Abstract
Endothelial apoptosis plays a major role in the development of cerebral vascular spasm after subarachnoid hemorrhage (SAH). C/EBP homologous protein (CHOP) orchestrates apoptosis in a variety of cell types in response to endoplasmic reticulum (ER) stress, implicated in the brain injury after SAH. However, the role of CHOP in the mechanism of cerebral vasospasm (CVS) after SAH remains unexplored. The aim of this study was to evaluate the effect of CHOP silencing on endothelial apoptosis and CVS following subarachnoid hemorrhage in the rat. The study was conducted on 65 rats and employed endovascular perforation model of SAH. CHOP siRNAs were injected 24 hrs prior to the hemorrhage. At 72 hours after SAH brains with basilar arteries (BA) were collected from euthanized rats for laboratory investigations. Triple fluorescence stain revealed expression of CHOP in cerebral vascular endothelia after SAH. Marked reduction of CHOP protein and the reduction of its downstream signaling effectors, bim and caspase-3, were found in BA with Western blot analysis. CHOP silencing reduced number of apoptotic endothelial cells in BA, and increased BA diameter after SAH. The amelioration of CVS was associated with reduced neuronal injury in cerebral tissues. In conclusion, CHOP siRNA treatment can effectively combat apoptotic mechanisms of cerebral vasospasm set in motion by subarachnoid bleeding.
Keywords: Apoptosis, bim, bcl-2, C/EBP homologous protein, caspase-3, cerebral vasospasm, siRNA, subarachnoid hemorrhage
Introduction
Apoptosis plays a major role in the development of vasospasm after SAH (Yan et al., 2008; Zhou et al., 2005). Although it had been pointed out that endoplasmic reticulum (ER) stress plays a major role in neurovascular apoptosis, its involvement in the mechanisms of cerebral vasospasm after SAH has not been addressed experimentally (Yan et al., 2008; Zhang et al., 2008). ER stress activates C/EBP homologous protein (CHOP) (Ma et al., 2002), which leads to apoptosis through several mechanisms including downregulation of antiapoptotic B-cell lymphoma-2 (bcl-2) protein (Matsumoto et al., 1996). CHOP dependent apoptosis also requires induction of B cell lymphoma-2 interacting mediator of cell death (bim) – one of the most powerful propapoptotic BH3-only member of bcl-2 family (O’Connor et al., 1998). In a variety of cellular stresses, CHOP acts through direct transcriptional induction of bim (Heath-Engel et al., 2008; Oyadomari and Mori, 2004; Puthalakath et al., 2007). Studies have shown that upon induction of ER stress bim translocates to the ER and promotes activation of caspases (Hetz, 2007). The aim of this study was to assess whether CHOP siRNA, after intracerebroventricular administration (Behlke, 2006) can eliminate CHOP in vascular tissues thus leading to a reduction of endothelial apoptosis and amelioration of cerebral vasospasm after SAH. Regarding the mechanism of treatment we hypothesized that bim, a major CHOP downstream target, is downregulated in the basilar artery in response to CHOP silencing while bcl-2 is overexpressed, which all in concert protect against endothelial cell death.
Material and methods
Animal model of SAH and experimental groups
A total of sixty five male Sprague Dawley rats weighing between 300 and 350 grams were randomly allocated to the following groups: sham surgery (n=10), untreated controls with SAH (n=14, two rats died), scramble siRNA injected group (n=15, three rats died) and rats with either of two sequences CHOP siRNAs injected ipsilaterally - into the left lateral cerebral ventricle at 24 hrs prior to the induction of SAH (siRNA −1 injected group (n=13, one rat died) and siRNA −2 injected group (n=13, one rat died)). SAH was induced by left sided endovascular perforation of middle cerebral artery with 4-0 nylon monofilament. At 72 hrs after surgery all rats were sacrificed to evaluate degree of vasospasm, examine cerebral basilar arteries for apoptosis and expression of CHOP and its downstream targets, and to detect histological injury in cerebral tissues. Basilar artery was collected because it is close to bleeding site and is sensitive to subarachnoid hemorrhage (SAH). Studies have shown that BA vasospasm forms an independent factor of poor neurological outcome in patients with SAH (Sviri et al., 2006). In addition BA is relatively easy to be obtained and the body of BA is big enough for researching. Thus BA appears representative for cerebral vascular spasm after SAH from the clinical standpoint and is very suitable for laboratory investigation techniques.
