeding. These findings complement our previous work showing that food intake and learned aspects of feeding are suppressed by VHPC leptin signaling (25).
The increased feeding response by VHPC GHSR1A stimulation was largely mediated by increased meal frequency, whereas higher doses increased both meal frequency and size. The meal size effect suggests that ghrelin signaling in the VHPC functions, in part, to reduce the effectiveness of satiation signals that arise during a meal, a notion consistent with recent data showing that the hippocampus is activated by gastric distention (53) and intragastric nutrients (54). We hypothesized that the increased meal frequency effect was based on VHPC ghrelin signaling augmenting spontaneous feeding episodes that arise in response to the presence of conditioned food-related environmental cues. To examine this possibility, we developed a “cue potentiated feeding” paradigm in which food-restricted rats were trained such that a discrete stimulus signaled meal access (Stimulus+) whereas another stimulus did not (Stimulus-). Other researchers have developed similar paradigms in which food-related cues stimulate excessive food intake in food-sated rats that would not otherwise eat (39, 41, 43, 44). Results showed that VHPC ghrelin increased meal initiation that followed the presentation of the Stimulus+ but not the Stimulus-. These findings provide novel information about the neuroendocrine systems mediating environmental cue-driven feeding. The relevance of these findings to human obesity is underscored by a recent report estimating that a substantial portion of increased per capita caloric intake since the 1970's is based on increased number of eating occasions (meals, snacks) (55), an effect that is potentially influenced by the increased pervasiveness of environmental cues associated with energy dense, rewarding food (56). That GHSR1A activation in the VHPC increased stimulus-induced meal initiation in ad libitum-fed animals suggests a nonhomeostatic function (food intake driven by factors other than metabolic need) for this system. Future work could examine whether this type of cue-driven feeding effect is unique to the VHPC or also involves GHSR signaling in other brain regions thought to control homeostatic (e.g., hypothalamus, brainstem) and nonhomeostatic (VTA) aspects of feeding.
GHSR1A signaling modulates rewarding aspects of feeding in paradigms that assess motivation to obtain palatable food, such as conditioned place preference (3) and PR lever pressing (36, 57). These motivational/reward augmenting effects likely involve altered dopaminergic signaling in the MRS structures as previous findings show that intra VTA ghrelin increases operant responding for sucrose (36) and central or peripheral ghrelin stimulates VTA/NAcc DA signaling [assessed from electrophysiology (12) and microdialysis (12, 28)]. Present results expand knowledge of the reward-associated neural circuitry mediating ghrelin's effects on feeding by showing that, 1) VHPC GHSR1A signaling elevates willingness to work for sucrose in a PR operant lever pressing paradigm, and 2) VHPC ghrelin delivery elevates pTH expression in the NAcc 60min after administration, likely indicating enhanced DA release from local terminals arising from the VTA. These findings are consistent with previous results showing that the VHPC projects directly to the NAcc shell (26, 27), and glutamatergic signaling in the VHPC has an acute stimulatory effect on NAcc DA release (58). The extent that VHPC ghrelin-mediated effects on NAcc DA signaling involve monosynaptic vs. polysynaptic communication is unknown. Further, given that CNS ghrelin signaling modulates the reinforcing properties of other primary reinforcers [e.g., alcohol (59), cocaine (60)], further work is needed to assess whether VHPC ghrelin signaling increases motivation for drugs of abuse. Indeed, several findings link VHPC neuronal activity with behavioral paradigms related to cocaine reward (61, 62).
