Abstract
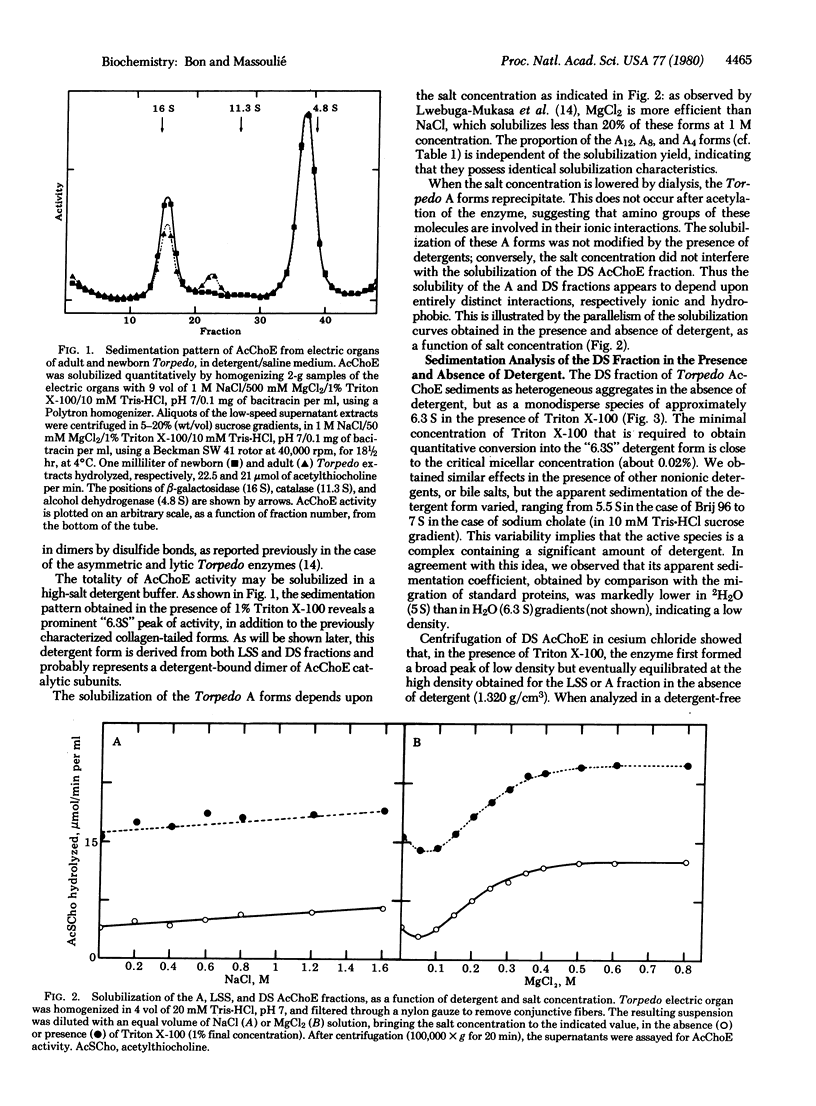
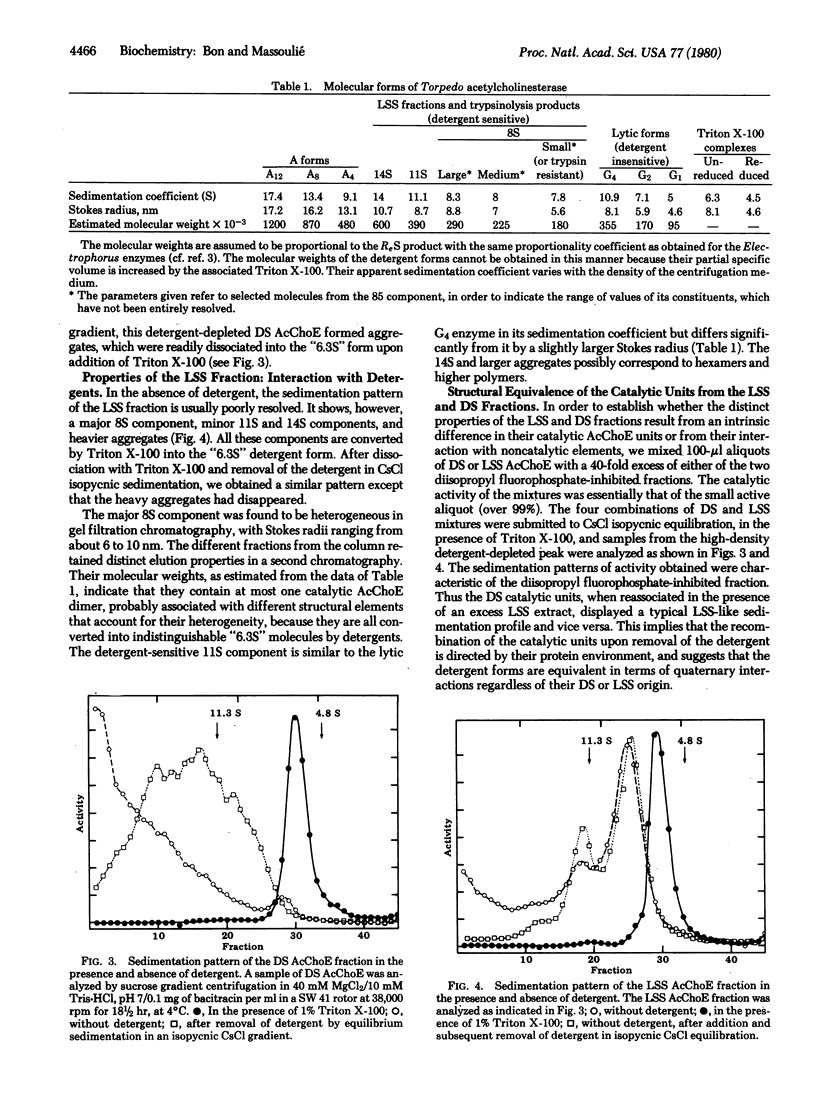
We have distinguished three fractions of acetylcholinesterase (AcChoE; acetylcholine acetylhydrolase, EC 3.1.1.7) from Torpedo marmorata electric organs, according to their solubilization characteristics. The low-salt-aggregating collagen-tailed forms are soluble in high-salt buffers; their hydrodynamic properties ae not modified in the presence of detergents. They constitute the A fraction, which amounts to about a third of the tissue's AcChoE activity. The low-salt-soluble (LSS) and detergent-soluble (DS) fractions are not sensitive to ionic strength and collagenase. In the presence of nonionic detergents or bile salts, both fractions behave as a monodisperse "6.3S" form, the properties of which have been investigated mostly in the case of Triton X-100. Disulfide bond reduction dissociates the detergent form into a smaller "5S" form. These two forms are thought to be, respectively, detergent-associated dimers and monomers. In the absence of detergent, the LSS fraction is polydisperse: it contains a major 8S component, 11S and 14S components, and faster-sedimenting aggregates, which appear to represent dimers, tetramers, and higher polymers. The heterogeneity of the 8S component in gel filtration suggests that it also contains variable noncatalytic elements. Upon removal of the detergent the DS fraction forms ill-defined aggregates. Trypsin induces quaternary rearrangements of part of the 8S component into 11S and 14S components, which are still convertible into the detergent form; therefore trypsin probably digests noncatalytic elements. Pronase and proteinase K, on the other hand, convert the enzyme into a dimeric form, G2, that does not interact with detergents, probably by cleaving a minor fragment of the subunit that is involved in hydrophobic interactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglister L., Silman I. Molecular structure of elongated forms of electric eel acetylcholinesterase. J Mol Biol. 1978 Nov 5;125(3):293–311. doi: 10.1016/0022-2836(78)90404-7. [DOI] [PubMed] [Google Scholar]
- Bon S., Cartaud J., Massoulié J. The dependence of acetylcholinesterase aggregation at low ionic strength upon a polyanionic component. Eur J Biochem. 1978 Apr;85(1):1–14. doi: 10.1111/j.1432-1033.1978.tb12207.x. [DOI] [PubMed] [Google Scholar]