Animal experiments complied with NIH Guide for the Care and Use of Laboratory Animals and were approved by the Loma Linda University Animal Care and Use Committee.
Treatment with CHOP siRNA
RNAs (Dharmacon) were injected in the sterile PBS intracerebroventricularly (i.c.v.) at a rate of 0.5 microL/min at 24 hrs before SAH. The following coordinates were used: 1.5 mm posterior, 1.0 mm lateral, and 3.2 mm below the horizontal plane of bregma. Irrelevant scrambled RNA was injected as control. siRNA injection site selection was based on the previous protocol with injection coordinates and the data verifying its effectiveness in vascular silencing (He et al., 2012; Suzuki et al., 2010). The intracerebroventricular (ICV) injection was associated with effective CHOP elimination from vascular tissues in the present study, as further demonstrated by Western Blot results. Studies utilizing ICV injections of siRNA after SAH have shown that siRNA can reach molecular targets in cerebral vascular endothelial cells with this approach (Yan et al., 2011). Collectively these data are indicative of high diffusion potential of siRNA administered via ICV route. Either of the two different CHOP siRNAs was administered. The first RNA sequence was: sense, 5′GGAAGAACUAGGAAACGGA; antisense, 5′UCCGUUUCCUAGUUCUUCC. The second siRNA sequence was: sense, 5′ CUGGGAAACAGCGCAUGAA; antisense, 5′ UUCAUGCGCUGUUUCCCAG (He et al., 2012)
Western blot analysis
Cerebral basilar arteries were recovered and homogenized in the RIPA buffer as previously described (Fathali et al., 2010). Protein extracts were resolved with the SDS-PAGE and transferred onto nitrocellulose membranes, which were blocked and subsequently incubated at 4°C overnight with primary antibodies including: CHOP (Millipore) bcl-2, bim (both from Santa Cruz Inc.), and cleaved caspase-3 (Cell Signaling), each diluted 1:500. This step was followed by 2-hr incubation with respective HRP-conjugated secondary antibodies (Santa Cruz; dilution 1:1000), and then by developing chemiluminescence of protein bands with ECL kit (Amersham). Chemiluminescence was recorded on autoradiography films and optical density of protein bands was quantified densitometrically with Image J software (NIH). Densitometry results were normalized to beta-actin and sham group data.
Nissl staining
The rats were euthanized and transcardially perfused with 10% buffered formalin, the brains recovered and postfixed by immersion in the same fixative overnight. Then brain samples were cryoprotected with 30% sucrose in PBS and frozen in liquid nitrogen, and cut into 10 microns thick sections with a cryostat. Nissl staining was done by dipping brain slices in 0.5% cresyl violet for 2 minutes followed by dehydration and coverslipping with Permount. Nissl stain slides were observed under the light microscope (Ostrowski et al., 2005).
Morphometric analysis
Brain sections encompassing basilar artery were stained with hematoxylin and eosin according to the routine protocol (Suzuki et al., 2009). Then histological photographs were serially captured with microscope camera under the final magnification X400. At the predetermined anatomical locations the diameter and thickness of basilar artery were measured using Image J software. Four measurements were used to calculate one score per rat while six rats were used to arrive at the mean values for morphometric parameters of the basilar artery.
Triple fluorescence staining
This combined staining was done as previously described (Fathali et al., 2010). Briefly, histological slices 10 microns thick were subjected to antigen retrieval by microwaving in the citrate buffer at pH 6.0 for 10 minutes. After incubation with blocking serum brain slices were treated with the primary anti-CHOP antibodies (Millipore) and anti Von Willebrand factor (VWF; Santa Cruz Inc.) at the dilution of 1:100 overnight at 4°C. Then primary antibodies were washed out with PBS and the incubation with secondary antibodies (Jackson Immunoresearch Labs), conjugated with AMCA (for CHOP) and Texas Red (for VWF) was carried out for 2 hrs at the room temperature. Primary antibodies were omitted for the negative immunostaining control. Then TUNEL with fluorescein was done on the same sections with a kit as described (Matchett et al., 2007). After coversliping with antifade reagent (Millipore), fluorescence in the brain sections was assessed under Olympus BX51 microscope. Images of histological preparations were captured in three composite channels and merged by means of Magna fire software (Optronics).