Our results show that feeding effects triggered by VHPC GHSR1A signaling involve intracellular PI3K-Akt signaling, a phenomenon demonstrated by others for hypothalamic leptin receptor signaling (63, 64). The GHSR1A is a rhodopsin-like G-protein coupled receptor that triggers intracellular second messengers through the activation of Gq proteins (65). Previous studies have shown that AMP-activated protein kinase (AMPK) is activated in the hypothalamus by ghrelin (66, 67). Ghrelin also initiates changes in hypothalamic mitochondrial respiration through uncoupling protein 2 (UCP2) and AMPK-dependent mechanisms (68) and elevates cAMP response-element binding protein (CREB) activity through a protein kinase A (PKA)-dependent mechanism (69). Our focus in the present report was on PI3K-Akt signaling as a recent report demonstrated that ghrelin activates Akt in the dorsal dentate gyrus of the hippocampal formation (DDG) and that enhanced water maze learning by ghrelin was blocked by DDG PI3K inhibition (29). Here, we extend these findings in several ways. Results show that the PI3K-Akt pathway is activated in the VHPC, that this activation is required for the food intake enhancing effects of VHPC-directed ghrelin, and that activation of this pathway is compromised by intake of “Western” diet. Others have demonstrated a similar type of “CNS ghrelin resistance” at the neuronal level [reduced activation of hypothalamic NPY neurons in diet-induced obese (DIO) mice (70)] and at the behavioral level [ghrelin augmented operant PR responding in normal weight but not DIO mice (71)]. Our findings show that diet-induced CNS ghrelin resistance can also occur at the intracellular signaling level. Further study is needed to assess whether other intracellular signaling pathways associated with GHSR1A activity are activated by ghrelin in the VHPC (e.g., AMPK, PKA-CREB) and whether DIO blunts the feeding effects of VHPC GHSR1A signaling, a phenomenon demonstrated for leptin signaling in the hypothalamus (72, 73). Data from Experiment 4 suggest that ghrelin's stimulatory effect on cue-potentiated feeding is preserved under certain conditions of HF diet maintenance. However, these rats were maintained on a HF diet for only ~3 weeks (vs. 4 weeks for Experiment 5a) and were food restricted for 10 of these days. A more systematic evaluation would be necessary before concluding whether the effects of HF diet intake on GHSR1A intracellular PI3K-Akt signaling are correlated with VHPC GHSR “resistance” at the behavioral level.
To our knowledge this is the first report to examine behavioral effects of VHPC GHSR1A activation; however, other researchers have assessed the effects of DHPC ghrelin delivery on various behavioral paradigms. DHPC ghrelin signaling has been shown to improve spatial memory performance in the Morris water maze paradigm (29). Carlini and colleagues reported that DHPC-directed ghrelin improves memory consolidation for aversive reinforcement in a step-down inhibitory avoidance paradigm and that this effect is blocked by co-administration of a serotonin reuptake inhibitor (74-76). These investigators also demonstrated that 1.5 and 3nmol ghrelin delivered to the DHPC significantly increased food intake vs. vehicle treatment (77). This contrasts with our results, as DHPC ghrelin delivery (at doses up to 1.5nmol) had no effect on feeding. The reasons for this discrepancy are not clear but could potentially be based on differences in rat strain (Sprague-Dawley vs. Wistar) or injection volume. Our volume per hemisphere (100nl) was 5-fold lower than the volume these investigators employed. Regardless, our results make a strong case that feeding effects by GHSR1A stimulation in the hippocampus are far more potent with VHPC compared to DHPC delivery.
Overall these findings establish the VHPC as a novel site of importance in the stimulation of food intake and other appetitive/rewarding behaviors by CNS ghrelin signaling. Taken together with our previous work (25), present results support the perspective that the VHPC modulates both anorectic and orexigenic processes related to the higher-order control of food intake through detection and processing of circulating energy status-relevant neuroendocrine signals. Results show that VHPC ghrelin signaling stimulates feeding by increasing the ability of environmental food-related cues to stimulate meal initiation and by increasing motivation to work for palatable food. Other results inform about the intracellular signaling and the downstream neuronal pathways mediating these effects. These findings are relevant to human obesity given the abundance of palatable yet nutritionally deplete foods, as well as the abundance environmental cues that are associated with these foods in modern Western cultures.
Supplementary Material
Figure 4.
Ghrelin delivered to the VHPC in ad libitum rats increased spontaneous meals initiated by a discrete cue that was previously associated with meal access when the rats were food deprived (Stimulus+), whereas this effect was not observed for a cue that was never paired with meal access (Stimulus-). Data are mean ± SEM, * = p < 0.05 vs. all other treatments.