- Bon S., Huet M., Lemonnier M., Rieger F., Massoulié J. Molecular forms of Electrophorus acetylcholinesterase. Molecular weight and composition. Eur J Biochem. 1976 Sep 15;68(2):523–530. doi: 10.1111/j.1432-1033.1976.tb10840.x. [DOI] [PubMed] [Google Scholar]
- Bon S., Massoulié J. Collagenase sensitivity and aggregation properties of Electrophorus acetylcholinesterase. Eur J Biochem. 1978 Aug 15;89(1):89–94. doi: 10.1111/j.1432-1033.1978.tb20899.x. [DOI] [PubMed] [Google Scholar]
- Bon S., Vigny M., Massoulié J. Asymmetric and globular forms of acetylcholinesterase in mammals and birds. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2546–2550. doi: 10.1073/pnas.76.6.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson S., Bon S., Vigny M., Massoulié J., Fardeau M. Distribution of acetylcholinesterase molecular forms in neural and non-neural sections of human muscle. FEBS Lett. 1979 Jan 15;97(2):348–352. doi: 10.1016/0014-5793(79)80119-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Weber M., Huchet M., Changeux J. P. Purification from Torpedo marmorata electric tissue of membrane fragments particularly rich in cholinergic receptor protein. FEBS Lett. 1972 Oct 1;26(1):43–47. doi: 10.1016/0014-5793(72)80538-6. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Raftery M. A. Fractionation and partial characterization of membrane particles from Torpedo californica electroplax. Biochemistry. 1973 Sep 11;12(19):3593–3597. doi: 10.1021/bi00743a003. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Hall E. R., Brodbeck U. Human erythrocyte membrane acetylcholinesterase. Incorporation into the lipid bilayer structure of liposomes. Eur J Biochem. 1978 Aug 15;89(1):159–167. doi: 10.1111/j.1432-1033.1978.tb20908.x. [DOI] [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Lappi S., Taylor P. Molecular forms of acetylcholinesterase from Torpedo californica: their relationship to synaptic membranes. Biochemistry. 1976 Apr 6;15(7):1425–1434. doi: 10.1021/bi00652a012. [DOI] [PubMed] [Google Scholar]
- McCann W. F., Rosenberry T. L. Identification of discrete disulfide-linked oligomers which distinguish 18 S from 14 S acetylcholinesterase. Arch Biochem Biophys. 1977 Sep;183(1):347–352. doi: 10.1016/0003-9861(77)90449-0. [DOI] [PubMed] [Google Scholar]
- Millar D. B., Christopher J. P., Burrough D. O. Evidence that eel acetylcholinesterase is not an integral membrane protein. Biophys Chem. 1978 Nov;9(1):9–14. doi: 10.1016/0301-4622(78)87010-0. [DOI] [PubMed] [Google Scholar]