TUNEL, DAB
Section were incubated with TUNEL reaction mixture as above, washed in PBS and incubated with anti-fluorescein-POD-conjugates for 30 minutes at room temperature followed by 10 minutes incubation with peroxidase substrate and DAB chromogen. After final wash, sections were dehydrated, cleared in xylenes and coverslipped under Permount.
Cell count
Cell count was conducted on the eight photographs of each analyzed anatomical region per animal, while six rats were included from each group for cell counting analysis (He et al., 2012).
Statistical Analysis
Quantitative data are expressed as means+/-SEM. Statistical significance was verified with ANOVA and Holm-Sidak post-hoc test. Probability level less than 0.05. was determined as significant. Non-parametric ANOVA with Dunn’s test was conducted where appropriate.
Results
CHOP elimination modulates apoptotic protein expression
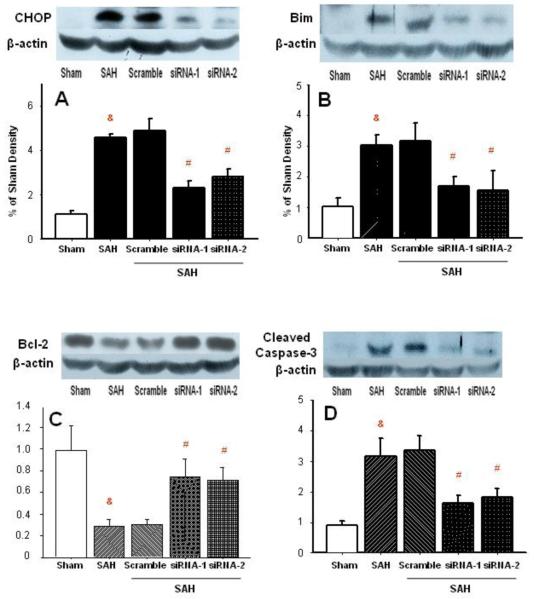
Western blot analysis showed that SAH increased CHOP level more than 4-fold. Scrambled RNA injection did not prevent this CHOP increase in the basilar artery (Fig. 1A). In either of the two siRNA groups CHOP protein increase was over 2-fold, which was significantly less than in the SAH group. In parallel, 3-fold increase in bim protein level of BA was noted in both SAH and scrambled RNA group. This increase was reduced to less than 2-fold with siRNA injections (Fig. 1B). The upregulation of bim expression after SAH was followed by that of the cleaved caspase-3 in BA. However, caspase-3 level was greatly reduced with silencing treatment (Fig. 1D).
Figure 1.
Western blot analysis of apoptotic proteins in the vascular tissues of basilar artery. CHOP protein was effectively eliminated with siRNA (panel A). Suppressed bim protein level accompanied CHOP decrease (panel B). Bcl-2 protein level showed increase in the group treated with CHOP siRNAs (panel C). Reduction of cleaved caspase-3 in BA of rats treated with CHOP siRNAs (panel D). Panels A-D: n=6 in each group (&p<0.05 vs. sham; #p<0.05 vs. SAH, ANOVA).
Bcl-2 protein was significantly reduced after untreated SAH or treated with scrambled RNA (Fig. 1C) to about 20% of control level (sham surgery). CHOP silencing brought partial normalization of bcl-2 levels, to nearly 80% of control. In addition there were no significant differences in bcl-2 levels between sham-operated control and silencing groups, regardless siRNA1 or siRNA2.
CHOP immunoreactivity is co localized with TUNEL in the basilar artery endothelia
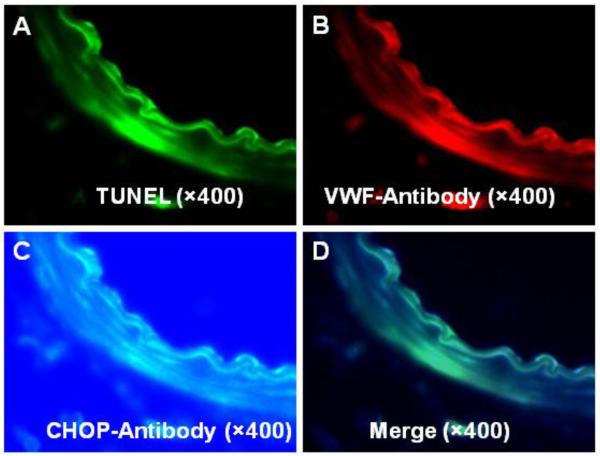
In the representative images of the triple fluorescence stain TUNEL positivity indicated presence of apoptosis (Fig. 2A) that co-localized with Von Willebrand factor fluorescence (Fig. 2B) and with that of CHOP (Fig. 2C), as demonstrated by white color on merged composite images (Fig. 2D).