Acknowledgements
The authors thank the following individuals for notable contributions to this work: Dr. Matthew R. Hayes, Amber Alhadeff, Jeffrey Chen, Jennifer Gilbert, Kalina Eneva, Elizabeth Schlesinger, Dr. Bart De Jonghe, Elizabeth Mietlicki-Baase, and Laura Rupprecht. This work was supported by the ECRG grant from The Obesity Society (SEK), and NIH grants DK089752 (SEK) and NIHDK21397 (HJG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 2.Davis JF, Choi DL, Clegg DJ, Benoit SC. Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav. 2011;103:39–43. doi: 10.1016/j.physbeh.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 5.Horvath TL, Castaneda T, Tang-Christensen M, Pagotto U, Tschop MH. Ghrelin as a potential anti-obesity target. Curr Pharm Des. 2003;9:1383–1395. doi: 10.2174/1381612033454748. [DOI] [PubMed] [Google Scholar]
- 6.Horvath TL, Diano S, Tschop M. Ghrelin in hypothalamic regulation of energy balance. Curr Top Med Chem. 2003;3:921–927. doi: 10.2174/1568026033452230. [DOI] [PubMed] [Google Scholar]
- 7.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 8.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 9.Faulconbridge LF, Grill HJ, Kaplan JM, Daniels D. Caudal brainstem delivery of ghrelin induces fos expression in the nucleus of the solitary tract, but not in the arcuate or paraventricular nuclei of the hypothalamus. Brain Res. 2008;1218:151–157. doi: 10.1016/j.brainres.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skibicka KP, Hansson C, Egecioglu E, Dickson SL. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol. 2012;17:95–107. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 2010;15:304–311. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–2279. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Guan X, Yu H, Palyha O, McKee K, Feighner S, Sirinathsinghji D, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 15.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 17.Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313:635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7:613–616. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Kanoski SE. Cognitive and neuronal systems underlying obesity. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. Journal of Comparative Neurology. 2006;497:101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36:1859–1870. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 27.Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- 28.McCallum SE, Taraschenko OD, Hathaway ER, Vincent MY, Glick SD. Effects of 18-methoxycoronaridine on ghrelin-induced increases in sucrose intake and accumbal dopamine overflow in female rats. Psychopharmacology (Berl) 2011;215:247–256. doi: 10.1007/s00213-010-2132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Xing T, Wang M, Miao Y, Tang M, Chen J, et al. Local infusion of through activation 5. ghrelin enhanced hippocampal synaptic plasticity and spatial memory of phosphoinositide 3-kinase in the dentate gyrus of adult rats. Eur J Neurosci. 2011;33:266–275. doi: 10.1111/j.1460-9568.2010.07491.x. [DOI] [PubMed] [Google Scholar]
- 30.Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol. 2008;198:511–521. doi: 10.1677/JOE-08-0160. [DOI] [PubMed] [Google Scholar]
- 31.Hayes MR, Skibicka KP, Bence KK, Grill HJ. Dorsal hindbrain 5'-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology. 2009;150:2175–2182. doi: 10.1210/en.2008-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 33.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 34.Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Azzara AV, Sokolnicki JP, Schwartz GJ. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. doi: 10.1016/s0031-9384(02)00883-1. [DOI] [PubMed] [Google Scholar]
- 38.Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, et al. Leptin Regulates Energy Balance and Motivation Through Action at Distinct Neural Circuits. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weingarten HP. Meal initiation controlled by learned cues: Basic behavioral properties. Appetite. 1984;5:147–158. doi: 10.1016/s0195-6663(84)80035-5. [DOI] [PubMed] [Google Scholar]
- 40.Weingarten HP. Stimulus control of eating: Implications for a two-factor theory of hunger. Appetite. 1985;6:387–401. doi: 10.1016/s0195-6663(85)80006-4. [DOI] [PubMed] [Google Scholar]
- 41.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating.[see comment]. Journal of Neuroscience. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiol Behav. 2007;90:362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic 3. circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weingarten HP, Martin GM. Mechanisms of conditioned meal initiation. Physiol Behav. 1989;45:735–740. doi: 10.1016/0031-9384(89)90287-4. [DOI] [PubMed] [Google Scholar]