- Morel N., Israel M., Manaranche R., Mastour-Frachon P. Isolation of pure cholinergic nerve endings from Torpedo electric organ. Evaluation of their metabolic properties. J Cell Biol. 1977 Oct;75(1):43–55. doi: 10.1083/jcb.75.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott P., Brodbeck U. Multiple molecular forms of acetylcholinesterase from human erythrocyte membranes. Interconversion and subunit composition of forms separated by density gradient centrifugation in a zonal rotor. Eur J Biochem. 1978 Jul 17;88(1):119–125. doi: 10.1111/j.1432-1033.1978.tb12428.x. [DOI] [PubMed] [Google Scholar]
- Ott P., Jenny B., Brodbeck U. Multiple molecular forms of purified human erythrocyte acetylcholinesterase. Eur J Biochem. 1975 Sep 15;57(2):469–480. doi: 10.1111/j.1432-1033.1975.tb02322.x. [DOI] [PubMed] [Google Scholar]
- Rieger F., Bon S., Massoulié J., Cartauld J., Picard B., Benda P. Torpedo marmorata acetylcholinesterase; a comparison with the Electrophorus electricus enzyme. Molecular forms, subunits, electron microscopy, immunological relationship. Eur J Biochem. 1976 Sep 15;68(2):513–521. doi: 10.1111/j.1432-1033.1976.tb10839.x. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L. Acetylcholinesterase. Adv Enzymol Relat Areas Mol Biol. 1975;43:103–218. doi: 10.1002/9780470122884.ch3. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Richardson J. M. Structure of 18S and 14S acetylcholinesterase. Identification of collagen-like subunits that are linked by disulfide bonds to catalytic subunits. Biochemistry. 1977 Aug 9;16(16):3550–3558. doi: 10.1021/bi00635a008. [DOI] [PubMed] [Google Scholar]
- Vigny M., Bon S., Massoulié J., Gisiger V. The subunit structure of mammalian acetylcholinesterase: catalytic subunits, dissociating effect of proteolysis and disulphide reduction on the polymeric forms. J Neurochem. 1979 Aug;33(2):559–562. doi: 10.1111/j.1471-4159.1979.tb05188.x. [DOI] [PubMed] [Google Scholar]
- Vigny M., Gisiger V., Massoulié J. "Nonspecific" cholinesterase and acetylcholinesterase in rat tissues: molecular forms, structural and catalytic properties, and significance of the two enzyme systems. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2588–2592. doi: 10.1073/pnas.75.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]