Figure 2.
Triple fluorescence of TUNEL (panel A), VWF (panel B) and CHOP (panel C) in the basilar artery after SAH. Merged images (panel D) demonstrate that CHOP colocalizes with TUNEL in endothelial cells of the basilar artery. (n=4 sham group; n=6 in other groups).
CHOP siRNA treatment reduces vascular narrowing and apoptosis in the basilar artery after SAH
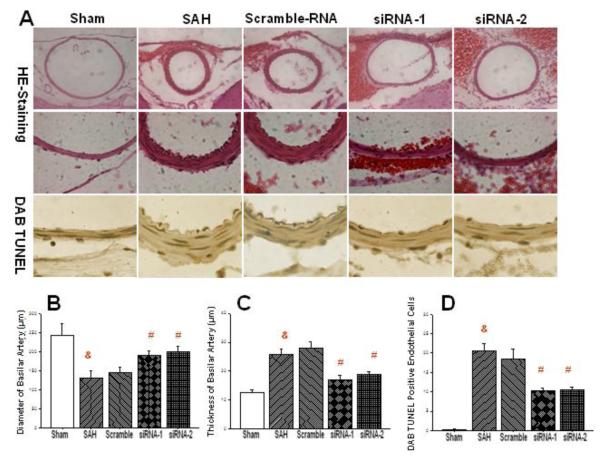
Upper panels in the Fig. 3A contain H&E stain photographs from low and high magnification which demonstrate reduced vascular diameter of basilar artery and the thickening of its vascular wall after SAH, when untreated or treated with scrambled RNA. Administration of siRNAs greatly increased the diameter of BA after SAH. Higher magnification reveals thickening of basilar arteries in SAH and scrambled RNA group but not in the siRNA treated group. The third row of panels in the Fig. 3A reveals marked abundance of TUNEL positive cells in the endothelial layer of basilar artery in the SAH and scrambled treatment groups which was however lessened in both siRNA groups. Bar graphs demonstrate around 50% reduction in the diameter of basilar artery after SAH (Fig. 3B). Scrambled RNA had no significant effect on the diameter of this artery after SAH while siRNA1 or siRNA2 largely restored BA diameter, to around 80% of its pre-SAH length. The thickness of the artery increased about twofold after SAH and even slightly above that in the scrambled RNA group. Significant reduction of the thickness was noted in the siRNA1- and siRNA2-treated rats (Fig. 3C). Quantitative cell count revealed no detectable TUNEL positive cells in the basilar arteries of sham operated rats (Fig. 3D) however the number of cells was around 20 per visual field in the SAH group and in the scrambled RNA group. Significant reduction of the apoptotic cell numbers by around 50% was brought by the treatment with either siRNA1 or siRNA2, with even strength.
Figure 3.
CHOP siRNA ameliorates narrowing of the basilar artery and reduces its thickness after SAH. Top panels show representative photographs of BA cross sections. The graph in the panel B shows a statistically significant reduction of BA diameter after CHOP silencing. Middle panels and a graph in the panel C illustrate reduced thickness of BA with either of siRNA treatments. Bottom row of histological panels show reduction of endothelial apoptosis consequent to CHOP silencing. Cell count analysis confirmed a significant reduction in the number of apoptotic endothelial cells with CHOP elimination (panel D) (&p<0.05 vs. sham; #p<0.05 vs. SAH, ANOVA). (n=4 sham group; n=6 in other groups).