- 45.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 46.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 47.Yao L, Fan P, Arolfo M, Jiang Z, Olive MF, Zablocki J, et al. Inhibition of aldehyde dehydrogenase-2 suppresses cocaine seeking by generating THP, a cocaine usedependent inhibitor of dopamine synthesis. Nat Med. 2010;16:1024–1028. doi: 10.1038/nm.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine selfadministration. J Neurosci. 2001;21:RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Lateral ventricular ghrelin and fourth ventricular ghrelin induce similar increases in food intake and patterns of hypothalamic gene expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1565–1569. doi: 10.1152/ajpregu.00785.2005. [DOI] [PubMed] [Google Scholar]
- 50.Faulconbridge LF, Grill HJ, Kaplan JM. Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes. 2005;54:1985–1993. doi: 10.2337/diabetes.54.7.1985. [DOI] [PubMed] [Google Scholar]
- 51.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience & Biobehavioral Reviews. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Min DK, Tuor UI, Chelikani PK. Gastric distention induced functional magnetic resonance signal changes in the rodent brain. Neuroscience. 2011;179:151–158. doi: 10.1016/j.neuroscience.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 54.Min DK, Tuor UI, Koopmans HS, Chelikani PK. Changes in differential functional magnetic resonance signals in the rodent brain elicited by mixed-nutrient or protein-enriched meals. Gastroenterology. 2011;141:1832–1841. doi: 10.1053/j.gastro.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 55.Duffey KJ, Popkin BM. Energy density, portion size, and eating occasions: contributions to increased energy intake in the United States, 1977-2006. PLoS Med. 2011;8:e1001050. doi: 10.1371/journal.pmed.1001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gallo AE. Food advertising in the United States. In: Frazao E, editor. America's eating habits: changes and consequences. United States Department of Agriculture; Washington, DC: 1999. pp. 173–180. [Google Scholar]
- 57.King SJ, Isaacs AM, O'Farrell E, Abizaid A. Motivation to obtain preferred foods is enhanced by ghrelin in the ventral tegmental area. Horm Behav. 2011;60:572–580. doi: 10.1016/j.yhbeh.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–957. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis KW, Wellman PJ, Clifford PS. Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept. 2007;140:148–152. doi: 10.1016/j.regpep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr., Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 65.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 66.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 67.Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- 68.Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuellar JN, Isokawa M. Ghrelin-induced activation of cAMP signal transduction and its negative regulation by endocannabinoids in the hippocampus. Neuropharmacology. 2011;60:842–851. doi: 10.1016/j.neuropharm.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:4745–4755. doi: 10.1210/en.2010-0556. [DOI] [PubMed] [Google Scholar]
- 71.Finger BC, Dinan TG, Cryan JF. Diet-induced obesity blunts the behavioural effects of ghrelin: studies in a mouse-progressive ratio task. Psychopharmacology (Berl) 2012;220:173–181. doi: 10.1007/s00213-011-2468-0. [DOI] [PubMed] [Google Scholar]
- 72.Munzberg H, Bjornholm M, Bates SH, Myers MG., Jr. Leptin receptor action and mechanisms of leptin resistance. Cell Mol Life Sci. 2005;62:642–652. doi: 10.1007/s00018-004-4432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 74.Carlini VP, Gaydou RC, Schioth HB, de Barioglio SR. Selective serotonin reuptake inhibitor (fluoxetine) decreases the effects of ghrelin on memory retention and food intake. Regul Pept. 2007;140:65–73. doi: 10.1016/j.regpep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Carlini VP, Ghersi M, Schioth HB, de Barioglio SR. Ghrelin and memory: differential effects on acquisition and retrieval. Peptides. 2010;31:1190–1193. doi: 10.1016/j.peptides.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 76.Carlini VP, Martini AC, Schioth HB, Ruiz RD, Fiol de Cuneo M, de Barioglio SR. Decreased memory for novel object recognition in chronically food-restricted mice is reversed by acute ghrelin administration. Neuroscience. 2008;153:929–934. doi: 10.1016/j.neuroscience.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 77.Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in egulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochemical Biophysical & Research Communications. 2004;313:635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.