Reduced neuronal injury accompanies amelioration of CVS with CHOP elimination
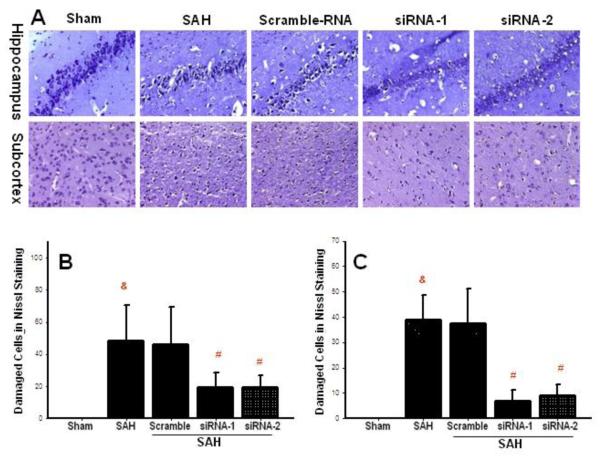
Representative photographs of Nissl stain show extensive damage of neuronal cells in the CA1 of the hippocampus and in the subcortical brain region on day 3 after SAH. In both structures cellular loss and presence of numerous dark neurons can be found (Fig. 4A). With siRNA treatment, however, only scarce dark neurons can be seen in the hippocampus (upper panels) and in the subcortex (lower panels). In the hippocampus the number of damaged cells exceeded 40 per visual field (Fig. 4B) after SAH and SAH with scramble RNA injection. Numbers of damaged cells were reduced to less than 20 in the two siRNA groups, which produced a significant difference with respect to SAH. In the subcortex cell count revealed nearly 40 damaged cells per visual field on average (Fig. 4C). Scrambled RNA had no effect on the number of damaged cells. In contrast CHOP siRNA injection very effectively reduced number of damaged cells in the subcortex, to less than 10 cells per visual field (Fig. 4C).
Figure 4.
Reduced neuronal injury accompanies reduced vasospasm with CHOP siRNA treatment. Representative photographs (panel A) and quantitative cell count in the hippocampus (panel B) and in the subcortex (panel C) show reduced number of damaged neurons with the treatment (&p<0.05 vs. sham; #p<0.05 vs. SAH, ANOVA). (n=4 sham group; n=6 in other groups).
Discussion
In the present study we have demonstrated elevated levels of CHOP in the basilar artery after SAH. CHOP silencing, however, by using two different siRNA sequences, effectively eliminated SAH-induced CHOP elevation in the vascular tissues. CHOP elimination was associated with reduction of bim and cleaved caspase-3 while it raised bcl-2 levels in basilar arteries of siRNA-treated rats. CHOP silencing resulted in the increased diameter, reduced thickness and lessened number of apoptotic cells in the vascular endothelia together with a reduction of neuronal damage in the hippocampus and subcortex.
Novelty of this work is several-fold. It embarks on revealing the role of CHOP and bim in mediating vascular endothelial apoptosis leading to arterial narrowing after SAH. To our knowledge this is the first study demonstrating such involvement. The significance of the present work is further supported by demonstrating that CHOP elimination targets mechanisms of CVS after SAH, which implicates viable therapeutic opportunities. In addition, this study shows a suitability of siRNA in targeting mediators of apoptosis in cerebral vasculature after SAH. Owing to this approach the study pioneers the effective use of siRNA against cerebral vasospasm following SAH. Only recently siRNA has been used as a molecular intervention in SAH but only to verify the role of mitogen-activated protein kinase (MAPK) phosphatase (MKP)-1 pathway in the anti-vasospastic effect of recombinant osteopontin (Suzuki et al., 2010).
Although the involvement of bcl-2 depletion and activation of caspase-3 in the mechanisms of apoptosis in the brain after SAH have been pointed out by earlier authors, the role of CHOP and bim has remained unexplored (Cheng et al., 2009; Yan et al., 2008). In the apoptotic machinery CHOP induces bim and represses bcl-2 (McCullough et al., 2001; Szegezdi et al., 2009). Consequently, in the brain vessels with CHOP siRNA treatment bim was reduced while bcl-2 rebounded, which resulted in the antiapoptotic effect. Bcl-2 can play antiapoptotic role at the level of miotochondria and ER where it reduces Ca2+ content or, alternatively, it blocks Ca2+ release upon cellular stress (Szegezdi et al., 2009). Furthermore overexpression of bcl-2 reduces capacitative calcium entry, the important mechanism of vasospasm alone (Zuccarello et al., 1996). The increased bcl-2 has been shown to reduce endothelial apoptosis and cerebral vasospasm in the rabbit model of SAH treated with EPO (Chen et al., 2009). However in order to fully demonstrate the role of CHOP elimination on the apoptosis in the hemorrhagic brain, other antiapoptotic proteins need to be examined, such as bcl-xl and bcl-w to which CHOP effector bim can bind as well (O’Connor et al., 1998). In addition the effect of CHOP elimination on bax and bak levels also warrants investigation (Gotoh et al., 2004). Nevertheless bim is considered to be one the most potent inducers of apoptosis amongst bcl-2 family, and has been linked to CHOP, hence investigated in this study (O’Connor et al., 1998). In accordance with the notion that apoptotic pathway underlying CVS ultimately relies on effector caspases, we observed elevated cleaved caspase-3 in the basilar arteries although it was reduced with CHOP siRNA treatment.
Through these mechanisms CHOP elimination could reduce apoptosis in the endothelia of cerebral vessels. Others have reported that targeted CHOP gene disruption potently reduces apoptosis in response to ER stress (Oyadomari and Mori, 2004). CHOP elimination with RNA interference also has been used by earlier authors to reduce cell death in a variety of cultured cells (Blaschke et al., 2004; Endo et al., 2007). As for the present study, following the salvage of endothelial cells from apoptotic death, the exposure to spasmodic substances in blood might has been reduced and thereby vasospasm could have been relieved in this mechanism. Indeed, we observed increased diameter of basilar artery in the treatment group. Noticeably, the thickening of arterial wall has been reduced significantly with the treatment despite thick perivascular blood deposits suggesting that the effect of CHOP siRNA did not depend on the favorable blood distribution or augmented blood clearance from the subarachnoid space in treatment group rats.
In addition this study strongly suggests that ER stress, as a main CHOP inducer, plays a major role in the development of CVS. ER stress in cerebral vessels after SAH may be initiated through several triggers including ROS and inflammatory cytokines (Zhang and Kaufman, 2008), which have been long known to be associated with development of CVS after SAH (Sercombe et al., 2002). However, it has been known that detrimental sequealae of CVS are enhanced by increased vulnerability of the brain that depends on the severity of primary hemorrhage (Frykholm et al., 2004). Especially because we administered CHOP prior to injury there is a possibility that reduced CVS could be at least in part due to alleviation of acute impact of SAH. On the other hand CHOP elimination specifically from vascular compartment could primarily trigger the reduction of CVS and consequently ameliorate neuronal injury. Thus, based on this study it cannot be ruled out that overall beneficial outcome stems also from interactions between early neuronal injury and CVS reductions with CHOP siRNA.
Alongside with the histologically confirmed CVS, in the Nissl stained preparations we have found damage in the subcortex and in the hippocampus, which forms a pattern of injury fairly consistent with the presence of cerebrovascular narrowing. It is known for the fact that cerebral vascular spasm, when abnormally prolonged, results in cortical and subcortical lesions and the hippocampal injury as well (Bendel et al., 2006). Noticeably, these changes were reversed with alleviation of vasospasm after CHOP siRNA administration.
Although siRNA based therapeutics remain in the nascent state a few instances in literatures point towards amelioration of behavioral deficits induced with siRNA treatments in cerebrovascular diseases including SAH (Li et al., 2012; Ma et al., 2011; Yan et al., 2011). Studies of siRNA for vasospasm after SAH are lacking, however. It has been proven by several authors that amelioration of CVS results in improved neurobehavior (Jeon et al., 2009). However endovascular perforation model of SAH is not very well suited to evaluate CVS-induced neurological deficit, due to overlapping neurological worsening caused by early neuronal injury. Thus the major strength of this present study stems from demonstrating that siRNA can reduce histological vasospasm after SAH. However it will be worth a while to test the effect of CHOP siRNA on arterial spasm-induced neurological deficit in an optimized animal model, such as rat double hemorrhage that exhibited significant neurobehavioral deficits even when minimal proximal large artery vasospasm was present (Jeon et al., 2009; Takata et al., 2008). Notably, in the study of early brain injury after SAH, also evaluating CHOP siRNA, we have found the acute beneficial effect of silencing on neurobehavior (He et al., 2012).
Although present findings may offer a new promise for clinical management of SAH, this study takes only one step towards translation. Indeed, several hurdles to clinical applicability of siRNA are ahead. In order to combat prolonged and insidious fashion of vasospasm formation, a slow release formulation of silencer may be needed. Clinical efficacy may suffer from difficulties in penetration of siRNA across biological membranes and hemorrhagic compartments as well. It seems that in order to overcome afore mentioned drawbacks of siRNA in the clinics, advancing technology of nanoparticle-encapsulated siRNA appears desirable (Lobovkina et al., 2011).
Thus the present study supports the effectiveness of intracerebroventricular route of siRNA administration for reaching targets in the cerebral vasculature, which results in neuroprotection, and as such, it carries implications for developing novel therapies of SAH. This present study tested the mechanisms of siRNA that targets particular mediator of endothelial damage leading to CVS after SAH. However for future therapy development there is a need of further experiments aimed at determination of CHOP expression profiles, posttreatment efficacy, clinical safety and lasting effects of CHOP elimination after SAH.
Conclusions
CHOP silencing reduces arterial narrowing after subarachnoid hemorrhage which results in histological protection. The mechanisms of vasospasm reduction involves a decrease in endothelial apoptosis owing to elimination of CHOP and its proapoptotic targets while enhancing the expression of bcl-2 in cerebrovascular tissues. The results of our study substantiate the concept of combating vasospasm with siRNA-based therapy.
Highlights maximum 85 characters per bullet point.
The study uses CHOP siRNA approach to reduce cerebral vasospasm after SAH.
CHOP elimination suppressed bim, while it upregulated bcl-2 in the in vascular tissues.
Endothelial apoptosis and narrowing of the basilar artery were reduced with siRNA.
Amelioration of vasospasm was accompanied by reduced neuronal damage in the hemorrhagic brain.
Acknowledgements
The study was partially supported by NIHNS53407 to John H. Zhang.
Abbreviations
- (BA)
basilar artery
- (bcl-2)
B-cell lymphoma-2
- (bim)
B cell lymphoma-2 interacting mediator of cell death
- (CHOP)
C/EBP homologous protein
- (CVS)
cerebral vasospasm
- (ER)
endoplasmic reticulum
- (SAH)
subarachnoid hemorrhage
- (siRNA)
small interfering ribonucleic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Behlke MA. Progress towards in vivo use of siRNAs. Mol. Ther. 2006;13:644–670. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendel P, Koivisto T, Hanninen T, Kolehmainen A, Kononen M, Hurskainen H, Pennanen C, Vanninen R. Subarachnoid hemorrhage is followed by temporomesial volume loss: MRI volumetric study. Neurology. 2006;67:575–582. doi: 10.1212/01.wnl.0000230221.95670.bf. [DOI] [PubMed] [Google Scholar]
- Blaschke F, Bruemmer D, Yin F, Takata Y, Wang W, Fishbein MC, Okura T, Higaki J, Graf K, Fleck E, Hsueh WA, Law RE. C-reactive protein induces apoptosis in human coronary vascular smooth muscle cells. Circulation. 2004;110:579–587. doi: 10.1161/01.CIR.0000136999.77584.A2. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang S, Shi J, Ai J, Hang C. Effects of recombinant human erythropoietin (rhEPO) on JAK2/STAT3 pathway and endothelial apoptosis in the rabbit basilar artery after subarachnoid hemorrhage. Cytokine. 2009;45:162–168. doi: 10.1016/j.cyto.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Cheng G, Wei L, Zhi-Dan S, Shi-Guang Z, Xiang-Zhen L. Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci. 2009;10:7. doi: 10.1186/1471-2202-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Murata K, Mukai M, Ishikawa O, Inoue M. Activation of insulin-like growth factor signaling induces apoptotic cell death under prolonged hypoxia by enhancing endoplasmic reticulum stress response. Cancer Res. 2007;67:8095–8103. doi: 10.1158/0008-5472.CAN-06-3389. [DOI] [PubMed] [Google Scholar]
- Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38:572–578. doi: 10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykholm P, Andersson JL, Langstrom B, Persson L, Enblad P. Haemodynamic and metabolic disturbances in the acute stage of subarachnoid haemorrhage demonstrated by PET. Acta Neurol. Scand. 2004;109:25–32. doi: 10.1034/j.1600-0404.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Terada K, Oyadomari S, Mori M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, Zhang JH. CHOP Silencing Reduces Acute Brain Injury in the Rat Model of Subarachnoid Hemorrhage. Stroke. 2012;43:484–490. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath-Engel HM, Chang NC, Shore GC. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene. 2008;27:6419–6433. doi: 10.1038/onc.2008.309. [DOI] [PubMed] [Google Scholar]
- Hetz CA. ER stress signaling and the BCL-2 family of proteins: from adaptation to irreversible cellular damage. Antioxid. Redox Signal. 2007;9:2345–2355. doi: 10.1089/ars.2007.1793. [DOI] [PubMed] [Google Scholar]
- Jeon H, Ai J, Sabri M, Tariq A, Shang X, Chen G, Macdonald RL. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci. 2009;10:103. doi: 10.1186/1471-2202-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Khatibi NH, Hu Q, Yan J, Chen C, Han J, Ma D, Chen Y, Zhou C. Transmembrane protein 166 regulates autophagic and apoptotic activities following focal cerebral ischemic injury in rats. Exp. Neurol. 2012;234:181–190. doi: 10.1016/j.expneurol.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Lobovkina T, Jacobson GB, Gonzalez-Gonzalez E, Hickerson RP, Leake D, Kaspar RL, Contag CH, Zare RN. In vivo sustained release of siRNA from solid lipid nanoparticles. ACS Nano. 2011;5:9977–9983. doi: 10.1021/nn203745n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J. Cereb. Blood Flow Metab. 2011;31:881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchett GA, Calinisan JB, Matchett GC, Martin RD, Zhang JH. The effect of granulocyte-colony stimulating factor in global cerebral ischemia in rats. Brain Res. 2007;1136:200–207. doi: 10.1016/j.brainres.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Minami M, Takeda K, Sakao Y, Akira S. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett. 1996;395:143–147. doi: 10.1016/0014-5793(96)01016-2. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Sercombe R, Dinh YR, Gomis P. Cerebrovascular inflammation following subarachnoid hemorrhage. Jpn. J. Pharmacol. 2002;88:227–249. doi: 10.1254/jjp.88.227. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH. Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann. Neurol. 2010;68:650–660. doi: 10.1002/ana.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Sozen T, Hasegawa Y, Chen W, Zhang JH. Caspase-1 inhibitor prevents neurogenic pulmonary edema after subarachnoid hemorrhage in mice. Stroke. 2009;40:3872–3875. doi: 10.1161/STROKEAHA.109.566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviri GE, Newell DW, Lewis DH, Douville C, Ghodke B, Chowdhary M, Lam AM, Haynor D, Zaaroor M, Britz GW. Impact of basilar artery vasospasm on outcome in patients with severe cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2006;37:2738–2743. doi: 10.1161/01.STR.0000244765.29502.85. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Macdonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am. J. Physiol. Cell Physiol. 2009;296:C941–953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- Takata K, Sheng H, Borel CO, Laskowitz DT, Warner DS, Lombard FW. Long-term cognitive dysfunction following experimental subarachnoid hemorrhage: new perspectives. Exp. Neurol. 2008;213:336–344. doi: 10.1016/j.expneurol.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Yan JH, Yang XM, Chen CH, Hu Q, Zhao J, Shi XZ, Luan LJ, Yang L, Qin LH, Zhou CM. Pifithrin-alpha reduces cerebral vasospasm by attenuating apoptosis of endothelial cells in a subarachnoid haemorrhage model of rat. Chin. Med. J. 2008;121:414–419. [PubMed] [Google Scholar]
- Yan J, Li L, Khatibi NH, Yang L, Wang K, Zhang W, Martin RD, Han J, Zhang J, Zhou C. Blood-brain barrier disruption following subarchnoid hemorrhage may be faciliated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Exp. Neurol. 2011;230:240–247. doi: 10.1016/j.expneurol.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Q, Li Z, Mei Y, Guo Y. The protection of Bcl-2 overexpression on rat cortical neuronal injury caused by analogous ischemia/reperfusion in vitro. Neurosci. Res. 2008;62:140–146. doi: 10.1016/j.neures.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Yamaguchi M, Colohan AR, Zhang JH. Role of p53 and apoptosis in cerebral vasospasm after experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2005;25:572–582. doi: 10.1038/sj.jcbfm.9600069. [DOI] [PubMed] [Google Scholar]
- Zuccarello M, Boccaletti R, Tosun M, Rapoport RM. Role of extracellular Ca2+ in subarachnoid hemorrhage-induced spasm of the rabbit basilar artery. Stroke. 1996;27:1896–1902. doi: 10.1161/01.str.27.10.1896. [DOI] [PubMed] [Google Scholar]